Abstract
Several biological changes characterize normal brain aging in humans. Although some of these age-associated neural alterations are also found in other species, overt volumetric decline of particular brain structures, such as the hippocampus and frontal lobe, has only been observed in humans. However, comparable data on the effects of aging on regional brain volumes have not previously been available from our closest living relatives, the chimpanzees. In this study, we used MRI to measure the volume of the whole brain, total neocortical gray matter, total neocortical white matter, frontal lobe gray matter, frontal lobe white matter, and the hippocampus in a cross-sectional sample of 99 chimpanzee brains encompassing the adult lifespan from 10 to 51 y of age. We compared these data to brain structure volumes measured in 87 adult humans from 22 to 88 y of age. In contrast to humans, who showed a decrease in the volume of all brain structures over the lifespan, chimpanzees did not display significant age-related changes. Using an iterative age-range reduction procedure, we found that the significant aging effects in humans were because of the leverage of individuals that were older than the maximum longevity of chimpanzees. Thus, we conclude that the increased magnitude of brain structure shrinkage in human aging is evolutionarily novel and the result of an extended lifespan.
Traits that distinguish humans from other primates include enlargement of the brain and increased longevity (1, 2). Consequently, humans are unique among animals in being susceptible to certain neuropathologies, such as Alzheimer's disease, in the later stages of life (3–5). Even in the absence of disease, however, healthy aging in humans is marked by variable degrees of neural deterioration and cognitive impairment (6). Diffuse amyloid-β deposits, dendritic attrition, reduced synapse numbers, loss of NMDA receptors, and degeneration of myelinated axons have all been observed to preferentially affect regions of the human cerebral cortex that are involved in learning, memory, and executive function (7, 8). These same changes also accompany normal senescence in other primate species and correlate with the disruption of cognition (3, 9, 10).
In addition to these microstructural and molecular changes, in vivo MRI studies of humans have shown that aging also involves whole-brain volumetric decline, as well as selective regional shrinkage (11–13). Within the cerebral cortex, the frontal lobe and hippocampus are especially vulnerable to age-associated atrophy (14–16). In contrast to humans, however, such dramatic age-related reduction in the size of these structures has not been observed in macaque monkeys based on manual volume tracing studies (17–19). More subtle volume loss of prefrontal cortex subregions (i.e., dorsolateral prefrontal cortex and anterior cingulate cortex) in macaques, however, has been reported (20, 21). Because macaque monkeys and humans are separated by ∼30 million y of independent evolution, these species differences raise the question of whether the more pronounced effects of neurodegenerative changes in human aging are unique and potentially related to an extended lifespan.
An improved understanding of the neurobiology of aging in great apes (i.e., chimpanzees, bonobos, gorillas, and orangutans) would help resolve this issue. Unfortunately, there is a paucity of information from these species, despite the fact that they comprise our closest living relatives and have the longest lifespan of any nonhuman primate, potentially extending into their 60s under medical care in captivity (22). Current evidence indicates that brain aging in great apes displays both similarities and differences from humans. For example, deposits of amyloid-β protein in the form of diffuse plaques and vascular lesions have been noted in the hippocampus and neocortex of aged chimpanzees, gorillas, and orangutans (23, 24), and there is evidence of humanlike neurofibrillary tangles from a 41-y-old female chimpanzee (25). Furthermore, one study of postmortem brain weight in chimpanzees found a minimal rate of decline over adulthood (26). Despite these similarities, however, the majority of gene expression changes in the neocortex during aging have been found to differ among humans, chimpanzees, rhesus macaques, and mice (27, 28), and humans are more vulnerable than great apes to age-related neurodegenerative diseases, such as Alzheimer's disease, Parkinson disease, and other forms of dementia (3).
In this study, we test the hypothesis that human brain aging patterns are a result of evolving a longer lifespan compared with the great apes. To do this, we examined whether chimpanzees display human-like age-related decline in the volume of regions of the cerebral cortex. We measured the total neocortical gray matter, total neocortical white matter, frontal lobe gray matter, frontal lobe white matter, and hippocampus in humans and chimpanzees from MRI using manual segmentation protocols that were similar in both species. The age ranges under investigation encompassed the adult lifespan for both species under natural conditions, extending into old age (29, 30).
Results
The volumes of regions of interest (ROI) were examined using multiple regressions in each species separately. Each model included a term for sex and either linear, quadratic, or cubic polynomial regression terms for age. The models for each ROI were compared to determine the best fit to the volumetric data by evaluating the statistical significance of the age terms.
Humans.
