Abstract
Great progress has been made in the field of insect olfaction in recent years. Receptors, neurons, and circuits have been defined in considerable detail, and the mechanisms by which they detect, encode, and process sensory stimuli are being unraveled. We provide a guide to recent progress in the field, with special attention to advances made in the genetic model organism Drosophila. We highlight key questions that merit additional investigation. We then present our view of how recent advances may be applied to the control of disease-carrying insects such as mosquitoes, which transmit disease to hundreds of millions of people each year. We suggest how progress in defining the basic mechanisms of insect olfaction may lead to means of disrupting host-seeking and other olfactory behaviors, thereby reducing the transmission of deadly diseases.
Keywords: olfactory coding, odorant receptor, olfactory circuits, vector biology, pheromone
The insect olfactory system has emerged as a prominent model in neuroscience. Investigation of its organization and function has revealed surprising answers to fundamental questions of how an animal detects, encodes, and processes sensory stimuli. The olfactory system is also of immense importance in the natural world, where it mediates attraction of insects to humans and thus underlies the transmission of disease to hundreds of millions of people each year.
Remarkable progress has been made over the past decade in elucidating mechanisms of insect olfaction, in many cases facilitated by the genetic tractability of the model organism Drosophila melanogaster. Here, we consider recent advances in the understanding of insect olfactory receptors, neurons, and circuits made in Drosophila and other insect species. We present our view that this emerging body of knowledge poises the field to make major contributions to the control of insect pests and vectors of disease, and we highlight strategies for olfactory-based vector control. We offer our perspective on the most critical challenges to fulfilling this technological promise and to solving the scientific problem of how olfactory input is translated into behavioral output.
Mechanisms of Insect Olfaction
Olfactory Organs.
Insects sense the volatile chemical world with antennae (Fig. 1). Additional organs such as maxillary palps also detect odors in many species. Olfactory organs are covered with sensory hairs called sensilla, each of which typically houses the dendrites of a few olfactory receptor neurons (ORNs) (Fig. 2 A and B) (1, 2). Olfactory sensilla fall into morphological classes, including long, single-walled sensilla and short, double-walled sensilla. The numbers of sensilla and ORNs per antenna vary dramatically among species. The moth Manduca sexta contains >100,000 antennal sensilla housing >250,000 ORNs, whereas ∼400 sensilla housing ∼1,200 ORNs are found in the D. melanogaster antenna (1, 3). Sexual dimorphism is striking in some species. For example, female Anopheles gambiae mosquitoes possess three to four times more antennal sensilla than males (2). Such dimorphism may reflect function: only female mosquitoes feed on blood, and they rely heavily on olfactory cues to locate their hosts (4).
Fig. 1.

Insect antennae. (Clockwise from upper left) Moth (Image courtesy of Geoffrey Attardo, Yale School of Public Health); Leconte's Scarab, Chrysina lecontei (Image courtesy of Alex Wild); nymph of Barytettix humphreysi (Image courtesy of Jeffrey C. Oliver); meloid beetle, Lytta magister (Image courtesy of Jeffrey C. Oliver); butterfly (Image courtesy of Geoffrey Attardo); beetle (Image courtesy of Geoffrey Attardo); ant (Image courtesy of Alex Wild); lubber grasshopper (Image courtesy of Geoffrey Attardo); bald-faced hornet, Dolichovespula maculate (Image courtesy of Gary Alpert, CDC/Harvard University). (Center) Mesquite bug nymph, Thasus neocalifornicus (Image courtesy of Alex Wild).
Fig. 2.
Morphology of and physiological recordings from olfactory sensilla. (A) Arrow indicates a single-walled trichoid sensillum from A. gambiae. (B) A double-walled grooved peg sensillum from A. gambiae. A and B are reprinted from ref. 179. (C) Single-sensillum recording method. An electrode is inserted in the lymph (L) of a sensillum, an odor stimulus is delivered, and action potentials are recorded from the ORNs. AC, accessory cells; EC, epidermal cells. Reprinted with permission from ref. 180. (D) Physiological recording. The bar above the trace indicates the 0.5-s odor stimulus. Action potentials of large amplitude derive from one ORN in the sensillum, and action potentials of smaller amplitude derive from the other ORN. In this trace, the ORN that produces large action potentials is excited by the odor.
Larvae of many insect species contain olfactory systems that are numerically simpler than their adult counterparts, perhaps reflecting the functional requirements of the two life stages. Adults often travel long distances to find food, mates, or oviposition sites; they may encounter olfactory stimuli intermittently and follow sparse odor gradients. By contrast, larvae typically hatch from eggs laid directly on or near a food source and do not navigate over long distances.
ORNs.
In adults, the clustering of a small number of ORNs in a sensillum allows convenient physiological analysis of the cellular basis of olfaction. By inserting an electrode into a sensillum, extracellular recordings of ORN responses to odors can be obtained (Fig. 2C). Each ORN in a sensillum produces an action potential with a characteristic relative amplitude, allowing identification of the ORNs that respond to a particular olfactory stimulus (Fig. 2D).
Such electrophysiological recordings have revealed that different ORNs respond to overlapping subsets of odorants (5–7) and that the different morphological classes of sensilla are functionally distinct. In many insect species, the ORNs of some single-walled sensilla respond to pheromones, whereas the neurons of others are sensitive to more general odorants, such as food odors (8). The double-walled sensilla are found in many insect orders, reflecting an ancient origin (9). They are often sensitive to polar compounds, including amines, carboxylic acids, and water vapor (10, 11).
Odorant Receptors.
The first insect odorant receptors (Ors) were identified just over a decade ago (12–14). Ors are unrelated in sequence to odorant receptors of mammals, fish, or Caenorhabditis elegans. The insect genomes characterized to date contain from 60 to 341 Or genes; D. melanogaster has 60 Or genes encoding 62 gene products through alternative splicing, whereas the red flour beetle, Tribolium castaneum, has 341 predicted Ors (15).
Each Or is expressed within a spatially restricted subpopulation of ORNs (12–14, 16). One exceptional receptor, formerly called Or83b and now called Orco, is expressed in most ORNs of both the adult and larval stages (12, 14, 16–21). The protein sequence of Orco is highly conserved among insect species (21–23), and orthologs from different species can substitute for one another functionally (23, 24). Orco forms a heteromer with Ors and is required for targeting of Ors to the ORN dendrites (21, 25, 26). More recently, a surprising role for Orco in signal transduction has been identified, as discussed below.
Insect Ors are seven-transmembrane-domain proteins and were long thought to be G protein-coupled receptors (GPCRs) like their counterparts in vertebrates and C. elegans. However, in addition to lacking sequence similarity to known GPCRs, their topology is inverted, with an intracellular N terminus and an extracellular C terminus (25, 27). Recent in vitro studies indicate that the Or–Orco heteromer functions as an odorant-gated ion channel (27–29). One of these studies provided evidence that Orco can function as a channel independent of the canonical Or, that it is stimulated by cyclic nucleotides, and that it can also signal through G proteins, albeit at a slower time scale (28). Although there are some in vivo data consistent with a role for G proteins in olfactory signaling (30), a systematic study of single-sensillum ORN physiology after genetic manipulations of G proteins did not find evidence that they contribute to odor sensitivity (31).
An intriguing theme in both vertebrate and invertebrate olfaction is that distinct classes of receptors continue to be identified. In insects, members of the Gustatory receptor (Gr) gene family (32) have been identified as coreceptors of CO2 in Drosophila (33, 34) and mosquitoes (35). CO2 signaling by the neurons that contain these Grs depends on G proteins, although neither the nature of the dependence nor the transduction mechanism has been defined (31). Two transient receptor potential (TRP) channels have been implicated in humidity detection in D. melanogaster (36); however, it will be important to resolve whether these channels are humidity receptors or components of downstream signaling machinery.
The most recently identified insect receptors for odorants are related to ionotropic glutamate receptors (IRs) (37). Several IRs are expressed in ORNs housed in the coeloconic sensilla of D. melanogaster, a sensillum class that, with rare exceptions (10), does not express Ors. Misexpression of two IRs conferred responses to odorants that evoked responses from coeloconic ORNs, supporting a role for IRs as receptors in these ORNs; IRs are likely to detect a variety of acids, aldehydes, and amines, including ammonia (37). The sequence similarity of IRs to ligand-gated ion channels suggested that they act as odor-gated ion channels, a hypothesis that has recently been supported by functional studies (38).
From Air to Receptor.
How do odorants reach receptors? Most odorants are hydrophobic and must traverse an aqueous lymph before binding their transmembrane receptors. Odorant binding proteins [OBPs; some are referred to as pheromone-binding proteins (PBPs)] are thought to bind and solubilize odorants in the aqueous environment of the sensillum. OBPs were first identified in the silk moth, Antheraea polyphemus (39), and large families of OBPs have since been identified in many other insects (40). The structure and binding mechanisms of OBPs of several species have been analyzed (41–44), and their expression patterns are diverse, with overlapping subsets of OBPs found in different sensilla (45).
