Abstract
Memory consolidation has been proposed as a function of sleep. However, sleep is a complex phenomenon characterized by several features including duration, intensity, and continuity. Sleep continuity is disrupted in different neurological and psychiatric conditions, many of which are accompanied by memory deficits. This finding has raised the question of whether the continuity of sleep is important for memory consolidation. However, current techniques used in sleep research cannot manipulate a single sleep feature while maintaining the others constant. Here, we introduce the use of optogenetics to investigate the role of sleep continuity in memory consolidation. We optogenetically targeted hypocretin/orexin neurons, which play a key role in arousal processes. We used optogenetics to activate these neurons at different intervals in behaving mice and were able to fragment sleep without affecting its overall amount or intensity. Fragmenting sleep after the learning phase of the novel object recognition (NOR) task significantly decreased the performance of mice on the subsequent day, but memory was unaffected if the average duration of sleep episodes was maintained at 62–73% of normal. These findings demonstrate the use of optogenetic activation of arousal-related nuclei as a way to systematically manipulate a specific feature of sleep. We conclude that regardless of the total amount of sleep or sleep intensity, a minimal unit of uninterrupted sleep is crucial for memory consolidation.
Keywords: nonrapid eye movement sleep, rapid eye movement sleep
Although much has been learned about how sleep is regulated, there is still no consensus on the function of sleep. One hypothesis is that sleep is needed for memory consolidation (1–5). Testing this hypothesis has been confounded by sleep deprivation methodologies which are drastic manipulations affecting the overall activity of neuronal networks, including cellular energy demand (6, 7), protein synthesis (8), levels of free radicals (9), and specific synaptic changes (10). In many cases sleep deprivation also induces a stress response (11, 12), which itself has been shown to affect memory (4, 13).
Some argue that sleep might support memory consolidation by merely providing passive isolation from sensory interruption (14, 15), a claim that cannot be excluded when external sensory stimulation (such as gentle handling or playing a tone) is the method used to disturb sleep. Traditional methods of sleep deprivation disrupt the composition of sleep [percentage of rapid eye movement (REM) and non-REM (NREM) sleep] at the same time as disrupting sleep quality, sleep continuity, and, in most cases, reducing total sleep duration (8, 16, 17). Thus, it has not been possible to study the role of a specific characteristic of sleep, such as sleep continuity, in memory consolidation.
Sleep continuity is affected by different neurological conditions, e.g., sleep apnea, aging, and alcoholism (18, 19). The occurrence of memory deficits in many of these conditions raised the question of whether the continuity of sleep is important for memory consolidation. Without the ability to distinguish between the effects of sleep continuity from that of other sleep characteristics, this question remains unanswered.
If sleep could be fragmented into shorter episodes without the use of sensory stimuli, without inducing stress, and without affecting sleep intensity or total duration, would memory consolidation be impaired? And if so, what is the minimal quantum of uninterrupted sleep required for memory consolidation?
To address these questions we used optogenetics. We optically stimulated hypocretin/orexin (Hcrt) neurons (20, 21), which participate in the regulation of sleep–wake transitions by setting a threshold for arousal (22, 23). Previous studies in our laboratory have shown that optogenetic stimulation of Hcrt neurons in sleeping mice increases the probability of sleep-to-wake transitions (24, 25). However, these stimulations are not effective in inducing arousal when mice are under sleep pressure (25), suggesting that optogenetic stimulation of these cells does not override the homeostatic regulation of sleep. This is important background information for the current study, which aims to maintain the homeostatic mechanisms of sleep regulation while modifying sleep continuity.
We have used scheduled optogenetic stimulation of the Hcrt cells to disrupt sleep continuity in mice immediately following training in the novel object recognition (NOR) task. By modifying the intervals between these stimulations, we were able to establish a model in which sleep was fragmented, but total sleep amounts, quality, and overall composition were intact. Comparing the effects of stimulation protocols of different frequencies on sleep and on memory consolidation reveals a minimal quantum of uninterrupted sleep required for memory consolidation in mice.
Results
Optogenetic Stimulation of Hcrt Neurons at 60-s Intervals Fragments Sleep Without Affecting Total Sleep Time.
Our aim was to establish a protocol of chronic fragmentation of sleep without affecting other features of sleep including total sleep time. Therefore, we compared different optogenetic stimulation patterns and examined their effects on sleep architecture and total sleep amounts. First, we transduced Hcrt neurons in 8-wk-old C57BL/6 mice using a lentivirus packed with the Hcrt::ChR2-mCherry cassette (ChR2; light sensitive Channelrhodopsin-2 cation channel; mCherry; Red fluorescent protein). As a control, we transduced mice with virus expressing the fluorescent proteins but lacking the ChR2 coding sequence (Hcrt::mCherry). On the basis of our previous data (24, 26), sufficient ChR2 expression is achieved after 3 wk (>95% of Hcrt neurons transduced with the lentivirus). We stimulated Hcrt neurons using a laser diode directed to the lateral hypothalamus (Fig. 1A). Each stimulus was 10 s of light pulses (20 Hz, 20 mW, 477 nm). Stimuli of these characteristics were previously reported to efficiently decrease latency to arousal compared with lower frequencies (24). Moreover, in vitro this stimulation protocol reliably induces an action potential with each pulse of light, whereas stimuli above 20 Hz do not (24). To determine the interstimulus interval that would enable sleep fragmentation without affecting total sleep time, we delivered the stimulations either every 30 s or every 60 s over 4 h beginning 1 h after light onset (stimulations start at 10:00 AM). Control mice that were transduced with virus not expressing ChR2, were stimulated at 60-s intervals.
