Abstract
The major mechanism for generating diversity of neuronal connections beyond their genetic determination is the activity-dependent stabilization and selective elimination of the initially overproduced synapses [Changeux JP, Danchin A (1976) Nature 264:705–712]. The largest number of supranumerary synapses has been recorded in the cerebral cortex of human and nonhuman primates. It is generally accepted that synaptic pruning in the cerebral cortex, including prefrontal areas, occurs at puberty and is completed during early adolescence [Huttenlocher PR, et al. (1979) Brain Res 163:195–205]. In the present study we analyzed synaptic spine density on the dendrites of layer IIIC cortico–cortical and layer V cortico–subcortical projecting pyramidal neurons in a large sample of human prefrontal cortices in subjects ranging in age from newborn to 91 y. We confirm that dendritic spine density in childhood exceeds adult values by two- to threefold and begins to decrease during puberty. However, we also obtained evidence that overproduction and developmental remodeling, including substantial elimination of synaptic spines, continues beyond adolescence and throughout the third decade of life before stabilizing at the adult level. Such an extraordinarily long phase of developmental reorganization of cortical neuronal circuitry has implications for understanding the effect of environmental impact on the development of human cognitive and emotional capacities as well as the late onset of human-specific neuropsychiatric disorders.
Keywords: association cortex, critical period, schizophrenia, synaptogenesis
Selective stabilization of developing synapses as a mechanism for specification of neuronal connections was proposed more than 4 decades ago (1). This hypothesis gained considerable support from the discovery that synaptic connections in the cerebral cortex of human and nonhuman primates initially are overproduced to about two times the adult number and are then pruned during puberty to reach the adult level at the onset of adolescence (2–5). The selective-elimination hypothesis basically assumes that during a period of overproduction of synapses neuronal activity tunes the molecular structure of individual synapses and determines which will be retained and which removed from the neural network (6, 7). Previous EM analyses in nonhuman primates revealed that synaptic elimination in the monkey prefrontal cortex occurs mainly by removal of asymmetric synapses on spines, whereas the number of symmetric synapses on dendritic shafts remains constant (5, 8).
The tempo of elimination of supranumerary synaptic spines and the identification of the end of this critical period are extremely important, because these factors are related to establishment of cognitive abilities and duration of the window for optimal acquisition of new language and mathematical skills as well as personality transformation from the developmental mode to adult status. In addition, the several leading hypotheses for the explanation of late-onset neuropsychiatric disorders, such as schizophrenia and drug- or stress-induced psychoses, implicate defective pruning of the initially overproduced synapses on dendritic spines (8–15). Furthermore, spine dysgenesis is the only detectable anatomical phenotype in some human cognitive disorders such as nonsyndromic mental retardation (16). Finally, this biomedically and socially important concept (9) is also the subject of continuing dialogue between proponents of selectionism versus constructionism (17).
The end of the critical period of synaptic spine elimination in the human cortex basically relies on the pioneering study of Huttenlocher and colleagues (2, 4). Thus, it usually is assumed tacitly that the period of synaptic overproduction in the human cerebral cortex is completed by the end of puberty (18), even though Huttenlocher's study contains only a single 19-y-old brain specimen in the age group between 15 and 32 y. In contrast, more recent studies using electroencephalography (15, 19), PET (20), and functional MRI (18, 21–26) have suggested that the dynamic changes in gray matter density and white matter integrity in the human association neocortex extend into the third decade of life (21, 23, 26, 27). These changes, observed by MRI, cannot be explained by a capacious increase in dendritic length, because the dendritic growth in the human neocortex is limited mainly to early childhood (28). Therefore, it has been assumed that functional plasticity probably reflects reorganization of circuitry, including synaptic elimination (29–32), essential for acquisition of the highest brain functions in humans, including affective modulation of emotional cues, self-conceptualization, mentalization, cognitive flexibility, and working memory (33–36). However, the cellular data supporting this assumption have been missing.
Results
To fill this gap in our knowledge, we analyzed the initial overproduction and subsequent elimination of dendritic spines on layer IIIc and layer V pyramidal neurons in the dorsolateral prefrontal cortex [Brodmann area 9 (28, 37)]. Our focus was on the prefrontal cortex because of the relevance of this region for late-onset, human-specific, neuropsychiatric disorders and the possible implications in understanding the mechanisms of environmental impacts such as education and training on prolonged development of human cognitive capacities (8–12, 38–41). Because dendritic spines are impregnated reliably by the rapid Golgi methods in postmortem brain tissue (Material and Methods), we decided to examine their development and measure their density on dendrites in well-preserved human tissue, including ages that were not analyzed in previous studies (Table S1).