The diversity of OBP expression patterns and large numbers of OBPs are reminiscent of odorant receptors; they suggest an interesting role in shaping the odor response profiles of ORNs within the sensilla that contain them. However, when individual odorant receptors were misexpressed in a sensillum that presumably contains a different complement of OBPs than the sensillum in which the receptors are endogenously expressed, the receptors conferred odor response profiles very similar to those observed in the endogenous sensillum (46, 47). These results suggested that odorant receptors are sufficient to confer the odor specificity of an ORN, at least for many receptors and many general odorants. However, OBPs seem likely to play roles in the dynamics of olfactory response and in olfactory sensitivity. Two recent studies have reported decreased electrophysiological responses to odorants when an OBP was targeted with RNAi (48, 49), and variations in behavioral responses to odorants have been associated with polymorphisms in OBP genes (50, 51).
OBPs may play especially critical roles in the reception of atypical odorants. One OBP, LUSH, is required for normal sensitivity to the pheromone cis-vaccenyl acetate (cVA) in Drosophila (43, 52, 53). cVA is highly hydrophobic and may be particularly dependent on LUSH to be solubilized. However, it is not clear how broadly such strong dependence applies to other insect pheromones. Some studies have reported responses to the silk moth pheromone bombykol without the cognate OBP (24, 54); others report that moth OBPs make a crucial contribution to pheromone sensitivity in a ligand-specific manner (55). Thus, the precise role of OBPs in odorant reception remains an intriguing problem in the field, one that merits extensive analysis of the physiological and behavioral effects of manipulating individual OBPs in vivo.
Like OBPs, sensory neuron membrane protein (SNMP) was first identified in the moth A. polyphemus (56). It is localized to the cilia and dendrites of ORNs, and its sequence is similar to that of CD36, a vertebrate receptor that binds both proteins and fatty acids. It was proposed to interact with odorant–OBP complexes and enhance the delivery of odorants to receptors. Recently, SNMP was shown to be required for the response of certain ORNs to cVA in Drosophila (53, 57).
Odor Coding.
How is odorant identity encoded by this repertoire of receptors and neurons? An ORN typically expresses a single Or along with the ubiquitous Orco in both adults (16–19, 47) and larvae (20, 58). Thus, the identity of an odorant may be encoded largely in the identity of the Ors that it activates and by extension, in the identity of the ORNs that express those Ors.
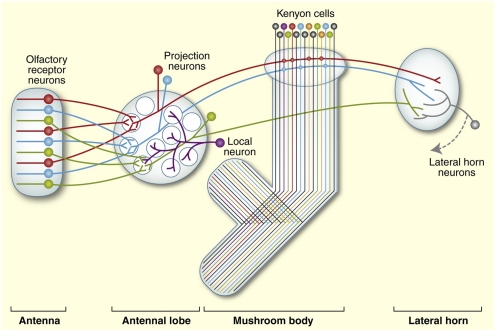
Although ORNs expressing a given Or are widely distributed across the antenna, their axons converge in the antennal lobe (AL) of the brain in spherical modules called glomeruli (59) (Fig. 3). ORNs expressing the same Or converge on a single glomerulus in Drosophila (16–18, 60), as in mammals (61), although at least in locusts, the pattern seems to be more complex (62). The organization of the larval olfactory circuit of Drosophila is similar to that of the adult, but because each Or is expressed in only one ORN, there is no convergence (63). In both adults and larvae, this anatomic organization suggests that odorant identity is encoded largely by the particular combination of glomeruli that are activated. Indeed, imaging studies in ants, moths, honey bees, flies, and other insects have confirmed that individual odorants generate complex and distinct patterns of activated glomeruli (61).
Fig. 3.
Olfactory system circuitry. ORNs expressing an individual odorant receptor (same color) send axons to an individual glomerulus in the antennal lobe. In the antennal lobe, the ORNs form synaptic connections with projection neurons, which send axons to Kenyon cells of the mushroom bodies and then to the lateral horn (red and blue axons), or directly to the lateral horn (green axon). ORNs also form synapses with local neurons in the antennal lobe. Reprinted from ref. 90 with permission from Elsevier.
Although some olfactory stimuli activate many classes of ORNs and their cognate glomeruli, other stimuli are more specific. Moth sex pheromones activate selectively tuned neurons on the male antenna and their cognate glomeruli (64, 65). Similarly, the D. melanogaster pheromone cVA strongly activates just one population of narrowly tuned ORNs and the cognate glomerulus (7, 66, 67). CO2 also activates strongly only one narrowly tuned ORN class and the corresponding glomerulus in D. melanogaster (6, 68). Such a coding strategy, in which an odorant activates a single narrowly tuned ORN class, is called a labeled line, and it may be used to encode odorants of particular biological significance. Indeed, moth pheromones robustly activate mating behavior (69), cVA acts in D. melanogaster mating, aggregation, and aggression (70), and CO2 is a component of D. melanogaster stress odor (dSO), which elicits an innate avoidance response (68, 71, 72).
Much insight into the molecular basis of odor coding has come from functional studies of insect odorant receptors. The Or repertoire of D. melanogaster has been analyzed in an in vivo expression system called the empty neuron. This system is based on a D. melanogaster deletion mutant that lacks one of its Or genes, thereby creating an empty neuron that expresses no endogenous functional receptor (47). Individual Or genes were systematically expressed in this neuron and were found in most cases to confer odor response profiles that matched those of individual ORN classes of the WT fly (46). The matches permitted the construction of a receptor to neuron map and provided evidence that one Or is sufficient to account for the response specificity of most ORNs. These misexpression experiments also showed that, in addition to the odor response spectrum, the spontaneous firing rate, temporal dynamics, and response mode (inhibitory vs. excitatory) of an ORN depend on the receptor that it expresses (46). Many intriguing questions arise as to how the structural features of an Or contribute to characteristics such as odorant specificity and temporal dynamics, and these questions will be an important direction for future work.
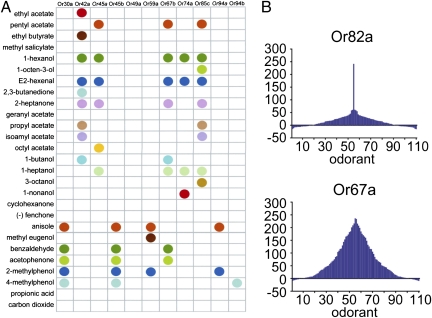
Several fundamental principles of olfactory coding by the Or repertoire were revealed by additional analysis in the empty neuron system (20, 46, 73, 74). Individual odorants activated subsets of receptors, consistent with a combinatorial model of odor coding (Fig. 4A). Individual receptors responded to overlapping subsets of odorants. Some receptors were broadly tuned, being strongly excited by a large proportion of the odorants tested, whereas others seemed more narrowly tuned, activated by just a few odorants (Fig. 4B). There was a smooth continuum in receptor-tuning breadth rather than a discrete division of receptors into specialists and generalists. These principles—combinatorial coding, variation in receptor-tuning breadth, and a continuum in tuning breadths across the receptor repertoire—were found to apply to both the adult and larval Or repertoire of Drosophila.
Fig. 4.
Odor coding by a receptor repertoire. (A) Combinatorial coding of odors by receptors of the Drosophila larva. Colored dots indicate a strong odor response, defined as ≥100 spikes/s to a 10−2 dilution in the empty neuron system. Reprinted from ref. 20 with permission from Elsevier. (B) Tuning curves for a narrowly tuned receptor, Or82a, and a broadly tuned receptor, Or67a. The 110 odorants are listed along the x axis according to the magnitudes of the responses that they elicit from each receptor. The odorants that elicit the strongest responses are placed near the center of the distribution, whereas those odorants eliciting weak responses are at the edges. The order of odors is different for the two receptors. Negative values represent inhibitory responses. Reprinted from ref. 73 with permission from Elsevier.
We note that, in the preceding analyses, Ors were expressed in the empty neuron and tested with a panel of general odorants. When certain moth or fly pheromone receptors were expressed in the empty neuron, responses were observed with the cognate pheromones, but stronger responses were observed when these receptors were expressed in a different Drosophila neuron that is sensitive to fly pheromones (54, 75, 76), consistent with a role for additional factors, including PBPs, in the detection of certain olfactory stimuli.
Central Processing of Olfactory Signals.
How is the primary representation of an odorant transformed by the downstream neuronal circuitry? The first relay in the olfactory circuit is in the antennal lobe, where the many ORNs that express a given Or converge in the same glomerulus (Fig. 3). At this location, ORNs synapse onto a smaller number of secondary neurons called projection neurons (PNs) (77). Electrophysiological studies have shown that many PNs are more broadly tuned than their cognate ORNs (67, 78, 79). This feature of PN tuning derives, in part, from excitatory interneurons with multiglomerular processes, which can transmit signals from an ORN in one glomerulus to a PN in another glomerulus (80, 81). The appearance of broader tuning also arises from properties of the ORN-PN synapse that preferentially amplify weak ORN responses; thus, PNs may respond strongly to many odorants that excite the cognate ORNs weakly (82).
