Among new patients entering HIV care from 1999 to 2009 in a North Carolina observational clinical cohort, Latinos initiated HIV care at lower CD4 cell counts and were more likely to have several specific AIDS-defining clinical conditions, compared with non-Latinos.
Abstract
(See the Editorial Commentary by Rio on pages 488–489.)
Background. Late diagnosis of human immunodeficiency virus (HIV) infection remains common despite advances in therapy and prognosis. The southeastern United States is a rapidly growing Latino settlement area where ethnic disparities may contribute to late presentation to care.
Methods. We assessed demographic and clinical factors between racial/ethnic groups at the time of HIV care initiation in the University of North Carolina Center for AIDS Research Clinical Cohort. We identified independent predictors of late presentation, defined as a CD4+ T lymphocyte (CD4) count <350 cells/mm3 or an AIDS-defining event (ADE), using log-linear binomial regression.
Results. During the period 1999–2009, 853 patients initiated HIV care, of whom 11% were Latino, 28% were white, and 61% were black. Median initial CD4 counts were lower for Latino patients (186 cells/mm3) than white patients (292 cells/mm3; P = .006) and black patients (302 cells/mm3; P = .02). Latino persons were more likely to be late presenters than white or black persons (76% vs 58%; P < .001) and accounted for 86%, 75%, and 50% of all presenting cases of active tuberculosis, histoplasmosis, and toxoplasmosis, respectively. Latino ethnicity, older age, male sex, and earlier entry year were independently associated with late presentation (P < .05 for all). In multivariable analyses, Latino persons were 1.29 times more likely to present to care late than white or black persons (95% confidence interval, 1.15–1.45).
Conclusions. Latinos are more likely to initiate HIV care later in the course of illness than are black and white persons and account for a majority of several ADEs. Strategies to improve earlier HIV testing among Latinos in new settlement areas are needed.
The importance of early diagnosis of human immunodeficiency virus (HIV) infection is highlighted by the profound reductions in morbidity and mortality with the receipt of highly active antiretroviral therapy (HAART) [1, 2] as well as the decreased opportunity for onward HIV transmission. Unfortunately, presentation late in the course of infection remains common [3, 4]. The epidemiology of HIV infection in the United States is also characterized by significant racial and ethnic disparities. US surveillance data from 1996 to 2005 indicated that 42% of Latino persons develop AIDS ≤1 year after diagnosis of HIV infection, compared with 39% of black persons and 37% of white persons [3]. However, these small national differences may not fully reflect the epidemiology of HIV infection among Latinos in specific regions because of the heterogeneity of the Latino population, which is the fastest growing US minority group, with origins in >20 countries. Notably, HIV risk behaviors, diagnostic rates, and interval progression from HIV diagnosis to AIDS among Latinos differ by country of birth [5].
Nontraditional Latino settlement areas, such as the southeastern United States, have seen rapid Latino population growth over the past 20 years [6, 7]. North Carolina experienced a 394% increase in the Latino population from 1990 to 2000 [6] and had the seventh largest population of Mexican-born immigrants among all US states in 2008 [8]. Latinos residing in rapid-growth southern states are more likely to be male, young, and foreign born and to have arrived in the United States after 1995, compared with states with long-established Latino communities, such as California and New York [6]. This population may be at increased risk for HIV infection because recent immigrants may lack social support systems and stable sexual networks that are found in traditional settlement areas [9]. Interrelated barriers to HIV prevention, such as culture, migration, poverty, and limited use of English [10], likely also influence testing and timing of presentation to care.
Understanding disparities at initial entry to HIV care among Latinos or other racial/ethnic groups in areas of rapid Latino growth is important to develop directed public health interventions. We compared demographic characteristics, transmission risk behaviors, and clinical factors among Latinos and non-Latinos at first presentation to HIV care in a nontraditional Latino settlement area and evaluated potential factors associated with late presentation to HIV care.
METHODS
Patients and Design
We performed a cross-sectional evaluation of adults and adolescents naive to HIV care who initiated HIV care for the first time during the period from 1 January 1999 through 31 December 2009 at the University of North Carolina (UNC) Infectious Diseases Clinic, located in a large, tertiary care facility in central North Carolina, and who participated in the UNC Center for AIDS Research HIV Clinical Cohort (UCHCC). An estimated 98% of HIV-infected patients in the clinic participate in the UCHCC, providing an accurate representation of the HIV clinic population. To enroll in the UCHCC, participants must be aged ≥18 years and must provide written informed consent in English or Spanish. Adolescents who enter care in the clinic can enroll in the UCHCC when they reach 18 years of age. This study was approved by the UNC Biomedical Institutional Review Board.