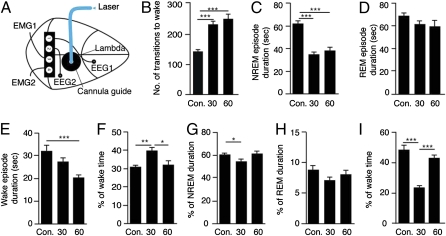
Fig. 1.
Optogenetic stimulation of Hcrt cells every 60 s disrupts sleep integrity but not total sleep time or the overall composition of sleep. Mice expressing ChR2 under the Hcrt promoter were stimulated with a blue laser diode (477 nm; 20 mW) through an optical fiber aimed at the lateral hypothalamus. Trains of 10 s (20 Hz, 15-ms light pulse) with 30- or 60-s intervals between the stimuli were used. Control mice expressing only the fluorescent marker were stimulated in 60-s intervals. (A) Schematic representation of the cannula placement and the placing of the EEG/EMG recording setup are also shown. Sleep was recorded during the time of stimulation (10:00 AM–2:00 PM). Intervals of 4 s were visually scored for wake, NREM, and REM sleep. Data are presented as total over the 4 h of stimulation from artifact-free intervals. (B) Number of transitions from sleep (NREM and REM) to wake. (C–E) The average duration in seconds of a single NREM (C), REM (D), or wake episode (E). (F–H) The percentage of total time spent in wake (F), NREM (G), or REM (H). (I) The percentage of time spent in wake during the first hour immediately after the stimulation session ended (2:00–3:00 PM). Values are represented as means ± SEM; n = 8–9 in each group. One-way ANOVA (factor “stimulation”) was followed by Tukey's multiple comparison test; *P < 0.05, **P < 0.01, ***P < 0.0001.
To achieve maximal sensitivity in quantifying microarousal events induced by the Hcrt stimulation, events of decreased electroencephalographic (EEG) delta power coupled with increased electromyographic (EMG) activity that lasted <2 s were defined as microarousals (an example is provided in Fig. S1) and counted as a transition to wake. We analyzed the degree of sleep fragmentation (indicated by the number of transitions to wake), NREM sleep, and REM sleep episode duration, the duration of wake, and the total amount of time spent in these vigilance states. We found that stimulation at both 30- or 60-s intervals significantly increased the number of transitions from both NREM and REM sleep to wake in the ChR2 mice [one-way ANOVA F(2,22) = 20.45, P < 0.0001; Tukey's post hoc comparisons P < 0.0001 for the 30 and the 60 s, each compared with the control; Fig. 1B]. The increased number of transitions to wakefulness was accompanied by a change in the mean NREM sleep episode duration, which was shortened in both protocols [one-way ANOVA F(2,22) = 28.45, P < 0.0001; P < 0.0001 for the 30 and the 60 s compared with the control; Fig. 1C]. The duration of REM sleep episodes was not significantly altered by the stimulation (one-way ANOVA P = 0.215; Fig. 1D). This is in agreement with other studies indicating that once REM is initiated, it may be less sensitive to interruption than NREM sleep (27). Along with the increase in the number of transitions to wake, stimulation of the ChR2 mice every 60 s reduced the duration of wake episodes [Fig. 1E; one-way ANOVA F(2,22) = 8.206, P = 0.0022; Tukey's post hoc comparisons P < 0.001 for the 60 s compared with control]. These changes of increased fragmentation and decreased wake duration offset each other, so the 60-s stimulation protocol did not affect the overall percentage of time spent awake in the ChR2 mice. In contrast, the 30-s stimulation protocol significantly increased the percentage of wake time [one-way ANOVA F(2,22) = 6.947, P = 0.046 and Tukey's post hoc comparisons; P < 0.01 for the 30 s compared with the control and P < 0.05 comparing between 30 and 60 s of ChR2 mice; Fig. 1F]. Thus, the overall amount of sleep and the relative composition of sleep were not affected in the ChR2 mice stimulated in the 60-s protocol, as the time spent in NREM and REM sleep remained unchanged (Fig. 1 G and H, respectively). Stimulations in the 30-s protocol significantly reduced the time spent in NREM (Fig. 1G), although the percentage of time spent in REM sleep was not significantly altered [Fig. 1H; NREM sleep analysis: one-way ANOVA F(2,22) = 6.109, P = 0.0078; Tukey's post hoc comparisons P < 0.05 for the 30 s compared with control and between 30 and 60 s of the ChR2 mice. REM: one-way ANOVA P = 0.248].