PN responses show complex temporal features that may encode odorant identity and intensity (83). Recent work has shown that PN dynamics are shaped largely by the temporal dynamics of ORN responses (84). The PN responses occur in a milieu of oscillatory, synchronized neuronal activity (85, 86). The oscillations are believed to arise from a feedback loop between PNs and inhibitory interneurons within the AL (77, 87, 88). One study showed that disrupting these oscillations impairs olfactory discrimination (89). The precise function of these oscillations is an outstanding question in the field, and there is a pressing need for additional studies to define the role of synchronized neuronal activity in olfactory behaviors.
From the antennal lobe, PNs send axons to the mushroom body (MB), a higher brain region associated with olfactory learning and memory, and the lateral horn (LH), a region associated with innate olfactory behaviors (Fig. 3) (90). In the MB, PNs synapse onto Kenyon cells (KCs). PNs from multiple glomeruli synapse onto an individual KC, suggesting a role for KCs as coincidence detectors that integrate information from multiple ORN classes (91). Consistent with this notion, KCs are much more narrowly tuned than their inputs. Their selectivity depends on strong inhibitory inputs that are overcome only by coincident excitatory inputs (92, 93).
An intriguing question is whether PN-KC projections are stereotyped or plastic, which might be expected for a region associated with learning and memory. It seems that there is broad, zonal stereotypy in the projection of PNs to the MB (94–96) but variability in PN-KC connectivity within these zones (97). This variability may be experience-dependent and may play a crucial role in olfactory learning and memory.
By contrast, PN projections to the LH seem to be more stereotyped (95, 98, 99). For example, PNs that respond to the D. melanogaster pheromone cVA project to the LH in a stereotyped and sexually dimorphic fashion (66), consistent with the innate behavioral responses that this odorant elicits.
Remarkably little is known about the olfactory circuit beyond the third-order neurons of the MB and LH. However, a recent study extended the mapping of an olfactory circuit in Drosophila (100). A cVA-responsive neural circuit was traced from the ORN across three synapses to the ganglia of the ventral nerve cord, where it likely initiates motor programs. This work invites a detailed definition of the behavior elicited by the circuit, and it sets an important precedent for the analysis of other insect olfactory circuits.
In addition to the feed-forward circuitry detailed above, centrifugal projections have been described in some insects. In the moth, serotonergic neurons project into the AL and may modulate PN responses in pheromone-responsive glomeruli, suggesting a mechanism by which sensitivity to olfactory cues may be subject to central control (101). Consistent with this proposed mechanism, exogenously applied serotonin modulates PN responses in D. melanogaster (102).
These results illustrate that, although great progress has recently been made in understanding the principles of olfactory circuitry, there are major limitations to our knowledge. Remaining elements in the circuit need to be defined anatomically. Detailed knowledge of connectivity is necessary to understand the flow of olfactory information, but it is not sufficient. The polarity, strength, and modulation of synapses within the circuit must be elucidated to understand the genesis of olfactory behavior.
From Stimulus to Behavior.
The mapping and functional definition of ORNs and odorant receptors has permitted precise genetic manipulations of the olfactory circuit in Drosophila. Such manipulations are elucidating the links between olfactory stimulus and behavioral response.
Olfactory input and output are tightly coupled by dedicated circuits in some cases. In the case of CO2 detection, silencing the CO2-sensitive ORNs abolished the innate avoidance response that they mediate (68, 71). Conversely, artificially stimulating the CO2-sensitive ORNs is sufficient to trigger the avoidance response (71, 72). Thus, one population of ORNs is both necessary and sufficient to elicit a robust behavioral response in this case. Likewise, the ORNs dedicated to cVA are necessary and sufficient to mediate male courtship behavior and male–male aggression (70, 103–105), and a population of IR-expressing ORNs is necessary and sufficient for the avoidance of certain acids (106).
Mechanistic insights into the tight coupling between olfactory input and behavioral output in these cases are emerging. Electrophysiological studies of PNs in the cVA-responsive glomerulus revealed that they are not more broadly tuned than their cognate ORNs in contrast to other classes of PNs (67). This finding is consistent with the segregation of cVA-responsive ORNs into a discrete processing pathway. Furthermore, projections of these PNs to the LH are sexually dimorphic as are subsequent elements of the circuit, which may explain the sex-specific behavioral responses of D. melanogaster to cVA (66).
Behavioral responses to some odorants are driven by multiple receptors expressed in multiple ORNs. Two Ors of the larval repertoire confer robust physiological responses to the fruit odorant ethyl acetate in the empty neuron system, but the two receptors differ in their sensitivity (74). Deletion of each Or revealed that the receptors with high and low thresholds for ethyl acetate mediate behavioral responses to high and low concentrations, respectively, of this odorant. In this case, by integrating the responses of multiple receptors, the animal can extend the dynamic range of the response and evaluate odor intensity more precisely. The use of multiple receptors for an odorant may, thereby, allow the insect to navigate up an odor gradient more effectively.
The combinatorial coding of odorants through the activation of multiple receptors and ORNs is supported by an analysis of three Ors in adult Drosophila (107). Polymorphisms in all three of these genes were associated with variation in behavioral response to benzaldehyde. Deletion analysis of other receptor genes, such as Or43b, suggested redundancy in the olfactory system: although a number of odorants excite Or43b-expressing ORNs, deletion of Or43b in adult Drosophila did not produce any discernible difference in behavior to >200 olfactory stimuli (108). Similarly, selective silencing of certain ORNs in Drosophila larvae resulted in subtle behavioral deficits (58).
These examples show that the circuit diagrams underlying responses to different odorants can vary markedly. An important direction for future work is to delineate these circuits in greater anatomical and physiological detail, which will facilitate our understanding of the mechanisms by which they drive behavior. Such understanding will also aid in defining the molecular and cellular basis of plasticity in these circuits (109, 110).
Olfaction in Vector Insects
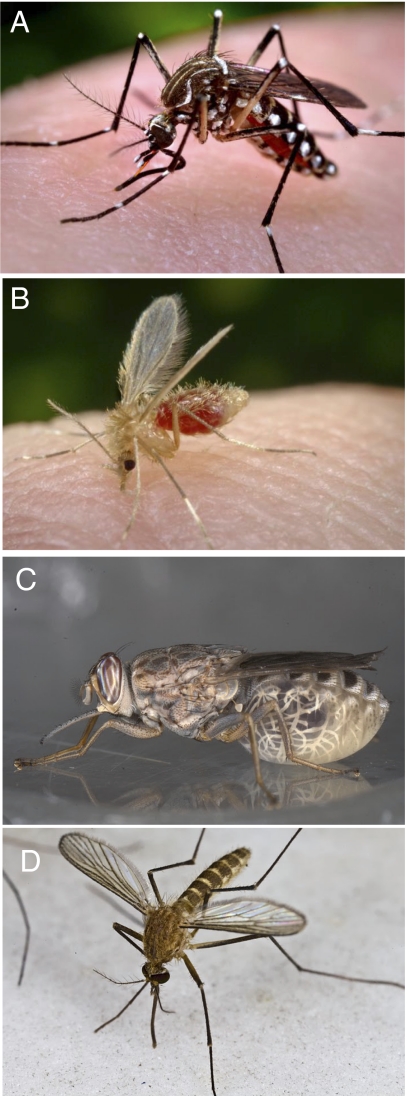
Hundreds of millions of people suffer from vector-borne diseases every year. These diseases include malaria, yellow fever, dengue, trypanosomiasis, and leishmaniasis, which are spread by mosquitoes, tsetse flies, sandflies, or other insects (Fig. 5). Insect vectors of disease rely on their sense of smell to locate hosts, find mates, and select egg-laying sites (4). Malaria-vector mosquitoes, for example, may fly upwind to host volatiles from up to 70 m away (111); triatomine bugs, the vectors of Chagas disease, leave their resting sites when they sense the CO2 exhaled by their sleeping hosts (112). Culex quinquefasciatus mosquitoes, vectors of filariasis and West Nile Virus, are attracted to oviposition sites by a pheromone released from maturing eggs that signals the suitability of the site (113). Progress in the understanding of olfaction in moths, locusts, and flies is rapidly advancing the study of olfaction in vector insects, and reciprocally, advances made in vector insects are making important contributions to our understanding of these other insect systems.
Fig. 5.
Insect vectors of disease. (A) A. aegypti is a vector of dengue fever and yellow fever (Image courtesy of James Gathany, CDC). (B) Phlebotomus papatasi, the sandfly, is a vector of Leishmaniasis (Image courtesy of James Gathany, CDC). (C) Tsetse fly Glossina morsitans morsitans is a vector of trypanosomiasis. [Image courtesy of Geoffrey Attardo (Reprinted from the cover of PLoS Neglected Tropical Diseases, March 12, 2008, volume 2, issue 3)]. (D) C. pipiens is a vector of West Nile virus. (Image courtesy of Geoffrey Attardo).
