Abstract
Wolf–Hirschhorn syndrome (WHS) is a malformation syndrome associated with growth retardation, mental retardation, and immunodeficiency resulting from a hemizygous deletion of the short arm of chromosome 4, called the WHS critical region (WHSC). The WHSC1 gene is located in this region, and its loss is believed to be responsible for a number of WHS characteristics. We identified WHSC1 in a genetic screen for genes involved in responding to replication stress, linking Wolf–Hirschhorn syndrome to the DNA damage response (DDR). Here, we report that the WHSC1 protein is a member of the DDR pathway. WHSC1 localizes to sites of DNA damage and replication stress and is required for resistance to many DNA-damaging and replication stress-inducing agents. Through its SET domain, WHSC1 regulates the methylation status of the histone H4 K20 residue and is required for the recruitment of 53BP1 to sites of DNA damage. We propose that Wolf–Hirschhorn syndrome results from a defect in the DDR.
Keywords: checkpoint, histone methylation, MMSET
Damage to DNA occurs continuously, threatening the integrity of our genetic material. The ability to repair DNA lesions is critical for proper organismal development and survival. In response to DNA damage or replication stress, a signal transduction cascade called the DNA damage response (DDR) is activated to maintain genomic integrity (1, 2). The ATM and ATR kinases are central components of the DDR. ATM is recruited and activated by the MRE11-RAD50-NBS1 complex at double-strand breaks (DSBs), where ATM phosphorylates the histone variant H2AX (3, 4). Phosphorylated H2AX then associates with the mediator protein MDC1, which leads to the activation of ubiquitination and sumoylation cascades required for the recruitment of the BRCA1 ubiquitin ligase and the mediator protein 53BP1 to promote DNA repair (5, 6). Unlike ATM, the ATR kinase is activated following its recruitment by its partner protein ATRIP to RPA-coated ssDNA regions generated at stalled or collapsed forks. RPA-ssDNA complexes also recruit the RAD17-RFC2-5 complex that loads the RAD1-RAD9-HUS1 (9-1-1) complex onto DNA. The 9-1-1 complex recruits RHINO (7) and the mediator protein TOPBP1 to further activate ATR (8, 9). ATM and ATR are known to control the phosphorylation of nearly 1,000 effector proteins involved in a wide number of cellular processes, such DNA repair, transcription, chromatin structure, splicing, metabolism, induction of apoptosis, and senescence.
The importance of the DDR in human physiology is underscored by the observation that dysregulation of DNA repair pathways can lead to a range of human disorders exhibiting developmental defects, neurological defects, immunodeficiency, and cancer (2, 3). The developmental and neurological defects are likely due to the requirement for extremely accurate DNA repair during the rapid expansion of progenitor cells occurring during embryonic development and for the maintenance of the homeostasis of the nervous system.
The number of syndromes associated with neurological and developmental defects related to the DDR has rapidly expanded in the last decade. Ataxia telangiectasia (A-T) is one of the most studied DNA repair deficiencies resulting in developmental and neurological impairment. A-T, which is caused by mutations in ATM, is characterized by progressive neurologic impairment, cerebellar ataxia, variable immunodeficiency, and a predisposition to malignancy (10). Other examples of DDR syndromes that affect the development and homeostasis of the nervous and immune system include the Nijmegen breakage syndrome and the ataxia-telangiectasia–like disorder, which are due to mutations in the DDR factors NBS1 and MRE11, respectively (11, 12). Mutations in ATR, and other components of the ATR pathway, have been identified in Seckel syndrome patients with microcephaly, facial dysphormism, and growth defects (12).