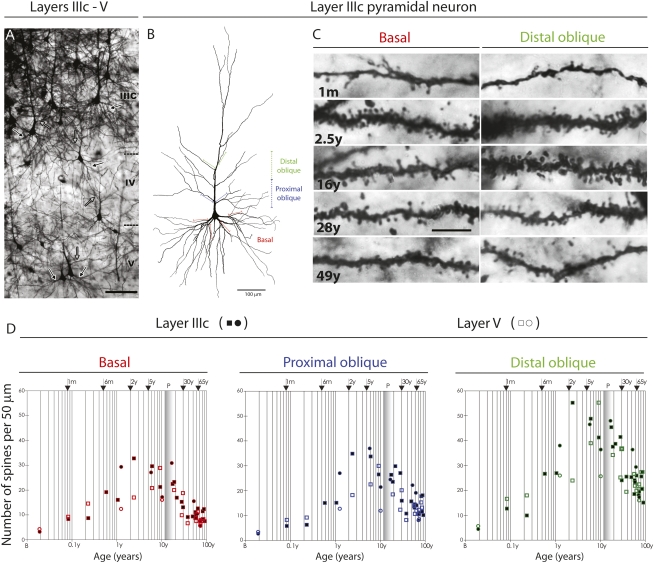
We selected to focus on the large pyramidal cells in layer IIIc and layer V (28), that form cortico–cortical and subcortical projections, respectively (Fig. 1A and Figs. S1 and S2). The dendrites were defined by their division into basal and oblique branches emanating from the main apical shaft (Fig. 1 A and B). In addition, the oblique branches were subdivided into proximal and distal groups, and all types were quantified separately (Fig. 1C). We found that the vast majority of spines (about 90% after the neonatal period) belong to the mushroom type characterized by a neck 1.5–3.5 μm long and up to 0.5 μm thick that expands into a bulb with a radius of 1–2 μm. The morphology of the head and neck of mushroom spines remained relatively constant except in the late adolescent stage (age 16–20 y), when spine heads become slightly larger. The second type, hair-like thin spines, which lack an obvious bulb and have terminal expansions slightly wider than the neck, were observed predominantly during the first postnatal month, and their percentage diminished afterward. The percentage of the third type of dendritic spines, stubby spines with a broad neck, was low on both oblique and basal dendrites at all ages analyzed (Fig. 1C).
Fig. 1.
(A) Representative low-magnification photographs of the rapid Golgi-impregnated layer IIIc and V pyramidal cells in the dorsolateral prefrontal cortex of a 16-y-old subject. Black arrows indicate basal dendrites, and gray arrows indicate oblique dendrites. (Scale bar: 100 μm.) (B) Neurolucida reconstruction of layer IIIc pyramidal neuron of a 49-y-old subject, illustrating sites selected for counting spines over a 50-μm length of apical distal oblique dendrites (green), apical proximal oblique dendrites (blue), and basal dendrites (red). (Scale bar: 100 μm.) (C) Representative high-power magnification images of rapid Golgi-impregnated layer IIIc pyramidal neurons from the dorsolateral prefrontal cortex showing basal dendrites (Left) and distal apical oblique dendrites (Right) during different stages: an infant 1 mo of age, a 2.5-y-old child, and 16-y-old, 28-y-old, and 49-y-old subjects. (Scale bar: 10 μm.) (D) Graphs representing number of dendritic spines per 50-μm dendrite segment on basal dendrites after the first bifurcation (red); apical proximal oblique dendrites originating within 100 μm from the apical main shaft (blue); and apical distal oblique dendrites originating within the second 100-μm segment from the apical main shaft (green) of layer IIIc (filled symbols) and layer V (open symbols) pyramidal cells in the dorsolateral prefrontal cortex. Squares represent males; circles represent females. The age in postnatal years is shown on a logarithmic scale. Puberty is marked by a shaded bar. B, birth (fourth postnatal day); P, puberty. Specification of tissue analyzed is given in Table S1, and the positions of sections on which pyramidal neurons were measured are indicated on the reconstructed pyramidal neuron shown in B.
Statistical analysis of interindividual differences in dendritic spine density (DSD), using the a posteriori Student–Newman–Keuls test for multiple comparisons, revealed that in all dendritic segments of both layer IIIc and layer V pyramidal neurons, the DSD increased significantly during infancy and reached its peak during childhood, when the DSD was, on average, more than two times higher than in the adults (Figs. 1D, 2 A–C, and Table S2). Importantly, although the DSD diminished gradually during late childhood and adolescence (9–22 y), it remained significantly higher throughout this period than in the adult (Figs. 1D and 2).
Fig. 2.
The DSD, as defined in Fig. 1, plotted at the linear scale to illustrate the dynamics of changes occurring during the 100-y human lifespan. Regression curves fit the distribution of data from the basal dendrites (A), apical proximal oblique dendrites (B), and apical distal oblique dendrites (C) of pyramidal cells from layer IIIc and V. In all cases the equation of the curves is a double exponential function in the form: y = a*exp(−bt)+c*exp (−dt)+e, where a, b, c, d, and e are fixed coefficients, and t is time in years (Table S3).
