Abstract
Understanding the processes underlying the origin of species is a fundamental goal of biology. It is widely accepted that speciation requires an interruption of gene flow between populations: ongoing gene exchange is considered a major hindrance to population divergence and, ultimately, to the evolution of new species. Where a geographic barrier to reproductive isolation is lacking, a biological mechanism for speciation is required to counterbalance the homogenizing effect of gene flow. Speciation with initially strong gene flow is thought to be extremely rare, and few convincing empirical examples have been published. However, using phylogenetic, karyological, and ecological data for the flora of a minute oceanic island (Lord Howe Island, LHI), we demonstrate that speciation with gene flow may, in fact, be frequent in some instances and could account for one in five of the endemic plant species of LHI. We present 11 potential instances of species divergence with gene flow, including an in situ radiation of five species of Coprosma (Rubiaceae, the coffee family). These results, together with the speciation of Howea palms on LHI, challenge current views on the origin of species diversity.
Keywords: Metrosideros, sympatric speciation, geography of speciation
Speciation with strong gene flow is controversial among evolutionary biologists (1–3). Unlike speciation without gene flow (e.g., allopatric speciation and polyploid speciation) (4), it requires both divergent natural selection and a mechanism to promote nonrandom mating (5–7). Theoretically, these conditions might coincide via a pleiotropic magic trait, through linkage disequilibrium between genes involved in local adaptation and assortative mating, or because habitat differences within a species’ range produce divergent genetic adaptations as well as plastic responses that confer reproductive isolation (e.g., environmentally controlled shifts in flowering time) (3, 5, 7–10). The most controversial incarnation of speciation occurs in sympatry, when gene exchange is high. Numerous definitions of sympatric speciation have been proposed since it was first outlined by Charles Darwin (11). Population genetic definitions focus on random mating with respect to location or habitat of the mating partners. For the biogeographic definition, which we adopt here, the absence of geographic isolation is the key criterion. The relative merits and disadvantages of both views continue to be debated (1–3, 7, 8, 12, 13).
In plants, evidence for the influence of sympatric speciation with strong gene flow remains sparse, with a single study on the Howea palms of Lord Howe Island (LHI) presenting the only conclusive evidence (14, 15). On the other hand, sympatric speciation via polyploidization is well known in plants and is thought to have contributed significantly to species diversity but is not thought to involve ongoing gene flow (16). Very few studies have attempted to quantify the frequency of speciation without geographic isolation, and those that have indicate that it is exceptionally rare (17–19). Kisel and Barraclough (12) demonstrated that the geographic scale required for speciation is related to the spatial scale of gene flow, and so, for organisms to speciate on small islands, they must have very restricted dispersal. In animals, speciation at small spatial scales has been ruled out for some taxa [e.g., in island birds (18) and Caribbean Anolis lizards (19)].
In this study, we conduct an empirical assessment of the frequency of sympatric speciation in plants. We use the flora of the remote LHI as a model system and a set of strict criteria to classify speciation events. Coyne and Orr (8) proposed four criteria for confirmation of a sympatric speciation event: (i) species must be sister taxa; (ii) an allopatric phase in their divergence must be highly unlikely; (iii) species must occur in sympatry; and (iv) species must demonstrate reproductive isolation. Additionally, the sister relationships recovered in phylogenetic reconstructions must not be an artifact of hybridization (8). These criteria, which we apply here, provide a consistent framework for diagnosing sympatric speciation events.
LHI presents an ideal setting to test the frequency of sympatric speciation in plants, because the geography and isolation of the island renders an allopatric phase highly unlikely (14, 15, 20). The product of a shield volcano which erupted 6.9 Mya (21), this small (<16 km2), subtropical island is located 600 km east of Australia. Apart from Ball's Pyramid (a sea stack 551 m high and 0.2 km2 at its base) situated 24 km southeast of LHI, there are no other islands in the vicinity (21, 22). It has been proposed that the small size of the island relative to probable rates of gene flow and the lack of any physical barriers mean that speciation events occurring within the confines of the island fulfill the second and third criteria of Coyne and Orr (8, 14, 15, 20). Currently, the topography of the island is heterogeneous. In the south, Mt. Lidgbird (777 m) and Mt. Gower (875 m) dominate the skyline and support various habitats (21, 23). This variation allows LHI to accommodate a remarkable diversity of species, given its size: 242 vascular plant species have been recorded, 90 of which are endemic (23, 24). The LHI flora has been subject to a thorough taxonomic treatment (24), and current species delimitations are likely to be indicative of complete, or nearly complete, reproductive isolation between close relatives.
Here we combine phylogenetic, karyological, and ecological evidence to assess the contribution of alternative modes of speciation to the composition of the LHI flora. In particular, we test the extent to which the hypothesis of sympatric speciation with gene flow in Howea palms on LHI may be generalized to other native flora. We conduct molecular phylogenetic analyses of genera with more than one native species on the island, through which sister species groups (i.e., potential sympatric speciation events) can be identified. We then evaluate sister species against a range of evidence from molecular dating, chromosome counts, and ecology to support or refute the sister species as products of speciation in the face of gene flow. Previous studies investigating macroevolutionary patterns of sympatric speciation often used co-occurrence of congeneric species as proxies for speciation events (e.g., 12, 18); our study, however, directly assesses the evolutionary relationships of closely related species within an entire plant community.
Results
The native flora of LHI comprises 242 species in 179 genera. In 139 genera only a single species is present on LHI; of these species, 42 are endemic to the island. The remaining 40 genera include between two and six LHI species. In 13 cases, all species are endemic; in 15, all are non-endemic; and the remaining 12 genera contain a mixture of endemic and non-endemic species (Dataset S1).