Wolf–Hirschhorn syndrome (WHS) is a malformation syndrome characterized by brain developmental defects, immunodeficiency, microcephaly, craniofacial phenotypes, and congenital heart defects (13). Defects in the production of immunogluobulin A and common variable immunodeficiency (CVID) are frequently associated with WHS (14). WHS results from a hemizygous deletion of the subtelomeric region of the short arm of chromosome 4. The Wolf–Hirschhorn syndrome candidate 1 gene (WHSC1/MMSET) is located in the WHS critical region and its loss is believed to be responsible for several phenotypes of the syndrome (15). In addition to its role in WHS, WHSC1 is also targeted by the t(4;14)(p16.3;q32.3) chromosomal translocation, which results in the overproduction of the WHSC1 protein in multiple myelomas (MMs) (16). Although studies have outlined the involvement of WHSC1 in development, the pathogenic role of WHSC1 in WHS and MM remains to be elucidated. WHSC1 is a SET domain histone methyltranferase containing proline-tryptophan-tryptophan-proline (PWWP), high-mobility group (HMG box) and plant homeodomain (PHD) domains (17). We identified WHSC1 in a genetic screen for proteins involved in responding to DNA replication stress. This finding, together with the fact that the protein domains of WHSC1 are commonly found in DDR factors and that WHSC1 is phosphorylated in response to DNA damage (18), prompted us to investigate whether WHSC1 is a member of the DDR machinery. Here, we report that the WHSC1 protein has a role in DNA damage response via regulation of the methylation of the K20 residue of histone H4 and the recruitment of 53BP1 to sites of DNA damage.
Results
Inactivation of WHSC1 Sensitizes Human Cells to DNA Damage and Replication Stress.
In an ongoing shRNA screen for genes involved in resistance to hydroxyurea (HU)-induced DNA replication stress, we identified the WHSC1 gene. To explore a possible role for WHSC1 in the DDR, we first investigated if WHSC1 depletion resulted in DNA damage sensitivity. HCT116 cells depleted with an shRNA targeting WHSC1 were compared with cells expressing the nontargeting firefly luciferase (FF) shRNA for their sensitivity to HU, mitomycin C (MMC), ionizing radiation (IR), camptothecin (CPT), and UV light. The viability of these cells after DNA damage treatment was measured using the CellTiter Blue reagent. WHSC1 depletion did not inhibit cell survival in the absence of DNA-damaging treatments. However, exposure of the cells to HU, MMC, IR, CPT, and UV caused a reduction in the viability of cells depleted for WHSC1 (Fig. 1 A and C).
Fig. 1.
WHSC1 is required for resistance to DNA damage and DNA replication stress. (A) HCT116 cells expressing FF shRNA (FFsh, blue columns), WHSC1 shRNA (WHSC1sh, red columns), or a combination of a WHSC1 shRNA and a WHCS1 cDNA resistant to the WHSC1 shRNA (WHSC1sh shRes, green columns) were treated with DNA-damaging agents as follows: 500 μM HU for 24 h, 50 nM MMC, 50 nM CPT for 24 h, 5 Gy irradiation or 30 J/m2 UV. Two days later, the viability of these treated cells was determined using CellTiter-Blue reagent (Promega). Experiments were repeated four times, and the average viabilities were plotted. Error bars represent the SD of four experiments. P < 0.005. (B) HCT116 cells transfected with siRNAs targeting FF (FFsiRNA, blue columns), WHSC1 (WHSC1siRNA, red columns), or a combination of a WHSC1 siRNA and a WHCS1 cDNA resistant to the WHSC1 siRNA (WHSC1siRNA siRes, green columns) were treated with 500 μM HU for 24 h or with 5 Gy irradiation. Two days later, the viability of these treated cells was determined using CellTiter-Blue reagent (Promega). Experiments were repeated four times, and the average viabilities were plotted. Error bars are as above. P < 0.005. (C) Depletion of WHSC1 by shRNA and siRNA, and the expression of the shRNA-resistant and siRNA-resistant WHSC1 protein. Extracts form the indicated cells were immunoblotted for WHSC1 protein.
To rule out off-target effects mediated by the WHSC1 shRNA, we confirmed the DNA damage sensitivity of WHSC1-depleted cells using an siRNA targeting a different site. HCT116 cells were transfected with nontargeting FF siRNA, a WHSC1 targeting siRNA, or a combination of the WHSC1-targeting siRNA and an siRNA-resistant wild-type WHSC1 expression plasmid. At 48 h posttransfection, cells were treated with 500 μM HU for 24 h or with 5 Gy IR, incubated for 48 h to allow recovery from the damage, and assayed for viability using the Cell Titer-Blue reagent. Both HU and IR treatments resulted in reduced cell viability, confirming the DNA damage sensitivity also observed in the WHSC1 shRNA-depleted cells. In addition, the siRNA-resistant WHSC1 rescued the damage sensitivity conferred by the siRNA (Fig. 1 B and C). We conclude from these observations that WHSC1 contributes to the resistance of human cells to DNA damage and DNA replication stress.
WHSC1 Localizes to Sites of DNA Damage.