Variables
Demographic, risk behavior, and clinical data were abstracted from medical records. The UCHCC has standardized data extraction methods from medical charts and institutional databases, as described elsewhere [11]. Medical information, including the earliest date of HIV-positive test results and prior illnesses, were extracted from UNC. Charts were reviewed to ensure accuracy of race/ethnicity data in our medical records. Geographic area of residence at entry was estimated using the zip code approximation of Rural-Urban Commuting Area Codes, a 2000 census-tract classification that uses urbanized area/cluster definitions of core population size with work commuting information to determine rural and urban status [12, 13]. We placed patients in 3 categories: urban, large rural/town (“micropolitan”), or small and isolated rural. Driving distances to clinic were estimated by calculating the distance from UNC to the centroid of patient residence zip codes using CDX Zipstream and Microsoft MapPoint 2010 software (Microsoft). HIV transmission risk groups were categorized as men who have sex with men (MSM), heterosexual, injection drug user (IDU), and persons with other or unknown risk. Patients who could be categorized as both IDU and MSM or as IDU and heterosexual were categorized as IDU.
Outcomes and Definitions
Primary outcomes were the CD4+ T lymphocyte (CD4) count, obtained closest to and within 90 days of entering care at UNC, and AIDS-defining events (ADEs) at entry. We defined late presenters as patients who had CD4 counts <350 cells/mm3 or ADEs [14]. The subset of these patients who had CD4 counts <200 cells/mm3 or ADEs at entry were defined as advanced presenters. Advanced presenters whose first HIV-positive test results occurred <6 months before entry to HIV care were classified as delayed testers. HIV RNA loads were only included if available within 90 days of entry to care and within 2 weeks after commencement of HAART. The ADEs were categorized on the basis of the Centers for Disease Control and Prevention 1993 classification system [15] and included conditions diagnosed ≤60 days after study entry.
Statistical Analyses
Differences in categorical variables by race/ethnicity and sex were examined with the Pearson’s χ2 test, and continuous variables were evaluated with the Kruskal-Wallis test. We calculated unadjusted prevalence ratios (PRs) and associated 95% confidence intervals (CIs) for the prevalence of late presentation by race/ethnicity, as well as other possible predictors of late care presentation. Multivariable analyses were fit using log-linear binomial regression to identify independent predictors of late presentation. We first fit a full model based on results of our bivariable analyses and then used backwards elimination to arrive at a final model that included only factors predictive of late care presentation on the basis of a 2-sided α of .05. We evaluated the following possible predictors in addition to race/ethnicity: age, sex, distance to care, urban/rural residence, transmission risk group, and year entering care. PRs were calculated instead of odds ratios to avoid overestimation of the relative risk because our outcome was common (>10%) [16]. All data were analyzed using SAS software, version 9.2 (SAS Institute).
RESULTS
We identified 1256 patients who entered HIV care for the first time during the period from 1 January 1999 through 31 December 2009, of whom 876 had their entry to care at UNC and thus had comprehensive initial data for analysis. Twenty-three patients were then excluded because of unknown race or a race/ethnicity other than black, white, or Latino. Our analysis was performed on these 853 patients, of whom 61% were black, 28% were white, and 11% were Latino, with a median age of 36 years. The 380 patients who had initiated care outside of the UNC system had similar demographic characteristics, although only 5% of these patients were Latino. Among the 853 patients in the analysis cohort, we did not find statistically significant differences in the racial/ethnic composition over the 11-year period; however, there were significant differences among these groups with respect to demographic characteristics and transmission risk at entry to care (Table 1). Latinos were significantly more likely to be younger than black and white persons, with a median age of 30 years (interquartile range [IQR], 26–37 years) for Latinos versus 36 years (IQR, 28–45 years) for black persons (P < .001) and 40 years (IQR, 30–46 years) for white persons (P < .001). More than 80% of patients entered care within 6 months of their reported date of diagnosis of HIV infection; however, black persons were more likely to have delayed entry by >6 months than were nonblack persons (20% vs 13%; P = .01). Black persons and Latinos had a similar breakdown of transmission risk group, with heterosexual risk predominating (54% and 60%, respectively), which differed significantly from white persons, among whom MSM risk predominated (53%; overall P < .001). Although the majority of our cohort resided in urban areas (64%), Latinos were more likely than black and white persons to live in rural areas (48%; overall P = .02).
Table 1.