To assess whether these stimulation protocols induced sleep debt, we examined sleep recovery after the stimulation sessions. We found sleep rebound in the ChR2 mice that were stimulated every 30 s, indicated by a decrease in the time spent awake. This decrease was limited to the first hour following the stimulation. Following the 60-s stimulation protocol, no significant change in sleep/wake time was seen even during the first hour after the stimulation [Fig. 1I; one-way ANOVA F(2,22) = 22.89, P < 0.0001 with Tukey's post hoc comparisons P < 0.0001 30 s compared with control and between 30 and 60 s]. Taken together, these findings demonstrated that the 60-s stimulation protocol disrupted sleep continuity, but it did not have an effect on total sleep time or on amounts of NREM and REM sleep. In contrast, the 30-s stimulation protocol had a small effect on total sleep time and the overall composition of sleep. Therefore, we focused on the 60-s protocol and further investigated its relevance as a tool to specifically disrupt sleep continuity.
The Quality of NREM and REM Sleep Is Not Affected by Hcrt Stimulation at 60-s Intervals.
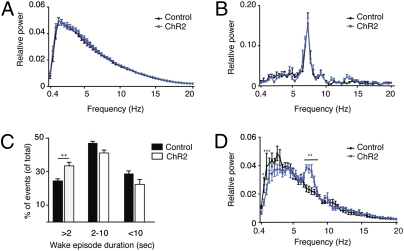
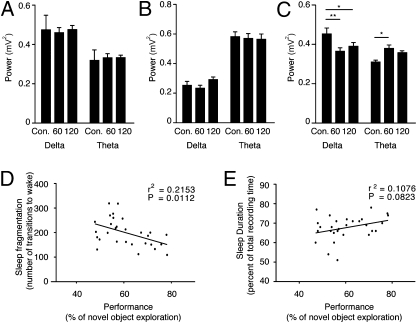
To examine whether the 60-s stimulation protocol had an effect on sleep quality, we analyzed the EEG power spectra of control and ChR2 mice during NREM and REM sleep. We performed fast Fourier transformation (FFT) analysis to obtain the EEG power of each frequency bin between 0.4 and 20 Hz and normalized it to total power. Analysis of NREM and REM sleep in the ChR2 mice showed no effect of the stimulation on the total EEG power or on any individual frequency bin (0.4–20 Hz; two-way ANOVA with Bonferroni correction; Fig. 2 A and B). The lack of a significant effect of sleep fragmentation on the quality of sleep can be partially explained by the nature of the stimulation-induced arousal events, which were mostly shorter than 2 s (Fig. 2C). Nevertheless, a nonsignificant decrease in the delta frequency band (1–4 Hz; P > 0.05) was present during NREM sleep (Fig. 2A). To determine whether this decrease represented another effect, which was masked in the overall NREM sleep analysis, we looked at the transitional stages as Hcrt neurons are known to modulate the threshold for transition to wake (22). We analyzed the EEG spectra during the different transitional stages, namely wake to NREM sleep, NREM to REM sleep, and NREM sleep to wake. We found a significant decrease in the delta frequency band and an increase in the theta frequency band of the ChR2 mice present only during the transition from NREM to REM sleep (Fig. 2D). As only around 8% of the NREM sleep events result in REM sleep, these transition state effects did not significantly affect the overall NREM spectral analyses, but they may represent an interesting function of Hcrt neurons, a question that is beyond the scope of the present study.
Fig. 2.
EEG power density during NREM and REM sleep is not affected by Hcrt stimulations every 60 s. We performed fast Fourier transformation (FFT) analysis of sleep for two groups: control (expressing only the fluorescent marker) and ChR2 mice, both stimulated every 60 s. The relative EEG power of each frequency bin was normalized to the total power (0.4–20 Hz). (A and B) The curves represent relative power densities (mean ± SEM) for NREM (A) and REM (B) sleep. (C) Distribution of arousal events by their duration. The duration of each arousal event was determined by specific scoring in 2-s intervals. These events were divided into three categories: events shorter than 2 s, events between 2 and 10 s, and events longer than 10 s. The percentage of each category out of the total events was determined and shown here as a comparison between control and ChR2-expressing mice, both stimulated at the 60-s paradigm. Two-way ANOVA (factors “stimulation” and “wake duration”) with Bonferroni correction was used to determine the significance of the data. (D) Relative power densities (mean ± SEM) for transitional stages between NREM and REM sleep (12 s of NREM before the presence of stable REM sleep). Two-way ANOVA (factors “stimulation” and “frequency”) with Bonferroni correction (n = 6–7 mice per group; *P < 0.05, **P < 0.01, ***P < 0.0001).
Taken together, these findings show that Hcrt stimulation at 60-s intervals induced frequent microarousals, which affected sleep integrity but did not change total sleep time, the total duration of the sleep stages, or sleep quality.
Stress-Related Factors Are Not Affected by Hcrt Stimulation at 60-s Intervals.
One of the major problems for studying the effects of sleep on memory consolidation is the need to distinguish the functional impact of absence of sleep from nonspecific effects of many sleep deprivation protocols (e.g., stress) that can affect memory (11, 12, 28). We examined stress indicators to exclude the possibility that stimulation of Hcrt neurons in our paradigm induces stress. We analyzed corticosterone (CORT) levels in the plasma of ChR2 mice and compared those to the levels in control mice, stimulated in the same 60-s protocol for 4 h. There was no significant difference between the two groups. As a control for our assay, we used plasma from mice restrained for 15 min [one-way ANOVA F(2,8) = 13.84, P = 0.0025; Tukey's post hoc comparisons; P < 0.01 restraint stress compared with the control; Fig. S2A].