Biogeographic Analyses of Source Regions for LHI Flora.
Several genera occurring on LHI are species rich and widespread. Biogeographic analyses were used to determine the most likely source regions for LHI species. In turn, this information established which regions are likely to harbor the sister species of LHI plants. The results (Dataset S1) show that dispersal from Australia is likely to have had a strong influence on the composition of LHI's flora (pAustralia = 0.38), with New Zealand, Norfolk Island, New Caledonia, and the Kermadec Islands also acting as significant source regions (pi = 0.15, 0.14, 0.10, and 0.03, respectively). Dispersal to LHI from these focal regions accounts for the majority of LHI species (pfocal regions = 0.81). The probability of a double colonization by two closely related species from a single region outside the focal regions is negligible ([pexcluding focal regions]2 ≤ 0.0009). As a result, we focused our phylogenetic sampling on these focal regions (SI Appendix, Table S1).
Evolutionary Relationships of LHI Species.
To assess the evolutionary relationships of co-occurring congeneric species on LHI, DNA sequence-based Bayesian analyses (25) were used to generate phylogenetic trees for 32 of the 40 genera with more than one species on LHI. Phylogenetic reconstruction for the remaining eight genera was not possible because material for the LHI species was unavailable. For the 32 genera, we analyzed 2,456 DNA sequences from GenBank and 294 new DNA sequences (SI Appendix, Tables S2–S31). This sampling included 518 species from the focal biogeographic regions (49% of the total for the 32 genera) and 545 species from other regions; all LHI species except Blechnum geniculatum were represented (SI Appendix, Table S1).
For seven genera (Coprosma, Geniostoma, Korthalsella, Melicope, Metrosideros, Peperomia, and Rytidosperma) data from both plastid (cpDNA) and nuclear ribosomal (nrDNA) genomes were available. In these cases BEAST analyses were applied to each data set separately. No hard incongruences in the placement of LHI taxa were evident (SI Appendix, Figs. S1–S7), and the data sets were combined for the final analyses. In the final analyses 18 sister relationships between LHI taxa were identified in 14 genera (SI Appendix, Figs. S8–S38). Molecular dating indicates that four of these divergence events (in Adiantum, Ophioglossum, Cheilanthes, and Pterostylis) are likely to have occurred before the formation of LHI. In three genera (Doodia, Macropiper, and Xylosma) the relationships between LHI species were not fully resolved. We found evidence for one instance of hybrid speciation (26) among endemic species. In the genus Myrsine, cloned DNA shows that M. mccomishii carries sequences derived from M. myrtillina and another species, potentially M. platystigma. The non-endemic species Calystegia affinis, a species found only on LHI and Norfolk Island, also possessed mixed nrDNA sequences similar to C. soldanella (native to both islands) and C. marginata.
Species sampling within the focal regions for phylogenetic reconstruction ranged from 7 to 100% (SI Appendix, Table S1). We argue that phylogenetic reconstructions in which LHI taxa are not recovered as sister species pairs (n = 17) probably represent a true picture of non-sister species relationships, regardless of their level of sampling. The median of the sampling for these phylogenetic trees was 60%. As a result, in phylogenetic trees containing <60% of the focal region species, the recognition of LHI taxa as sister species may be unreliable.
Contributions of Speciation Modes to LHI Flora.
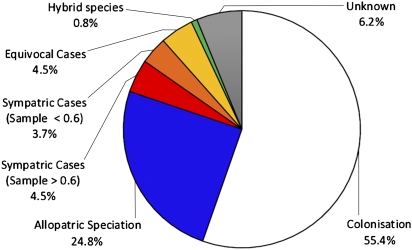
Each native species on the island was assigned to one of the following six categories (Materials and Methods): (i) sympatric speciation (Fig. 1A); (ii) allopatric speciation (Fig. 1C); (iii) colonization without speciation (Fig. 1D); (iv) hybrid species; (v) equivocal cases (Fig. 1 E–H); and (vi) unknown mode of divergence. The sympatric speciation results are reported in two categories of phylogenetic sampling (Fig. 2). We have calculated that at least 4.5% of the flora (seven in situ cladogenetic speciation events; Table 1) may be the product of sympatric speciation, that is, with sampling of congeneric taxa from the focal regions ≥60% (Fig. 2). Second, we have evaluated that as many as 8.2% of LHI species (12 in situ speciation events; Table 1) could be the result of sympatric speciation, i.e., for any level of phylogenetic sampling. Colonization followed by no speciation accounts for 55.4% of the species on the island, and allopatric speciation has contributed to the evolution of 24.8% of the island's plant species (Fig. 2). Equivocal cases represented 4.5% of the flora (Fig. 2).
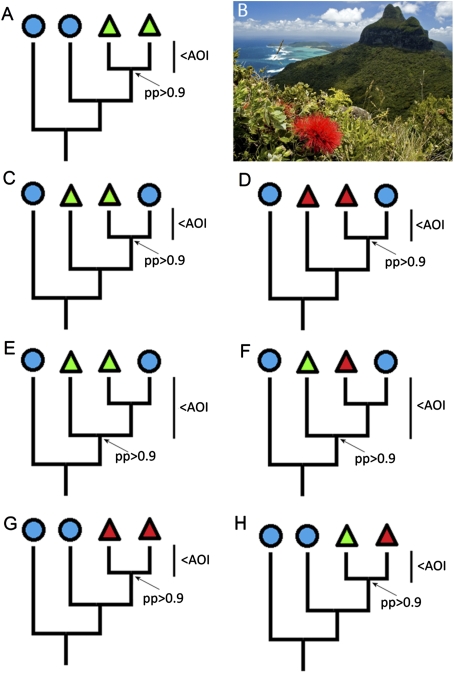
Fig. 1.