Demographic and Risk Behavior Characteristics of HIV-Infected Individuals Entering HIV Care Between 1999 and 2009
| Race/ethnicity |
||||
| Variable | Latino | White | Black | P a |
| No. (%) of subjects | 97 (11) | 236 (28) | 520 (61) | |
| Year entering care | .06 | |||
| 1999–2001 | 31 (32) | 72 (31) | 209 (40) | |
| 2002–2005 | 31 (32) | 83 (35) | 169 (33) | |
| 2006–2009 | 35 (36) | 81 (34) | 142 (27) | |
| Median age, years bcd (IQR) | 30 (26–37) | 40 (30–46) | 36 (28–45) | <.001 |
| Median year of diagnosis cd (IQR) | 2003 (2001–2006) | 2003 (2000–2006) | 2002 (2000–2005) | .002 |
| Time from diagnosis to entry d | .04 | |||
| <6 months | 86 (89) | 204 (86) | 418 (80) | |
| ≥6 months | 11 (11) | 32 (14) | 102 (20) | |
| Sex cd | .002 | |||
| Male | 74 (76) | 179 (76) | 336 (65) | |
| Female | 23 (24) | 57 (24) | 184 (35) | |
| Residence bc | .02 | |||
| Urban | 51 (52) | 163 (69) | 329 (63) | |
| Large rural city/town | 31 (32) | 56 (24) | 151 (29) | |
| Small and isolated rural | 15 (16) | 17 (7) | 40 (8) | |
| Distance to clinic, km bc | <.001 | |||
| <97 (60 miles) | 86 (89) | 163 (69) | 342 (66) | |
| ≥97 (60 miles) | 11 (11) | 73 (31) | 178 (34) | |
| Transmission risk group bcd | <.001 | |||
| Heterosexual | 58 (60) | 85 (36) | 279 (54) | |
| MSM | 31 (32) | 126 (53) | 154 (30) | |
| IDU | 2 (2) | 19 (8) | 60 (11) | |
| Other/unknown | 6 (6) | 6 (3) | 27 (5) | |
NOTE. Data are no. (%) of patients, unless otherwise indicated. IQR, interquartile range; MSM, men who have sex with men; IDU, injection drug user.
P values reflect Pearson’s χ2 test results among all groups and categories.
Statistically significant difference (P < .05) between Latino and white subjects.
Statistically significant difference (P < .05) between Latino and black subjects.
Statistically significant difference (P < .05) between white and black subjects.
Generally, Latinos presented with more advanced HIV than did white or black persons, but there were substantial differences for men and women in all racial/ethnic groups; therefore, we present results stratified by sex (Table 2). The overall median CD4 count at HIV care initiation was 286 cells/mm3 (IQR, 63–482 cells/mm3); it was significantly lower for Latinos, at 186 cells/mm3 (IQR, 57–340 cells/mm3), compared with 292 cells/mm3 (IQR, 86–496 cells/mm3) for white persons (P = .006) and 302 cells/mm3 (IQR, 56–505 cells/mm3) for black persons (P = .02). Latinos were more likely to be late presenters, but no difference was seen between groups in advanced presenter status. These trends in late presentation are largely due to differences among men. Latino men were significantly more likely than white and black men to be late presenters (both P = .001) and were also significantly more likely than black men to be advanced presenters (P = .03) and to have an ADE (P = .02) (Table 2). Latinas were not significantly different than black or white women in any of the aforementioned clinical characteristics, although Latinas were more likely to be pregnant at the time of entry (overall P < .001). Pregnant women had higher median CD4 cell counts (430 cells/mm3) than nonpregnant women (313 cells/mm3; P = .002) or men (254 cells/mm3; P < .001). However, pregnant Latinas had lower median CD4 counts than did pregnant non-Latinas (361 vs 461 cells/mm3; P = .09). Although no statistically significant differences among groups were seen among delayed testers overall, fewer black women were delayed testers, compared with white women. Viral loads also differed marginally among the racial/ethnic groups but were substantially different between sexes.
Table 2.
Clinical Characteristics at Entry to HIV Care Between 1999 and 2009 Stratified by Race/Ethnicity and Sex
| Males |
Females |
Total |
|||||||
| Variable | Latino | White | Black | Latino | White | Black | Latino | White | Black |
| (n = 74) | (n = 179) | (n = 336) | (n = 23) | (n = 57) | (n = 184) | (n = 97) | (n = 236) | (n = 520) | |
| Median CD4+ cell count, cells/mm3a (IQR) | 169bc (60–312) | 249 (79–484) | 279 (53–454) | 294 (39–432) | 352 (150–547) | 338 (67–549) | 186bc (57–340) | 292 (86–496) | 302 (56–505) |
| Median log viral loadd (IQR) | 5.1c (4.6–5.6) | 5.1 (4.5–5.7) | 4.9 (4.1–5.5) | 4.3 (3.6–5.0) | 4.5 (4.0–5.2) | 4.5 (3.8–5.2) | 4.9 (4.3–5.4) | 4.9 (4.3–5.6) | 4.7 (4.0–5.4) |
| AIDS-defining event | 27 (36)c | 49 (27) | 78 (23) | 6 (26) | 9 (16) | 38 (21) | 33 (34)c | 58 (25) | 116 (22) |
| Late presentatione | 60 (81)bc | 105 (59) | 204 (61) | 13 (59) | 28 (49) | 97 (53) | 73 (76)bc | 133 (56) | 301 (58) |
| Advanced presentationf | 43 (58)c | 82 (46) | 149 (44) | 7 (32) | 19 (33) | 73 (40) | 50 (52) | 101 (43) | 222 (43) |
| Delayed testingg | 37 (86) | 67 (82) | 120 (81) | 6 (86) | 19 (100) | 52 (71)h | 43 (86) | 86 (85) | 172 (77) |
| Pregnant on entry | … | … | … | 9 (39)bc | 10 (18) | 17 (9) | … | … | … |
NOTE. Data are no. (%) of patients, unless otherwise indicated. IQR, interquartile range.