The open field test characterizes the behavioral responses of mice that are placed in a novel arena: a stressful experience, and can therefore reveal individual differences in fear and anxiety (29). We analyzed the open field behavior of ChR2 mice to test for indications of anxiety. Mice that are more anxious tend to spend more time at the boundaries of an arena and have fewer center crossings. ChR2 mice that were stimulated every 60 s for 4 h did not show any change in their open field behavior compared with controls stimulated in the same 60-s protocol for 4 h. As a comparison, wild-type mice that had been restrained for 15 min demonstrated a clear change in the overall distance traveled [one-way ANOVA F(2,15) = 14.84, P = 0.0003; Tukey's post hoc comparisons; P < 0.001 restraint stress compared with the control; Fig. S2B] and center crossings [F(2,15) = 8.048, P = 0.0042; P < 0.01 restraint stress compared with the control; Fig. S2C]. Taken together, our findings show that optogenetic stimulation of Hcrt neurons at intervals of 60 s produced microfragmentation of sleep, without compromising total sleep time or the general composition of sleep. This approach did not involve any external sensory input to induce sleep fragmentation and did not increase CORT levels or affect behavior in the open field.
Hcrt Stimulation at 60-s Intervals Impairs Memory Consolidation in the Novel Object Recognition Task.
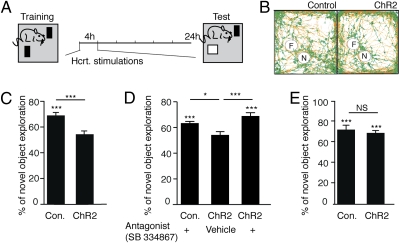
We assessed memory consolidation using the NOR paradigm (Fig. 3A), which takes advantage of an animal's tendency to explore novelty and does not require exposure to aversive stimuli. In this task, the mouse is trained in an arena with two similar objects. The mice usually explore the two objects equally, and mice that demonstrate over 65% preference for one object in the training session are excluded from the experiment (such object bias was evident in less than 10% of the examined control and ChR2 mice). Twenty-four hours after the training session, the mouse is returned to the arena where one of the familiar objects has been replaced with a new object. If the mouse spends more time during this testing session with the novel object, it is considered an indication that the mouse remembered the other, familiar object. The effects of sleep on memory consolidation in this task have been extensively characterized (30, 31) and suggest that 3 to 6 h of sleep during the light period immediately following training is crucial for memory consolidation (30, 32). In the present study, the training session took place 30 min after light onset (9:30 AM; Fig. 3A). We stimulated a group of ChR2 and control mice every 60 s during their first 4 h of sleep following the training (10:00 AM–2:00 PM). Optogenetic Hcrt stimulations significantly reduced the performance of the ChR2 mice; they did not explore the novel object longer than the familiar one as the controls did [Student's t test; t(16) = 4.115; P = 0.0008; Fig. 3 B and C]. This change in performance was not due to an increase in the motor activity of the ChR2 mice [Student's t test; t(16) = 0.1174; P = 0.908] or the speed of movement [Student's t test; t(16) = 0.5684; P = 0.5777]. To examine whether this decrease in performance was Hcrt dependent, we injected ChR2 and control mice with the Hcrt Receptor1 antagonist (SB334867; 10 mg/kg) immediately after their training, before the stimulation session. Mice in the control group showed no effect of the antagonist on their performance, distinguishing between the novel and familiar objects. ChR2 mice that were injected with vehicle continued to show a memory deficit, whereas ChR2 mice receiving the HcrtR1 antagonist did not [one-way ANOVA F(2,19) = 10.17, P = 0.001; Tukey's post hoc comparisons; P < 0.05 vehicle ChR2 mice and P < 0.001 antagonist ChR2 mice both compared with the control; Fig. 3D].
Fig. 3.
Hcrt stimulation every 60 s impairs performance in the novel object recognition task via a sleep-dependent mechanism. (A) Schematic representation of the behavioral paradigm. At the training session on the first day (9:30 AM) mice (ChR2 mice and the controls) were given 10 min to adapt to an open field arena followed by 5 min to explore two similar objects placed in the same open arena. At the end of the session, the optic fibers were inserted and mice were stimulated during their resting phase for 4 h every 60 s. At the testing session 24 h later, mice were reexamined in the same arenas with one of the previously encountered objects replaced with a novel one. (B) Representative videographs of the overall pathway of a mouse in a testing session determined automatically by the Viewpoint Videotrack system. Red lines represent movements and green dots indicate pauses in movement. Differences in exploration of the novel (N) and the familiar (F) object are evident (the intensity of green dots around the circled areas) in the control but not in the ChR2 mice (stimulated every 60 s). (C–E) Percentage of time spent exploring each object (novel or familiar) was determined. Student's t test was used to determine whether they are significantly different (for each group of mice; n = 8–10 per group). Significance is indicated by asterisks above the statistically significant groups. Difference in novel object explorations between groups is indicated above the lines connecting statistically significant groups (Student's t test in C and E and one-way ANOVA in D). (C) Novel object exploration in the control and ChR2 mice stimulated every 60 s (mean ± SEM). (D) Exploration of novel object in three groups of mice (control or ChR2 mice) that were injected immediately after the acquisition session with either the Hcrt receptor 1 antagonist (SB334867, 10 mg/kg i.p.) or vehicle alone as indicated in the figure. All mice were stimulated in the same paradigm of 60-s intervals (n = 7–8 per group). (E) Dark phase; control and ChR2 mice were exposed to the training session with dark onset (9:30 PM) instead of the onset of light as shown in C. The testing session took place again, 24 h later (n = 6–7 per group). ***P < 0.001.