(A and C–H) Possible speciation scenarios on islands. Triangles represent native island species, with green for endemic species and red for non-endemics. Circles represent species not found on the island. Divergences are shown relative to the age of the island (AOI). Posterior probabilities (pp) are indicated for nodes of interest. (A) Sympatric speciation. (C) Allopatric speciation. (D) Colonization without speciation. (E–H) Equivocal scenarios. (B) Metrosideros nervulosa growing on Mt. Gower, LHI.
Fig. 2.
Origins of the LHI flora. Half of the colonization events did not lead to speciation. When speciation did occur (i.e., among endemic species), 12–22% of the resulting species are the products of sympatric divergences. At least 3.7% of all native LHI species are derived from speciation with gene flow events, a subset of sympatric speciation events, equivalent to 10% of the endemic flora (not shown on chart). Phylogenetic trees are given in SI Appendix, Figs. S8–S38.
Table 1.
Putative products of sympatric speciation
| Species | Node age (million y)* | Approximate elevation range (meters) | Principal habitat | 2n† |
| Coprosma huttoniana (0.80‡) | 2.08 | 437–857 | Abundant tree in cloud forests | 44 |
| C. lanceolaris | 5.04 | 131–852 | Common along wet cliff faces | 44 |
| C. putida | 1.34 | 0–860 | Understory tree in lowland forest | 44 |
| C. inopinata | 4.05 | 760–780 | Rare cliff dwelling species | — |
| C. sp. nov. | 1.34 | 142–532 | Exposed north facing sites | 44 |
| Howea forsteriana (1.00‡) | 1.08 | 0–400 | Common on calcarenite soil | 32 |
| H.belmoreana | 1.08 | 0–500 | Restricted to volcanic soil | 32 |
| Metrosideros nervulosa (1.00‡) | 3.53 | 57–875 | Exposed sites above 350m, summits | 22 |
| M. sclerocarpa | 3.53 | 10–481 | Wet valleys below 350m | 22 |
| Polystichum moorei (1.00‡) | 4.76 | 0–50, 400 | Under basalt overhangs | — |
| P. whiteleggei | 4.76 | 300–600 | Flanks of mountain summits | — |
| Alyxia squamulosa (0.16‡) | 2.19 | 600–875 | Mountain summits | — |
| A. lindii | 2.19 | 0–300 | Common in northern hills | — |
| Asplenium milnei (0.54‡) | 6.47 | 0–875 | Lowland mixed forest | — |
| A. pteridoides | 1.09 | 600–875 | Forest at high altitudes | — |
| A. surrogatum | 1.09 | 50–875 | Southern wet areas | — |
| Geniostoma huttonii (0.16‡) | 4.37 | 500–800 | Remote ridges of mountains | — |
| G. petiolosum | 4.37 | 0–500 | Sheltered lowland mixed forest | — |
| Grammitis nudicarpa (0.48‡) | 2.93 | 700–875 | Densely shaded areas of summits | — |
| G. diminuta | 2.93 | 200–875 | Cloud forest | — |
All species are endemic.
*Values shown are upper 95% CI for time since divergence from nearest relative.
†Species chromosome number.
‡Proportion of members of each genus occurring in the five focal regions that were accounted for in this study.
Gene Flow and the Geological History of LHI.
We evaluated the potential for geographic isolation to occur in angiosperms on LHI by reconstructing the spatial extent of LHI in the past. At its largest, when sea levels were 100 m lower than they are now, LHI measured 690 km2, and Ball's Pyramid measured 231 km2. During these periods of low sea level the maximum distance from one point on LHI to another on Ball's Pyramid was 57 km, and the minimum distance between LHI and Ball's Pyramid was 4 km.
At the maximum scale of LHI and Ball's Pyramid in the past (i.e., maximum distance of 57 km), we found that the average fixation index (FST, mean = 0.11, median = 0.07) for wind-dispersed plants falls well below the level considered necessary for neutral divergence (FST = 0.20) (12). For such plants, speciation within the island pair may reasonably be considered sympatric. For plants dispersed by other means, average FST is high enough at the same scale to support isolation by distance (FST, mean = 0.24, median = 0.20), suggesting that speciation could have been facilitated by spatially restricted gene flow.
Genetic and Ecological Variation in Metrosideros and Coprosma on LHI.
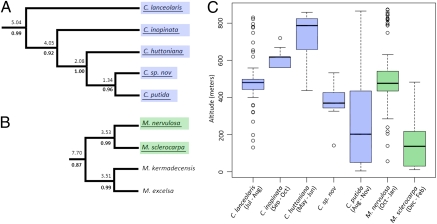
Two genera (Metrosideros and Coprosma) with well-sampled phylogenetic trees (100% and 80%, respectively) were investigated in more detail. A single sympatric speciation event was found in Metrosideros (Fig. 3B), whereas an in situ radiation with four speciation events was detected in Coprosma (Fig. 3A). Multiple accessions of each LHI species of Metrosideros and Coprosma were sequenced, confirming that are they distinct not only morphologically (24) but also genetically (Coyne and Orr's criterion 4), as shown by the parsimony phylogenetic reconstructions of these samples in SI Appendix, Figs. S39 and S40. Conventional cytological techniques were used to determine the chromosome numbers of endemic Metrosideros and Coprosma species (Table 1), indicating polyploidization was not involved in these speciation events. Metrosideros sclerocarpa occurs predominantly in wet environments surrounding creeks, whereas M. nervulosa (Fig. 1B) grows at higher elevations on exposed ridges and in the cloud forest (mean altitudes for these species are significantly different; P < 0.0001; Welch's t test; Fig. 3C). The flowering period for M. nervulosa (October–January) precedes that of M. sclerocarpa (December–February) (24). In Coprosma, species distributions are staggered along an altitudinal gradient (P < 0.0001; one-way ANOVA; Fig. 3C). Field observations indicate that flowering periods in these Coprosma are largely nonoverlapping: C. huttoniana flowers first (in May and June), C. lanceolaris second (in July and August), and C. inopinata flowers in September and October, with C. putida spanning its more distant relatives, flowering from August–November. Within each Metrosideros and Coprosma species, individuals at lower elevations flower slightly earlier in the season.