A. gambiae: A Vector of Malaria.
A. gambiae is the major vector of malaria in sub-Saharan Africa, where this disease killed >700,000 people in 2009 (114). A nocturnal blood-feeder, A. gambiae relies heavily on olfactory cues to locate its preferred host, humans (4). A. gambiae are strongly and preferentially attracted to the particular blend of volatiles emitted from humans (115), and olfactory sensilla responsive to human volatiles have been identified through electrophysiological studies (11, 116). Given the devastating impact of this mosquito's olfactory behaviors, there is great interest in elucidating the molecular mechanisms that underlie them.
A family of 79 A. gambiae Odorant receptor (AgOr) genes was identified by virtue of similarity to the fruit fly odorant receptors (117, 118). Functional characterization of the AgOrs was carried out in the empty neuron system (119). Despite ∼250 million y of evolutionary distance between the insect lineages, two AgOrs were successfully expressed in D. melanogaster. Both AgOrs responded to aromatic odorants, one of which, 4-methylphenol, is a component of human sweat and oviposition sites (115). The AgOr repertoire was subsequently examined systematically in the empty neuron and in Xenopus oocytes (35, 120–122). Interestingly, some of the most narrowly tuned AgOrs responded strongly to components of human sweat and oviposition site volatiles.
The systematic characterization of both the A. gambiae and D. melanogaster Or repertoires in the empty neuron system allowed a unique opportunity to compare them. Odorants are differentially encoded by the two species in ways that seem consistent with their ecological needs (120). For example, no AgOr was narrowly tuned to esters or aldehydes, which dominate the headspace of many fruits. By contrast, of the most narrowly tuned fruit fly Ors, in most cases, the strongest responses are to esters or a compound that contains an ester group.
A number of Ors are expressed in A. gambiae larvae (123). The role of AgOrco in larval olfactory behavior has been validated by RNAi analysis (124). Recently, orthologs of the D. melanogaster IRs have been identified in A. gambiae and are expressed in larvae (124, 125), and a role for one AgIR in the larval response to an amine was shown using RNAi (124). Because the IRs likely mediate the responses of double-walled sensilla in adult antennae to other polar compounds such as ammonia and lactic acid (37), known mosquito attractants, there is great interest in characterizing the IRs of the adult mosquito antenna.
There are three A. gambiae orthologs of the two coexpressed Grs that mediate CO2 reception in D. melanogaster (33, 35, 126, 127). All three contribute to CO2 detection in the empty neuron system (35). The CO2-sensitive Grs are expressed in the A. gambiae maxillary palp (33, 35), consistent with physiological studies in other mosquito species (128, 129).
A number of accessory olfactory proteins have been identified in A. gambiae, including a family of OBPs (130–132). RNAi experiments have shown a role for AgamOBP1 in the response to indole (48), an oviposition site compound and human volatile. A G protein, Gαq (133), and arrestins (134) have been identified. An SNMP ortholog has also been identified (135), inviting a renewed search for hydrocarbon pheromones, which have not been identified in this species. Chemical communication among A. gambiae could be a fertile topic of investigation that could generate not only scientific interest but also means of controlling this major vector of human disease.
C. quinquefasciatus: A Vector of Filariasis and West Nile Encephalitis.
The mosquito C. quinquefasciatus is common in tropical and subtropical regions throughout the world. It is a vector of filariasis, a disfiguring and debilitating disease that infects over 100 million people worldwide and can cause elephantiasis (136). It is also a vector of encephalitis viruses such as West Nile. Host odors are attractive to C. quinquefasciatus in behavioral studies (115, 137), and certain olfactory sensilla are activated by host volatiles (128, 137–140).
The olfactory basis of C. quinquefasciatus oviposition behavior is of special interest. Gravid females deposit large numbers of eggs in one location, thereby providing a ready target for insect control. An oviposition pheromone, released by maturing eggs, attracts gravid females and increases egg deposits (113). Certain aromatic compounds released from decaying organic matter in the mosquito's preferred oviposition sites have similar effects (141, 142). Olfactory sensilla responsive to these volatiles have been identified (128, 138, 140).
The first olfactory protein identified in C. quinquefasciatus was CquiOBP1 (143). More recently, a family of 53 OBPs was identified bioinformatically, and many of its members are expressed in olfactory tissues (144). CquiOBP1 binds the oviposition pheromone as well as other odorants (138, 145). In a recent study, RNAi knockdown of CquiOBP1 resulted in a reduced electrophysiological response to oviposition pheromone and some, but not all, of the nonpheromonal odorants that bind to this OBP (49). This result provides an interesting opportunity to investigate the role of an OBP in the behavioral response to nonpheromonal odorants, an unresolved question in the field.
The first Or to be identified in C. quinquefasciatus was CqOr7 (146), now called CqOrco. The C. quinquefasciatus genome project subsequently facilitated the bioinformatic identification of the entire Or family (147). Two canonical Ors, CqOr2 and CqOr10, are expressed in olfactory organs and were found in a Xenopus expression system to be narrowly tuned to the oviposition volatiles indole and 3-methylindole (147, 148). Other CqOrs and three orthologs of the Grs required for CO2 detection in Drosophila (126) await functional analysis.
A. aegypti: A Vector of Yellow Fever and Dengue.
A. aegypti is the vector of yellow fever and dengue, diseases that infect 200,000 and 50 million individuals each year, respectively (Fig. 5A). A. aegypti feeds principally at dusk and dawn, relying heavily on olfactory cues to locate its blood-meal hosts (149). A. aegypti also relies on volatile cues to locate oviposition sites, preferring water infused with organic material (150). The olfactory organs of A. aegypti have been characterized anatomically and physiologically, and sensilla that respond to host volatiles and oviposition site volatiles have been identified (115). However, despite the global impact of the diseases that it transmits, remarkably little is known about the molecular basis of olfaction in A. aegypti.
The A. aegypti genome project facilitated the identification of 131 AaOrs and 34 OBPs (151, 152) in addition to putative CO2-sensitive Grs (126). Before this work, only the ortholog of Orco (153) and a small number of OBPs (154, 155) had been identified. The crystal structure of AaegOPB1 has been solved (156), and the odorant binding profile of AaegOBP22 has been characterized (157). However, the contribution that these OBPs make to odorant reception in vivo has not been determined. Four odorant receptors, AaOr2, AaOr8, AaOr9, and AaOr10, have been functionally characterized in heterologous expression systems, and responses to host volatiles were found (158, 159). The need for additional investigation of olfaction in A. aegypti is pressing, because the incidence of dengue has increased 30-fold in the past 50 y (160), likely caused, in part, by global climate change and range expansion of its vectors.
Multiplicity of Vector Insects and Diseases.
There are many more diseases transmitted by insect vectors, including sleeping sickness (transmitted by tsetse flies), river blindness (transmitted by flies of the Simulium genus), and Chagas disease (transmitted by triatomine bugs). For these and other vector insects, olfactory cues play a role in locating hosts and in other critical aspects of the life cycle. In these species, olfactory behavior, anatomy, and physiology have been examined, but the molecular mechanisms of olfaction remain largely unexplored. Genome projects are currently underway and should accelerate investigation of tsetse flies and the Chagas disease vector Rhodnius prolixus as well as the Lyme disease vector Ixodes scapularis.
One particularly intriguing problem is the molecular and cellular basis of differences in host preferences among related vector insects. For example, some species of Anopheles are anthropophilic, whereas others are classified as zoophilic (4). Why does A. gambiae have a strong preference for human odor, whereas A. quadriannulatus does not? It will be interesting to determine whether differences in the response spectra of odorant receptors underlie such behavioral preferences.
Perspective: From Basic Science to Technology
The great advances of the last decade in defining basic mechanisms and principles of insect olfaction have provided an exciting opportunity. The molecular and cellular insight has laid a foundation for the development of olfactory-based insect control technology. The timing is auspicious: there has been renewed interest in controlling the insect vectors of disease, because other approaches, including vaccine and drug development, continue to encounter major challenges. There is added urgency to vector control efforts because of the predicted effects of climate change on the geographical distribution of many of these insects (161). Olfactory behaviors, particularly host seeking and oviposition, offer opportunities to disrupt the disease-transmission process. In this section, we consider how recent advances can be applied to the problem of vector control and how some limitations might be overcome through basic research.
Molecular Targets for Olfactory-Based Vector Control Strategies.
Work of the past decade has identified molecular targets that may be useful in developing insect control strategies. A number of Ors, Grs, and IRs are promising targets for manipulating the olfactory-guided behaviors of insects. Compounds that excite or inhibit these receptors and that are inexpensive, stable, and nontoxic could provide effective and environmentally friendly means of controlling insect vectors and pests. The identification of molecular targets may greatly increase the efficiency of screens for activators of either attraction or avoidance circuits; high-throughput cell-based expression systems can be used to screen large chemical libraries and rapidly identify candidate compounds.
Certain odorant receptors may be prime targets. More than 20 AgOrs are activated by human volatiles (119–121). Among these receptors are several that are specifically and sensitively tuned to components of human odor and may report the presence of a blood-meal host. The three C. quinquefasciatus Ors that respond to oviposition site or host volatiles (137, 147, 148) and an A. aegypti Or that is narrowly tuned to a host volatile (158) provide additional examples of receptors whose function may drive a critical behavior, and they may be prime targets for the development of behavior-modifying compounds.