Next we wanted to exclude the possibility that the effect on memory consolidation was mediated by Hcrt activity independent of its disruption of sleep continuity. A previous study showed that memory consolidation in the NOR task depends on sleep during the light phase but not during the dark phase when mice are typically more active (31). We therefore repeated the experiment during the dark phase beginning the training 30 min after dark onset (9:30 PM) followed by the 4 h of stimulation (10:00 PM–2:00 AM). We used ChR2 mice and control mice, both stimulated every 60 s. These stimulations during the dark phase had no effect on memory performance, as the mice were able to distinguish between the novel and the familiar objects [Student's t test; t(11) = 0.4515; P = 0.6604; Fig. 3E].
Memory Consolidation Is Impaired by Hcrt Stimulation at 60-s Intervals but Unaffected at 120-s Intervals.
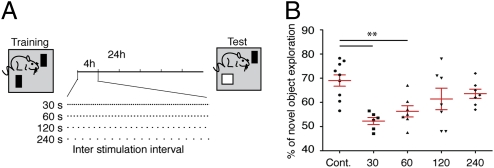
As shown in Fig. 1, stimulations every 30 s had a different effect on the number of wake episodes and on the total wake duration compared with stimulations every 60 s. We therefore wanted to extend our range of stimulation intervals to 120 and 240 s (Fig. 4A) and compare the effects on memory. The 30-s stimulation protocol, similar to the 60-s intervals, impaired performance in the NOR task. In contrast, the 120- and 240-s stimulation protocols did not impair memory consolidation [one-way ANOVA F(4,33) = 6.331, P = 0.0007; Tukey's post hoc comparisons; P < 0.01 for the 30- and 60-s stimulation compared with the control; Fig. 4B]. This result suggested that a property of sleep required for memory consolidation can be quantitatively characterized through a comparison of sleep architecture in the 60-s and 120-s stimulation protocols.
Fig. 4.
Hcrt stimulation at different intervals differentially affects performance in the novel object recognition task. (A) Schematic representation of the behavioral paradigm. The same paradigm as described in Fig. 4 was used but Hcrt stimulations were introduced in four different intervals (schematic view). (B) Percentage of novel object exploration (out of the total time) for each mouse is shown (n = 6–9 per group). The red lines represent means ± SEM; one-way ANOVA (**P < 0.01).
The Main Difference Between the 60- and 120-s Stimulation Protocols Is in the Degree of Sleep Fragmentation.
Comparison of the EEG/EMG traces of mice stimulated at 60-s or 120-s intervals indicated that neither stimulation protocol affected the total sleep time, shown as no change in percentage of wake (Table S1). Similarly, neither protocol had a significant effect on total percentage of time spent in REM sleep or on the duration of each REM sleep episode (Table S1). The EEG spectral power during NREM and REM sleep was similar in the 60- and the 120-s stimulation protocols (Fig. 5 A and B). The change we previously observed during the transitional stages from NREM to REM sleep (Fig. 2C) in the 60-s stimulation protocol was evident in the 120-s protocol as well (Fig. 5C). The main difference between the effects of the two protocols was in the extent of sleep fragmentation, which was reflected in a change in the mean duration of NREM sleep episodes, but not in the overall time spent in NREM sleep.
Fig. 5.
Intact sleep is crucial for memory consolidation in the novel object recognition task in mice. Quantification of the total EEG power in the delta (0.4–4 Hz) and theta (4–9 Hz) frequency bands for control and ChR2 mice stimulated at 60-s intervals and ChR2 mice stimulated every 120 s during NREM (A), REM (B), and transitional stages (C) between NREM and REM (12 s of NREM before the presence of stable REM). Two-way ANOVA (factors “stimulation” and “frequency”) with Bonferroni correction was used to determine the significance of the data. Correlation between the sleep duration (D) or fragmentation of sleep (E) (represented by the number of sleep interruptions) and performance on the test phase is represented by percentage of novel object exploration for the pooled control and ChR2 mice stimulated every 30, 60, and 120 s (n = 29).
Although the 120-s stimulation protocol fragmented sleep, significantly increasing the number of wake events, it did so to a lesser extent than the 60-s stimulation protocol (Table S1 and Fig. S3). The NREM sleep bout duration decreased in both protocols compared with control (62 ± 2.9 s in the control; 38 ± 3.5 s in the 60 s and 45 ± 4.3 s in the 120 s), representing 62 and 73% of the control bout duration in the 60- and 120-s paradigms, respectively. To verify that the integrity of sleep is indeed correlated with the impact on memory performance, we plotted performance as a function of the sleep integrity (number of transitions to wake). We found a significant correlation between the two factors (Fig. 5D). For comparison, the correlation (within the same population of mice) between total sleep time and memory performance was not significant (Fig. 5E). Furthermore, average duration of wake episodes was not significantly correlated with the performance (r2 = 0.01287; P = 0.7398). However, percentage of short wake episodes (<2 s) significantly correlated with performance (r2 = 0.4175; P = 0.0038), indicating the crucial role of uninterrupted sleep for memory consolidation.