Fig. 3.
Ecological divergence in Coprosma and Metrosideros. (A and B) Phylogenetic subtrees with endemic LHI species underlined (full trees are shown in SI Appendix, Figs. S14 and S26). Numbers above branches are the upper 95% CI for the node ages. Numbers below branches are posterior probabilities. (C) Box plot depicting altitudinal distributions, showing the median (in bold type), interquartile range (box), and 1.5 times the interquartile range (bars); circles represent outliers (C. lanceolaris, n = 101; C. inopinata, n = 33; C. huttoniana, n = 46; C. sp. nov., n = 16; C. putida, n = 159; M. nervulosa, n = 99; M. sclerocarpa, n = 78). Flowering periods for each species are given in brackets.
Finally, to determine if sympatric speciation events may have played a widespread role in the evolution of Metrosideros and Coprosma, the age–range correlation approach of Barraclough and Vogler (17) was applied to the Metrosideros and Coprosma phylogenetic trees. In both genera the patterns recovered are consistent with occurrences of both sympatric (e.g., on LHI) and allopatric speciation at the geographic scale applied (SI Appendix, Fig. S41).
Discussion
Based on the relatedness of endemic LHI species and the timing of their divergences, it is plausible that at least seven sympatric speciation events have taken place on the island. Accounting for 4.5% on the island's flora, these events include four speciation events in the genus Coprosma and one each in Metrosideros, Howea, and the fern genus Polystichum. Polyploid speciation was not evident in Metrosideros and Coprosma, making them good candidates for speciation with gene flow; however, polyploid speciation could not be ruled out in Polystichum. For Metrosideros and Coprosma more detailed phylogenetic and ecological data were collected, including evidence of shifts in elevational ranges and flowering times which may have played important roles in the divergences observed. Ecological divergence and phenological differences have been described previously in the speciation of Howea forsteriana and H. belmoreana (14), and theoretical models support the potential for speciation with strong gene flow in this case (27). In addition to the seven well-supported cases, a further five speciation events (two in angiosperms and three in ferns) also may have occurred recently on LHI. Limited phylogenetic sampling within the latter genera leaves the possibility that these LHI species may not be each other's closest relatives. However, their consideration provides an upper bound to the prevalence of sympatric speciation and speciation in the face of gene flow within the groups studied. If one is willing to accept that these events have occurred on LHI, it is evident that sympatric speciation has played a greater role in the evolution of the LHI flora than may have been expected from studies of other organisms (18, 19, 28). Nevertheless, other processes account for the larger proportion of the islands species, leaving sympatric events in the minority (Fig. 2).
The identified speciation events represent beguiling cases for potential sympatric speciation and speciation with gene flow. However, a number of issues must be considered before accepting them as such. Hybridization, within-island allopatric divergence, multiple colonizations, and extinction all present potential problems when interpreting the data presented here.
Detecting Hybridization.
A caveat of Coyne and Orr's criteria is that the phylogenetic pattern defining sister species pairs must be genuine and not the result of hybridization between distant relatives. The multiple nuclear sequences found in Myrsine mccomishii point to a role for hybrid speciation in the evolution of Myrsine on LHI. This pattern was not observed in any other endemic species group. However, for the majority of genera, either nuclear or plastid DNA regions were available, but not both, and so it is not possible to rule out hybridization or chloroplast capture (8). When data from both genomes were available (i.e., for Coprosma, Geniostoma, and Metrosideros), congruence between nuclear and plastid genomes indicates that postcolonization hybridization is unlikely to have generated these patterns, although we cannot eliminate this possibility completely.
Geographic Isolation Within LHI.
Concerns have been raised that the LHI system does not rule out geographic isolation of populations within the island, because the island may have been larger in the past (29). Studies of the geology and bathymetry indicate that the volcanic areas of LHI currently above sea level were eroded rapidly into their current state within 1–2 million years, after of the initial eruption and since then have been buffered from significant wave erosion by the presence of coral reefs (22, 30, 31). These studies also indicate that neither LHI nor Ball's Pyramid has subsided, but changes in sea level during glacial periods would have increased the terrestrial extent of both to the limits of the sea mounts upon which they sit (22, 30, 31). It is questionable whether these increases in size would have generated greater ecological diversity (20) or habitats suitable for many of the species discussed here, because these species are largely restricted to volcanic soils. If sea level fluctuations during the Pleistocene had led to within-island allopatric speciation, we would expect to observe a correspondence between these climatic events and the divergence times of species pairs (Table 1 and SI Appendix, Figs. S8–S38). Although estimates of divergence time indicate these speciation events took place within the lifetime of LHI, the confidence intervals (CIs) are too large (commonly >2 million years) to confirm whether the speciation events coincided with glacial periods.
