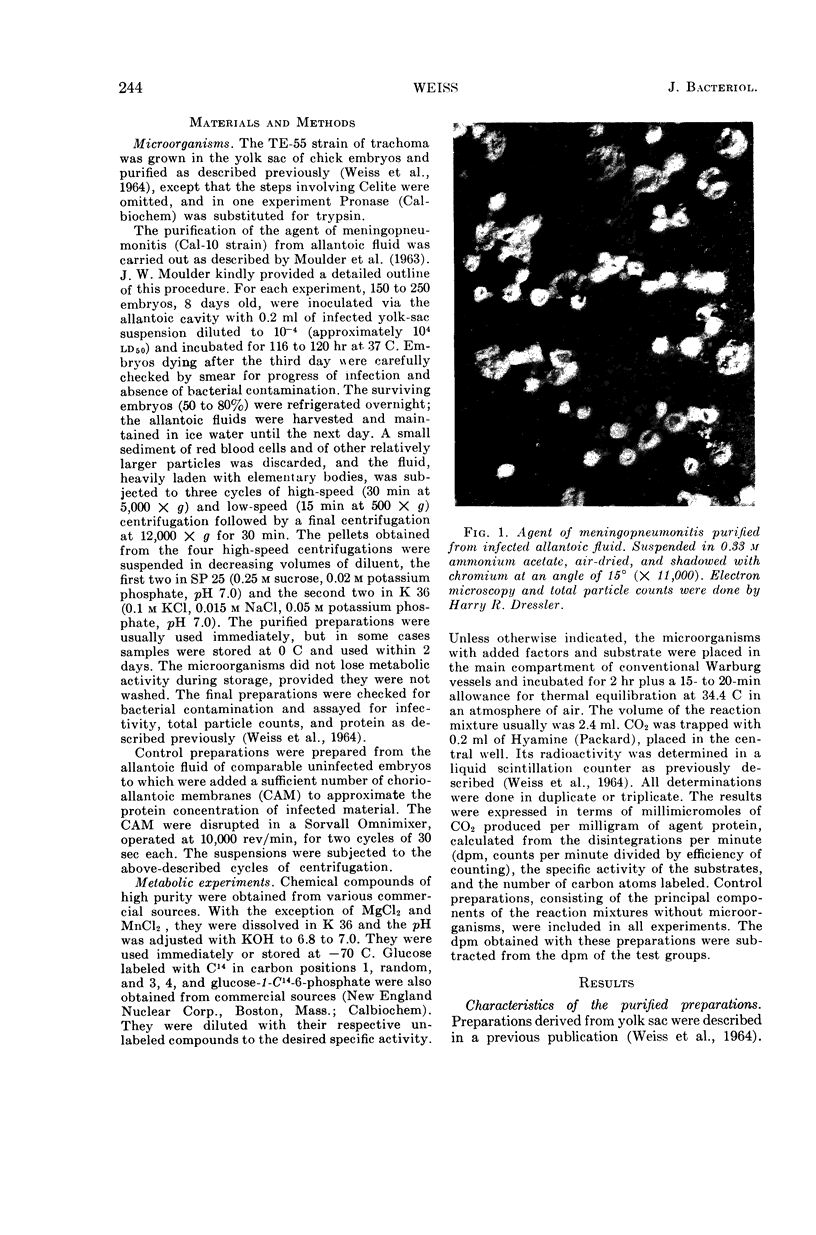
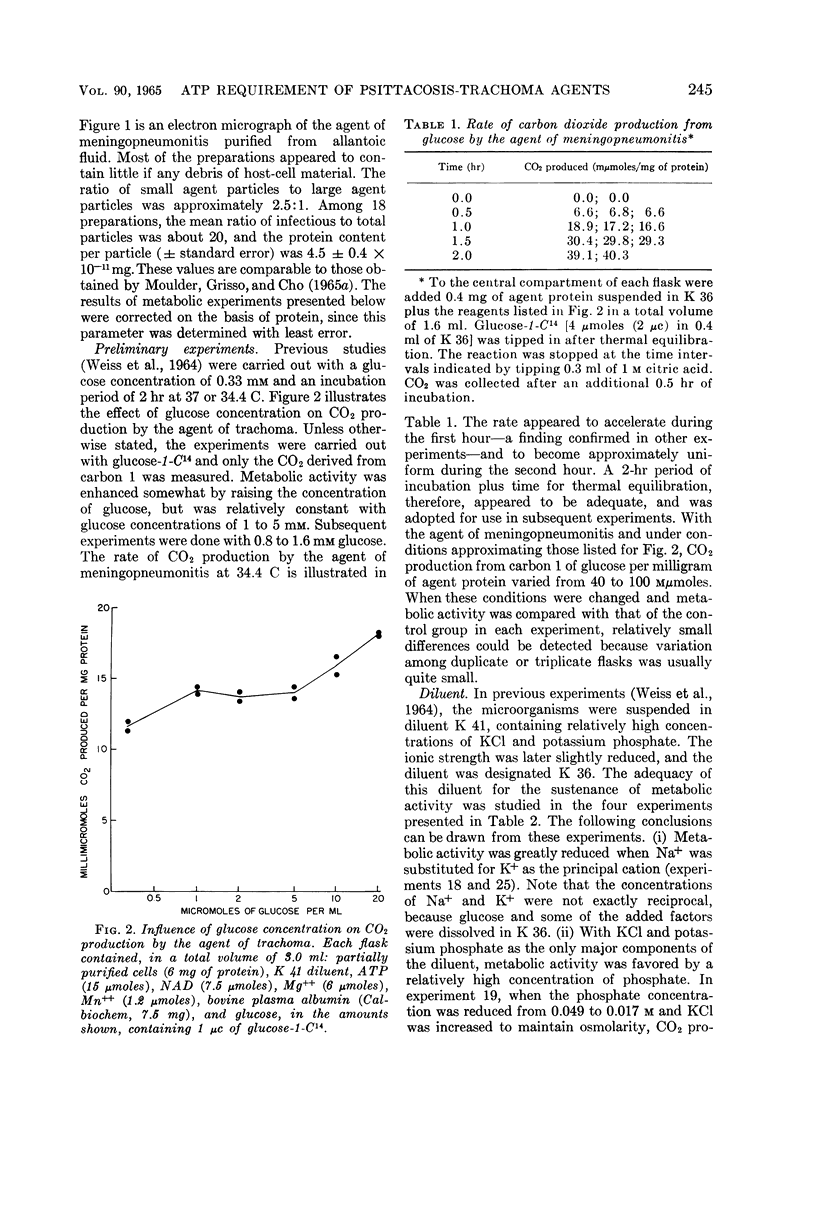
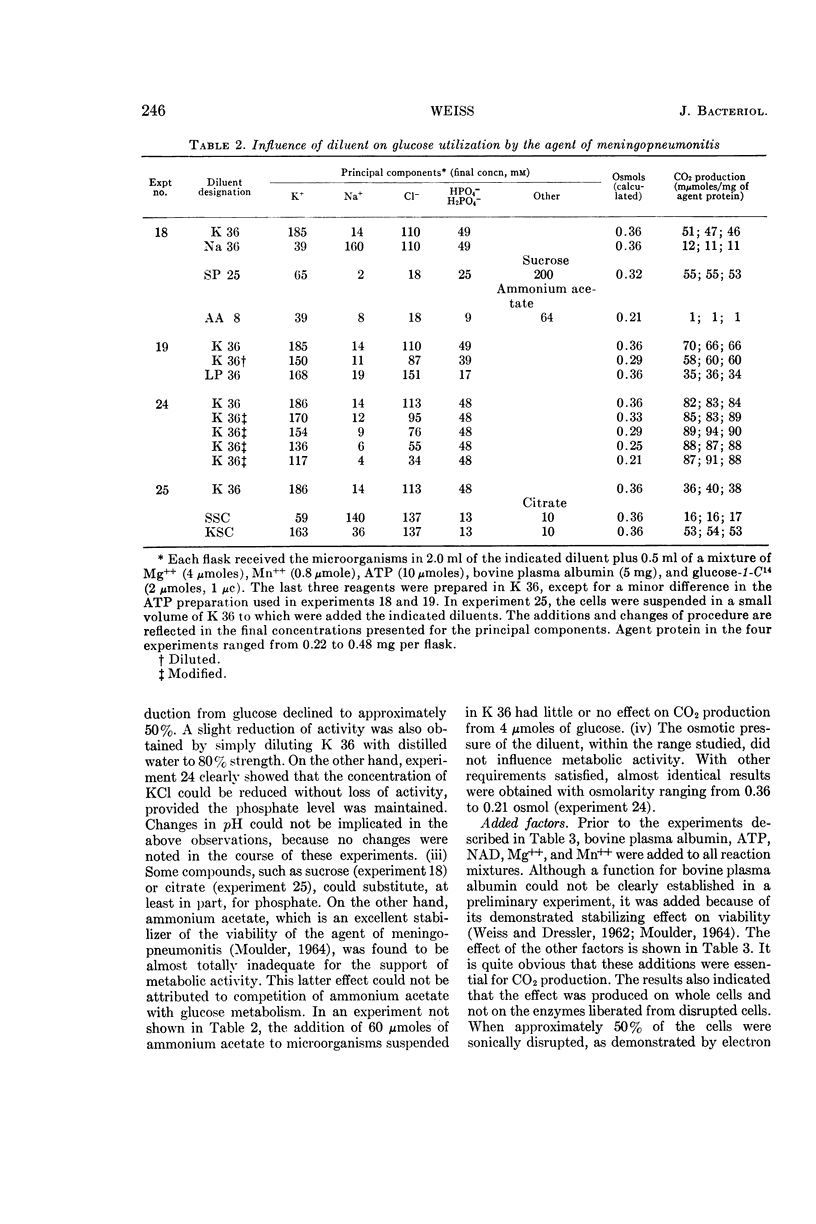
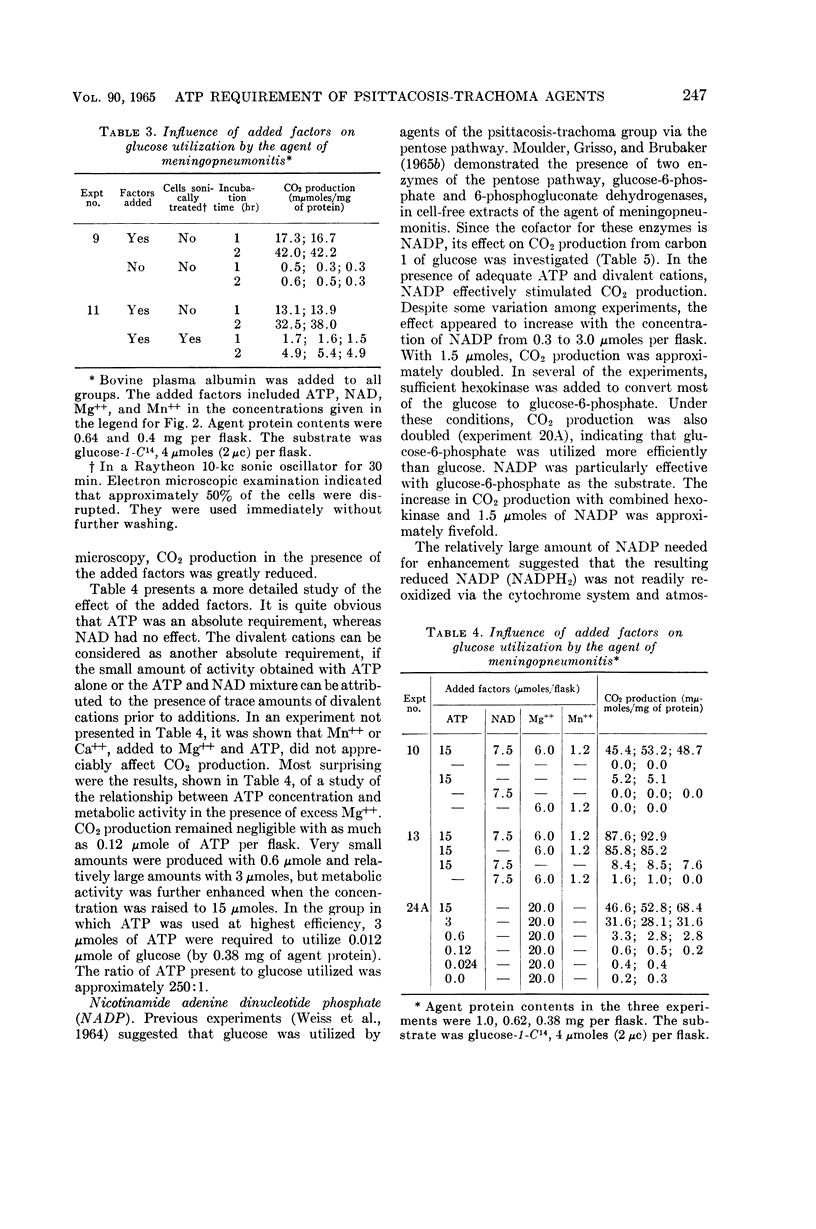
Abstract
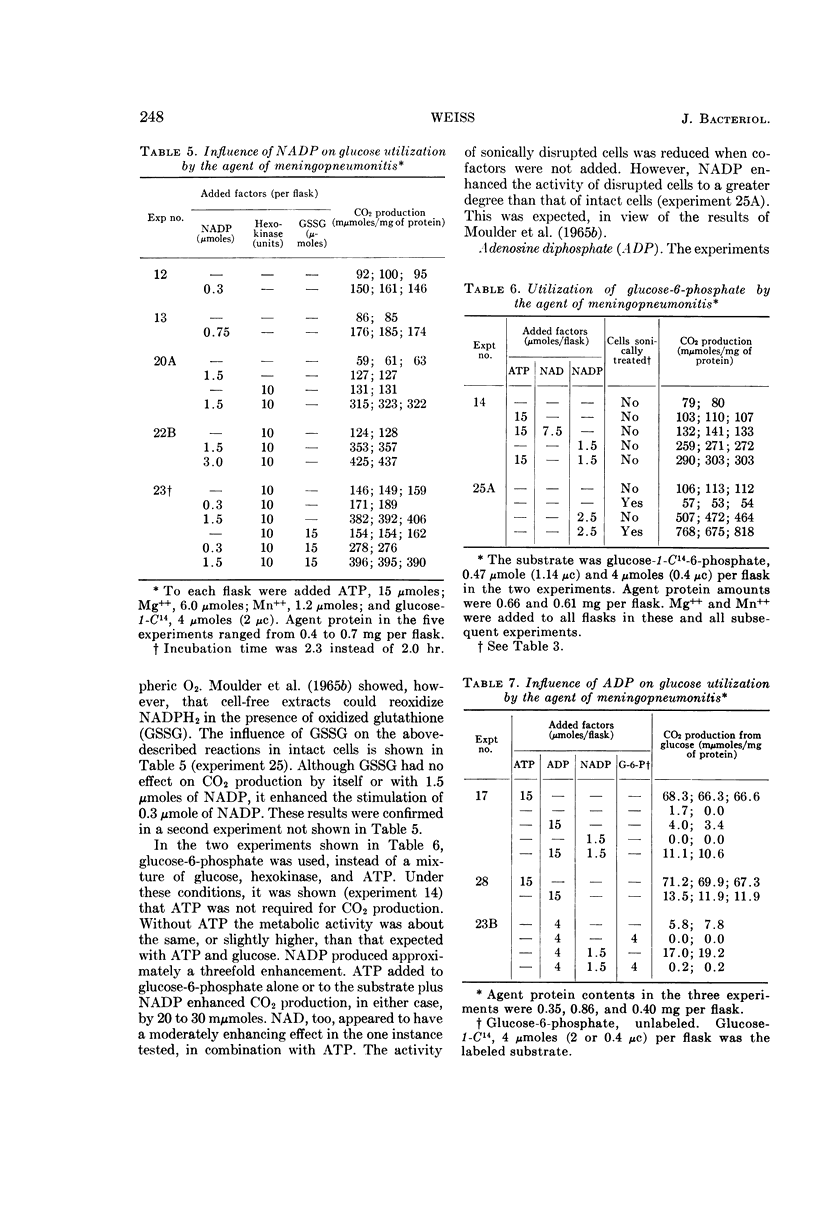
Weiss, Emilio (Naval Medical Research Institute, Bethesda, Md.). Adenosine triphosphate and other requirements for the utilization of glucose by agents of the psittacosis-trachoma group. J. Bacteriol. 90:243–253. 1965.—The agent of meningopneumonitis cultivated in the allantoic cavity of chick embryos and purified by differential centrifugations was employed for most of the studies of the requirements for glucose utilization. The evolution of C14O2 from glucose-1-C14 was used as the criterion of metabolic activity in most experiments. The rate of glucose utilization increased somewhat during the first hour of incubation at 34.4 C and became approximately constant during the second hour. Changes in glucose concentration from 1 to 5 mm did not appreciably affect metabolic activity. More vigorous CO2 production was obtained when the ratio of K+-Na+ was >1 and, under certain conditions, when the concentration of inorganic phosphate was relatively high (0.05 m). Glucose utilization was entirely dependent on added adenosine triphosphate (ATP) and Mg++. The effect of ATP was greatly reduced when the microorganisms were partially disrupted with sonic energy. Adenosine diphosphate (ADP) could be substituted for ATP, but the activity was reduced to less than 20%. ATP was not required when glucose-6-phosphate was substituted for glucose. With ADP and glucose, glucose-6-phosphate was an effective competitor of glucose utilization. Nicotinamide adenine dinucleotide phosphate (NADP) enhanced CO2 production from carbon 1, but not from other carbons, with glucose and, especially, glucose-6-phosphate as substrates. ATP and NADP produced the above-described effects only when their concentrations were comparable to those of the substrates. These concentrations always exceeded the amount of CO2 produced (0.05 to 0.5 μmole/mg of agent protein). The concentration of NADP could be reduced when oxidized glutathione was added. Diphosphothiamine had no effect on CO2 production. Qualitatively similar results were obtained with the agent of trachoma purified from yolk sac. These experiments furnish evidence that agents of the psittacosistrachoma group, despite their enzymatic capabilities, require an exogenous source of energy.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A. Reversible metabolic swelling of bacterial protoplasts. J Biol Chem. 1959 Feb;234(2):383–388. [PubMed] [Google Scholar]
- BALIS M. E., LARK C. T., LUZZATI D. Nucleotide utilization by Escherichia coli. J Biol Chem. 1955 Feb;212(2):641–645. [PubMed] [Google Scholar]
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of the toxicity and hemolytic activity of typhus rickettsiae by starvation. J Bacteriol. 1957 Nov;74(5):637–645. doi: 10.1128/jb.74.5.637-645.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of typhus Rickettsiae. I. Inactivation by freezing. J Gen Physiol. 1954 Nov 20;38(2):169–179. doi: 10.1085/jgp.38.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., ALLEN E. G. Reversible inactivation of typhus rickettsiae at O C. J Bacteriol. 1957 Jan;73(1):56–62. doi: 10.1128/jb.73.1.56-62.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BOVARNICK M. R., SCHNEIDER L. The incorporation of glycine-1-C14 by typhus rickettsiae. J Biol Chem. 1960 Jun;235:1727–1731. [PubMed] [Google Scholar]