Discussion
We have established a method to modify sleep continuity without inducing sensory stimulation or altering sleep duration, intensity, and composition. We used optogenetic stimulation of Hcrt neurons during sleep to fragment sleep at different intervals. Comparing the effects of 60-s and 120-s stimulation protocols on sleep and on memory performance revealed a minimal quantum of uninterrupted sleep required for memory consolidation.
The continuity of sleep is an especially intriguing feature as it poses a major challenge from an evolutionary point of view; continuous, undisrupted sleep exposes the organism to prolonged periods of inactivity and sensory unresponsiveness to the surrounding environment. As these are significant disadvantages, the evolutionary selection in favor of continuous sleep over fragmented episodes indicates that the continuity of sleep may be relevant to specific brain functions.
Hcrt neurons are thought to participate in the homeostatic regulation of sleep by increasing the probability of arousal without inducing immediate waking. Targeting this relatively subtle inducer of arousal enables the homeostatic sleep regulatory mechanisms to integrate the extrinsic pressure for awakening induced by Hcrt stimulation with the intrinsic pressures for specific sleep intensity and composition (23, 33). This utilization of an endogenous mechanism of sleep regulation provides an explanation for the relatively specific effect on sleep continuity that we observe in the present study, compared with previous reports (8, 16, 17).
The effects of the stimulation were dependent on Hcrt signaling, shown here by the use of the HcrtR1 antagonist SB334867 before the photostimulation, which inhibits the photostimulation effect for both NREM and REM sleep-to-wake transitions (24, 25). However, it is important to emphasize that SB334867, although known mostly as a HcrtR1 antagonist can also block HcrtR2, although less effectively.
Our data suggest that fragmentation of sleep induced by Hcrt stimulation causes impairment of memory consolidation. An alternative explanation is that the Hcrt has a direct effect on the memory consolidation processes and is not acting through its effects on sleep continuity. We believe this possibility is unlikely because it has been shown that elevating Hcrt levels by i.v. injection improves learning and memory despite sleep deprivation (34), and application of Hcrt to a hippocampal slice preparation increases long term potentiation (35). Thus, any direct effects of Hcrt on learning would be more likely to augment than to impair it.
Hcrt stimulations did not impair memory when delivered during the dark phase of the daily rhythms. A possible explanation of this result is that the activity of the Hcrt cells was already maximal during the dark phase, and the stimulation did not significantly increase it. However, it has been shown that optogenetic stimulation of Hcrt cells can induce arousal during both light and dark cycles (25). Our study does not explain why the effects of Hcrt stimulation were circadian phase dependent; however, these results are compatible with previous findings (31).
The chronic stimulation of Hcrt neurons resulted in a stable frequency of arousal events throughout the 4 h of stimulation. Nevertheless, it is possible that other aspects were affected throughout this chronic stimulation paradigm, such as changes in blood flow or heating of the stimulated brain region. Although the control mice received exactly the same stimulation protocol, we cannot exclude the possibility that the expression of ChR2 changed the sensitivity of these neurons to local heating or other effect resulting from the stimulation. Moreover, a number of variables including hormones and neurotransmitters change as a result of any sleep manipulation. These variables could potentially affect memory consolidation. We focused on stress and specifically on CORT (as an indicator of stress), because it has been shown to increase with prolonged sleep disruption (4, 11, 12). We did not see a change in CORT levels after 4 h of optogenetic sleep fragmentation. However, because CORT levels fluctuate during sleep, we cannot exclude temporal changes in CORT levels within the 4 h of stimulation.
In addition to the fragmentation of sleep, we observed an effect of the stimulations on the spectral profile of the EEG during transitional stages between NREM and REM sleep. These changes are not likely to account for the effect on memory consolidation, as they were evident in both the 60- and the 120-s stimulation patterns, which differentially affected memory. Nevertheless these results imply that additional features of sleep can be systematically studied by modulating different parameters of the stimulations.
None of the examined stimulation paradigms induced a significant effect on REM sleep. This is possibly due to the fact that REM sleep constitutes a relatively small amount of total sleep time and therefore the Hcrt stimulations that occurred during REM episodes were correspondingly limited. However, even in the 30-s stimulation protocol in which there was a statistically higher probability that stimulations coincided with REM bouts, we still did not observe REM sleep fragmentation. We assume that REM bouts are simply more resistant to interruption than are NREM bouts. It has been shown previously that once REM sleep is initiated, it is more stable to interference than NREM sleep (27).
The nature of the arousal events induced by the optogenetic Hcrt stimulation emphasizes the importance of sleep continuity for memory consolidation. Most of the arousal events were shorter than 2 s, and the mice immediately recovered the lost slow wave activity during subsequent NREM sleep. Although similar microarousal events occur also in normal sleep, the increase in their frequency as a result of the stimulations, correlated with the decrease in memory performance. The correlation between sleep continuity and memory performance was statistically significant, whereas the correlation between sleep duration and memory performance was not. We do not interpret these results to imply that sleep duration is not important for learning and memory. This study was not designed to test that proposition, and the range of sleep durations in our study was very small as our aim was to maintain sleep duration intact.