Four broad classes of odorants may be useful in insect control. First, odorants that activate some receptors may drive attraction behaviors and could be used as lures in traps. Odorants identified in electrophysiological screens of tsetse olfactory organs have been used in this manner (162). A blend of electrophysiologically active odorants and visual cues was highly attractive to tsetse flies, and the traps have been used successfully in Africa. Similarly, volatiles from oviposition site material that elicited robust electrophysiological responses in C. quinquefasciatus attracted gravid females and increased egg deposits (138, 141). Recently, an electrophysiologically active host odor has been shown to be effective in trapping C. quinquefasciatus in the field (137).
Second, some odorants may activate receptors that drive avoidance circuits. There is evidence in C. quinquefasciatus that the repellent effect of N,N-diethyl-3-methylbenzamide (DEET) is caused by the activation of a particular ORN class, which presumably activates an avoidance circuit (139). If the cognate receptor for this ORN can be identified, the development of new repellents could be significantly advanced.
Third, some odorants may inhibit excitatory responses elicited by attractive human odors. Such compounds may be useful as masking agents that could be applied topically for personal protection. Indeed, one study suggested that the repellent effect of DEET is mediated through such a mechanism (163), although there is evidence for other mechanisms (139). Recently, an odorant that inhibits the Gr-mediated response to CO2 in D. melanogaster was shown to abolish the avoidance response to this volatile (71). Turner and Ray (71) also identified an odorant that inhibits the excitatory ORN response to CO2 in C. quinquefasciatus, and it will be of interest to determine whether such inhibitors reduce the behavioral response of this mosquito to CO2.
Finally, compounds that alter the temporal dynamics of an ORN response could be useful in insect control. The importance of temporal dynamics in odor coding has been described above, and manipulations that alter the temporal structure of an odorant response affect olfactory behavior (89). Compounds that generate unusually prolonged responses, for example, may disrupt host-seeking behavior. When the silk moth Bombyx mori was exposed to a structural analog of the mating pheromone bombykol that causes a persistent response in the bombykol-sensitive ORN, the normal attractive response to this pheromone was abolished (164).
The coreceptor Orco could, in principle, be a useful target for manipulating insect behavior on account of its essential role in the olfactory response of many ORNs (21, 25, 26). The phylogenetic conservation of Orco sequence and function (23) suggests that compounds that affect it in one vector species may also affect orthologs in other vector species. A concern, however, is the potential of such compounds to disrupt the behavior of beneficial insects as well. Insects are essential to the pollination of many crops and are vital to ecosystems; thus, species-specific disruption strategies may be preferable. The functional comparison of A. gambiae and D. melanogaster Ors identified a number of narrowly tuned mosquito-specific receptors (120). Such receptors might be exploited to manipulate olfactory behavior in a species-specific manner.
OBPs constitute another class of potential targets for modifying olfactory behaviors. Because RNAi knockdown of an OBP resulted in decreased electrophysiological responses to some odorants (48, 49), it will be interesting to examine the behavioral effects of such manipulations from the standpoint of technology as well as science. Targeting of OBPs that bind pheromones deserves special attention; precedent for this approach comes from the phenotypic effects observed from genetic disruption of LUSH (52) and CquiOBP1, which binds the oviposition pheromone of C. quinquefasciatus (49, 145). Screens for molecules that interact with such OBPs could yield agents that disrupt mating or oviposition behavior.
Rapid increases in the power of genomics and proteomics seem likely to identify additional targets. A recent analysis of the proteome of the D. melanogaster antenna by MS found that nearly one-third of the identified proteins were of unknown function, many of which may represent olfactory signaling components (165). As such technologies become less expensive, they may be applied to nonmodel insects more readily.
Black Box of Olfactory-Guided Insect Behavior.
One of the greatest hurdles in developing olfactory-based insect control technology is also one of the most fascinating scientific challenges. It is difficult to predict how the activation or inhibition of a particular olfactory receptor or neuron will affect a particular olfactory behavior. Even in the highly tractable model organism D. melanogaster, the link between olfactory stimulus and behavioral output is difficult to predict, despite some success in certain cases (74).
It is even more challenging to predict the behavioral effects of olfactory stimuli in vector insects such as A. gambiae. A blend of carboxylic acids has been variously shown to be attractive to female A. gambiae (166), to have no effect (167), and to be attractive only when presented combined with ammonia and CO2 (168). The difficulty in drawing simple conclusions about the relationship between an odor stimulus and the behavior that it drives may be caused, in part, by the plasticity of olfactory behaviors and the sensitivity of the behaviors to other factors that are difficult to control. Experimental design often varies between studies, and in laboratory-based studies, the limited numbers of available blood-feeding insects impose difficulties in obtaining a robust database.
These difficulties illustrate one of the greatest challenges in the field of insect olfaction: the pressing need to establish new paradigms for measuring olfactory behavior. Ideally, such paradigms should measure robust behaviors that simulate those the olfactory system has evolved to drive in its natural environment. Furthermore, they should maximize the information that can be garnered from the sometimes limited number of animals available. Automated tracking of the movements of individual animals has improved markedly in recent years and reduces the numbers of animals needed for study (169). Automated tracking technology has been used to analyze the behavior of larval (124) and adult-stage mosquitoes (170, 171) and could be adapted to monitor the behavior of other vector insects.
Although insects in their natural environment are exposed to complex mixtures of odorants, laboratory studies have generally used monomolecular odor stimuli. To understand and control insect behavior in nature, it will be necessary to devote increased attention to the mechanisms by which complex odor stimuli are encoded and processed. Although information about odorants in mixtures is known to be integrated in the antennal lobe (172–174), studies in moths (175, 176) and beetles (177) have provided evidence that it is also integrated at the periphery, and a recent study showed in detail how odor mixtures can be encoded in the magnitude and temporal dynamics of ORN responses (178). Additional consideration of odor mixtures should be a high priority in studies of insect olfactory coding and behavior.
Behavioral studies may also benefit enormously from an improved understanding of the neural circuitry underlying olfactory behaviors. In most cases, only the first few neurons have been anatomically mapped and functionally characterized, and the precise role of downstream neurons in transforming the olfactory signal into behavioral output remains largely unknown.
Diversity in Insect Olfactory Systems.
Additional deconstruction of the olfactory circuit will no doubt improve our understanding of olfactory behavior and aid in the development of insect control strategies. Rapid progress in delineating the circuitry and mechanisms of olfaction is being made in the genetically tractable system D. melanogaster. An important question, however, is the extent to which conclusions from one insect apply to others. Insects are enormously diverse, having had hundreds of millions of years in which to fill a vast number of different ecological niches. Analysis of a wide variety of insect systems is therefore essential to determine the extent of diversity in the domain of insect olfaction. It will be of special interest to determine how the receptors, neurons, and circuits of the olfactory system have evolved to meet the needs of different species.
Historically, breakthroughs in understanding of insect olfaction have come from diverse insects, and many systems continue to offer unique advantages to the field. Investigation of the differences among these systems promises to reveal interesting biological principles and facilitate progress to technological goals, including the control of insect pests. If such differences can be exploited for the control of specific vector insects, the potential impact on global health is enormous.