- Bolton E. BIOSYNTHESIS OF NUCLEIC ACID IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Aug;40(8):764–772. doi: 10.1073/pnas.40.8.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bovarnick M. R., Schneider L. ROLE OF ADENOSINE TRIPHOSPHATE IN THE HEMOLYSIS OF SHEEP ERYTHROCYTES BY TYPHUS RICKETTSIAE. J Bacteriol. 1960 Sep;80(3):344–354. doi: 10.1128/jb.80.3.344-354.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRAENKEL D. G., FALCOZ-KELLY F., HORECKER B. L. THE UTILIZATION OF GLUCOSE 6-PHOSPHATE BY GLUCOKINASELESS AND WILD-TYPE STRAINS OF ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Nov;52:1207–1213. doi: 10.1073/pnas.52.5.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN P. J. Cocarboxylase and adenosine triphosphate as growth factors for Hemophilus piscium. Arch Biochem. 1951 Jan;30(1):100–102. [PubMed] [Google Scholar]
- GRIFFIN P. Further studies on the nutrition of Hemophilus piscium. Yale J Biol Med. 1952 Apr;24(5):411–418. [PMC free article] [PubMed] [Google Scholar]
- Gingrich W., Schlenk F. Codehydrogenase I and Other Pyridinium Compounds as V-Factor for Hemophilus influenzae and H. parainfluenzae. J Bacteriol. 1944 Jun;47(6):535–550. doi: 10.1128/jb.47.6.535-550.1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOKIN L. E., HOKIN M. R. BIOLOGICAL TRANSPORT. Annu Rev Biochem. 1963;32:553–578. doi: 10.1146/annurev.bi.32.070163.003005. [DOI] [PubMed] [Google Scholar]
- LESLEY S. M., GRAHAM A. F. The utilization of desoxyribonucleotide phosphorus by Escherichia coli. Can J Microbiol. 1956 Feb;2(1):17–27. doi: 10.1139/m56-004. [DOI] [PubMed] [Google Scholar]
- LICHTENSTEIN J., BARNER H. D., COHEN S. S. The metabolism of exogenously supplied nucleotides by Escherichia coli. J Biol Chem. 1960 Feb;235:457–465. [PubMed] [Google Scholar]
- Landman O. E., Spiegelman S. ENZYME FORMATION IN PROTOPLASTS OF BACILLUS MEGATERIUM. Proc Natl Acad Sci U S A. 1955 Oct 15;41(10):698–704. doi: 10.1073/pnas.41.10.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULDER J. W., GRISSO D. L., BRUBAKER R. R. ENZYMES OF GLUCOSE CATABOLISM IN A MEMBER OF THE PSITTACOSIS GROUP. J Bacteriol. 1965 Mar;89:810–812. doi: 10.1128/jb.89.3.810-812.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOULDER J. W., GRISSO D. L., CHO G. J. ENDOGENOUS METABOLISM OF PROTEIN AND RIBONUCLEIC ACID IN A MEMBER OF THE PSITTACOSIS GROUP. J Infect Dis. 1965 Jun;115:254–262. doi: 10.1093/infdis/115.3.254. [DOI] [PubMed] [Google Scholar]