The rate of decrease in DSD varied among dendritic segments. The highest DSD values for all segments in layer IIIc pyramidal neurons (Fig. S2) were reached by the age of 2.5–7 y (Fig. 1 C and D, Fig. S2, and Table S2). Although a significant decline of DSD in basal and proximal apical oblique dendrites started at age 7–9 y, the DSD of distal apical oblique dendrites did not decrease significantly until age 17 y (Figs. 1 C and D and 2 A–C). In all dendritic segments of layer IIIc pyramidal neurons, the DSD decreased to an adult level by age 30 y and remained stable thereafter (Figs. 1D and 2 and Table S2). In almost all subjects older than 30 y (n = 16), DSD values for all segments of layer IIIc pyramidal neurons were significantly lower than in subjects of a younger age group (15 m to 28 y; n = 11). Despite the relatively smaller sample and interindividual variability, the DSD on all segments of layer V pyramids clearly displayed the highest values between age 7 and 9 y before beginning to decline. Thus, the overall developmental course of the DSD was similar in both layer IIIc and layer V pyramidal neurons, and the DSD in both types attained the stable adult value around age 30 y (Figs. 1D and 2).
During the peak in number of dendritic spines (i.e., between 2 and 19 y of age), the DSD values were 25–40% higher in layer IIIc than in layer V pyramidal neurons (Fig. 2); the difference was larger on the apical proximal oblique dendrites (P = 0.04) than on apical distal oblique (P = 0.10) or basal dendrites (P = 0.12). Because both types of neurons attain the adult-like total dendritic length during the third postnatal year (28), our findings suggest that the number of overproduced spines is consistently higher on layer IIIc than on layer V pyramidal neurons. This difference probably reflects a more recent evolutionary history of layer IIIc pyramidal neurons, which in primates represent a major source of highly expanded ipsi- and contralateral associative cortico–cortical connections (28, 42–46).
The average DSD value remained relatively constant between 38 and 65 y of age for a particular segment type (Fig. 2). However, on layer IIIc neurons, the DSD was most prominent in the distal apical oblique dendrites (Fig. 2 and Table S2); those neurons display DSD values that are ∼20% higher in the adult prefrontal cortex than in layer V pyramidal neurons (P = 0.18 for apical distal oblique dendrites, P = 0.05 for apical proximal oblique dendrites, and P = 0.10 for basal dendrites) (Fig. 2). It should be noted that both basal and apical oblique dendrites of layer IIIc pyramid neurons are ∼10% longer than those of layer V neurons (28). Thus, the total number of dendritic spines remains greater in layer IIIc than in layer V pyramidal neurons both during development and in the adult prefrontal cortex.
Discussion
The present study provides three findings concerning maturation of the human prefrontal cortex: (i) the period of overproduction and elimination of dendritic spines on pyramidal neurons in this area extends to the third decade of life; (ii) the pruning of supernumerary dendritic spines is more pronounced in layer IIIc cortico–cortical neurons than in comparable segments of layer V subcortically projecting neurons; and (iii) for layer IIIc neurons, spine pruning begins earlier in basal and proximal apical oblique dendrites than in distal apical oblique dendrites.
Previous data from human and nonhuman primates showed somewhat higher synaptic overproduction in supragranular than in infragranular layers (8), results that are consistent with the data obtained in the present study. Furthermore, recent data on identified neurons obtained from both the rhesus monkey (47) and human (48) showed marked regional differences in the number of spines grown and pruned in the basal dendritic tree of layer III pyramidal neurons. The spine formation and elimination between sensory, association, and executive cortex in these studies displayed a similar pattern. However, the number of spines in the adult cortex was related to functional hierarchy, as was the number of spines overproduced, being highest in the prefrontal cortex and lowest in the primary sensory regions (47, 48).
In the present study we have not performed the regional comparison because of the lack of appropriate material. However, there is converging evidence that dynamics of synaptic overproduction and elimination differ among cytoarchitectonic areas both in humans and in nonhuman primates (2, 8, 47–49). Most of these studies indicate that the prefrontal cortex undergoes the largest overproduction and the slowest rate of elimination of all areas, an observation which is explained by the late evolutionary emergence of the prefrontal cortex (47). Although the present study provides data only for the prefrontal cortex, it reveals a difference in the rate of spine formation and elimination between two evolutionarily different pyramidal cell populations and their dendrites situated in the different layers within the same region. Thus, our findings support the notion that different types of microcircuitry may have different rates of synaptic formation and elimination. These findings are in line with the finding that reorganization of intracortical excitatory synaptic systems in macaque prefrontal cortex continues after puberty, when the cortico–cortical synapses reach maturity (49). Therefore, we propose that the most extensive and protracted overproduction in humans is related to the associative and intracortical excitatory network that becomes more represented across the functional hierarchy and markedly prominent in the areas of highest order, such as the prefrontal cortex (28, 43, 45, 46, 50).