Kisel and Barraclough (12) evaluated the scale at which two populations can diverge into separate species with limited opposing gene flow. Our analyses of their data indicate that species with wind-mediated seed or pollen dispersal are likely to have higher gene flow over large distances than plants exploiting other modes of dispersal. This result has important implications for speciation on LHI, because it is possible that populations of plants without wind dispersal may have diverged as the result of geographic isolation. Among our potential cases of sympatric speciation on LHI, the well-sampled angiosperm groups possess some form of wind dispersal (pollen for Howea and Coprosma and seed for Metrosideros). In these groups, geographic distance between individuals is unlikely to have restricted gene flow. The dispersal mechanism of several other taxa remains unknown, with the exception of Alyxia (insect pollinated and bird dispersed) and Geniostoma (bird-mediated seed and pollen dispersal). Therefore, divergence with gene flow restricted by geographic distance between populations cannot be excluded in Alyxia and Geniostoma.
The two mountains on LHI may act as habitat islands for montane species, a possible source of geographic isolation within the island. Among the potential cases of sympatric speciation (Table 1), all the montane species have been observed on both Mt. Lidgbird and Mt. Gower; an indication that this form of isolation is unlikely to have driven these speciation events. However, at this stage it is unclear whether mountaintop populations of the same species (e.g., Alyxia squamulosa) have differentiated genetically.
Currently, vegetation on Ball's Pyramid is sparse. The exposed, sheer cliff faces of the rock spire are inhospitable to most LHI species. Plant life is limited to the endemic shrub Melaleuca howeana (the host plant of the recently rediscovered LHI stick insect, Dryococelus australis), two herb species (Achyranthes aspera and Tetragonia tetragonioides), a sedge (Cyperus lucidus), and a grass (Sporobolus virginicus), all of which also occur on LHI (24, 32). Genetic analyses of these populations would provide direct evidence for the level of isolation of LHI and Ball's Pyramid and shed further light on the potential for allopatric speciation in this system.
Multiple Colonizations as an Alternative to Sympatric Speciation.
Double colonization, rather than in situ speciation, could potentially explain the existence of endemic sister species on LHI. Given enough genetic data, it should be possible to distinguish the phylogenetic pattern generated by in situ speciation, i.e., [(LHI endemic A, LHI endemic B), source population C] from that arising from two independent colonizations from the same source population, i.e., A (B, C). Unfortunately, this distinction may be disrupted by extinction of the source population, failure to sample the source population, or postcolonization hybridization. None of these factors can be ruled out conclusively. Nevertheless, there is some evidence that multiple colonizations by close relatives are unlikely (i.e., double colonization by very close relatives is apparently infrequent among non-endemic species; only the highly vagile Paspalum species have diverged within the last 6.9 million years).
It is possible to assess whether multiple colonization may have had a significant impact on our results. It is reasonable to assume that all non-endemic LHI species have been introduced via separate colonization events. Each endemic species could have resulted from either a unique colonization or an in situ divergence. For the two groups (endemic species versus nonendemic species), we calculated the number of species that co-occur with at least one congeneric species on LHI. If we adopt a null hypothesis that in situ divergence has not taken place, we would expect the frequency of non-endemic congeneric species (FCON) to be similar to the frequency of endemic congeneric species (FCOE). If sympatric speciation has occurred, FCOE should be higher than FCON because of the additive effects from multiple colonizations and in situ speciation events. We found that FCOE is significantly higher than FCON (P < 0.01; two-way z-test): 34.2% of the 152 non-endemic species but 56.7% of the 90 endemic species are accompanied by a congeneric species. We suggest that the higher FCOE is the result of sympatric speciation events among endemic species.
Extinction.
Extinction could have two possible consequences for our results. As mentioned previously, the sister species of an LHI endemic may have existed elsewhere and subsequently gone extinct, resulting in the spurious detection of sister relationships and overestimation of the rate of sympatric speciation. The effect of this occurrence probably is limited, because double colonization of the island is unlikely. Second, species that evolved via sympatric speciation may have gone extinct subsequently, leaving an underestimate of this mode of speciation. Unfortunately, without direct evidence (e.g., fossils), it is impossible to quantify these effects precisely.
Speciation with Gene Flow in Coprosma and Metrosideros.
Polyploidization is well documented in plants and may have acted as an isolating mechanism in the LHI taxa (Table 1) (16, 26). Polyploidization has not been recorded in Metrosideros but had been confirmed in 12 Coprosma species (33) before this study. We were able to exclude polyploid speciation in both genera on LHI (Table 1), suggesting that speciation in these groups has occurred despite high levels of gene flow. Polyploidization has been ruled out previously in the Howea palms (14).
Ecological and altitudinal separation of species is observed in Metrosideros and Coprosma (Table 1 and Fig. 3). The genus Coprosma possesses low rates of concerted evolution, leaving hybrid species with copies of nuclear genes from both parent species (34). A single accession identified morphologically as C. huttoniana growing at 600 m (the lower limit of this species’ range) does possess both C. huttoniana and C. putida copies of nrDNA, suggesting hybridization between these species does occur in sympatric zones but is unlikely to be responsible for the origin of C. huttoniana as a species.