- MOULDER J. W., NOVOSEL D. L., TRIBBY I. C. DIAMINOPIMELIC ACID DECARBOXYLASE OF THE AGENT OF MENINGOPNEUMONITIS. J Bacteriol. 1963 Mar;85:701–706. doi: 10.1128/jb.85.3.701-706.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ORMSBEE R. A., WEISS E. TRACHOMA AGENT: GLUCOSE UTILIZATION BY PURIFIED SUSPENSIONS. Science. 1963 Nov 22;142(3595):1077–1078. doi: 10.1126/science.142.3595.1077. [DOI] [PubMed] [Google Scholar]
- WEISS E., DRESSLER H. R. Investigation of the stability of the trachoma agent. Ann N Y Acad Sci. 1962 Mar 5;98:250–260. doi: 10.1111/j.1749-6632.1962.tb30549.x. [DOI] [PubMed] [Google Scholar]
- WEISS E., MYERS W. F., DRESSLER H. R., CHUN-HOON H. GLUCOSE METABOLISM BY AGENTS OF THE PSITTACOSIS-TRACHOMA GROUP. Virology. 1964 Apr;22:551–562. doi: 10.1016/0042-6822(64)90076-5. [DOI] [PubMed] [Google Scholar]
- WEISS E., MYERS W. F., SUITOR E. C., Jr, NEPTUNE E. M., Jr Respiration of a rickettsialike microorganism, Wolbachia persica. J Infect Dis. 1962 Mar-Apr;110:155–164. doi: 10.1093/infdis/110.2.155. [DOI] [PubMed] [Google Scholar]