Acknowledgments
This work was funded by National Institutes of Health grants (to J.R.C.) and the Foundation for the National Institutes of Health through Grand Challenges in Global Health Initiative Grant GCGH 121.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Shanbhag SR, Muller B, Steinbrecht RA. Atlas of olfactory organs of Drosophila melanogaster—1. Types, external organization, innervation and distribution of olfactory sensilla. Int J Insect Morphol Embryol A. 1999;28:377–397. [Google Scholar]
- 2.McIver SB. Sensilla of mosquitoes (Diptera: Culicidae) J Med Entomol. 1982;19:489–535. doi: 10.1093/jmedent/19.5.489. [DOI] [PubMed] [Google Scholar]
- 3.Sanes JR, Hildebrand JG. Structure and development of antennae in a moth, Manduca sexta. Dev Biol. 1976;51:280–299. [PubMed] [Google Scholar]
- 4.Zwiebel LJ, Takken W. Olfactory regulation of mosquito-host interactions. Insect Biochem Mol Biol. 2004;34:645–652. doi: 10.1016/j.ibmb.2004.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields VDC, Hildebrand JG. Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2001;186:1135–1151. doi: 10.1007/s003590000165. [DOI] [PubMed] [Google Scholar]
- 6.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 7.Clyne P, Grant A, O'Connell R, Carlson JR. Odorant response of individual sensilla on the Drosophila antenna. Invert Neurosci. 1997;3:127–135. doi: 10.1007/BF02480367. [DOI] [PubMed] [Google Scholar]
- 8.de Bruyne M, Baker TC. Odor detection in insects: Volatile codes. J Chem Ecol. 2008;34:882–897. doi: 10.1007/s10886-008-9485-4. [DOI] [PubMed] [Google Scholar]
- 9.Steinbrecht RA. Structure and function of insect olfactory sensilla. Ciba Found Symp. 1996;200:158–174. doi: 10.1002/9780470514948.ch13. [DOI] [PubMed] [Google Scholar]
- 10.Yao CA, Ignell R, Carlson JR. Chemosensory coding by neurons in the coeloconic sensilla of the Drosophila antenna. J Neurosci. 2005;25:8359–8367. doi: 10.1523/JNEUROSCI.2432-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qiu YT, van Loon JJA, Takken W, Meijerink J, Smid HM. Olfactory coding in antennal neurons of the malaria mosquito, Anopheles gambiae. Chem Senses. 2006;31:845–863. doi: 10.1093/chemse/bjl027. [DOI] [PubMed] [Google Scholar]
- 12.Gao Q, Chess A. Identification of candidate Drosophila olfactory receptors from genomic DNA sequence. Genomics. 1999;60:31–39. doi: 10.1006/geno.1999.5894. [DOI] [PubMed] [Google Scholar]
- 13.Vosshall LB, Amrein H, Morozov PS, Rzhetsky A, Axel R. A spatial map of olfactory receptor expression in the Drosophila antenna. Cell. 1999;96:725–736. doi: 10.1016/s0092-8674(00)80582-6. [DOI] [PubMed] [Google Scholar]
- 14.Clyne PJ, et al. A novel family of divergent seven-transmembrane proteins: Candidate odorant receptors in Drosophila. Neuron. 1999;22:327–338. doi: 10.1016/s0896-6273(00)81093-4. [DOI] [PubMed] [Google Scholar]
- 15.Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol. 2009;71:307–332. doi: 10.1146/annurev.physiol.010908.163209. [DOI] [PubMed] [Google Scholar]
- 16.Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell. 2000;102:147–159. doi: 10.1016/s0092-8674(00)00021-0. [DOI] [PubMed] [Google Scholar]
- 17.Couto A, Alenius M, Dickson BJ. Molecular, anatomical, and functional organization of the Drosophila olfactory system. Curr Biol. 2005;15:1535–1547. doi: 10.1016/j.cub.2005.07.034. [DOI] [PubMed] [Google Scholar]
- 18.Fishilevich E, Vosshall LB. Genetic and functional subdivision of the Drosophila antennal lobe. Curr Biol. 2005;15:1548–1553. doi: 10.1016/j.cub.2005.07.066. [DOI] [PubMed] [Google Scholar]
- 19.Goldman AL, Van der Goes van Naters W, Lessing D, Warr CG, Carlson JR. Coexpression of two functional odor receptors in one neuron. Neuron. 2005;45:661–666. doi: 10.1016/j.neuron.2005.01.025. [DOI] [PubMed] [Google Scholar]
- 20.Kreher SA, Kwon JY, Carlson JR. The molecular basis of odor coding in the Drosophila larva. Neuron. 2005;46:445–456. doi: 10.1016/j.neuron.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 21.Larsson MC, et al. Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron. 2004;43:703–714. doi: 10.1016/j.neuron.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 22.Pitts RJ, Fox AN, Zwiebel LJ. A highly conserved candidate chemoreceptor expressed in both olfactory and gustatory tissues in the malaria vector Anopheles gambiae. Proc Natl Acad Sci USA. 2004;101:5058–5063. doi: 10.1073/pnas.0308146101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jones WD, Nguyen TAT, Kloss B, Lee KJ, Vosshall LB. Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr Biol. 2005;15:R119–R121. doi: 10.1016/j.cub.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Nakagawa T, Sakurai T, Nishioka T, Touhara K. Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science. 2005;307:1638–1642. doi: 10.1126/science.1106267. [DOI] [PubMed] [Google Scholar]
- 25.Benton R, Sachse S, Michnick SW, Vosshall LB. Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 2006;4:e20. doi: 10.1371/journal.pbio.0040020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neuhaus EM, et al. Odorant receptor heterodimerization in the olfactory system of Drosophila melanogaster. Nat Neurosci. 2005;8:15–17. doi: 10.1038/nn1371. [DOI] [PubMed] [Google Scholar]
- 27.Smart R, et al. Drosophila odorant receptors are novel seven transmembrane domain proteins that can signal independently of heterotrimeric G proteins. Insect Biochem Mol Biol. 2008;38:770–780. doi: 10.1016/j.ibmb.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 28.Wicher D, et al. Drosophila odorant receptors are both ligand-gated and cyclic-nucleotide-activated cation channels. Nature. 2008;452:1007–1011. doi: 10.1038/nature06861. [DOI] [PubMed] [Google Scholar]
- 29.Sato K, et al. Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature. 2008;452:1002–1006. doi: 10.1038/nature06850. [DOI] [PubMed] [Google Scholar]
- 30.Kain P, et al. Reduced odor responses from antennal neurons of G(q)α, phospholipase Cβ, and rdgA mutants in Drosophila support a role for a phospholipid intermediate in insect olfactory transduction. J Neurosci. 2008;28:4745–4755. doi: 10.1523/JNEUROSCI.5306-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yao CA, Carlson JR. Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila. J Neurosci. 2010;30:4562–4572. doi: 10.1523/JNEUROSCI.6357-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clyne PJ, Warr CG, Carlson JR. Candidate taste receptors in Drosophila. Science. 2000;287:1830–1834. doi: 10.1126/science.287.5459.1830. [DOI] [PubMed] [Google Scholar]
- 33.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 34.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lu T, et al. Odor coding in the maxillary palp of the malaria vector mosquito Anopheles gambiae. Curr Biol. 2007;17:1533–1544. doi: 10.1016/j.cub.2007.07.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu L, et al. Drosophila hygrosensation requires the TRP channels water witch and nanchung. Nature. 2007;450:294–298. doi: 10.1038/nature06223. [DOI] [PubMed] [Google Scholar]
- 37.Benton R, Vannice KS, Gomez-Diaz C, Vosshall LB. Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell. 2009;136:149–162. doi: 10.1016/j.cell.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abuin L, et al. Functional architecture of olfactory ionotropic glutamate receptors. Neuron. 2011;69:44–60. doi: 10.1016/j.neuron.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vogt RG, Riddiford LM. Pheromone binding and inactivation by moth antennae. Nature. 1981;293:161–163. doi: 10.1038/293161a0. [DOI] [PubMed] [Google Scholar]
- 40.Pelosi P, Zhou JJ, Ban LP, Calvello M. Soluble proteins in insect chemical communication. Cell Mol Life Sci. 2006;63:1658–1676. doi: 10.1007/s00018-005-5607-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wojtasek H, Leal WS. Conformational change in the pheromone-binding protein from Bombyx mori induced by pH and by interaction with membranes. J Biol Chem. 1999;274:30950–30956. doi: 10.1074/jbc.274.43.30950. [DOI] [PubMed] [Google Scholar]
- 42.Horst R, et al. NMR structure reveals intramolecular regulation mechanism for pheromone binding and release. Proc Natl Acad Sci USA. 2001;98:14374–14379. doi: 10.1073/pnas.251532998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Laughlin JD, Ha TS, Jones DNM, Smith DP. Activation of pheromone-sensitive neurons is mediated by conformational activation of pheromone-binding protein. Cell. 2008;133:1255–1265. doi: 10.1016/j.cell.2008.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mao Y, et al. Crystal and solution structures of an odorant-binding protein from the southern house mosquito complexed with an oviposition pheromone. Proc Natl Acad Sci USA. 2010;107:19102–19107. doi: 10.1073/pnas.1012274107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pikielny CW, Hasan G, Rouyer F, Rosbash M. Members of a family of Drosophila putative odorant-binding proteins are expressed in different subsets of olfactory hairs. Neuron. 1994;12:35–49. doi: 10.1016/0896-6273(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 46.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell. 2004;117:965–979. doi: 10.1016/j.cell.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 47.Dobritsa AA, van der Goes van Naters W, Warr CG, Steinbrecht RA, Carlson JR. Integrating the molecular and cellular basis of odor coding in the Drosophila antenna. Neuron. 2003;37:827–841. doi: 10.1016/s0896-6273(03)00094-1. [DOI] [PubMed] [Google Scholar]