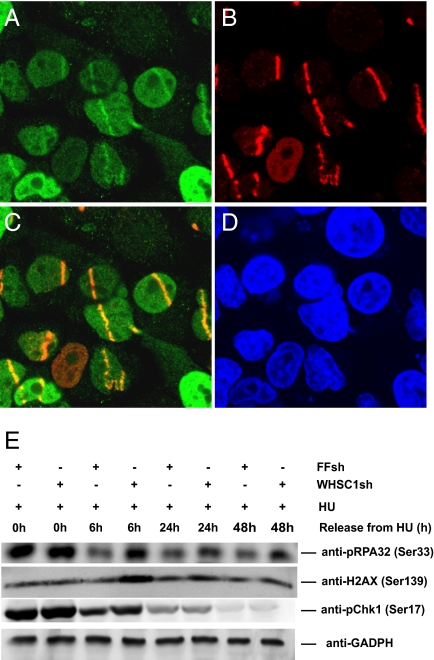
To further explore the role of WHSC1 in the DDR, we investigated whether WHSC1 is recruited to sites of DNA damage. We expressed an N-terminal GFP-WHSC1 fusion in U2OS cells and assayed for recruitment to DNA lesions induced by UV laser microirradiation following sensitization of genomic DNA by BrdU treatment. BrdU-labeled cells were irradiated and subsequently fixed with formaldehyde and immunostained with antibodies against GFP to allow the detection of GFP-WHSC1. WHSC1 showed a pan-nuclear staining in interphase cells and was associated with the mitotic chromosomes. However, within 45 min of microirradiation, WHSC1 was recruited to sites of DNA damage where it colocalized with γH2AX, a marker of sites of DNA damage (Fig. 2). This finding suggests that WHSC1 is likely to play a direct role in DNA damage sensing and/or repair.
Fig. 2.
WHSC1 localizes to sites of DNA damage and is required for recovery from DNA replication stress. (A–D) U2OS cells expressing GFP-WHSC1 were laser microirradiated, incubated for 45 min, and processed for immunofluorescence staining of GFP-WHSC1 (A) and γ-H2AX (B). The merged image (C) shows the colocalization of p-H2AX and GFP-WHSC1 at the DNA damage sites. Nuclei were stained with DAPI (D). (E) DDR activation in HCT116 cells expressing FF and WHSC1 hairpins upon HU treatment. Cells were treated with 500 μM HU for 24 h and released from the HU block for the indicated times. Released cells were collected, lysed in the presence of phosphatase inhibitors, and processed for Western blotting. The phosphorylation status of RPA32, Chk1, and H2AX were analyzed, and GADPH was used as a loading control.
WHSC1 Depletion Causes Prolonged DNA Damage-Induced RPA, H2AX Phosphorylation.
Prompted by the evidence suggesting a link between WHSC1 and DNA damage, we asked whether WHSC1 might be involved in DDR signaling. Upon activation, ATR activates CHK1 by phosphorylation on Ser317 and Ser345. We followed CHK1 activation upon HU treatment via monitoring its phosphorylation on Ser317 in the presence and absence of WHSC1 in HCT116 cells by Western blot. Cells were treated with 500 μM HU for 24 h and released into HU-free media. Samples were collected at the indicated times. No significant difference in the kinetics of CHK1 activation was observed. However, analysis of the kinetics of CHK1 phosphorylation after release from the HU block revealed that CHK1 activation persisted longer in WHSC1-depeted cells and was higher at 6 h after HU release compared with controls. Together, these observations suggest that DDR activation is intact in the absence of WHSC1, but that the DNA damage signaling generated by replication stress persists longer after release from the stress. This indicates that cells lacking WHSC1 generate more DNA damage than control cells upon replication stress, repair the DNA damage much less efficiently, or both.
To explore whether more DNA damage is generated after replication stress in the absence of WHSC1, we examine two reporters of DNA damage in the same experiment as above. The first reporter was the phosphorylation status of RPA. In response to replication stress, the 32-kDa subunit of RPA (RPA32) becomes phosphorylated by ATR/ATRIP on residue S33. Phosphorylation of S33 is an indicator of the presence of DNA damage and the activation status of ATR/ATRIP. We observed that RPA32 phosphorylation persisted longer in WHSC1-depleted cells compared with the control shRNA-expressing cells up to 48 h postremoval of HU, indicating a prolonged DNA damage response activation.