CD4+ cell count within 90 days of entry not available for 3 patients.
Statistically significant difference (P < .05) between Latino and white patients.
Statistically significant difference (P < .05) between Latino and black patients.
Viral load within 90 days of entry or 2 weeks of starting antiretroviral therapy not available for 18 patients.
Late presenters were patients with a CD4+ cell count <350 cells/mm3 or an AIDS-defining event.
Advanced presenters were patients with a CD4+ cell count <200 cells/mm3 or an AIDS-defining event.
Delayed testers were advanced presenters with a first HIV-positive test result <6 months before entry.
Statistically significant difference (P < .05) between white and black patients.
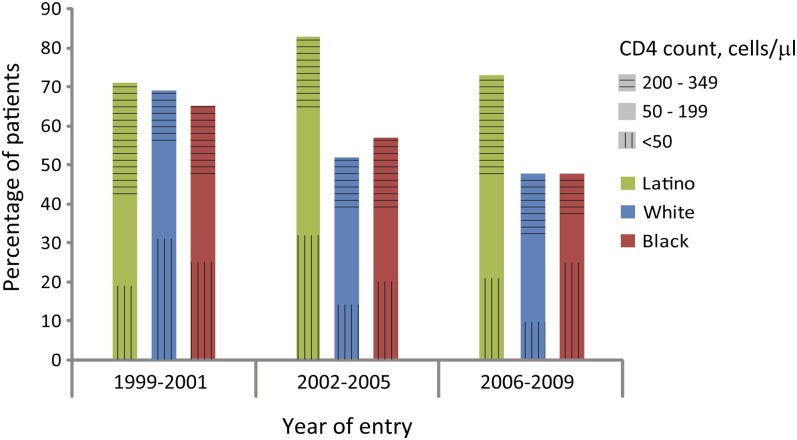
In unadjusted analyses, Latino ethnicity, older age, male sex, and earlier calendar year of entry were associated with late presentation (Table 3). All of these variables remained independent predictors in the multivariable regression model, which was adjusted for all variables. Latinos (PR, 1.31; 95% CI, 1.14–1.50), men (PR, 1.12; 95% CI, 1.01–1.23), and older patients (PR, 1.08; 95% CI, 1.05–1.12 per 10-year increase in age) were more likely to present late. Over the 11-year time period, a decreasing proportion of black and white patients entered care with CD4 counts <350 cells/mm3, whereas little change was seen among Latinos (Figure 1).
Table 3.
Predictors of Presenting to HIV Care Between 1999 and 2009 With a CD4+ Cell Count <350 Cells/mm3 or an AIDS-Defining Event
| Variable | Unadjusted PR (95% CI) | P | Adjusted PRa (95% CI) | P |
| Race/ethnicity | ||||
| Latino | 1.35 (1.15–1.58) | <.001 | 1.31 (1.14–1.50) | <.001 |
| Black | 1.03 (.90–1.18) | .65 | 1.01 (.92–1.12) | .78 |
| White | Referent | Referent | ||
| Age, per 10-year increase | 1.11 (1.07–1.15) | <.001 | 1.08 (1.05–1.12) | <.001 |
| Sex | ||||
| Male | 1.18 (1.04–1.35) | .01 | 1.12 (1.01–1.23) | .03 |
| Female | Referent | Referent | ||
| Transmission risk group | ||||
| MSM | 0.90 (.80–1.01) | .08 | NA | |
| Non-MSM | Referent | NA | ||
| Distance, per 16 km (10 miles) | 1.01 (.99–1.02) | .28 | NA | |
| Residence | ||||
| Small/isolated rural | 1.15 (.96–1.38) | .12 | NA | |
| Large rural | 1.06 (.94–1.20) | .32 | NA | |
| Urban | Referent | NA | ||
| Year entering care | NA | |||
| 1999–2001 | 1.28 (1.11–1.47) | <.001 | 1.14 (1.02–1.27) | .02 |
| 2002–2005 | 1.14 (.98–1.33) | .09 | 1.09 (.97–1.22) | .16 |
| 2006–2009 | Referent | Referent |
NOTE. CI, confidence interval; MSM, men who have sex with men; NA, not applicable; PR, prevalence ratio.
Based on a log-linear binomial regression model including race/ethnicity, age, sex, and year entering care.
Figure 1.
Proportion of patients by race/ethnicity presenting with CD4+ cell counts <350 cells/mm3 by time period entering care. Categorized by CD4+ cell count distribution.