Taken together, the previous data and the present findings strongly indicate that anatomical (23, 24, 26–28, 31) and functional changes (15, 18, 19, 21, 22, 25, 35) in the prefrontal cortex observed in vivo during late adolescence and young adulthood reflect the dynamic reorganization of synaptic circuitry rather than solely activity-dependent molecular tuning of the stable synaptic connections. Experimental studies performed in developing and adult rodents indicate that dendritic spines in the cerebral cortex are remarkably plastic initially but gradually become very stable, with the majority lasting throughout the entire lifespan (51–55). Analysis of the human frontal cortex also shows significant changes in synapse-associated molecules during the period of growth and strengthening of synaptic elements in childhood (32). Therefore, molecular tuning of synaptic strength during the formative years may be a major mechanism for the environmental effect on structural reorganization, including elimination of supernumerary spines and synapses (1, 6, 7). This hypothesis is supported by the finding that a peak in the expression of genes regulating neuronal development, including those that are associated with schizophrenia, occurs between age 15 and 25 y (56). Finally, comparative analysis of mRNA expression in the prefrontal cortex shows that the dramatic changes in transcriptome profiles in the human brain are delayed relative to nonhuman primates (57). Thus, our data on spine overproduction and elimination are not in contradiction to the molecular changes in the synaptic membranes. It is likely that molecular changes occur in each phase, but after the period of synaptic stabilization molecular tuning becomes the predominant way of interacting with the environment (51, 52, 54).
Although the molecular mechanisms that regulate prolonged reorganization of dendritic spines are not well understood, there are indications that they reflect the changes in dopaminergic innervation. In both human and nonhuman primates, the dopaminergic input, together with glutamatergic synapses, terminates predominantly on the dendrites of layer IIIc pyramidal neurons of the prefrontal cortex (8, 29, 42, 58), where it modulates neuronal activity (59). The magnitude of dopaminergic innervation in the monkey and human cortex, including gene expression of the D1-receptor that is essential for the bidirectional modulation of synaptic plasticity in the medial prefrontal cortex (60), increases up to young adulthood (61) and reaches its highest level during adolescence and young adulthood (62–64). These data led to the hypothesis that an increase in dopaminergic innervation in the prefrontal cortex is associated with an increase in modulation of glutamatergic synapses located on the dendritic spine and involved in synaptic stabilization (65). Furthermore, dopamine–glutamate interaction on dendritic spines of pyramidal neurons in the prefrontal cortex during synaptic elimination also might be involved in protecting vulnerable subjects from developing schizophrenia (66). It may be significant that the largest postnatal increase in the level of catechol-o-methyltransferase enzyme activity (which alters extracellular dopamine levels in the prefrontal cortex) occurs between the second and the fourth decade (67), supporting the hypothesis that the dopamine–glutamate interaction is involved in regulating synaptic elimination in the human prefrontal cortex.
It may seem paradoxical that the period during which learning and acquisition of new knowledge are highest in the human coincides with a net decrease rather than an increase in the number of synapses. The protracted postadolescent period of synaptic elimination and increase in dopaminergic innervation of the prefrontal cortex (61) may be linked to human-specific cognitive functions and circuitry specializations (58) that are a product of cooperation between genetic endowment and environment, as postulated by the selective-stabilization hypothesis (1, 3, 8). The prolonged developmental plasticity in the associative frontal cortex in human allows an unprecedented opportunity for acquisition of the highest level of cognitive abilities (27, 39, 50, 68–70) but also is susceptible to the formation of abnormal circuitry that is manifested in late-expressed neuropsychiatric disorders (10, 71–74).
Materials and Methods
Subjects.
Thirty-two subjects, ranging in age from a 1-wk-old term newborn to 91 y, were studied quantitatively (Table S1). None of the subjects had a clinical history of neurological disorder or a neuropathological alteration detected at autopsy. All analyzed subjects lived under standard environmental and socioeconomic conditions. Details on subject age, sex, postmortem delay, and cause of death as described in autopsy and medical records are given in Table S1. In two subjects (19 and 22 y old) death was caused by suicide, but personal and clinical records did not point to any specific psychiatric disorders. The brains were collected with the approval of the Ethical Committee of the University of Zagreb School of Medicine in compliance with Croatian law. The prefrontal cortex tissue was studied in sections from the Zagreb Collection located at the Croatian Institute for Brain Research (75).
The time interval between death and fixation of the tissue (i.e., the postmortem delay) was <8 h for early postnatal cases, <13 h for infants, <16 h for children, and <20 h for adults. All analyzed subjects died without preagonal state, so that the postmortem delay actually represents the interval in which neuron death took place. No staining artifacts caused by postmortem delay described for rapid Golgi staining were detected in the cases that were studied quantitatively.
Tissue Preparation.