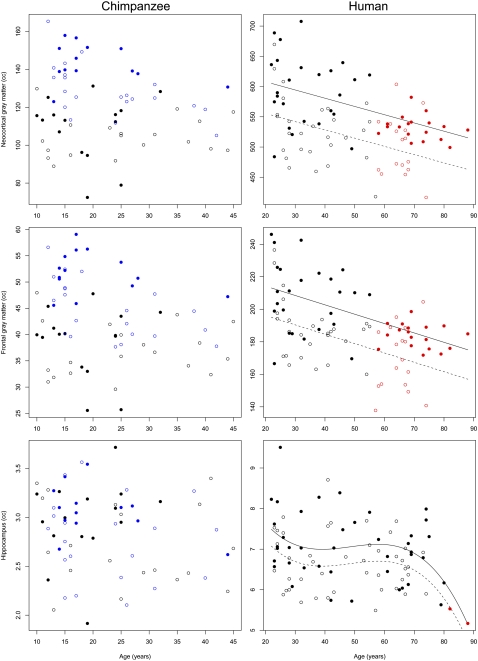
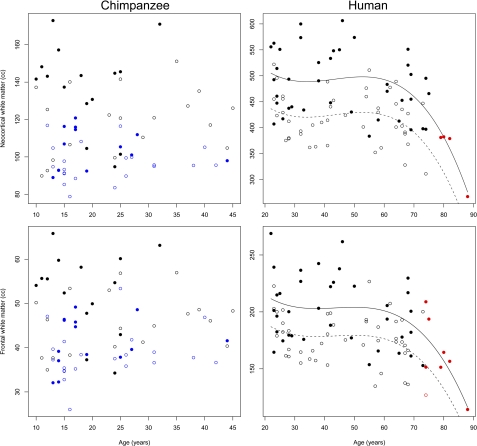
As has been shown in other studies, the humans in our sample exhibited a significant aging effect for all brain structure volumes that were measured (Table 1). Linear models were the best fit for the reduction of neocortical gray matter volume (significance of age effect: P < 0.001) and frontal lobe gray matter volume (significance of age effect: P < 0.001) with age (Fig. 1). The decline in total neocortical white-matter volume was best fit by a cubic age function (significance of age effect: P < 0.001), as was the decline in frontal lobe white matter (significance of age effect: P < 0.001) (Fig. 2). Hippocampus volume loss was best characterized by a cubic model (significance of age effect: P = 0.003) (Fig. 1). Additionally, there was a main effect of sex for all ROIs: males had larger brain structure volumes than females. To determine whether the frontal lobe and hippocampus ROIs showed a differential rate of age-related change in volume compared with the rest of the brain, we examined frontal lobe gray matter as a fraction of total neocortical gray matter, frontal lobe white matter as a fraction of total neocortical white matter, and the ratio of hippocampus volume over total neocortical gray matter. All ratios were logged before inclusion in regression models. These relative measures of frontal lobe gray matter, frontal lobe white matter, and hippocampus also showed decreases that were associated with aging (Table 2 and Fig. S1).
Table 1.
Proportional reduction of sum of squared residuals resulting from the addition of age effects to multiple regression models
| Brain region | Age model | Age effects in chimpanzees (n = 69) | Age effects in humans (n = 87) |
| Whole brain | Linear | 0.012 (0.369) | — |
| Quadratic | 0.021 (0.505) | — | |
| Cubic | 0.042 (0.435) | — | |
| Neocortical gray matter | Linear | 0.027 (0.184) [20] | 0.223 (<0.001*) |
| Quadratic | 0.039 (0.284) [29] | 0.224 (<0.001*) | |
| Cubic | 0.061 (0.265) [36] | 0.231 (<0.001*) | |
| Frontal lobe gray matter | Linear | 0.028 (0.175) [17] | 0.277 (<0.001*) |
| Quadratic | 0.057 (0.153) [23] | 0.283 (<0.001*) | |
| Cubic | 0.071 (0.196) [29] | 0.288 (<0.001*) | |
| Hippocampus | Linear | 0.010 (0.416) [54] | 0.076 (0.011*) |
| Quadratic | 0.013 (0.649) [67] | 0.093 (0.018*) | |
| Cubic | 0.034 (0.530) [53] | 0.153 (0.003*) | |
| Neocortical white matter | Linear | 0.004 (0.627) [32] | 0.131 (<0.001*) |
| Quadratic | 0.004 (0.881) [28] | 0.237 (<0.001*) | |
| Cubic | 0.064 (0.241) [28] | 0.295 (<0.001*) | |
| Frontal lobe white matter | Linear | 0.014 (0.348) [28] | 0.153 (<0.001*) |
| Quadratic | 0.014 (0.628) [30] | 0.219 (<0.001*) | |
| Cubic | 0.055 (0.307) [32] | 0.259 (<0.001*) |
Proportional reduction of sum of squared residuals resulting from the addition of age effects to multiple regression models, where a value of 1 corresponds to a perfect fit between model and data, and a value of 0 indicates no improvement of model fit by including age effects. Before the addition of age effects, brain-region size is dependent on sex in humans, and is dependent on sex and MRI scan type in chimpanzees. P values given in parentheses correspond to F-tests to evaluate whether the addition of age effects significantly improve model fits. Boldface indicates best-fit model following procedure described in Materials and Methods; an asterisk indicates the age effect is significant at α = 0.05. Numbers in brackets are sample sizes derived from a power analysis: they indicate the minimum sample size required in the chimpanzee sample to detect as significant (at α = 0.05) an effect size as small as the effect size in the associated human model. Note that the chimpanzee sample exceeds those minimum sample sizes in all cases. All models reported here do not include interaction terms. Full models that included interactions between sex and age variables were also run; in all but two cases interaction terms were insignificant. For the cubic models for neocortical gray matter and neocortical white matter in humans, there was a significant interaction effect between age and sex for both models, although interactions between sex and higher order polynomials of age were not significant. For chimpanzee models, a categorical MRI scan type variable was also included (1.5 T vs. 3 T); in full models, interactions between MRI scan type and age were insignificant in all cases except the linear models for neocortical gray matter and frontal lobe gray matter. In the models reported here (with no interaction effects), the MRI scan-type coefficient was significant for all models except for the hippocampus and whole-brain models.