As a second reporter, we analyzed the kinetics of γH2AX formation after exposure to HU in HCT116 cells. Following HU treatment, H2AX becomes phosphorylated in an ATR-dependent manner to generate γH2AX. As seen in Fig. 2E, γH2AX is clearly detected after 24 h of HU treatment at the same level in the presence of FF and WHSC1 hairpins. However, following the release of cells into HU-free media, in the absence of WHSC1 the level of γH2AX increased at 6 h and slowly decreased throughout the 48-h time period. In contrast, the control cells showed no significant increase of γH2AX after release. The increased H2AX phosphorylation seen in WHSC1-depleted cells, together with the RPA and CHK1 phosphorylation data, suggest that WHSC1 acts to allow recovery from DNA replication stress. Part of this increased H2AX signal could be due to apoptosis (19), but in that case the H2AX staining is pan-nuclear as opposed to focal, which we have not observed. Furthermore, the increase of H2AX phosphorylation 6 h after release from the HU block suggests that cells lacking WHSC1 create more DNA damage in the process of replication stress recovery than WT cells, and this damage persists.
CHK2 Activation Is Impaired in the Absence of WHSC1 Protein.
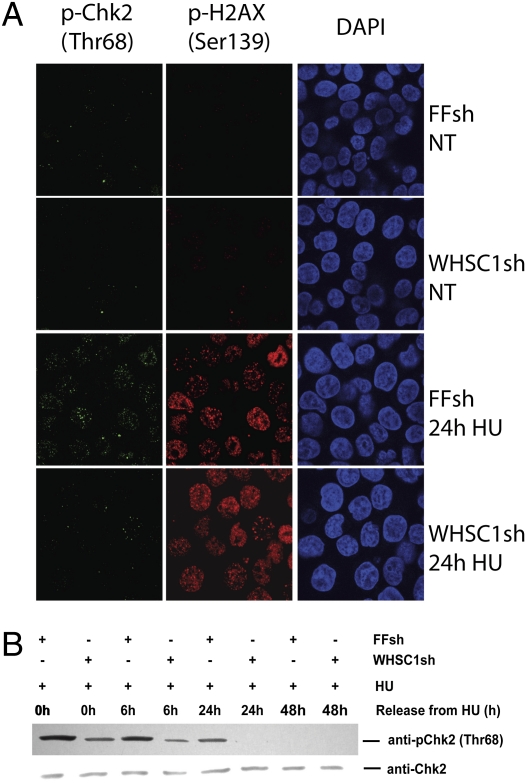
To further investigate the integrity of the DDR in the absence of WHSC1, we examined the activation of CHK2. HCT116 cells were treated with 500 μM HU for 24 h and released to HU-free media. As shown in Fig. 3A, HU treatment resulted in the detection of Thr68 CHK2 phosphorylation in nuclear foci in WT cells. However, the signal of CHK2 Thr68 foci showed a significantly diminished intensity in the absence of WHSC1, suggesting that HU-induced CHK2 phosphorylation depends upon WHSC1. Immunofluorescence with phosphoantibodies is potentially problematic due to cross-reaction with other phosphoepitopes. To provide further support, we performed Western blotting for CHK2 p-Thr68. In control cells, CHK2 became phosphorylated on Thr68, and this phosphorylation gradually diminished over time after release into HU-free media. In the WHSC1-depleted cells, CHK2 activation was significantly impaired at zero time and at all time points after release, becoming undetectable by 24 h. Throughout the course of the experiment, the amount of total CHK2 protein recovered in the extracts remained unchanged. These data indicate that WHSC1 promotes CHK2 phosphorylation in response to DNA replication stress.
Fig. 3.
Chk2 activation in response to DNA replication stress requires WHSC1. (A) HCT116 cells expressing FF and WHSC1 shRNAs were treated with 500 μM HU for 24 h and processed for immunofluorescence staining of p-Chk2 (Thr68) and p-H2AX. Nuclei were stained with DAPI. (B) Chk2 activation in HCT116 cells expressing FF and WHSC1 hairpins upon HU treatment. Cells were treated with 500 μM HU for 24 h and released from the treatment for the indicated times. Released cells were collected, lysed in the presence of phosphatase inhibitors, and immunoblotted. The phosphorylation status of Chk2 was analyzed.
WHSC1 Is Required for 53BP1 Localization and HU-Induced Methylation of the K20 Residue of Histone H4.