The parts of the prefrontal cortex examined included the superior and middle frontal gyrus, mainly defined as the frontal granular and magnopyramidal Brodmann's area 9 (76). Blocks of tissue (1 cm3) were sectioned perpendicular to the long axis of the frontal gyrus, from the right hemisphere in the majority of cases. The classical rapid Golgi method was used, as described previously (28, 46). The pia was not removed. After sectioning, the cortical tissue was immersed immediately in rapid Golgi solution (0.3% osmium tetroxide and 3% potassium dichromate) and kept in the dark. After 7 d, the dichromate solution was replaced by 1% silver nitrate for 2 d. Then the tissue was dehydrated and embedded rapidly in 8% celloidin. After embedding, a microtome was used to section the blocks serially into coronal sections 160–200 μm in thickness. This thickness was chosen as a compromise to have many dendrites and good microscopic clarity. Nissl-stained sections from adjacent blocks were cut at 30 μm to check and additionally ensure that the neurons quantified were taken from Brodmann's area 9 (37).
Quantitative Analysis.
The criteria for cell selection for quantitative analysis included clear impregnation of the finest dendrites and dendritic spines visible on all parts of the dendritic tree, without smooth segments and at least 10 well-impregnated neurons per layer. The entire measured part of the dendritic segments had to be sharply visible with a 40× objective without moving the microscope in z direction (depth). The use of these criteria for quantification resulted in the inclusion of only 32 of 109 subjects analyzed.
The measurements were performed using a 63×-oil immersion objective with a long working distance (Olympus 1.4 N.A.). The cases were coded, so that investigators were not aware of the subjects’ age, sex, or medical history. Large layer IIIc pyramidal neurons were always located within the 200-μm–wide zone above layer IV. Layer V was detected in counterstained Nissl sections and in Golgi sections as a 200-μm–thick layer below the transparent layer IV. Only neurons of typical pyramidal morphology were analyzed (28, 45, 46, 77). Modified pyramidal neurons were not included in the analysis.
Dendritic spine density was analyzed on apical side branches (oblique dendrites) and basal dendrites (Fig. 1 A and B). Data in Table S2 give spine numbers on (i) the most proximal 50-μm length of the first-order basal dendrite, (ii) the first 50-μm length in side branches of apical dendrites (oblique dendrites), which were divided into two groups: proximal oblique dendrites, originating from the apical dendrite segment at up to 100-μm distance from soma, and (iii) distal oblique dendrites, originating in the segment of apical dendrite at a distance of 100–200 μm from soma (Fig. 1B).
Statistical Analysis.
The SPSS package was used for statistical analysis. The spine number was tested separately for each layer and each segment with one-way ANOVA with parametric and nonparametric analyses with age as a main effect (28). In the statistical analysis every subject represents a separate age. The a posteriori Student-Newman-Keuls test for multiple comparisons was applied to determine which subjects were significantly different. P values <0.05 were considered statistically significant. Statistical analysis with parametric and nonparametric procedures showed comparable results.
DSD was plotted at the linear scale to illustrate the dynamics of changes occurring during the 100-y human lifespan (Fig. 2) and to obtain regression curves fitting the distribution of data from the basal, apical proximal oblique, and apical distal oblique dendrites of the pyramidal cells from layer IIIc and V. In all cases the equation of the curves was a double exponential function in the form: y = a*exp(−bt)+c*exp(−dt) + e, where a, b, c, d, and e are fixed coefficients and t is time in years (Table S3).
Supplementary Material
Acknowledgments
We thank J. Arellano for insightful discussions and A. Bernacchia for help with regression curves in Fig. 2. This work was supported by grants from the Ministry of Science, Education, and Sports of the Republic of Croatia (to Z.P., M.J., I.K., and G.Š.), the Unity Through Knowledge Fund (to I.K.), the National Institutes of Health, and the Kavli Institute for Neuroscience at Yale University (to P.R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105108108/-/DCSupplemental.