- 48.Biessmann H, et al. The Anopheles gambiae odorant binding protein 1 (AgamOBP1) mediates indole recognition in the antennae of female mosquitoes. PLoS ONE. 2010;5:e9471. doi: 10.1371/journal.pone.0009471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pelletier J, Guidolin A, Syed Z, Cornel AJ, Leal WS. Knockdown of a mosquito odorant-binding protein involved in the sensitive detection of oviposition attractants. J Chem Ecol. 2010;36:245–248. doi: 10.1007/s10886-010-9762-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Arya GH, et al. Natural variation, functional pleiotropy and transcriptional contexts of odorant binding protein genes in Drosophila melanogaster. Genetics. 2010;186:1475–1485. doi: 10.1534/genetics.110.123166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang P, Lyman RF, Mackay TF, Anholt RR. Natural variation in odorant recognition among odorant-binding proteins in Drosophila melanogaster. Genetics. 2010;184:759–767. doi: 10.1534/genetics.109.113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xu PX, Atkinson R, Jones DNM, Smith DP. Drosophila OBP LUSH is required for activity of pheromone-sensitive neurons. Neuron. 2005;45:193–200. doi: 10.1016/j.neuron.2004.12.031. [DOI] [PubMed] [Google Scholar]
- 53.Benton R, Vannice KS, Vosshall LB. An essential role for a CD36-related receptor in pheromone detection in Drosophila. Nature. 2007;450:289–293. doi: 10.1038/nature06328. [DOI] [PubMed] [Google Scholar]
- 54.Syed Z, Ishida Y, Taylor K, Kimbrell DA, Leal WS. Pheromone reception in fruit flies expressing a moth's odorant receptor. Proc Natl Acad Sci USA. 2006;103:16538–16543. doi: 10.1073/pnas.0607874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grosse-Wilde E, Svatos A, Krieger J. A pheromone-binding protein mediates the bombykol-induced activation of a pheromone receptor in vitro. Chem Senses. 2006;31:547–555. doi: 10.1093/chemse/bjj059. [DOI] [PubMed] [Google Scholar]
- 56.Rogers ME, Sun M, Lerner MR, Vogt RG. Snmp-1, a novel membrane protein of olfactory neurons of the silk moth Antheraea polyphemus with homology to the CD36 family of membrane proteins. J Biol Chem. 1997;272:14792–14799. doi: 10.1074/jbc.272.23.14792. [DOI] [PubMed] [Google Scholar]
- 57.Jin X, Ha TS, Smith DP. SNMP is a signaling component required for pheromone sensitivity in Drosophila. Proc Natl Acad Sci USA. 2008;105:10996–11001. doi: 10.1073/pnas.0803309105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fishilevich E, et al. Chemotaxis behavior mediated by single larval olfactory neurons in Drosophila. Curr Biol. 2005;15:2086–2096. doi: 10.1016/j.cub.2005.11.016. [DOI] [PubMed] [Google Scholar]
- 59.Tolbert LP, Hildebrand JG. Organization and synaptic ultrastructure of glomeruli in the antennal lobes of the moth Manduca sexta - a study using thin-sections and freeze-fracture. Proc R Soc Lond B Biol Sci. 1981;213:279–301. [Google Scholar]
- 60.Gao Q, Yuan BB, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci. 2000;3:780–785. doi: 10.1038/77680. [DOI] [PubMed] [Google Scholar]
- 61.Wilson RI, Mainen ZF. Early events in olfactory processing. Annu Rev Neurosci. 2006;29:163–201. doi: 10.1146/annurev.neuro.29.051605.112950. [DOI] [PubMed] [Google Scholar]
- 62.Ignell R, Anton S, Hansson BS. The antennal lobe of orthoptera - anatomy and evolution. Brain Behav Evol. 2001;57:1–17. doi: 10.1159/000047222. [DOI] [PubMed] [Google Scholar]
- 63.Ramaekers A, et al. Glomerular maps without cellular redundancy at successive levels of the Drosophila larval olfactory circuit. Curr Biol. 2005;15:982–992. doi: 10.1016/j.cub.2005.04.032. [DOI] [PubMed] [Google Scholar]
- 64.Christensen TA, Hildebrand JG. Male-specific, sex pheromone-selective projection neurons in the antennal lobes of the moth Manduca sexta. J Comp Physiol A. 1987;160:553–569. doi: 10.1007/BF00611929. [DOI] [PubMed] [Google Scholar]
- 65.Kaissling KE, Hildebrand JG, Tumlinson JH. Pheromone receptor cells in the male moth Manduca sexta. Arch Insect Biochem Physiol. 1989;10:273–279. [Google Scholar]
- 66.Datta SR, et al. The Drosophila pheromone cVA activates a sexually dimorphic neural circuit. Nature. 2008;452:473–477. doi: 10.1038/nature06808. [DOI] [PubMed] [Google Scholar]
- 67.Schlief ML, Wilson RI. Olfactory processing and behavior downstream from highly selective receptor neurons. Nat Neurosci. 2007;10:623–630. doi: 10.1038/nn1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suh GSB, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 69.van der Goes van Naters W, Carlson JR. Insects as chemosensors of humans and crops. Nature. 2006;444:302–307. doi: 10.1038/nature05403. [DOI] [PubMed] [Google Scholar]
- 70.Wang LM, Anderson DJ. Identification of an aggression-promoting pheromone and its receptor neurons in Drosophila. Nature. 2010;463:227–231. doi: 10.1038/nature08678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Turner SL, Ray A. Modification of CO2 avoidance behaviour in Drosophila by inhibitory odorants. Nature. 2009;461:277–281. doi: 10.1038/nature08295. [DOI] [PubMed] [Google Scholar]
- 72.Suh GSB, et al. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol. 2007;17:905–908. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 73.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 74.Kreher SA, Mathew D, Kim J, Carlson JR. Translation of sensory input into behavioral output via an olfactory system. Neuron. 2008;59:110–124. doi: 10.1016/j.neuron.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van der Goes van Naters W, Carlson JR. Receptors and neurons for fly odors in Drosophila. Curr Biol. 2007;17:606–612. doi: 10.1016/j.cub.2007.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Syed Z, Kopp A, Kimbrell DA, Leal WS. Bombykol receptors in the silkworm moth and the fruit fly. Proc Natl Acad Sci USA. 2010;107:9436–9439. doi: 10.1073/pnas.1003881107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matsumoto SG, Hildebrand JG. Olfactory mechanisms in the moth Manduca sexta—response characteristics and morphology of central neurons in the antennal lobes. Proc R Soc Lond B Biol Sci. 1981;213:249–277. [Google Scholar]
- 78.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science. 2004;303:366–370. doi: 10.1126/science.1090782. [DOI] [PubMed] [Google Scholar]
- 79.Bhandawat V, Olsen SR, Gouwens NW, Schlief ML, Wilson RI. Sensory processing in the Drosophila antennal lobe increases reliability and separability of ensemble odor representations. Nat Neurosci. 2007;10:1474–1482. doi: 10.1038/nn1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shang YH, Claridge-Chang A, Sjulson L, Pypaert M, Miesenböck G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell. 2007;128:601–612. doi: 10.1016/j.cell.2006.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the Drosophila antennal lobe. Neuron. 2007;54:89–103. doi: 10.1016/j.neuron.2007.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kazama H, Wilson RI. Homeostatic matching and nonlinear amplification at identified central synapses. Neuron. 2008;58:401–413. doi: 10.1016/j.neuron.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stopfer M, Jayaraman V, Laurent G. Intensity versus identity coding in an olfactory system. Neuron. 2003;39:991–1004. doi: 10.1016/j.neuron.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 84.Raman B, Joseph J, Tang J, Stopfer M. Temporally diverse firing patterns in olfactory receptor neurons underlie spatiotemporal neural codes for odors. J Neurosci. 2010;30:1994–2006. doi: 10.1523/JNEUROSCI.5639-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Laurent G, Wehr M, Davidowitz H. Temporal representations of odors in an olfactory network. J Neurosci. 1996;16:3837–3847. doi: 10.1523/JNEUROSCI.16-12-03837.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tanaka NK, Ito K, Stopfer M. Odor-evoked neural oscillations in Drosophila are mediated by widely branching interneurons. J Neurosci. 2009;29:8595–8603. doi: 10.1523/JNEUROSCI.1455-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.MacLeod K, Laurent G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science. 1996;274:976–979. doi: 10.1126/science.274.5289.976. [DOI] [PubMed] [Google Scholar]
- 88.Bazhenov M, et al. Model of transient oscillatory synchronization in the locust antennal lobe. Neuron. 2001;30:553–567. doi: 10.1016/s0896-6273(01)00284-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stopfer M, Bhagavan S, Smith BH, Laurent G. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390:70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 90.Masse NY, Turner GC, Jefferis GS. Olfactory information processing in Drosophila. Curr Biol. 2009;19:R700–R713. doi: 10.1016/j.cub.2009.06.026. [DOI] [PubMed] [Google Scholar]
- 91.MacLeod K, Bäcker A, Laurent G. Who reads temporal information contained across synchronized and oscillatory spike trains? Nature. 1998;395:693–698. doi: 10.1038/27201. [DOI] [PubMed] [Google Scholar]