The observation that Chk1 activation remained intact in the absence of WHSC1, but that Chk2 activation was impaired even in the presence of increased RPA and H2AX phosphorylation, suggested that ATR was functional but the ATM pathway may be impaired. Thus, we examined the consequences of WHSC1 depletion in the ATM portion of the DDR. p53-binding protein 1 (53BP1) is a mediator/adaptor of the DNA damage response and is recruited to nuclear structures termed foci in response to double-strand breaks in an ATM-dependent manner. The recruitment of 53BP1to foci requires multiple protein–protein interactions and posttranslational modifications, and is not understood completely.
To investigate if 53BP1 localization is regulated by WHSC1, we depleted WHSC1 in HCT116 cells and examined HU-induced 53BP1 foci formation. Cells were treated with 500 μM HU for 24 h and processed for immunostaining to visualize 53BP1 and γH2AX foci formation. In cells expressing the FF hairpin, 24-h HU treatment resulted in the appearance of clear 53BP1and γH2AX foci. However, in cells lacking full WHSC1 function, reduced numbers of 53BP1foci were detectable after HU treatment. This result suggests that WHSC1 is required for either the recruitment or the retention of 53BP1 at DNA damage sites (Fig. 4A).
Fig. 4.
WHSC1 controls histone H4K20 methylation and 53BP1 localization in response to DNA replication stress. (A) Impaired localization of 53BP1 to sites of replication stress in the absence of WHSC1. HCT116 cells expressing FF, WHSC1 shRNAs, or WHSC1 shRNA together with an shRNA-resistant WT or SET mutant WHSC1 cDNA were treated with 500 μM HU for 24 h and processed for immunostaining to visualize p-H2AX (red) and 53BP1(green). Nuclei were stained with DAPI. (B) WHSC1 regulates the methylation status of H4K20 upon DNA replication stress. HCT116 cells expressing FF or WHSC1 shRNAs were treated with 500 μM HU. Cells were released into HU-free media and collected, lysed at the indicated times, and immunoblotted to determine H4K20 methylation. GADPH was used as a loading control.
53BP1 is recruited to sites of DNA damage in part through its Tudor domain, which binds methylated histone residues at the sites of damage. Methyl-histone–mediated recruitment of 53BP1to DNA-damaged sites has been described but remains controversial. One of the residues implicated in this recruitment is the dimethylated histone H4 lysine 20 (H4K20me2). However, a role for H4K20me3 in the recruitment of 53BP1 to sites of damage has not been ruled out. WHSC1 has been previously shown to have histone methyltransferase activity through its SET domain. To examine whether the methyltransferase activity of WHSC1 plays a role in the recruitment of 53BP1to damage foci, we generated a point mutant in the WHSC1 SET domain (Y1092A), in a residue known to be essential for histone methyltransferase activity. shRNA-resistant forms of the WT and SET domain mutant WHSC1 gene were expressed, infected with shRNA WHSC1 virus to deplete endogenous WHSC1. These cells were examined for 53BP1 recruitment to foci in response to HU. WT WHSC1 rescued the 53BP1 foci formation defect, whereas the SET domain mutant did not. Taken together, these results indicate that WHSC1 plays a role in the recruitment of 53BP1 to DNA damage foci via an activity associated with its histone methyltransferase SET domain, and also rule out a possible off-target effect on 53BP1 localization of the WHSC1 hairpin used in this study.
To further investigate how the methyltransferase activity of WHSC1 impacts the methylation status of H4K20, we followed the methylation status of the H4K20 residue by Western blot in HU-treated, WHSC1-depleted, and control cells. As shown in Fig. 4B, in control cells, 2 h of 500-μM HU treatment caused the complete disappearance of the bulk K20me2 form of H4 that coincided with the appearance of significantly enhanced H4K20 trimethylation. In WHSC1-depleted cells, we did not observe an HU-induced change in the methylation status of H4K20, suggesting that WHSC1 mediates H4K20 trimethylation upon DNA replication stress.
Discussion
Rapidly accumulating evidence suggests that in addition to their apparent role in carcinogenesis, the DNA damage response play an important role in human development and neuronal homeostasis (2, 3). The Wolf–Hirschhorn syndrome candidate 1 gene (WHSC1/MMSET) is located on the short arm of chromosome 4, deleted in WHS, and likely accounts for a number of the characteristic phenotypes of the syndrome. WHS is a developmental syndrome with neurological and immunological impairment. Although it shares phenotypes in common with a number of DDR-defective syndromes, no link has been drawn between proteins affected by the 4p deletion and DDR pathways. In this study we identify WHSC1 as an integral component of the DDR.