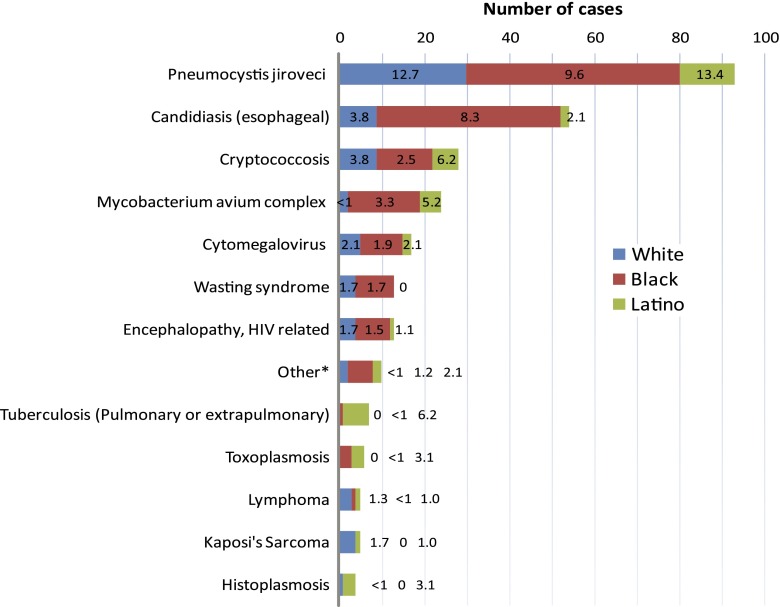
A total of 279 ADEs were diagnosed ≤60 days after care entry among 207 patients (24% of cohort), with a higher prevalence among Latinos versus non-Latinos (34% vs 23%; P = .02). Only 14 patients (1.6%) presented with an ADE and a CD4 count >200 cells/mm3, the majority of which were due to esophageal candidiasis (43%) and Kaposi’s sarcoma (22%). Overall, 7% of the cohort had ≥2 ADEs (10.3% of Latinos vs 6.2% of non-Latinos; P = .13). Pneumocystis jiroveci infection was the most frequently reported ADE, and Latinos had the highest prevalence (13.4%) (Figure 2). Latinos accounted for the majority of cases of active tuberculosis (6 of 7), toxoplasmosis (3 of 6), and histoplasmosis (3 of 4), although the overall reported numbers were small. Prevalence of active tuberculosis on entry was 6.2% for Latinos.
Figure 2.
AIDS-defining events diagnosed at presentation to care by race/ethnicity. Figure represents total number of cases and overall prevalence (%) within each race/ethnicity. *Other conditions include coccidioidomycosis (n = 1), chronic cryptosporidiosis (n = 3), chronic herpes simplex ulcers (n = 1), recurrent pneumonia (n = 1), progressive multifocal leukoencephalopathy (n = 3), and recurrent Salmonella septicemia (n = 1).
DISCUSSION
Significant clinical differences existed on first presentation to HIV care among Latinos versus both non-Latino black and white patients in our clinic cohort located in the southeastern United States. Latino participants were younger, yet they had significantly lower median CD4 cell counts, which were most pronounced among men. Moreover, Latinos were more likely to present with specific ADEs and were 1.4 times more likely than non-Latinos to present with a CD4 count <350 cells/mm3 or an ADE, after controlling for other factors, including age, sex, and year entering care. No statistically significant clinical differences at HIV care entry were seen between black and white patients. In addition to Latino ethnicity, we found that older age and male sex were independently associated with late presentation. To our knowledge, our study is the first detailed description of differences among ethnic groups in a nontraditional Latino settlement area in the southeastern United States, a region with the largest number of US persons living with AIDS and a substantial proportion of HIV-infected persons residing in nonurban areas [17].
Although many studies have not observed significant immunologic differences between racial/ethnic groups at entry to care or diagnosis [4, 18–20], other investigators have reported similar findings of lower CD4 counts among Latinos [21, 22]. Only one of these focused on nontraditional Latino settlements; the other examined immigrant-rich areas. Among patients entering care from 1996 to 2001 in Nebraska, a nontraditional Latino settlement area, Latinos had lower median CD4 counts, compared with both white and black persons (220 vs 372 and 318 cells/mm3, respectively), and were more likely to have an opportunistic infection [21]. In 5 US clinics along the Mexican border, a location with an increasing HIV incidence and migrant population, the median CD4 counts at entry were lower for Latinos than non-Latinos (246 vs 376 cells/mm3) [22]. At 2 of these clinics, Latinos were twice as likely to present with AIDS after adjusting for age, sex, and mode of exposure. Although both of these studies included antiretroviral therapy–naive patients, they did not restrict to patients initiating HIV care. Despite our use of more recent data, we observed even lower CD4 counts among Latinos compared with both these studies. In addition, we found no effect of rural residence and distance to care on late presentation—variables that were not examined in these prior studies among Latinos. Finally, the substantial age difference between Latinos and non-Latinos in our study was not similarly described.