For Metrosideros and Coprosma, we mapped species distributions along transects throughout the island and confirmed that species are found in close proximity. However, species within each genus display some habitat and phenological differentiation. The difference in ecology between Metrosideros species has interesting parallels with the bog and mountain ecotypes found within the Hawaiian Metrosideros polymorpha complex (35). For Metrosideros and Coprosma the available data are consistent with a scenario of ecological speciation under which colonization and local adaptation to new habitats leads to postzygotic reproductive isolation through reduced fitness of migrants and hybrids. Prezygotic isolation via altitudinal shifts in flowering time, either as a plastic or genetic adaptation, may precipitate population differentiation (9, 14, 15, 36). Although the populations are physically in parapatry along this gradient, unrestricted pollen and seed dispersal means that the frequency of encounters between the populations will be high, characteristic of sympatric speciation (3). Ecological (Table 1) and phenological shifts also are found in Alyxia (A. lindii, November–February; A. squamulosa, October–January) and Geniostoma (G. huttonii, January–March; G. petiolosum, September–December). The coincidence of ecological and phenological shifts is common in plants and is an indication that the factors promoting sympatric speciation in plants on LHI may not be unique to this system. Rather, they may be typical of any ecologically complex, isolated island.
The age–range correlation data indicate that allopatry played a role when Coprosma and Metrosideros colonized numerous Pacific islands and presumably speciated via anagenesis, whereas sympatric speciation has occurred within islands and continents. Age–range correlation methods have a number of inherent problems (37), and the results of this analysis should be approached with caution. However, this analysis further indicates that sympatric speciation is likely to have been a feature of the evolution of Metrosideros and Coprosma beyond LHI.
Conclusion
After decades of debate as to whether speciation can occur in the absence of geographic isolation (4–6, 10, 38), we have shown that it may occur frequently in plants on LHI. The speciation events in Metrosideros and Coprosma provide compelling evidence for at least five cases of speciation despite strong ongoing gene flow. The association between flowering time and altitude that is likely to have driven speciation in LHI's Metrosideros and Coprosma species may be widespread in other angiosperms and locations. For the other examples we present here (Table 1), more information is desirable. Nevertheless, we have set an upper bound for the frequency of sympatric speciation on LHI and a lower bound for the frequency of speciation with gene flow. Our results for plants are contrary to those found in insular animals (18, 19), suggesting that speciation in the face of strong gene flow may be a botanical specialty.
Materials and Methods
Biogeographic Analyses.
We coded the presence or absence of all native LHI species in 24 biogeographic regions (Dataset S1). Regions were defined as described by van Balgooy (39) with minor modifications (SI Appendix, Table S32). Endemic species (n = 90) or those present in more than 10 regions (n = 30) were considered as uninformative. Data for 122 indigenous species were used to calculate the probability that each region (i) is the source of a single species selected at random from the LHI species pool (pi, Eq. 1).
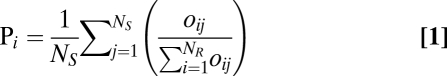 |
where Ns is the number of species included, NR is the number of source regions, and oi,,j is the presence (1) or absence (0) of species j in region i.
Molecular Phylogenetics.
In total 2,456 DNA sequences were downloaded from GenBank, and an additional 294 sequences were generated for this study using standard PCR and sequencing protocols (SI Appendix, Materials and Methods). Applying specific models of evolution for each gene region [assessed using MrModeltest v2.2 (40)], phylogenetic tree searches were conducted using BEAST v1.5.2 (25). Monte Carlo Markov chains for each tree search were run until the effective sample of all estimated parameters exceeded 200 (calculated using Tracer v1.5). When sister relationships were found among LHI species (SI Appendix, Figs. S8–S38), divergence times were estimated using molecular dating (25), calibrated with fossils and/or previously published divergence estimates (SI Appendix, Table S33). Details of tree search parameters are given in SI Appendix, Table S34, and calibration points are given in SI Appendix, Table S33. Maximum parsimony analyses were carried out as detailed in SI Appendix, Materials and Methods.
Categorization of Speciation Events.
Each species was assigned to one of six speciation categories using strict criteria. (i) Sympatric speciation, dependent on fulfillment of the following criteria: (a) LHI species are sister species in the phylogenetic tree; (b) both species are endemic; (c) the species relationship is strongly supported, i.e., by ≥0.9 posterior probability (41); (d) the upper 95% CI for the divergence time falls within the age of the island, i.e., 6.9 million years (Fig. 1). (ii) Allopatric speciation, i.e., endemics with no sister species on LHI. (iii) Colonization without speciation, i.e., non-endemic with no sister species on LHI. (iv) Hybrid species, i.e., species possessing mixed nrDNA sequences. (v) Events meeting a subset of the sympatric speciation criteria (c, d plus either criterion a or that at least one species is endemic) were considered as equivocal. (vi) Unknown mode of divergence, i.e., species with congeners present on LHI but without phylogenetic data to determine the evolutionary relationships of the species. In equivocal cases the phylogenetic results cannot distinguish between divergences in allopatry or in sympatry, either because of poor resolution in the phylogenetic trees or because the species are not endemic, and so their location of origin is ambiguous. The species derived from potential sympatric speciation events, the timing of their divergence, and the level of phylogenetic sampling are listed in Table 1.
Geological Reconstruction of LHI.
To explore the spatial extent of LHI in the past and to assess the possibility of allopatric speciation within the island, we used ArcGIS V9.2 (ESRI, 2009) to generate a digital elevation model for the current extent of LHI and Ball's Pyramid, as well as for the submerged shelves to 100 m below current sea level based on recent bathymetric measurements (30).
Gene Flow at the Spatial Scale of LHI.
Kisel and Barraclough (12) presented a meta-analysis of data describing FST at different spatial scales for various organisms. Using the methods of Kisel and Barraclough (12), their angiosperm data have been reanalyzed here in two partitions to reflect the potential differences in the spatial scale of gene flow for angiosperms using differing modes of pollination and seed dispersal (42). These divisions are (i) angiosperms with some form of wind-mediated pollen or seed dispersal and (ii) plants with no form of wind dispersal.