The involvement of WHSC1 in the DDR is based on multiple lines of evidence. First, WHSC1 was identified in a HU sensitivity screen, suggesting a role in the DNA replication stress response. Depletion of WHSC1 resulted in increased levels of DNA damage induced by HU, slower recovery kinetic from replication stress, and, as a consequence, increased Chk1 activation. Second, WHSC1 depletion sensitizes cells to a wide variety of genotoxic agents, such as camptothecan, mitomycin C, IR, and UV light. Finally, like many DDR factors directly involved in controlling events at the sites of DNA damage, WHSC1 accumulates at the sites of DNA damage induced by laser microirradiation. Together, these observations strongly suggest that WHSC1 is an integral member of the DDR network.
The mechanism of WHSC1’s role in response to DNA damage is not completely clear, but a clue to its function comes from its domain structure. WHSC1 has a histone methyltransferase SET domain, and several proteins containing this domain have been implicated in the DDR. Lysine 20 of histone H4 is mono-, di-, or trimethylated in vivo, but the regulation and significance of the different methylation stages is poorly understood. It is believed that H4K20 methylation plays an important role in the DDR by contributing to the recruitment of 53BP1 to double-stranded breaks (20, 21). However, much uncertainty surrounds the role of H4K20 methylation in the focal recruitment of 53BP1 upon replication stress. Our study shows that upon HU treatment, H4K20 becomes trimethylated in bulk, and this trimethylation is dependent upon WHSC1. It has been recently shown that replication stress induces the relocalization of 53BP1 into nuclear foci (22). According to our observations, the methyl transferase activity of WHSC1 is essential for 53BP1 localization to sites of replication stress. Consistent with this finding, we found that WHSC1 was also required for CHK2 phosphorylation upon replication stress. The defect in CHK2 activation in the absence of WHSC1 is consistent with the dysregulation of 53BP1 recruitment to sites of replication stress, given that 53BP1−/− cells have impaired CHK2 activation. During the course of the study, WHSC1 was also found to control 53BP1 localization in response to IR (23).
Our findings suggest that control of trimethylation of the H4K20 residue by WHSC1 may be required for 53BP1 recruitment to sites of DNA replication stress. Alternatively, a WHSC1 methylation substrate other than H4K20 could be responsible for the recruitment. Although it has been shown in vitro that 53BP1 binds H4K20me2 and does not bind H4K20me3 (21), it is possible that in vivo, in the context of a replication stress, the trimethylation marks function in the initial 53BP1 recruitment directly or indirectly. One unexplained observation by Jackson and coworkers (24), and also observed here, is that the bulk H4K20me2 appears to be converted to H4K20me3 in response to HU-induced replication stress. If 53BP1 requires H4K20me2, converting it to H4K20me3 should interfere with 53BP1 localization. Instead, if we deplete WHSC1, which allows H4K20me2 to be maintained in the presence of replication stress, 53BP1 no longer localizes to replication stress foci. Either the abundance of H4K20me2 at sites of replication stress behaves differently from bulk H4K20me2, or the requirements for 53BP1 localization after replication stress are different from those previously thought.
This study describes a role for the SET methyltransferase domain of WHSC1 in the localization of the mammalian DDR factor 53BP1 to chromosomal sites experiencing replicational stress, and the activation of the Chk2 kinase. The discovery of a WHSC1-dependent H4K20 trimethylation upon replication stress uncovers an added level of complexity in replicational stress response processes. We propose that WHSC1’s role in the DNA damage and DNA replication stress response is a cause of the developmental and neurological impairment observed in Wolf–Hirschhorn syndrome patients, thereby linking WHS to other known DDR syndromes, such as Seckel and A-T.
Experimental Procedures
Cell Culture, Plasmids, and Antibodies.