Different lines of evidence suggest a role for sleep continuity in memory consolidation. Sleep continuity is one of the main factors affected in various pathological conditions that impact memory, including Alzheimer's (19) and other age-related cognitive deficits (18, 36). Patients with sleep disorders such as obstructive sleep apnea experience sleep fragmentation and memory deficits, although their overall sleep duration is normal (37). These pathological conditions have wide-ranging effects beyond the disruption of sleep continuity; therefore, they cannot be considered direct evidence of a causal relationship. However, the present study suggests that the compromised continuity of sleep, or potentially the arousal events themselves, may account for some of the memory deficits associated with these pathological conditions.
One interpretation of the role of sleep continuity in memory consolidation is related to the replay phenomenon. The transfer of information from the hippocampus to the neocortex occurring during sleep (38) may bring the memory into a labile state. This is supported by recent studies showing that interference with the replay sequence without affecting sleep results in impaired memory consolidation (39, 40). We suggest that sleep interruption may similarly cause memory loss if it interferes with the replay or the replay-related events.
Taken together, we demonstrate a nonstressful, nonpharmacological means of manipulating a specific feature of animal sleep through the activation of endogenous arousal mechanisms. Using an optogenetic strategy, we were able to modulate a single property of sleep, leaving other aspects of sleep undisturbed. We show that sleep continuity independent of sleep amount is critical for learning and memory in mice and suggest the existence of a minimal length of continuous NREM sleep necessary for memory consolidation.
Materials and Methods
Full methods and associated references are presented in SI Materials and Methods. The optogenetic methods used were previously described (24). Preparations of the animals for EEG/EMG recording, methods of data acquisition and processing, and subsequent off-line analysis were as described by Colas et al. (36).
Supplementary Material
Acknowledgments
We thank Patricia Bonnavion for her help with the analysis of stress, Grace Hagiwara for help with the animal care, Bayarasaikhan Chuluun for her help with behavioral testing, and Jana Schaich Borg for her helpful comments on the manuscript.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015633108/-/DCSupplemental.
References
- 1.Frank MG, Benington JH. The role of sleep in memory consolidation and brain plasticity: Dream or reality? Neuroscientist. 2006;12:477–488. doi: 10.1177/1073858406293552. [DOI] [PubMed] [Google Scholar]
- 2.Graves L, Pack A, Abel T. Sleep and memory: A molecular perspective. Trends Neurosci. 2001;24:237–243. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- 3.Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: off-line memory reprocessing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- 4.Wagner U, Born J. Memory consolidation during sleep: Interactive effects of sleep stages and HPA regulation. Stress. 2008;11:28–41. doi: 10.1080/10253890701408822. [DOI] [PubMed] [Google Scholar]
- 5.Walker MP, Stickgold R. Sleep-dependent learning and memory consolidation. Neuron. 2004;44:121–133. doi: 10.1016/j.neuron.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 6.Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11:163–178. doi: 10.1016/j.smrv.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franken P, Gip P, Hagiwara G, Ruby NF, Heller HC. Glycogen content in the cerebral cortex increases with sleep loss in C57BL/6J mice. Neurosci Lett. 2006;402:176–179. doi: 10.1016/j.neulet.2006.03.072. [DOI] [PubMed] [Google Scholar]
- 8.Mackiewicz M, Naidoo N, Zimmerman JE, Pack AI. Molecular mechanisms of sleep and wakefulness. Ann N Y Acad Sci. 2008;1129:335–349. doi: 10.1196/annals.1417.030. [DOI] [PubMed] [Google Scholar]
- 9.Ramanathan L, Gulyani S, Nienhuis R, Siegel JM. Sleep deprivation decreases superoxide dismutase activity in rat hippocampus and brainstem. Neuroreport. 2002;13:1387–1390. doi: 10.1097/00001756-200208070-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tononi G, Cirelli C. Sleep and synaptic homeostasis: A hypothesis. Brain Res Bull. 2003;62:143–150. doi: 10.1016/j.brainresbull.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 11.Hairston IS, et al. Sleep deprivation elevates plasma corticosterone levels in neonatal rats. Neurosci Lett. 2001;315:29–32. doi: 10.1016/s0304-3940(01)02309-6. [DOI] [PubMed] [Google Scholar]
- 12.McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: Allostasis and allostatic load. Metabolism. 2006;55(10) Suppl 2:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 13.Tartar JL, et al. Experimental sleep fragmentation and sleep deprivation in rats increases exploration in an open field test of anxiety while increasing plasma corticosterone levels. Behav Brain Res. 2009;197:450–453. doi: 10.1016/j.bbr.2008.08.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Payne JD, et al. The role of sleep in false memory formation. Neurobiol Learn Mem. 2009;92:327–334. doi: 10.1016/j.nlm.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siegel JM. The REM sleep-memory consolidation hypothesis. Science. 2001;294:1058–1063. doi: 10.1126/science.1063049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tartar JL, et al. Hippocampal synaptic plasticity and spatial learning are impaired in a rat model of sleep fragmentation. Eur J Neurosci. 2006;23:2739–2748. doi: 10.1111/j.1460-9568.2006.04808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van Der Werf YD, et al. Sleep benefits subsequent hippocampal functioning. Nat Neurosci. 2009;12:122–123. doi: 10.1038/nn.2253. [DOI] [PubMed] [Google Scholar]