Fig. 1.
Gray-matter ROIs versus age in chimpanzees and humans. Open symbols are females, closed symbols are males; for humans, solid line indicates best-fit curve superimposed on males, dashed line indicates best fit curve superimposed on females. Blue datapoints are individuals scanned at 3 T, all other individuals scanned at 1.5 T. Red datapoints are those individuals which are at the cutoff age or older (Table 3).
Fig. 2.
White-matter ROIs versus age in chimpanzees and humans. Symbols and lines follow Fig. 1.
Table 2.
Proportional reduction of sum of squared residuals associated with addition of age effects for analyses of relative brain-region size
| Brain region | Age model | Age effects in chimpanzees (n = 99) | Age effects in humans (n = 87) |
| Relative frontal lobe gray matter | Linear | 0.001 (0.811) | 0.109 (0.002*) |
| Quadratic | 0.004 (0.844) | 0.135 (0.002*) | |
| Cubic | 0.012 (0.782) | 0.141 (0.006*) | |
| Relative hippocampus | Linear | 0.007 (0.429) | 0.019 (0.206) |
| Quadratic | 0.012 (0.585) | 0.059 (0.079) | |
| Cubic† | 0.023 (0.538) | ||
| Males | — | 0.181 (0.048*) | |
| Females | — | 0.176 (0.050*) | |
| Relative frontal lobe white matter | Linear‡ | 0.053 (0.038*) | |
| 1.5 T | 0.000 (0.913) | — | |
| 3 T | 0.097 (0.073) | — | |
| PM 3 T | 0.237 (0.007*) | — | |
| Quadratic‡ | 0.065 (0.061) | ||
| 1.5 T | 0.016 (0.788) | — | |
| 3 T | 0.109 (0.167) | — | |
| PM 3 T | 0.365 (0.003*) | — | |
| Cubic | 0.132 (0.004*) | 0.079 (0.079) |
Proportional reduction of sum of squared residuals associated with addition of age effects (as explained in Table 1) for analyses of relative brain region size. Before the addition of age effects, relative brain-region size is dependent on sex in humans, and is dependent on sex and MRI scan type in chimpanzees. All of the models reported here do not include interaction terms. Abbreviations follow Table 1. Boldface indicates best-fit model following procedure described in Materials and Methods; an asterisk indicates the age effect is significant at α = 0.05; em-dash indicates no data.
†Significant interaction between sex and age; regression model run separately within each sex. PM, postmortem.
‡Significant interaction between MRI scan type and age variables; regression model run separately within each MRI scan type.
Chimpanzees.
In vivo MRI of 69 chimpanzees revealed that total brain volume does not vary significantly with age from 10 to 45 y, and there is no interaction effect between sex and age (P = 0.369) (Table 1 and Fig. S2). In contrast to humans, the total neocortical gray matter, frontal lobe gray matter, and hippocampus did not exhibit age-related changes in volume (Fig. 1 and Fig. S3). In addition, neither the volume of the neocortical white matter nor frontal lobe white matter displayed significant associations with age (Fig. 2, Table 1, and Fig. S4). All ROIs exhibited a significant main effect of sex in chimpanzees: males had larger volumes for all brain structures than females. Because the relative measures of frontal lobe gray matter, frontal lobe white matter, and hippocampus represent the proportional size of each structure within an individual, chimpanzee brains that were scanned postmortem and subject to fixation could also be included in these analyses. Based on the combined sample of in vivo and postmortem chimpanzee brains (n = 99; age range = 10–51 y), there was a significant age effect for relative frontal lobe white matter volume, but this measure did not decline over the lifespan (Table 2 and Fig. S1). No other relative measures of brain structure volumes showed a significant effect of age.
Chimpanzees may differ from humans in the variability of ROI size for a given age or sex. Therefore, we performed power analyses to determine the minimum sample of chimpanzees required to find statistical significance at α = 0.05 for an age effect equal to or greater than the age effect in humans for each regression model (effect size is measured as the proportional reduction of sum of squared residuals associated with the addition of age effects to a regression model without age effects; see SI Materials and Methods for more detail). According to these power analyses, the minimum sample sizes required to detect humanlike age effects for all ROIs were lower than the chimpanzee sample size (Table 1). Thus, if aging effects in the chimpanzee sample were similar to humans, it is very likely that they would have been detected by our method. Moreover, although the current study is based on a comparatively small cross-sectional sample of chimpanzees, the prospect of obtaining longitudinal brain aging data for chimpanzees in the foreseeable future is remote; therefore, we regard our conclusions about chimpanzee brain aging to be justified relative to the human data in our analysis.
Human Longevity.