Ecological Analyses.
The species occurrences of Coprosma and Metrosideros were recorded along 15 random transects of LHI using an eTrex Summit HC (Garmin Ltd.). All known altitudinal records of C. inopinata were included. Flowering times, altitudinal ranges, and ecological preferences were recorded from field surveys carried out since 1980 and drawn from previously published data (23, 24). A one-way ANOVA was used to test for differences between the mean altitudes occupied by Coprosma species, and Welch's t test was used to compare species means for Metrosideros.
Age–Range Correlation Analyses.
Distribution maps of each species were drawn in ArcGIS V9.2 using dot maps and published descriptions of species’ ranges (references are contained in SI Appendix, Materials and Methods). The presence/absence of each species in quarter-degree squares then was extracted. The range overlap for each node in the respective phylogenetic trees was calculated and plotted against node age using the R statistical package (43) and an R script provided by T. Barraclough.
Supplementary Material
Acknowledgments
For help and advice we thank Tim Barraclough, Hank and Sue Bower, Elizabeth Brown, Mark Chase, Jerry Coyne, Aaron Davis, Céline Devaux, Christo Haselden, Ilia Leitch, Jackie Lighten, Eve Lucas, Seb Perceau-Wells, Ally Phillimore, Martyn Powell, Rhian Smith, Alfried Vogler, Peter Weston, Larry Wilson, the Lord Howe Island Board, the New South Wales National Parks and Wildlife Service, Mount Tomah Botanic Garden, the Royal Botanic Garden Trust Sydney, the editor and two anonymous reviewers. We thank the European Research Council, the Natural Environment Research Council (United Kingdom), the Leverhulme Trust, and the Royal Society for funding.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
Data deposition: The new DNA sequences reported in this paper have been deposited in the GenBank database (accession nos. JF950649–JF950942).
See Commentary on page 12975.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106085108/-/DCSupplemental.
References
- 1.Fitzpatrick BM, Fordyce JA, Gavrilets S. What, if anything, is sympatric speciation? J Evol Biol. 2008;21:1452–1459. doi: 10.1111/j.1420-9101.2008.01611.x. [DOI] [PubMed] [Google Scholar]
- 2.Butlin RK, Galindo J, Grahame JW. Review. Sympatric, parapatric or allopatric: The most important way to classify speciation? Philos Trans R Soc Lond B Biol Sci. 2008;363:2997–3007. doi: 10.1098/rstb.2008.0076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallet J, Meyer A, Nosil P, Feder JL. Space, sympatry and speciation. J Evol Biol. 2009;22:2332–2341. doi: 10.1111/j.1420-9101.2009.01816.x. [DOI] [PubMed] [Google Scholar]
- 4.Mayr E. Animal Species and Evolution. Cambridge, Massachusetts: Harvard Univ Press; 1963. [Google Scholar]
- 5.Dieckmann U, Doebeli M. On the origin of species by sympatric speciation. Nature. 1999;400:354–357. doi: 10.1038/22521. [DOI] [PubMed] [Google Scholar]
- 6.Coyne JA. Genetics and speciation. Nature. 1992;355:511–515. doi: 10.1038/355511a0. [DOI] [PubMed] [Google Scholar]
- 7.Gavrilets S. Perspective: Models of speciation: What have we learned in 40 years? Evolution. 2003;57:2197–2215. doi: 10.1111/j.0014-3820.2003.tb00233.x. [DOI] [PubMed] [Google Scholar]
- 8.Coyne JA, Orr HA. Speciation. 1st Ed. Massachusetts: Sinauer Associates; 2004. p. 545. [Google Scholar]
- 9.Devaux C, Lande R. Selection on variance in flowering time within and among individuals. Evolution. 2010;64:1311–1320. doi: 10.1111/j.1558-5646.2009.00895.x. [DOI] [PubMed] [Google Scholar]
- 10.Tregenza T, Butlin RK. Speciation without isolation. Nature. 1999;400:311–312. doi: 10.1038/22419. [DOI] [PubMed] [Google Scholar]
- 11.Darwin C. On the Origin of Species by Means of Natural Selection or the Preservation of Favoured Races in the Struggle for Life. London: J. Murray; 1859. [PMC free article] [PubMed] [Google Scholar]
- 12.Kisel Y, Barraclough TG. Speciation has a spatial scale that depends on levels of gene flow. Am Nat. 2010;175:316–334. doi: 10.1086/650369. [DOI] [PubMed] [Google Scholar]
- 13.Fitzpatrick BM, Fordyce JA, Gavrilets S. Pattern, process and geographic modes of speciation. J Evol Biol. 2009;22:2342–2347. doi: 10.1111/j.1420-9101.2009.01833.x. [DOI] [PubMed] [Google Scholar]
- 14.Savolainen V, et al. Sympatric speciation in palms on an oceanic island. Nature. 2006;441:210–213. doi: 10.1038/nature04566. [DOI] [PubMed] [Google Scholar]
- 15.Babik W, et al. How sympatric is speciation in the Howea palms of Lord Howe Island? Mol Ecol. 2009;18:3629–3638. doi: 10.1111/j.1365-294X.2009.04306.x. [DOI] [PubMed] [Google Scholar]