The primary factor driving our findings of lower CD4 counts among Latinos may be a large number of foreign-born Latinos. An estimated 53% of Latinos in North Carolina are foreign born, compared with 38% in the United States in general [8]. Prior studies that did not find significant immunologic differences among racial/ethnic groups were often characterized by smaller sample sizes [18, 20] or perhaps included Latinos from both immigrant-rich and US-born populations [4, 19, 23]. In a study from northern California that included a large immigrant population comprising mostly Latinos, immigrant status but not Latino ethnicity was the only factor independently associated with an opportunistic infection at diagnosis [23].
The immigrant-rich nature of the North Carolina Latino population is reflected in its younger age structure [6] and certain clinical characteristics found in our study. We found that Latinos accounted for 86% of active tuberculosis cases among all patients initiating HIV care. Foreign-born persons with HIV are at risk of reactivating opportunistic infections endemic to their originating countries, such as tuberculosis. Nationally, Latinos comprised 23% of tuberculosis cases among HIV-infected individuals in 2005 [24]. Surveillance data from southern California showed that Latinos accounted for 82% of cases of concurrent HIV infection and tuberculosis in 2007, compared with 42% in 1993, a change attributed to an increase in cases in Mexican-born immigrants [25]. In addition, the majority of histoplasmosis cases in our study occurred among Latinos, which also is indicative of migration, because histoplasmosis is not endemic to North Carolina.
Initial entry to medical care in the later stages of HIV disease may either be due to a delay in diagnosis or a delay in accessing care. Factors associated with delays in seeking medical care after HIV testing may differ from factors associated with late testing [26]. We found that >80% of white and Latino persons and >75% of black persons who entered care with an AIDS diagnosis had their initial HIV positive test ≤6 months prior to entering care. These observations suggest that late testing accounts for the majority of late entries into care, rather than a delay in accessing treatment, and are similar to findings in other studies [26–28]. In a study at the Veterans Health Administration (VA), where equal access is available, more than one-half of the HIV-infected patients presented with AIDS, and no difference was seen between patients who had received prior VA health care or not, suggesting a larger contribution from late testing [27]. A limitation of our data lies in our information on the date of the first positive HIV test result. Although we extracted these data from multiple sources, including North Carolina state testing data, patients might not have disclosed previous testing in a private clinic or outside the state.
Potential factors associated with late HIV testing have been investigated in both Latino and non-Latino populations using various definitions of late testing/diagnosis. Older age [5, 26, 29–33] and male sex [5, 18, 32, 33] have been recognized as independently associated factors in several studies; this is consistent with our results. Older persons may have a lower perception of HIV risk, resulting in late testing. Foreign-born status is also independently associated with late testing in several studies, both in the United States and Europe [23, 29–34]. In a US surveillance study of Latinos, a short interval in progression from the initial HIV diagnosis to AIDS was also associated with birth in Mexico or Central America, high-risk heterosexual contact, and IDU [5]. However, among interviews of Latinos with AIDS in Los Angeles, California, only completion of the interview in Spanish was associated with late testing after adjusting for age, education, country of birth, and IDU status [35]. Thus, acculturation factors, such as English proficiency, may be a principal barrier to late testing for immigrant Latinos.
Notably, heterosexual risk was the predominate mode of transmission reported among Latinos in our study, but only 24% of subjects were Latina. This finding may indicate underreporting of MSM risk due to the high rate of social stigma surrounding homosexuality among Latinos [36, 37]. Alternatively, the higher proportion of men in our study may reflect the demographic structure of the immigrant Latino population in North Carolina, which is majority male [38]; these men may have female partners residing in their originating countries.
We lack specific migration history on our Latino patients, preventing direct assessment of the effect of immigration on HIV clinical characteristics and entry to care. Gathering these data, as well as information on acculturation markers, such as language, alcohol and substance abuse, sexual partnerships, insurance, and legal status, may offer further insight into reasons for delayed entry to care among Latinos. This knowledge would be instrumental in informing targeted intervention programs not only in North Carolina but also potentially in other nontraditional Latino settlement areas. Given the high rates of late presentation seen among all groups in our cohort, there clearly is a continued need for a comprehensive public health program focusing on early testing for everyone, as well as for understanding the barriers between Latino immigrants and full use of public health resources.
Acknowledgments
Financial support. This work was supported by the National Institute of Allergy and Infectious Diseases (T32 AI007001-33), the University of North Carolina at Chapel Hill Center for AIDS Research (P30 AI50410), and the National Center for Research Resources (UL1RR025747).
Potential conflicts of interest. J. J. E. reports that he has received consulting fees from Merck & Company, Bristol-Myers Squibb, GlaxoSmithKline, and Gilead Sciences; research support from Merck & Company and GlaxoSmithKline; and payment for development of education presentations from Clinical Care Options and viralEd. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed in the Acknowledgments section.