Because humans and chimpanzees differ in their maximum longevity, we sought to examine the impact of an elongated lifespan in humans on the observed results. To do this, we applied an iterative age-range reduction procedure to the human sample (see SI Materials and Methods for details). For each significant model, the datapoint from the oldest individual in the series was removed and model coefficients were recalculated. This procedure was repeated until the P value of the model was no longer significant, providing a cutoff point at which the statistical effect of age on the brain structure volume was no longer evident. Results of this analysis are shown in Figs. 1 and 2 and Table 3. Notably, all cutoff ages for the best-fit models in humans are substantially above the oldest age represented in the chimpanzee sample. For frontal lobe white matter, neocortical white matter, and hippocampus, the cutoff ages are in the seventh or eighth decade of life, and for frontal lobe gray matter and neocortical gray matter the cutoff ages are in the late fifth decade; all are well above the maximum potential age known for chimpanzees in the wild.
Table 3.
Cutoff ages for age effects in humans
| Brain region | Age model | Cutoff age | Reduced sample n | Reduced sample P |
| Human neocortical gray matter | Linear | 58 (57) | 53 (52) | 0.078 (0.092) |
| Quadratic | 61 (27) | 57 (16) | 0.051 (0.101) | |
| Cubic | 65 (–) | 63 (–) | 0.071 (–) | |
| Human frontal lobe gray matter | Linear | 57 (–) | 52 (–) | 0.058 (–) |
| Quadratic | 50 (–) | 46 (–) | 0.051 (–) | |
| Cubic | 41 (–) | 34 (–) | 0.080 (–) | |
| Human neocortical white matter | Linear | 74 (74) | 79 (79) | 0.145 (0.125) |
| Quadratic | 79 (74) | 83 (79) | 0.057 (0.215) | |
| Cubic | 79 (74) | 83 (79) | 0.056 (0.206) | |
| Human frontal lobe white matter | Linear | 73 (69) | 77 (74) | 0.057 (0.108) |
| Quadratic | 74 (74) | 79 (79) | 0.106 (0.083) | |
| Cubic | 74 (74) | 79 (79) | 0.119 (0.090) | |
| Human hippocampus | Linear | 82 (82) | 85 (85) | 0.071 (0.066) |
| Quadratic | 88 (88) | 86 (86) | 0.090 (0.086) | |
| Cubic | 82 (82) | 85 (85) | 0.159 (0.148) |
When datapoints older than or equal to the cutoff ages are removed, age is no longer significantly associated with brain region size. Boldface values indicate the best-fit models identified in Table 1. Cutoff ages and associated P values are identified using the two methods described in Materials and Methods; values in parentheses are for the method using degrees-of-freedom based on the full human sample size (maximum age = 88, n = 87). A dash indicates that the age effect remains significant even at the smallest possible sample size for which model parameters could be calculated (n = 7, with one brain at age 22 and six brains at age 23) when the model is evaluated as if it had the full sample size of n = 87.
Discussion
We found that neurological aging in chimpanzees does not involve the same magnitude and pattern of widespread volumetric loss that characterizes humans. These phylogenetic differences suggest that humans may be uniquely vulnerable to age-related neurodegeneration, pointing to compromises that have been struck in the evolution of an enlarged brain and an extended lifespan.
Brain Aging in Humans and Chimpanzees Compared.
In normal human aging, decline in cognitive abilities affects numerous domains, including fluid reasoning, mental processing speed, episodic memory, and spatial ability (31). Furthermore, studies of human postmortem brains have demonstrated degenerative changes in the microstructure of the neocortex with age. Specifically, dendritic arborization of pyramidal neurons and the number of synapses in higher-order association areas of the neocortex and hippocampus progressively deteriorate after ∼50 y of age (32–34). Neuronal perikarya in the cerebral cortex also shrink in volume over the course of adulthood in humans (35). Healthy brain aging, however, is not accompanied by substantial loss of neurons in the cerebral cortex of humans or other primates (8, 36–38).
The severity of microstructural changes ultimately leads to gross volumetric decline in particular brain regions in the course of normal human aging. Our current results and those of other cross-sectional studies demonstrate that neocortical gray-matter volume decreases at a linear rate with age, whereas the hippocampus and cerebral white matter are relatively stable until approximately the sixth or seventh decade, after which there is a nonlinear acceleration of shrinkage (2, 15, 39, 40). This general pattern has also been observed in longitudinal data (14, 41). More subtle age-related alterations in the size of specific regions of the prefrontal cortex (i.e., anterior cingulate, orbitofrontal, and lateral prefrontal cortices) have been reported to occur earlier in life, preceding the shrinkage of the total frontal lobe and whole neocortex gray matter (42).
In contrast to what is known from humans, extremely little data exist concerning age-related variation in brain morphology and cognition of chimpanzees or other great apes. A prior study of brain mass in chimpanzees over the lifespan found a minimal decline with age (26); we did not observe such a correlation in our data. This discrepancy may be explained by methodological differences between studies. Herndon and colleagues (26) analyzed the mass of 42 postmortem brains ranging in age at death from 7 to 59, whereas we analyzed data from 69 in vivo MRI scans from individuals ages 10 to 45 y. Thus, our sample may not represent effects on brain size among the most geriatric chimpanzees under captive care. Indeed, after removal of the four individuals in their sample above age 45, reanalysis does not indicate a significant relationship between brain mass and age (Pearson correlation: r = −0.131, P = 0.433, n = 38). Because chimpanzees in the wild usually die before they reach age 45 (29), this finding suggests that brain size remains static throughout most of adulthood under natural conditions. In contrast, modern human foragers without access to medical services can potentially live into their 80s (30), yet overall brain size has been shown to decrease in humans between age 25 and 75, with an accelerated rate of reduction after the age of 50 (13).