HCT116 and U2OS cells were grown in DMEM supplemented with 10% (vol/vol) FBS (Invitrogen), 100 units/mL penicillin, and 0.1 mg/mL streptomycin WHSC1 in pDONR223 was recombined into appropriate recipient vectors using LR Clonase (Invitrogen). Mutagenesis was performed with the QuikChange Site-Directed Mutagenesis Kit using the following primers: shRNA-resistant WHSC1: ccccaccatacaagcacatcaaggtgaataaaccgtatggaaaagtccagatctacac; siRNA-resistant WHSC1: ctggggcgcaccaaacaattgcacgtgatactggcgtg; WHSC1 SET Y1092A: gggagaatttgttaacgaggccgttggggagctgatcgac. The antibodies used in this study were as follows: P-Chk1 (S317) Cell Signaling, no. 2344S; H4K20me2 Cell Signaling no. 9759S; H4K20me3 Millipore 07-463; WHSC1 Abcam ab28470-50; P-H2AX Millipore 05-636; 53BP1 Bethyl A300-272A; Phospho-Chk2(T68) Cell Signaling no. 2661S; GAPDH Santa Cruz sc-25778; Phospho-RPA32 Bethyl 800-338-9579. Plasmid DNA and siRNA transfections were performed using Lipofectamine 2000 or RNAiMAX, respectively, as suggested by the manufacturer, with the final siRNA concentration of 20 nM. WHSC1-targeting siRNA and shRNA sequences used in this study were as follows: siRNA: uauagcugugaaggaguggaucugc (10620319; Invitrogen); shRNA: ttcccgtaaggcttattacct.
Laser-Induced Damage and Immunofluorescence.
Laser microirradiation and immunofluorescent studies were performed as described previously (25, 26).
Sensitiviy to DNA Damaging Agents.
WHSC1-silenced and -nontargeting siRNA or shRNA (FF)-transfected HCT116 cells were plated onto 96-well plates and treated with MMS (methyl methanesulfonate), Camptothecin, HU, and UV as indicated. Two days later, the viability of the cells was determined using the CellTiter-Blue reagent (Promega), and the average of four experiments was plotted.
Western Blots and Immunoprecipitation.
HCT116 cells were transfected with different combinations of expression plasmids, shRNA-expressing plasmid, or siRNA as indicated. At 48 h later, the cultures were split, treated with 500 μM HU for 24 h, and released into HU-free media for another 6 h. Cells were collected at the indicated times and lysed in buffer A [10 mM Tris·HCl (pH 7.6), 1 mM EDTA, 400 mM NaCl, 15% glycerol, 0.5% Nonidet P-40, phosphatase inhibitor cocktail (4906837001; Roche), and Complete Mini Protease Inhibitor Tablet (1 836 153; Roche)].
Acknowledgments
We thank the members of the S.J.E. laboratory for advice and reagents. This work was supported by grants from the National Institutes of Health and the Department of Defense. S.J.E., I.H., and A.C. are long-term postdoctoral fellows of the European Molecular Biology Organization. S.J.E. is an investigator with the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
References
- 1.Harper JW, Elledge SJ. The DNA damage response: Ten years after. Mol Cell. 2007;28:739–745. doi: 10.1016/j.molcel.2007.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–1078. doi: 10.1038/nature08467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciccia A, Elledge SJ. The DNA damage response: Making it safe to play with knives. Mol Cell. 2010;40:179–204. doi: 10.1016/j.molcel.2010.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee JH, Paull TT. ATM activation by DNA double-strand breaks through the Mre11-Rad50-Nbs1 complex. Science. 2005;308:551–554. doi: 10.1126/science.1108297. [DOI] [PubMed] [Google Scholar]
- 5.Stucki M, Jackson SP. gammaH2AX and MDC1: Anchoring the DNA-damage-response machinery to broken chromosomes. DNA Repair (Amst) 2006;5:534–543. doi: 10.1016/j.dnarep.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 6.Huen MS, et al. RNF8 transduces the DNA-damage signal via histone ubiquitylation and checkpoint protein assembly. Cell. 2007;131:901–914. doi: 10.1016/j.cell.2007.09.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cotta-Ramusino C, et al. A DNA damage response screen identifies RHINO, a 9-1-1 and TopBP1 interacting protein required for ATR signaling. Science. 2011;332:1313–1317. doi: 10.1126/science.1203430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang XH, Zou L. Checkpoint and coordinated cellular responses to DNA damage. Results Probl Cell Differ. 2006;42:65–92. [PubMed] [Google Scholar]