References
- 1.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 2.Hogg RS, Yip B, Chan KJ, et al. Rates of disease progression by baseline CD4 cell count and viral load after initiating triple-drug therapy. JAMA. 2001;286:2568–77. doi: 10.1001/jama.286.20.2568. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. Late HIV testing—34 states, 1996–2005. MMWR Morb Mortal Wkly Rep. 2009;58:661–5. [PubMed] [Google Scholar]
- 4.Althoff KN, Gange SJ, Klein MB, et al. Late presentation for human immunodeficiency virus care in the United States and Canada. Clin Infect Dis. 2010;50:1512–20. doi: 10.1086/652650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Espinoza L, Hall HI, Selik R, Hu X. Characteristics of HIV infection among Hispanics, United States 2003–2006. J Acquir Immune Defic Syndr. 2008;49:94–101. doi: 10.1097/QAI.0b013e3181820129. [DOI] [PubMed] [Google Scholar]
- 6.Kochhar R, Suro R, Tafoya S. The New Latino South: the context and consequences of rapid population growth. Washington, DC: Pew Hispanic Center; 2005. [Google Scholar]
- 7.Suro R, Singer A. Latino growth in Metropolitan America: changing patterns, new locations. Washington, DC: The Brookings Institution, Center on Urban and Metropolitan Policy and Pew Hispanic Center; 2002. [Google Scholar]
- 8.Pew Hispanic Center. Statistical profiles of the Hispanic and foreign-born population in the United States, 2008. Available at: http://pewhispanic.org/datasets/ Accessed 31 October 2010. [Google Scholar]
- 9.Painter TM. Connecting the dots: when the risks of HIV/STD infection appear high but the burden of infection is not known—the case of male Latino migrants in the southern United States. AIDS Behav. 2008;12:213–26. doi: 10.1007/s10461-007-9220-0. [DOI] [PubMed] [Google Scholar]
- 10.Organista KC, Carrillo H, Ayala G. HIV prevention with Mexican migrants: review, critique, and recommendations. J Acquir Immune Defic Syndr. 2004;37(Suppl 4):S227–39. doi: 10.1097/01.qai.0000141250.08475.91. [DOI] [PubMed] [Google Scholar]
- 11.Napravnik S, Eron JJ, Jr, McKaig RG, Heine AD, Menezes P, Quinlivan E. Factors associated with fewer visits for HIV primary care at a tertiary care center in the southeastern U.S. AIDS Care. 2006;18(Suppl 1):S45–50. doi: 10.1080/09540120600838928. [DOI] [PubMed] [Google Scholar]
- 12.Rural Health Research Center (RHRC) Rural-urban commuting area codes (RUCAs) version 2.0. Available at: http://depts.washington.edu.libproxy.lib.unc.edu/uwruca/. Accessed 20 April 2010. [Google Scholar]
- 13.Hall SA, Kaufman JS, Ricketts TC. Defining urban and rural areas in US epidemiologic studies. J Urban Health. 2006;83:162–75. doi: 10.1007/s11524-005-9016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antinori A, Coenen T, Costagiola D, et al. Late presentation of HIV infection: a consensus definition. HIV Med. 2010;12:61–4. doi: 10.1111/j.1468-1293.2010.00857.x. [DOI] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. 1993 Revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. MMWR Recomm Rep. 1992;41:1–19. [PubMed] [Google Scholar]
- 16.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003;157:940–3. doi: 10.1093/aje/kwg074. [DOI] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention. HIV/AIDS surveillance report, 2007. Atlanta, GA: Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 18.Gay CL, Napravnik S, Eron JJ., Jr Advanced immunosuppression at entry to HIV care in the southeastern United States and associated risk factors. AIDS. 2006;20:775–8. doi: 10.1097/01.aids.0000216380.30055.4a. [DOI] [PubMed] [Google Scholar]
- 19.Dybul M, Bolan R, Condoluci D, et al. Evaluation of initial CD4 T cell counts in individuals with newly diagnosed human immunodeficiency virus infection, by sex and race, in urban settings. J Infect Dis. 2002;185:1818–21. doi: 10.1086/340650. [DOI] [PubMed] [Google Scholar]
- 20.Mayben JK, Kramer JR, Kallen MA, Franzini L, Lairson DR, Giordano TP. Predictors of delayed HIV diagnosis in a recently diagnosed cohort. AIDS Patient Care STDS. 2007;21:195–204. doi: 10.1089/apc.2006.0097. [DOI] [PubMed] [Google Scholar]
- 21.Swindells S, Cobos DG, Lee N, et al. Racial/ethnic differences in CD4 T cell count and viral load at presentation for medical care and in follow-up after HIV-1 infection. AIDS. 2002;16:1832–4. doi: 10.1097/00002030-200209060-00020. [DOI] [PubMed] [Google Scholar]