- 16.Rieseberg LH, Willis JH. Plant speciation. Science. 2007;317:910–914. doi: 10.1126/science.1137729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barraclough TG, Vogler AP. Detecting the geographical pattern of speciation from species-level phylogenies. Am Nat. 2000;155:419–434. doi: 10.1086/303332. [DOI] [PubMed] [Google Scholar]
- 18.Coyne JA, Price TD. Little evidence for sympatric speciation in island birds. Evolution. 2000;54:2166–2171. doi: 10.1111/j.0014-3820.2000.tb01260.x. [DOI] [PubMed] [Google Scholar]
- 19.Losos JB, Schluter D. Analysis of an evolutionary species-area relationship. Nature. 2000;408:847–850. doi: 10.1038/35048558. [DOI] [PubMed] [Google Scholar]
- 20.Stuessy TF. Evolutionary biology: Sympatric plant speciation in islands? Nature. 2006;443:E12–E13, discussion E12–E13. doi: 10.1038/nature05216. [DOI] [PubMed] [Google Scholar]
- 21.McDougall I, Embleton BJJ, Stonea DB. Origin and evolution of Lord Howe Island, southwest Pacific Ocean. Aust J Earth Sci. 1981;28:155–176. [Google Scholar]
- 22.Woodroffe CD, Kennedy DM, Brooke BP, Dickson ME. Geomorphological evolution of Lord Howe Island and carbonate production at the latitudinal limit to reef growth. J Coast Res. 2006;22:188–201. [Google Scholar]
- 23.Pickard J. Vegetation of Lord Howe Island. Cunninghamia. 1983;1:133–265. [Google Scholar]
- 24.Green PS. Flora of Australia. Vol. 49. Canberra, Australia: Australian Government Publishing Service; 1994. Oceanic Islands 1. [Google Scholar]
- 25.Drummond AJ, Rambaut A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol Biol. 2007;7:214. doi: 10.1186/1471-2148-7-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mallet J. Hybrid speciation. Nature. 2007;446:279–283. doi: 10.1038/nature05706. [DOI] [PubMed] [Google Scholar]
- 27.Gavrilets S, Vose A. Case studies and mathematical models of ecological speciation. 2. Palms on an oceanic island. Mol Ecol. 2007;16:2910–2921. doi: 10.1111/j.1365-294X.2007.03304.x. [DOI] [PubMed] [Google Scholar]
- 28.Phillimore AB, et al. Sympatric speciation in birds is rare: Insights from range data and simulations. Am Nat. 2008;171:646–657. doi: 10.1086/587074. [DOI] [PubMed] [Google Scholar]
- 29.Stuessy TF. Evolutionary biology: Sympatric plant speciation in islands? Nature. 2006;443:E12. doi: 10.1038/nature05216. discussion E12–E13. [DOI] [PubMed] [Google Scholar]
- 30.Kennedy D. Carbonate sedimentation on subtropical shelves around Lord Howe Island and Balls Pyramid, Southwest Pacific. Mar Geol. 2002;188:333–349. [Google Scholar]
- 31.Brooke B. Quaternary calcarenite stratigraphy on Lord Howe Island, southwestern Pacific Ocean and the record of coastal carbonate deposition. Quat Sci Rev. 2003;22:859–880. [Google Scholar]
- 32.Priddel D, Carlile N, Humphrey M, Fellenberg S, Hiscox D. Rediscovery of the ‘extinct’ Lord Howe Island stick-insect (Dryococelus australis (Montrouzier)) (Phasmatodea) and recommendations for its conservation. Biodivers Conserv. 2003;12:1391–1403. [Google Scholar]
- 33.Beuzenberg E. Contributions to a chromosome atlas of the New Zealand flora – 24 Coprosma (Rubiaceae) NZ J Bot. 1983;21:9–12. [Google Scholar]
- 34.Wichman SR, Wright SD, Cameron EK, Keeling DJ, Gardner RC. Elevated genetic heterogeneity and Pleistocene climatic instability: Inferences from nrDNA in New Zealand Coprosma (Rubiaceae) J Biogeogr. 2002;29:943–954. [Google Scholar]
- 35.Wright ME, Ranker TA. Dispersal and habitat fidelity of bog and forest growth forms of Hawaiian Metrosideros (Myrtaceae) Bot J Linn Soc. 2010;162:558–571. [Google Scholar]
- 36.Schluter D. The Ecology of Adaptive Radiation. Oxford: Oxford Univ Press; 2000. [Google Scholar]
- 37.Fitzpatrick BM, Turelli M. The geography of mammalian speciation: Mixed signals from phylogenies and range maps. Evolution. 2006;60:601–615. [PubMed] [Google Scholar]
- 38.Bush GL. Sympatric speciation in animals: New wine in old bottles. Trends Ecol Evol. 1994;9:285–288. doi: 10.1016/0169-5347(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 39.van Balgooy MMJ. Plant geography of the Pacific based on a census of phanerogam genera. Blumea. 1971;6(Suppl):1–222. [Google Scholar]
- 40.Nylander JAA. Uppsala, Sweden: Program distributed by the author; 2004. MrModeltest v2.2. [Google Scholar]
- 41.Erixon P, Svennblad B, Britton T, Oxelman B. Reliability of Bayesian posterior probabilities and bootstrap frequencies in phylogenetics. Syst Biol. 2003;52:665–673. doi: 10.1080/10635150390235485. [DOI] [PubMed] [Google Scholar]
- 42.García-Verdugo C, Forrest AD, Fay MF, Vargas P. The relevance of gene flow in metapopulation dynamics of an oceanic island endemic, Olea europaea subsp. guanchica. Evolution. 2010;64:3525–3536. doi: 10.1111/j.1558-5646.2010.01091.x. [DOI] [PubMed] [Google Scholar]
- 43.R Development Core Team . R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.