References
- 1.Changeux JP, Danchin A. Selective stabilisation of developing synapses as a mechanism for the specification of neuronal networks. Nature. 1976;264:705–712. doi: 10.1038/264705a0. [DOI] [PubMed] [Google Scholar]
- 2.Huttenlocher PR, Dabholkar AS. Regional differences in synaptogenesis in human cerebral cortex. J Comp Neurol. 1997;387:167–178. doi: 10.1002/(sici)1096-9861(19971020)387:2<167::aid-cne1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 3.Rakic P, Bourgeois JP, Eckenhoff MF, Zecevic N, Goldman-Rakic PS. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986;232:232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- 4.Huttenlocher PR. Synaptic density in human frontal cortex—developmental changes and effects of aging. Brain Res. 1979;163:195–205. doi: 10.1016/0006-8993(79)90349-4. [DOI] [PubMed] [Google Scholar]
- 5.Bourgeois JP, Goldman-Rakic PS, Rakic P. Synaptogenesis in the prefrontal cortex of rhesus monkeys. Cereb Cortex. 1994;4:78–96. doi: 10.1093/cercor/4.1.78. [DOI] [PubMed] [Google Scholar]
- 6.Rakic P, Riley KP. Overproduction and elimination of retinal axons in the fetal rhesus monkey. Science. 1983;219:1441–1444. doi: 10.1126/science.6828871. [DOI] [PubMed] [Google Scholar]
- 7.Purves D, Lichtman JW. Elimination of synapses in the developing nervous system. Science. 1980;210:153–157. doi: 10.1126/science.7414326. [DOI] [PubMed] [Google Scholar]
- 8.Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: Implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 9.Bizzi E, Scolnick E, Desimone R. Advances in brain sciences: Implication for therapy. Bull Am Acad Sci. 2010;63:15–22. [Google Scholar]
- 10.Weinberger DR. Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry. 1987;44:660–669. doi: 10.1001/archpsyc.1987.01800190080012. [DOI] [PubMed] [Google Scholar]
- 11.Bennett A O MR. Dual constraints on synapse formation and regression in schizophrenia: Neuregulin, neuroligin, dysbindin, DISC1, MuSK and agrin. Aust N Z J Psychiatry. 2008;42:662–677. doi: 10.1080/00048670802203467. [DOI] [PubMed] [Google Scholar]
- 12.Garber K. Neuroscience. Autism's cause may reside in abnormalities at the synapse. Science. 2007;317:190–191. doi: 10.1126/science.317.5835.190. [DOI] [PubMed] [Google Scholar]
- 13.Hutsler JJ, Zhang H. Increased dendritic spine densities on cortical projection neurons in autism spectrum disorders. Brain Res. 2010;1309:83–94. doi: 10.1016/j.brainres.2009.09.120. [DOI] [PubMed] [Google Scholar]
- 14.Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol. 2010;92:370–385. doi: 10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 15.Feinberg I, Campbell IG. Sleep EEG changes during adolescence: An index of a fundamental brain reorganization. Brain Cogn. 2010;72:56–65. doi: 10.1016/j.bandc.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 16.Dierssen M, Ramakers GJ. Dendritic pathology in mental retardation: From molecular genetics to neurobiology. Genes Brain Behav. 2006;5(Suppl 2):48–60. doi: 10.1111/j.1601-183X.2006.00224.x. [DOI] [PubMed] [Google Scholar]
- 17.Quartz SR, Sejnowski TJ. The neural basis of cognitive development: A constructivist manifesto. Behav Brain Sci. 1997;20:537–556, discussion 556–596. doi: 10.1017/s0140525x97001581. [DOI] [PubMed] [Google Scholar]
- 18.Casey BJ, Jones RM, Hare TA. The adolescent brain. Ann N Y Acad Sci. 2008;1124:111–126. doi: 10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitford TJ, et al. Brain maturation in adolescence: Concurrent changes in neuroanatomy and neurophysiology. Hum Brain Mapp. 2007;28:228–237. doi: 10.1002/hbm.20273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chugani HT. A critical period of brain development: Studies of cerebral glucose utilization with PET. Prev Med. 1998;27:184–188. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- 21.Dosenbach NU, et al. Prediction of individual brain maturity using fMRI. Science. 2010;329:1358–1361. doi: 10.1126/science.1194144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luna B, Padmanabhan A, O'Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain Cogn. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Asato MR, Terwilliger R, Woo J, Luna B. White matter development in adolescence: A DTI study. Cereb Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gogtay N, et al. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci USA. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jolles DD, van Buchem MA, Crone EA, Rombouts SA. A comprehensive study of whole-brain functional connectivity in children and young adults. Cereb Cortex. 2011;21:385–391. doi: 10.1093/cercor/bhq104. [DOI] [PubMed] [Google Scholar]
- 26.Knickmeyer RC, et al. Maturational trajectories of cortical brain development through the pubertal transition: Unique species and sex differences in the monkey revealed through structural magnetic resonance imaging. Cereb Cortex. 2010;20:1053–1063. doi: 10.1093/cercor/bhp166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaw P, et al. Intellectual ability and cortical development in children and adolescents. Nature. 2006;440:676–679. doi: 10.1038/nature04513. [DOI] [PubMed] [Google Scholar]