The current analysis of chimpanzees and previous studies of macaque monkeys have not found a humanlike decline in total neocortical gray matter, frontal lobe gray matter, or hippocampus volume (17, 18, 21). Although functionally significant alterations in cortical morphology during aging are known to occur in nonhuman primates because of microstructural changes in dendritic systems and synaptic densities (8, 20, 21), our current results indicate that humans are unique in the severity of structural degeneration of neocortical gray matter in elderly individuals, being so extensive and widespread that it is detectable as overt volume loss.
Cerebral white matter in humans also has been shown to undergo steep volumetric reduction after middle age (ages 40–50) (40, 43–45). Because brain aging in primates involves degeneration of both axons and sheaths of myelinated nerve fibers (10), the resulting disruption of connectivity may explain the eventual loss of integrative coactivation among higher-order processing systems in the neocortex of the elderly (6). However, in contrast to humans, our analyses indicated that white matter volume in chimpanzees remains fairly stable with age. It is notable that the corpus callosum of chimpanzees has also been reported to maintain its cross-sectional area over adulthood (46), whereas in humans it decreases in size during normal aging (47). Taken together, these findings suggest that age-dependent changes in white matter may involve pathological processes that differ between humans and chimpanzees.
Evolutionary Implications.
Aging in humans and chimpanzees differ from each other in several important ways. By their mid-30s, chimpanzees in the wild are physically frail, have heavily worn teeth, experience weight loss, and display reduced activity level (29, 48). Nonetheless, females can retain fertility almost until the end of life, despite indications of lengthened cycles and oocyte depletion (48, 49). Somatic aging in humans progresses more slowly than in chimpanzees (50), yet reproductive senescence in women, which is marked by menopause, occurs well before the end of life, at ∼50 y of age (51). Thus, the age at which women's reproductive capacity ends is similar to the age at which chimpanzees and other great apes naturally die.
One hypothesized benefit of an elongated postreproductive lifespan in humans is that elderly grandmothers and other allocare helpers can provision and nurture dependent offspring, helping to subsidize the high caloric requirements to grow a large brain and reduce weaning time (2, 50, 52). Although the benefits of extended longevity might increase fitness through intergenerational cooperative breeding to support brain growth in children, the results of the current study suggest that these adaptive solutions also create a unique setting in which humans undergo more progressive neurological aging compared with our close relatives, the chimpanzees. The molecular machinery of neurons has been shown to accrue pathological modifications in an age-dependent fashion, including the accumulation of ubiquitinated protein aggregates and reduced signaling through the insulin/IGF1 pathway (6). In addition, the reduced transcription of mitochondrial genes is of particular relevance to human cortical aging (27). Defective mitochondrial function may impair the efficiency of the electron transport chain, thereby increasing the generation and release of damaging reactive oxygen species. The accumulation of nuclear DNA damage during the aging process affects postmitotic cells, such as neurons of the central nervous system in a regional and cell type-specific manner (53, 54).
In this regard, age-related mitochondrial dysfunction may have its greatest impact on cells that have the highest bioenergetic demand, such as the pyramidal neurons of the cerebral cortex. Microarray studies have demonstrated that increased expression of metabolic energy genes distinguishes the human neocortex from that of chimpanzees and other great apes (55). Consistent with this idea, age-dependent up-regulation of oxidative-stress response and DNA damage repair genes have been found in studies of human prefrontal cortex (28). Progressive oxidative damage can silence specific gene promoters, epigenetically transitioning them to a more repressive transcriptional state and leading to impairment of neuronal function (27).
In humans, the compounded effect of a large brain with a metabolically expensive neocortex and a long lifespan appear to amplify the effects of cellular aging processes that are common to other mammals, resulting in more pronounced pathogenesis. Furthermore, these interactions may explain the unique vulnerability of human neocortical gray matter and hippocampus volume to such striking deterioration with age. Although an enlarged brain and extended lifespan have conferred decisive fitness benefits to humans, ultimately these adaptations come at a cost. These factors combine in the later stages of life to beset many of the elderly of our species with the effects of intensified neurodegeneration.
Materials and Methods
Subjects and MRI Scanning.
Chimpanzees.
In vivo MRIs, using either a 1.5 T or 3 T scanner, were obtained from 69 captive chimpanzees ranging in age from 10 to 45 y, including 29 males (10–44 y of age) and 40 females (10–45 y of age). All of the chimpanzees were members of a captive colony housed at Yerkes National Primate Research Center in Atlanta, Georgia; this research was approved by their institutional review board. Only individuals over 10 y of age were used in this study to ensure that our sample represented subjects that had reached sexual maturity and had completed juvenile brain growth (26, 56). For analysis of relative ROI volumes, we included an additional 30 postmortem chimpanzee brains (10–51 y of age), which had been scanned at 3 T. These postmortem brains were not used in analyses of absolute volumetric changes because of concerns with unknown shrinkage artifacts from fixation.
Humans.