- 9.Zou L, Elledge SJ. Sensing DNA damage through ATRIP recognition of RPA-ssDNA complexes. Science. 2003;300:1542–1548. doi: 10.1126/science.1083430. [DOI] [PubMed] [Google Scholar]
- 10.Biton S, Barzilai A, Shiloh Y. The neurological phenotype of ataxia-telangiectasia: Solving a persistent puzzle. DNA Repair (Amst) 2008;7:1028–1038. doi: 10.1016/j.dnarep.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 11.Katyal S, McKinnon PJ. DNA strand breaks, neurodegeneration and aging in the brain. Mech Ageing Dev. 2008;129:483–491. doi: 10.1016/j.mad.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerzendorfer C, O'Driscoll M. Human DNA damage response and repair deficiency syndromes: Linking genomic instability and cell cycle checkpoint proficiency. DNA Repair (Amst) 2009;8:1139–1152. doi: 10.1016/j.dnarep.2009.04.018. [DOI] [PubMed] [Google Scholar]
- 13.Battaglia A, Carey JC. Wolf–Hirschhorn syndrome and the 4p-related syndromes. Am J Med Genet C Semin Med Genet. 2008;148C:241–243. doi: 10.1002/ajmg.c.30189. [DOI] [PubMed] [Google Scholar]
- 14.Hanley-Lopez J, Estabrooks LL, Stiehm R. Antibody deficiency in Wolf–Hirschhorn syndrome. J Pediatr. 1998;133:141–143. doi: 10.1016/s0022-3476(98)70194-5. [DOI] [PubMed] [Google Scholar]
- 15.Hirschhorn K, Cooper HL. Chromosomal aberrations in human disease. A review of the status of cytogenetics in medicine. Am J Med. 1961;31:442–470. doi: 10.1016/0002-9343(61)90128-0. [DOI] [PubMed] [Google Scholar]
- 16.Keats JJ, Reiman T, Belch AR, Pilarski LM. Ten years and counting: So what do we know about t(4;14)(p16;q32) multiple myeloma. Leuk Lymphoma. 2006;47:2289–2300. doi: 10.1080/10428190600822128. [DOI] [PubMed] [Google Scholar]
- 17.Nimura K, et al. Histone H3 lysine 36 trimethyltransferase links Nkx2-5 to Wolf–Hirschhorn syndrome. Nature. 2009;460:287–291. doi: 10.1038/nature08086. [DOI] [PubMed] [Google Scholar]
- 18.Matsuoka S, et al. ATM and ATR substrate analysis reveals extensive protein networks responsive to DNA damage. Science. 2007;316:1160–1166. doi: 10.1126/science.1140321. [DOI] [PubMed] [Google Scholar]
- 19.Lu C, et al. Cell apoptosis: Requirement of H2AX in DNA ladder formation, but not for the activation of caspase-3. Mol Cell. 2006;23:121–132. doi: 10.1016/j.molcel.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Greeson NT, Sengupta R, Arida AR, Jenuwein T, Sanders SL. Di-methyl H4 lysine 20 targets the checkpoint protein Crb2 to sites of DNA damage. J Biol Chem. 2008;283:33168–33174. doi: 10.1074/jbc.M806857200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Botuyan MV, et al. Structural basis for the methylation state-specific recognition of histone H4-K20 by 53BP1 and Crb2 in DNA repair. Cell. 2006;127:1361–1373. doi: 10.1016/j.cell.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lukas C, et al. 53BP1 nuclear bodies form around DNA lesions generated by mitotic transmission of chromosomes under replication stress. Nat Cell Biol. 2011;13:243–253. doi: 10.1038/ncb2201. [DOI] [PubMed] [Google Scholar]
- 23.Pei H, et al. MMSET regulates histone H4K20 methylation and 53BP1 accumulation at DNA damage sites. Nature. 2011;470:124–128. doi: 10.1038/nature09658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tjeertes JV, Miller KM, Jackson SP. Screen for DNA-damage-responsive histone modifications identifies H3K9Ac and H3K56Ac in human cells. EMBO J. 2009;28:1878–1889. doi: 10.1038/emboj.2009.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bekker-Jensen S, et al. Spatial organization of the mammalian genome surveillance machinery in response to DNA strand breaks. J Cell Biol. 2006;173:195–206. doi: 10.1083/jcb.200510130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smogorzewska A, et al. Identification of the FANCI protein, a monoubiquitinated FANCD2 paralog required for DNA repair. Cell. 2007;129:289–301. doi: 10.1016/j.cell.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]