- 28.Petanjek Z, Judaš M, Kostović I, Uylings HB. Lifespan alterations of basal dendritic trees of pyramidal neurons in the human prefrontal cortex: A layer-specific pattern. Cereb Cortex. 2008;18:915–929. doi: 10.1093/cercor/bhm124. [DOI] [PubMed] [Google Scholar]
- 29.Gonzalez-Burgos G, et al. Functional maturation of excitatory synapses in layer 3 pyramidal neurons during postnatal development of the primate prefrontal cortex. Cereb Cortex. 2008;18:626–637. doi: 10.1093/cercor/bhm095. [DOI] [PubMed] [Google Scholar]
- 30.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sowell ER, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 32.Webster MJ, Elashoff M, Weickert CS. Molecular evidence that cortical synaptic growth predominates during the first decade of life in humans. Int J Dev Neurosci. 2011;29:225–236. doi: 10.1016/j.ijdevneu.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 33.Leppänen JM, Nelson CA. Tuning the developing brain to social signals of emotions. Nat Rev Neurosci. 2009;10:37–47. doi: 10.1038/nrn2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blakemore SJ. The social brain in adolescence. Nat Rev Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- 35.Sebastian C, Viding E, Williams KD, Blakemore SJ. Social brain development and the affective consequences of ostracism in adolescence. Brain Cogn. 2010;72:134–145. doi: 10.1016/j.bandc.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 36.Germine LT, Duchaine B, Nakayama K. Where cognitive development and aging meet: Face learning ability peaks after age 30. Cognition. 2011;118:201–210. doi: 10.1016/j.cognition.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: I. Remapping of areas 9 and 46 using quantitative criteria. Cereb Cortex. 1995;5:307–322. doi: 10.1093/cercor/5.4.307. [DOI] [PubMed] [Google Scholar]
- 38.Beckman M. Neuroscience. Crime, culpability, and the adolescent brain. Science. 2004;305:596–599. doi: 10.1126/science.305.5684.596. [DOI] [PubMed] [Google Scholar]
- 39.Kishiyama MM, Boyce WT, Jimenez AM, Perry LM, Knight RT. Socioeconomic disparities affect prefrontal function in children. J Cogn Neurosci. 2009;21:1106–1115. doi: 10.1162/jocn.2009.21101. [DOI] [PubMed] [Google Scholar]
- 40.Dehaene S, Changeux JP. A hierarchical neuronal network for planning behavior. Proc Natl Acad Sci USA. 1997;94:13293–13298. doi: 10.1073/pnas.94.24.13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hill J, et al. Similar patterns of cortical expansion during human development and evolution. Proc Natl Acad Sci USA. 2010;107:13135–13140. doi: 10.1073/pnas.1001229107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- 43.Elston GN, et al. Specializations of the granular prefrontal cortex of primates: Implications for cognitive processing. Anat Rec A Discov Mol Cell Evol Biol. 2006;288:26–35. doi: 10.1002/ar.a.20278. [DOI] [PubMed] [Google Scholar]
- 44.Dehaene S, Kerszberg M, Changeux JP. A neuronal model of a global workspace in effortful cognitive tasks. Proc Natl Acad Sci USA. 1998;95:14529–14534. doi: 10.1073/pnas.95.24.14529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Elston GN, Benavides-Piccione R, Elston A, Manger PR, Defelipe J. Pyramidal cells in prefrontal cortex of primates: Marked differences in neuronal structure among species. Front Neuroanat. 2011;5:2. doi: 10.3389/fnana.2011.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeba M, Jovanov-Milosević N, Petanjek Z. Quantitative analysis of basal dendritic tree of layer III pyramidal neurons in different areas of adult human frontal cortex. Coll Antropol. 2008;32(Suppl 1):161–169. [PubMed] [Google Scholar]
- 47.Elston GN, Oga T, Fujita I. Spinogenesis and pruning scales across functional hierarchies. J Neurosci. 2009;29:3271–3275. doi: 10.1523/JNEUROSCI.5216-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: A quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- 49.Woo TU, Pucak ML, Kye CH, Matus CV, Lewis DA. Peripubertal refinement of the intrinsic and associational circuitry in monkey prefrontal cortex. Neuroscience. 1997;80:1149–1158. doi: 10.1016/s0306-4522(97)00059-6. [DOI] [PubMed] [Google Scholar]
- 50.Jacobs B, et al. Regional dendritic and spine variation in human cerebral cortex: A quantitative Golgi study. Cereb Cortex. 2001;11:558–571. doi: 10.1093/cercor/11.6.558. [DOI] [PubMed] [Google Scholar]