- 22.Carabin H, Keesee MS, Machado LJ, et al. Estimation of the prevalence of AIDS, opportunistic infections, and standard of care among patients with HIV/AIDS receiving care along the US-Mexico border through the Special Projects of National Significance: a cross-sectional study. AIDS Patient Care STDS. 2008;22:887–95. doi: 10.1089/apc.2007.0176. [DOI] [PubMed] [Google Scholar]
- 23.Levy V, Prentiss D, Balmas G, et al. Factors in the delayed HIV presentation of immigrants in northern California: implications for voluntary counseling and testing programs. J Immigr Minor Health. 2007;9:49–54. doi: 10.1007/s10903-006-9015-9. [DOI] [PubMed] [Google Scholar]
- 24.Centers for Disease Control and Prevention. Reported HIV status of tuberculosis patients—United States, 1993–2005. MMWR Morb Mortal Wkly Rep. 2007;56:1103–6. [PubMed] [Google Scholar]
- 25.Rodwell TC, Barnes RF, Moore M, et al. HIV-tuberculosis coinfection in southern California: evaluating disparities in disease burden. Am J Public Health. 2010;100(Suppl 1):S178–85. doi: 10.2105/AJPH.2009.170142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Girardi E, Aloisi MS, Arici C, et al. Delayed presentation and late testing for HIV: demographic and behavioral risk factors in a multicenter study in Italy. J Acquir Immune Defic Syndr. 2004;36:951–9. doi: 10.1097/00126334-200408010-00009. [DOI] [PubMed] [Google Scholar]
- 27.Gandhi NR, Skanderson M, Gordon KS, Concato J, Justice AC. Delayed presentation for human immunodeficiency virus (HIV) care among veterans. Med Care. 2007;45:1105–9. doi: 10.1097/MLR.0b013e3181271476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krawczyk CS, Funkhouser E, Kilby JM, Kaslow RA, Bey AK, Vermund SH. Factors associated with delayed initiation of HIV medical care among infected persons attending a southern HIV/AIDS clinic. South Med J. 2006;99:472–81. doi: 10.1097/01.smj.0000215639.59563.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Espinoza L, Hall HI, Hu X. Increases in HIV diagnoses at the US-Mexico border, 2003–2006. AIDS Educ Prev. 2009;21:19–33. doi: 10.1521/aeap.2009.21.5_supp.19. [DOI] [PubMed] [Google Scholar]
- 30.Mugavero MJ, Castellano C, Edelman D, Hicks C. Late diagnosis of HIV infection: the role of age and sex. Am J Med. 2007;120:370–3. doi: 10.1016/j.amjmed.2006.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Delpierre C, Dray-Spira R, Cuzin L, et al. Correlates of late HIV diagnosis: implications for testing policy. Int J STD AIDS. 2007;18:312–7. doi: 10.1258/095646207780749709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang B, Chan SK, Mohammad N, et al. Late HIV diagnosis in Houston/Harris County, Texas, 2000–2007. AIDS Care. 2010;22:766–74. doi: 10.1080/09540120903431348. [DOI] [PubMed] [Google Scholar]
- 33.Borghi V, Girardi E, Bellelli S, et al. Late presenters in an HIV surveillance system in Italy during the period 1992–2006. J Acquir Immune Defic Syndr. 2008;49:282–6. doi: 10.1097/QAI.0b013e318186eabc. [DOI] [PubMed] [Google Scholar]
- 34.Schwarcz S, Hsu L, Dilley JW, Loeb L, Nelson K, Boyd S. Late diagnosis of HIV infection: trends, prevalence, and characteristics of persons whose HIV diagnosis occurred within 12 months of developing AIDS. J Acquir Immune Defic Syndr. 2006;43:491–4. doi: 10.1097/01.qai.0000243114.37035.de. [DOI] [PubMed] [Google Scholar]
- 35.Wohl AR, Tejero J, Frye DM. Factors associated with late HIV testing for Latinos diagnosed with AIDS in Los Angeles. AIDS Care. 2009;21:1203–10. doi: 10.1080/09540120902729957. [DOI] [PubMed] [Google Scholar]
- 36.Diaz RM, Ayala G, Bein E, Henne J, Marin BV. The impact of homophobia, poverty, and racism on the mental health of gay and bisexual Latino men: findings from 3 US cities. Am J Public Health. 2001;91:927–32. doi: 10.2105/ajph.91.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garland JM, Andrade AS, Page KR. Unique aspects of the care of HIV-positive Latino patients living in the United States. Curr HIV/AIDS Repo. 2010;7:107–16. doi: 10.1007/s11904-010-0049-1. [DOI] [PubMed] [Google Scholar]
- 38.Pew Hispanic Center. Demographic profile of Hispanics in North Carolina, 2008. Available at: http://pewhispanic.org/states/?stateid=NC. Accessed 31 October 2010. [Google Scholar]