For comparison with the chimpanzees, we used in vivo 1.5 T MRI data of 87 adult humans from individuals aged 22 to 88 y of age. All subjects gave informed consent in accordance with relevant institutional and federal ethical regulations. The details of the human sample have been reported previously (13). Subjects included 43 men (22–88 y of age) and 44 women (23–74 y of age). All were right-handed (assessed by the Oldfield–Geschwind handedness inventory; mean score = 95, SD = 11), healthy, and with no history of neurological or psychiatric illness. Subjects older than 60 y were assessed by interview on a case-by-case basis for general health status and medication use. None had a clinical history of heart disease, hypertension, diabetes, or any other common age-associated disease.
All brain MRIs from both species were screened for the presence of visible pathology. Full details of the age and sex distribution of the sample can be found in Dataset S1 and details about the MRI scanning procedure can be found in SI Materials and Methods.
Volumetric Measurements.
Measurements of neocortical gray matter, neocortical white matter, frontal lobe gray matter, frontal lobe white matter, and hippocampus were performed for each hemisphere using similar manual segmentation protocols in both species. Before tracing ROIs, brains were realigned, but not resized, along a plane running through the AC–PC line. This realignment limited right–left rotation, and ensured that coronal slices used in the tracing of ROIs were perpendicular to a uniformly and anatomically defined axis of the brain in all subjects. Details about the volumetric measurements can be found in SI Materials and Methods.
Statistical Methods.
Human regression models all included sex and age terms, with three versions of the regression model including a single age term (linear), and additional second-order polynomial age term (quadratic), or an additional second- and third-order polynomial term (cubic). For chimpanzees, each regression model included an additional term for MRI scan type (in vivo 1.5 T, in vivo 3 T, or postmortem 3 T). Models reported here do not include interaction effects, although full models including interactions between sex and all age variables (as well as between scan type and all age variables for chimpanzees) were run to determine whether interaction effects were significant. To test whether age affects were significantly associated with brain-region size, F ratios were used to compare models including the age terms to models without age (i.e., with only sex or sex and scan type as independent variables). In the case of linear models, the resulting P value was identical to the P value for the age coefficient; in the case of quadratic and cubic models the resulting P value corresponds to combined significance of adding all of the age terms to the model. A power analysis was used to determine the minimum sample size required in the chimpanzee sample to detect human-like age-related decreases in brain region size as significant at α = 0.05. An iterative age range reduction procedure was applied to the human sample to determine the extent to which associations between brain-region size and age were driven by the oldest individuals in the sample. See SI Materials and Methods for more information about the statistical methods.
Supplementary Material
Acknowledgments
We thank Dr. Shannon C. McFarlin for many insightful discussions related to this research and Hanna Damasio and Joel Bruss for help with recruitment and analysis of the human subjects. This work was supported by grants from the National Science Foundation (BCS-0515484, BCS-0549117, BCS-0827531, DGE-0801634), the National Institutes of Health (NS42867), the James S. McDonnell Foundation (22002078), the Mathers Foundation, and the Yerkes Center (RR000165). J.S.A. was supported by National Institute of Neurological Disorders and Stroke Program Project Grant NS 19632.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016709108/-/DCSupplemental.
References
- 1.Sherwood CC, Subiaul F, Zawidzki TW. A natural history of the human mind: Tracing evolutionary changes in brain and cognition. J Anat. 2008;212:426–454. doi: 10.1111/j.1469-7580.2008.00868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allen JS, Bruss J, Damasio H. The aging brain: The cognitive reserve hypothesis and hominid evolution. Am J Hum Biol. 2005;17:673–689. doi: 10.1002/ajhb.20439. [DOI] [PubMed] [Google Scholar]
- 3.Hof PR, et al. Comparative neuropathology of brain aging in primates. In: Erwin JM, Hof PR, editors. Aging in Nonhuman Primates. Vol 31. Basel: Karger; 2002. pp. 130–154. [Google Scholar]
- 4.Walker LC, Cork LC. The neurobiology of aging in nonhuman primates. In: Terry RD, Katzman R, Bick KL, Sisodia SS, editors. Alzheimer Disease. 2nd Ed. Philadelphia: Lippincott Williams and Wilkins; 1999. pp. 233–243. [Google Scholar]
- 5.Finch CE, Sapolsky RM. The evolution of Alzheimer disease, the reproductive schedule, and apoE isoforms. Neurobiol Aging. 1999;20:407–428. doi: 10.1016/s0197-4580(99)00053-6. [DOI] [PubMed] [Google Scholar]