- 51.Yang G, Pan F, Gan WB. Stably maintained dendritic spines are associated with lifelong memories. Nature. 2009;462:920–924. doi: 10.1038/nature08577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 53.Bloss EB, et al. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lin YC, Koleske AJ. Mechanisms of synapse and dendrite maintenance and their disruption in psychiatric and neurodegenerative disorders. Annu Rev Neurosci. 2010;33:349–378. doi: 10.1146/annurev-neuro-060909-153204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arellano JI, Espinosa A, Fairén A, Yuste R, DeFelipe J. Non-synaptic dendritic spines in neocortex. Neuroscience. 2007;145:464–469. doi: 10.1016/j.neuroscience.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 56.Harris LW, et al. Gene expression in the prefrontal cortex during adolescence: Implications for the onset of schizophrenia. BMC Med Genomics. 2009;2:28. doi: 10.1186/1755-8794-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Somel M, et al. Transcriptional neoteny in the human brain. Proc Natl Acad Sci USA. 2009;106:5743–5748. doi: 10.1073/pnas.0900544106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raghanti MA, et al. Cortical dopaminergic innervation among humans, chimpanzees, and macaque monkeys: A comparative study. Neuroscience. 2008;155:203–220. doi: 10.1016/j.neuroscience.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Henze DA, González-Burgos GR, Urban NN, Lewis DA, Barrionuevo G. Dopamine increases excitability of pyramidal neurons in primate prefrontal cortex. J Neurophysiol. 2000;84:2799–2809. doi: 10.1152/jn.2000.84.6.2799. [DOI] [PubMed] [Google Scholar]
- 60.Huang YY, Simpson E, Kellendonk C, Kandel ER. Genetic evidence for the bidirectional modulation of synaptic plasticity in the prefrontal cortex by D1 receptors. Proc Natl Acad Sci USA. 2004;101:3236–3241. doi: 10.1073/pnas.0308280101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wahlstrom D, White T, Luciana M. Neurobehavioral evidence for changes in dopamine system activity during adolescence. Neurosci Biobehav Rev. 2010;34:631–648. doi: 10.1016/j.neubiorev.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weickert CS, et al. Postnatal alterations in dopaminergic markers in the human prefrontal cortex. Neuroscience. 2007;144:1109–1119. doi: 10.1016/j.neuroscience.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 63.Lambe EK, Krimer LS, Goldman-Rakic PS. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in prefrontal cortex of rhesus monkey. J Neurosci. 2000;20:8780–8787. doi: 10.1523/JNEUROSCI.20-23-08780.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosenberg DR, Lewis DA. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: A tyrosine hydroxylase immunohistochemical analysis. J Comp Neurol. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- 65.Goldman-Rakic PS, Muly EC, 3rd, Williams GV. D(1) receptors in prefrontal cells and circuits. Brain Res Brain Res Rev. 2000;31:295–301. doi: 10.1016/s0165-0173(99)00045-4. [DOI] [PubMed] [Google Scholar]
- 66.Tan HY, Callicott JH, Weinberger DR. Prefrontal cognitive systems in schizophrenia: Towards human genetic brain mechanisms. Cogn Neuropsychiatry. 2009;14:277–298. doi: 10.1080/13546800903091665. [DOI] [PubMed] [Google Scholar]
- 67.Tunbridge EM, et al. Catechol-o-methyltransferase enzyme activity and protein expression in human prefrontal cortex across the postnatal lifespan. Cereb Cortex. 2007;17:1206–1212. doi: 10.1093/cercor/bhl032. [DOI] [PubMed] [Google Scholar]
- 68.Nelson CA, 3rd, et al. Cognitive recovery in socially deprived young children: The Bucharest Early Intervention Project. Science. 2007;318:1937–1940. doi: 10.1126/science.1143921. [DOI] [PubMed] [Google Scholar]
- 69.Uylings HBM. In: Series in Cognitive Neuroscience and Language Learning and Processing. Gullberg M, Indefrey P, editors. Oxford: Blackwell; 2006. pp. 59–90. [Google Scholar]
- 70.Raizada RD, Kishiyama MM. Effects of socioeconomic status on brain development, and how cognitive neuroscience may contribute to levelling the playing field. Front Hum Neurosci. 2010;4:3. doi: 10.3389/neuro.09.003.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eluvathingal TJ, et al. Abnormal brain connectivity in children after early severe socioemotional deprivation: A diffusion tensor imaging study. Pediatrics. 2006;117:2093–2100. doi: 10.1542/peds.2005-1727. [DOI] [PubMed] [Google Scholar]
- 72.Diamond A, Barnett WS, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Changeux JP. Nicotinic receptors and nicotine addiction. C R Biol. 2009;332:421–425. doi: 10.1016/j.crvi.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 74.Penzes P, Cahill ME, Jones KA, VanLeeuwen JE, Woolfrey KM. Dendritic spine pathology in neuropsychiatric disorders. Nat Neurosci. 2011;14:285–293. doi: 10.1038/nn.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Judaš M, et al. The Zagreb Collection of human brains: A unique, versatile, but underexploited resource for the neuroscience community. Ann N Y Acad Sci. 2011;1225(Suppl 1):E105–E130. doi: 10.1111/j.1749-6632.2011.05993.x. [DOI] [PubMed] [Google Scholar]
- 76.Rajkowska G, Goldman-Rakic PS. Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex. 1995;5:323–337. doi: 10.1093/cercor/5.4.323. [DOI] [PubMed] [Google Scholar]
- 77.DeFelipe J, Fariñas I. The pyramidal neuron of the cerebral cortex: Morphological and chemical characteristics of the synaptic inputs. Prog Neurobiol. 1992;39:563–607. doi: 10.1016/0301-0082(92)90015-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.