- 18.Oosterman JM, van Someren EJ, Vogels RL, Van Harten B, Scherder EJ. Fragmentation of the rest-activity rhythm correlates with age-related cognitive deficits. J Sleep Res. 2009;18:129–135. doi: 10.1111/j.1365-2869.2008.00704.x. [DOI] [PubMed] [Google Scholar]
- 19.Widner B, Ledochowski M, Fuchs D. Sleep disturbances and tryptophan in patients with Alzheimer's disease. Lancet. 2000;355:755–756. doi: 10.1016/S0140-6736(05)72169-3. [DOI] [PubMed] [Google Scholar]
- 20.de Lecea L, et al. The hypocretins: Hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakurai T, et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 22.Sutcliffe JG, de Lecea L. The hypocretins: Setting the arousal threshold. Nat Rev Neurosci. 2002;3:339–349. doi: 10.1038/nrn808. [DOI] [PubMed] [Google Scholar]
- 23.Mochizuki T, et al. Behavioral state instability in orexin knock-out mice. J Neurosci. 2004;24:6291–6300. doi: 10.1523/JNEUROSCI.0586-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adamantidis AR, Zhang F, Aravanis AM, Deisseroth K, de Lecea L. Neural substrates of awakening probed with optogenetic control of hypocretin neurons. Nature. 2007;450:420–424. doi: 10.1038/nature06310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carter ME, Adamantidis A, Ohtsu H, Deisseroth K, de Lecea L. Sleep homeostasis modulates hypocretin-mediated sleep-to-wake transitions. J Neurosci. 2009;29:10939–10949. doi: 10.1523/JNEUROSCI.1205-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang F, Aravanis AM, Adamantidis A, de Lecea L, Deisseroth K. Circuit-breakers: Optical technologies for probing neural signals and systems. Nat Rev Neurosci. 2007;8:577–581. doi: 10.1038/nrn2192. [DOI] [PubMed] [Google Scholar]
- 27.Benington JH, Heller HC. REM-sleep timing is controlled homeostatically by accumulation of REM-sleep propensity in non-REM sleep. Am J Physiol. 1994;266:R1992–R2000. doi: 10.1152/ajpregu.1994.266.6.R1992. [DOI] [PubMed] [Google Scholar]
- 28.Mongrain V, et al. Separating the contribution of glucocorticoids and wakefulness to the molecular and electrophysiological correlates of sleep homeostasis. Sleep. 2010;33:1147–1157. doi: 10.1093/sleep/33.9.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ramos A. Animal models of anxiety: Do I need multiple tests? Trends Pharmacol Sci. 2008;29:493–498. doi: 10.1016/j.tips.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Palchykova S, Winsky-Sommerer R, Meerlo P, Dürr R, Tobler I. Sleep deprivation impairs object recognition in mice. Neurobiol Learn Mem. 2006;85:263–271. doi: 10.1016/j.nlm.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 31.Palchykova S, Winsky-Sommerer R, Tobler I. Sleep deprivation in the dark period does not impair memory in OF1 mice. Chronobiol Int. 2009;26:682–696. doi: 10.1080/07420520902926025. [DOI] [PubMed] [Google Scholar]
- 32.Graves LA, Heller EA, Pack AI, Abel T. Sleep deprivation selectively impairs memory consolidation for contextual fear conditioning. Learn Mem. 2003;10:168–176. doi: 10.1101/lm.48803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.de Lecea L, Sutcliffe JG, Fabre V. Hypocretins/orexins as integrators of physiological information: Lessons from mutant animals. Neuropeptides. 2002;36:85–95. doi: 10.1054/npep.2002.0892. [DOI] [PubMed] [Google Scholar]
- 34.Deadwyler SA, Porrino L, Siegel JM, Hampson RE. Systemic and nasal delivery of orexin-A (Hypocretin-1) reduces the effects of sleep deprivation on cognitive performance in nonhuman primates. J Neurosci. 2007;27:14239–14247. doi: 10.1523/JNEUROSCI.3878-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Selbach O, et al. Orexins/hypocretins cause sharp wave- and theta-related synaptic plasticity in the hippocampus via glutamatergic, gabaergic, noradrenergic, and cholinergic signaling. Neuroscience. 2004;127:519–528. doi: 10.1016/j.neuroscience.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 36.Colas D, Cespuglio R, Sarda N. Sleep wake profile and EEG spectral power in young or old senescence accelerated mice. Neurobiol Aging. 2005;26:265–273. doi: 10.1016/j.neurobiolaging.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 37.Engleman HM, Kingshott RN, Martin SE, Douglas NJ. Cognitive function in the sleep apnea/hypopnea syndrome (SAHS) Sleep. 2000;23(Suppl 4):S102–S108. [PubMed] [Google Scholar]
- 38.Diekelmann S, Born J. The memory function of sleep. Nat Rev Neurosci. 2010;11:114–126. doi: 10.1038/nrn2762. [DOI] [PubMed] [Google Scholar]
- 39.Ego-Stengel V, Wilson MA. Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus. 2010;20:1–10. doi: 10.1002/hipo.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Girardeau G, Benchenane K, Wiener SI, Buzsáki G, Zugaro MB. Selective suppression of hippocampal ripples impairs spatial memory. Nat Neurosci. 2009;12:1222–1223. doi: 10.1038/nn.2384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.