- 6.Bishop NA, Lu T, Yankner BA. Neural mechanisms of ageing and cognitive decline. Nature. 2010;464:529–535. doi: 10.1038/nature08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uylings HB, de Brabander JM. Neuronal changes in normal human aging and Alzheimer's disease. Brain Cogn. 2002;49:268–276. doi: 10.1006/brcg.2001.1500. [DOI] [PubMed] [Google Scholar]
- 8.Hof PR, Morrison JH. The aging brain: Morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 9.Peters A, Sethares C, Luebke JI. Synapses are lost during aging in the primate prefrontal cortex. Neuroscience. 2008;152:970–981. doi: 10.1016/j.neuroscience.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowley MP, Cabral H, Rosene DL, Peters A. Age changes in myelinated nerve fibers of the cingulate bundle and corpus callosum in the rhesus monkey. J Comp Neurol. 2010;518:3046–3064. doi: 10.1002/cne.22379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sowell ER, et al. Mapping cortical change across the human life span. Nat Neurosci. 2003;6:309–315. doi: 10.1038/nn1008. [DOI] [PubMed] [Google Scholar]
- 12.Kruggel F. MRI-based volumetry of head compartments: Normative values of healthy adults. Neuroimage. 2006;30(1):1–11. doi: 10.1016/j.neuroimage.2005.09.063. [DOI] [PubMed] [Google Scholar]
- 13.Allen JS, Bruss J, Brown CK, Damasio H. Normal neuroanatomical variation due to age: The major lobes and a parcellation of the temporal region. Neurobiol Aging. 2005;26:1245–1260. doi: 10.1016/j.neurobiolaging.2005.05.023. discussion 1279–1282. [DOI] [PubMed] [Google Scholar]
- 14.Raz N, et al. Regional brain changes in aging healthy adults: General trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- 15.Jernigan TL, et al. Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging. 2001;22:581–594. doi: 10.1016/s0197-4580(01)00217-2. [DOI] [PubMed] [Google Scholar]
- 16.Tisserand DJ, et al. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–669. [PubMed] [Google Scholar]
- 17.Shamy JL, et al. Hippocampal volume is preserved and fails to predict recognition memory impairment in aged rhesus monkeys (Macaca mulatta) Neurobiol Aging. 2006;27:1405–1415. doi: 10.1016/j.neurobiolaging.2005.07.019. [DOI] [PubMed] [Google Scholar]
- 18.O'Donnell KA, Rapp PR, Hof PR. Preservation of prefrontal cortical volume in behaviorally characterized aged macaque monkeys. Exp Neurol. 1999;160:300–310. doi: 10.1006/exnr.1999.7192. [DOI] [PubMed] [Google Scholar]
- 19.Herndon JG, Tigges J, Klumpp SA, Anderson DC. Brain weight does not decrease with age in adult rhesus monkeys. Neurobiol Aging. 1998;19:267–272. doi: 10.1016/s0197-4580(98)00054-2. [DOI] [PubMed] [Google Scholar]
- 20.Alexander GE, et al. Age-related regional network of magnetic resonance imaging gray matter in the rhesus macaque. J Neurosci. 2008;28:2710–2718. doi: 10.1523/JNEUROSCI.1852-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shamy JL, et al. Volumetric correlates of spatiotemporal working and recognition memory impairment in aged rhesus monkeys. Cereb Cortex. 2011;21:1559–1573. doi: 10.1093/cercor/bhq210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erwin JM, Hof PR, Ely JJ, Perl DP. One gerontology: Advancing understanding of aging through studies of great apes and other primates. In: Erwin JM, Hof PR, editors. Aging in Nonhuman Primates. Interdisciplinary Topics in Gerontology. Vol 31. Basel: Karger; 2002. pp. 1–21. [Google Scholar]
- 23.Kimura N, et al. Senile plaques in an aged western lowland gorilla. Exp Anim. 2001;50(1):77–81. doi: 10.1538/expanim.50.77. [DOI] [PubMed] [Google Scholar]
- 24.Gearing M, Tigges J, Mori H, Mirra SS. Beta-Amyloid (A beta) deposition in the brains of aged orangutans. Neurobiol Aging. 1997;18(2):139–146. doi: 10.1016/s0197-4580(97)00012-2. [DOI] [PubMed] [Google Scholar]
- 25.Rosen RF, et al. Tauopathy with paired helical filaments in an aged chimpanzee. J Comp Neurol. 2008;509:259–270. doi: 10.1002/cne.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Herndon JG, Tigges J, Anderson DC, Klumpp SA, McClure HM. Brain weight throughout the life span of the chimpanzee. J Comp Neurol. 1999;409:567–572. [PubMed] [Google Scholar]
- 27.Loerch PM, et al. Evolution of the aging brain transcriptome and synaptic regulation. PLoS ONE. 2008;3:e3329. doi: 10.1371/journal.pone.0003329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fraser HB, Khaitovich P, Plotkin JB, Pääbo S, Eisen MB. Aging and gene expression in the primate brain. PLoS Biol. 2005;3:e274. doi: 10.1371/journal.pbio.0030274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill K, et al. Mortality rates among wild chimpanzees. J Hum Evol. 2001;40:437–450. doi: 10.1006/jhev.2001.0469. [DOI] [PubMed] [Google Scholar]
- 30.Blurton Jones NG, Hawkes K, O'Connell JF. Antiquity of postreproductive life: Are there modern impacts on hunter-gatherer postreproductive life spans? Am J Hum Biol. 2002;14:184–205. doi: 10.1002/ajhb.10038. [DOI] [PubMed] [Google Scholar]
- 31.Whalley LJ, Deary IJ, Appleton CL, Starr JM. Cognitive reserve and the neurobiology of cognitive aging. Ageing Res Rev. 2004;3:369–382. doi: 10.1016/j.arr.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Anderson B, Rutledge V. Age and hemisphere effects on dendritic structure. Brain. 1996;119:1983–1990. doi: 10.1093/brain/119.6.1983. [DOI] [PubMed] [Google Scholar]
- 33.de Brabander JM, Kramers RJK, Uylings HBM. Layer-specific dendritic regression of pyramidal cells with ageing in the human prefrontal cortex. Eur J Neurosci. 1998;10:1261–1269. doi: 10.1046/j.1460-9568.1998.00137.x. [DOI] [PubMed] [Google Scholar]
- 34.Jacobs B, Driscoll L, Schall M. Life-span dendritic and spine changes in areas 10 and 18 of human cortex: A quantitative Golgi study. J Comp Neurol. 1997;386:661–680. [PubMed] [Google Scholar]
- 35.Stark AK, et al. The effect of age and gender on the volume and size distribution of neocortical neurons. Neuroscience. 2007;150(1):121–130. doi: 10.1016/j.neuroscience.2007.06.062. [DOI] [PubMed] [Google Scholar]
- 36.Pakkenberg B, Gundersen HJG. Neocortical neuron number in humans: Effect of sex and age. J Comp Neurol. 1997;384:312–320. [PubMed] [Google Scholar]
- 37.Peters A, Morrison JH, Rosene DL, Hyman BT. Feature article: Are neurons lost from the primate cerebral cortex during normal aging? Cereb Cortex. 1998;8:295–300. doi: 10.1093/cercor/8.4.295. [DOI] [PubMed] [Google Scholar]
- 38.Pakkenberg B, et al. Aging and the human neocortex. Exp Gerontol. 2003;38(1–2):95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- 39.Walhovd KB, et al. Consistent neuroanatomical age-related volume differences across multiple samples. Neurobiol Aging. 2011;32:916–932. doi: 10.1016/j.neurobiolaging.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Courchesne E, et al. Normal brain development and aging: Quantitative analysis at in vivo MR imaging in healthy volunteers. Radiology. 2000;216:672–682. doi: 10.1148/radiology.216.3.r00au37672. [DOI] [PubMed] [Google Scholar]
- 41.Fjell AM, et al. One-year brain atrophy evident in healthy aging. J Neurosci. 2009;29:15223–15231. doi: 10.1523/JNEUROSCI.3252-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pieperhoff P, et al. Deformation field morphometry reveals age-related structural differences between the brains of adults up to 51 years. J Neurosci. 2008;28:828–842. doi: 10.1523/JNEUROSCI.3732-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartzokis G, et al. Age-related changes in frontal and temporal lobes in men: A magnetic resonance imaging study. Arch Gen Psychiatry. 2001;58:461–465. doi: 10.1001/archpsyc.58.5.461. [DOI] [PubMed] [Google Scholar]
- 44.Guttmann CR, et al. White matter changes with normal aging. Neurology. 1998;50:972–978. doi: 10.1212/wnl.50.4.972. [DOI] [PubMed] [Google Scholar]
- 45.Walhovd KB, et al. Effects of age on volumes of cortex, white matter and subcortical structures. Neurobiol Aging. 2005;26:1261–1270. doi: 10.1016/j.neurobiolaging.2005.05.020. discussion 1275–1278. [DOI] [PubMed] [Google Scholar]
- 46.Hopkins WD, Phillips KA. Cross-sectional analysis of the association between age and corpus callosum size in chimpanzees (Pan troglodytes) Dev Psychobiol. 2010;52(1):133–141. doi: 10.1002/dev.20421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hasan KM, et al. Diffusion tensor quantification of the human midsagittal corpus callosum subdivisions across the lifespan. Brain Res. 2008;1227:52–67. doi: 10.1016/j.brainres.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Finch CE, Stanford CB. Meat-adaptive genes and the evolution of slower aging in humans. Q Rev Biol. 2004;79(1):3–50. doi: 10.1086/381662. [DOI] [PubMed] [Google Scholar]
- 49.Caro TM, et al. Termination of reproduction in nonhuman and human female primates. Int J Primatol. 1995;16:205–220. [Google Scholar]
- 50.Hawkes K. Grandmothers and the evolution of human longevity. Am J Hum Biol. 2003;15:380–400. doi: 10.1002/ajhb.10156. [DOI] [PubMed] [Google Scholar]
- 51.Gosden RG. Biology of the Menopause: The Causes and Consequences of Ovarian Aging. London, UK: Academic Press; 1985. [Google Scholar]
- 52.Burkart JM, Hrdy SB, van Schaik CP. Cooperative breeding and human cognitive evolution. Evol Anthropol. 2009;18(5):175–186. [Google Scholar]
- 53.Kirkwood TB, Austad SN. Why do we age? Nature. 2000;408:233–238. doi: 10.1038/35041682. [DOI] [PubMed] [Google Scholar]
- 54.Mandavilli BS, Rao KS. Neurons in the cerebral cortex are most susceptible to DNA-damage in aging rat brain. Biochem Mol Biol Int. 1996;40:507–514. doi: 10.1080/15216549600201073. [DOI] [PubMed] [Google Scholar]
- 55.Preuss TM, Cáceres M, Oldham MC, Geschwind DH. Human brain evolution: Insights from microarrays. Nat Rev Genet. 2004;5:850–860. doi: 10.1038/nrg1469. [DOI] [PubMed] [Google Scholar]
- 56.Harvey PH, Martin RD, Clutton-Brock TH. Life histories in comparative perspective. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Strusaker TT, editors. Primate Societies. Chicago: University of Chicago Press; 1987. pp. 181–196. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.