- 92.Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol. 2008;99:734–746. doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- 93.Perez-Orive J, et al. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 94.Tanaka NK, Awasaki T, Shimada T, Ito K. Integration of chemosensory pathways in the Drosophila second-order olfactory centers. Curr Biol. 2004;14:449–457. doi: 10.1016/j.cub.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 95.Jefferis GS, et al. Comprehensive maps of Drosophila higher olfactory centers: Spatially segregated fruit and pheromone representation. Cell. 2007;128:1187–1203. doi: 10.1016/j.cell.2007.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin HH, Lai JSY, Chin AL, Chen YC, Chiang AS. A map of olfactory representation in the Drosophila mushroom body. Cell. 2007;128:1205–1217. doi: 10.1016/j.cell.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 97.Murthy M, Fiete I, Laurent G. Testing odor response stereotypy in the Drosophila mushroom body. Neuron. 2008;59:1009–1023. doi: 10.1016/j.neuron.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Marin EC, Jefferis GS, Komiyama T, Zhu HT, Luo LQ. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- 99.Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- 100.Ruta V, et al. A dimorphic pheromone circuit in Drosophila from sensory input to descending output. Nature. 2010;468:686–690. doi: 10.1038/nature09554. [DOI] [PubMed] [Google Scholar]
- 101.Kloppenburg P, Hildebrand JG. Neuromodulation by 5-hydroxytryptamine in the antennal lobe of the sphinx moth Manduca sexta. J Exp Biol. 1995;198:603–611. doi: 10.1242/jeb.198.3.603. [DOI] [PubMed] [Google Scholar]
- 102.Dacks AM, Green DS, Root CM, Nighorn AJ, Wang JW. Serotonin modulates olfactory processing in the antennal lobe of Drosophila. J Neurogenet. 2009;23:366–377. doi: 10.3109/01677060903085722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ha TS, Smith DP. A pheromone receptor mediates 11-cis-vaccenyl acetate-induced responses in Drosophila. J Neurosci. 2006;26:8727–8733. doi: 10.1523/JNEUROSCI.0876-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kurtovic A, Widmer A, Dickson BJ. A single class of olfactory neurons mediates behavioural responses to a Drosophila sex pheromone. Nature. 2007;446:542–546. doi: 10.1038/nature05672. [DOI] [PubMed] [Google Scholar]
- 105.Ronderos DS, Smith DP. Activation of the T1 neuronal circuit is necessary and sufficient to induce sexually dimorphic mating behavior in Drosophila melanogaster. J Neurosci. 2010;30:2595–2599. doi: 10.1523/JNEUROSCI.4819-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ai M, et al. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Rollmann SM, et al. Odorant receptor polymorphisms and natural variation in olfactory behavior in Drosophila melanogaster. Genetics. 2010;186:687–697. doi: 10.1534/genetics.110.119446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Elmore T, Ignell R, Carlson JR, Smith DP. Targeted mutation of a Drosophila odor receptor defines receptor requirement in a novel class of sensillum. J Neurosci. 2003;23:9906–9912. doi: 10.1523/JNEUROSCI.23-30-09906.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou S, Stone EA, Mackay TF, Anholt RR. Plasticity of the chemoreceptor repertoire in Drosophila melanogaster. PLoS Genet. 2009;5:e1000681. doi: 10.1371/journal.pgen.1000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Larkin A, et al. Central synaptic mechanisms underlie short-term olfactory habituation in Drosophila larvae. Learn Mem. 2010;17:645–653. doi: 10.1101/lm.1839010. [DOI] [PubMed] [Google Scholar]
- 111.Gillies MT, Wilkes TJ. A comparison of the range of attraction of animal baits and of carbon dioxide for some West African mosquitoes. Bull Entomol Res. 1969;59:441–456. doi: 10.1017/s0007485300003412. [DOI] [PubMed] [Google Scholar]
- 112.Guerenstein PG, Lazzari CR. Host-seeking: How triatomines acquire and make use of information to find blood. Acta Trop. 2009;110:148–158. doi: 10.1016/j.actatropica.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 113.Laurence BR, Pickett JA. An oviposition attractant pheromone in Culex quinquefasciatus Say (Diptera: Culicidae) Bull Entomol Res. 1985;75:283–290. [Google Scholar]
- 114.World Health Organization . World Malaria Report: 2010. 2010. (WHO Press, Geneva, Switzerland) [Google Scholar]
- 115.Takken W, Knols BGJ. Odor-mediated behavior of Afrotropical malaria mosquitoes. Annu Rev Entomol. 1999;44:131–157. doi: 10.1146/annurev.ento.44.1.131. [DOI] [PubMed] [Google Scholar]
- 116.Meijerink J, van Loon JJA. Sensitivities of antennal olfactory neurons of the malaria mosquito, Anopheles gambiae, to carboxylic acids. J Insect Physiol. 1999;45:365–373. doi: 10.1016/s0022-1910(98)00135-8. [DOI] [PubMed] [Google Scholar]
- 117.Fox AN, Pitts RJ, Robertson HM, Carlson JR, Zwiebel LJ. Candidate odorant receptors from the malaria vector mosquito Anopheles gambiae and evidence of down-regulation in response to blood feeding. Proc Natl Acad Sci USA. 2001;98:14693–14697. doi: 10.1073/pnas.261432998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fox AN, Pitts RJ, Zwiebel LJ. A cluster of candidate odorant receptors from the malaria vector mosquito, Anopheles gambiae. Chem Senses. 2002;27:453–459. doi: 10.1093/chemse/27.5.453. [DOI] [PubMed] [Google Scholar]
- 119.Hallem EA, Nicole Fox A, Zwiebel LJ, Carlson JR. Olfaction: Mosquito receptor for human-sweat odorant. Nature. 2004;427:212–213. doi: 10.1038/427212a. [DOI] [PubMed] [Google Scholar]
- 120.Carey AF, Wang GR, Su CY, Zwiebel LJ, Carlson JR. Odorant reception in the malaria mosquito Anopheles gambiae. Nature. 2010;464:66–71. doi: 10.1038/nature08834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang GR, Carey AF, Carlson JR, Zwiebel LJ. Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2010;107:4418–4423. doi: 10.1073/pnas.0913392107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kwon HW, Lu T, Rützler M, Zwiebel LJ. Olfactory responses in a gustatory organ of the malaria vector mosquito Anopheles gambiae. Proc Natl Acad Sci USA. 2006;103:13526–13531. doi: 10.1073/pnas.0601107103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xia YF, et al. The molecular and cellular basis of olfactory-driven behavior in Anopheles gambiae larvae. Proc Natl Acad Sci USA. 2008;105:6433–6438. doi: 10.1073/pnas.0801007105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu C, et al. Distinct olfactory signaling mechanisms in the malaria vector mosquito Anopheles gambiae. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Croset V, et al. Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 2010;6:e1001064. doi: 10.1371/journal.pgen.1001064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Robertson HM, Kent LB. Evolution of the gene lineage encoding the carbon dioxide receptor in insects. J Insect Sci. 2009;9:19. doi: 10.1673/031.009.1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hill CA, et al. G protein-coupled receptors in Anopheles gambiae. Science. 2002;298:176–178. doi: 10.1126/science.1076196. [DOI] [PubMed] [Google Scholar]
- 128.Syed Z, Leal WS. Maxillary palps are broad spectrum odorant detectors in Culex quinquefasciatus. Chem Senses. 2007;32:727–738. doi: 10.1093/chemse/bjm040. [DOI] [PubMed] [Google Scholar]
- 129.Grant AJ, Wigton BE, Aghajanian JG, O'Connell RJ. Electrophysiological responses of receptor neurons in mosquito maxillary palp sensilla to carbon dioxide. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1995;177:389–396. doi: 10.1007/BF00187475. [DOI] [PubMed] [Google Scholar]
- 130.Li ZX, Pickett JA, Field LM, Zhou JJ. Identification and expression of odorant-binding proteins of the malaria-carrying mosquitoes Anopheles gambiae and Anopheles arabiensis. Arch Insect Biochem Physiol. 2005;58:175–189. doi: 10.1002/arch.20047. [DOI] [PubMed] [Google Scholar]
- 131.Biessmann H, Walter MF, Dimitratos S, Woods D. Isolation of cDNA clones encoding putative odourant binding proteins from the antennae of the malaria-transmitting mosquito, Anopheles gambiae. Insect Mol Biol. 2002;11:123–132. doi: 10.1046/j.1365-2583.2002.00316.x. [DOI] [PubMed] [Google Scholar]
- 132.Xu PX, Zwiebel LJ, Smith DP. Identification of a distinct family of genes encoding atypical odorant-binding proteins in the malaria vector mosquito, Anopheles gambiae. Insect Mol Biol. 2003;12:549–560. doi: 10.1046/j.1365-2583.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 133.Rützler M, Lu T, Zwiebel LJ. Gα encoding gene family of the malaria vector mosquito Anopheles gambiae: Expression analysis and immunolocalization of AGαq and AGαo in female antennae. J Comp Neurol. 2006;499:533–545. doi: 10.1002/cne.21083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Walker WB, Smith EM, Jan T, Zwiebel LJ. A functional role for Anopheles gambiae Arrestin1 in olfactory signal transduction. J Insect Physiol. 2008;54:680–690. doi: 10.1016/j.jinsphys.2008.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Vogt RG, et al. The insect SNMP gene family. Insect Biochem Mol Biol. 2009;39:448–456. doi: 10.1016/j.ibmb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 136.World Health Organization Lymphatic Filariasis: Epidemiology. 2011. Available at http://www.who.int/lymphatic_filariasis/epidemiology/en/. Accessed February 22, 2010.
- 137.Syed Z, Leal WS. Acute olfactory response of Culex mosquitoes to a human- and bird-derived attractant. Proc Natl Acad Sci USA. 2009;106:18803–18808. doi: 10.1073/pnas.0906932106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Leal WS, et al. Reverse and conventional chemical ecology approaches for the development of oviposition attractants for Culex mosquitoes. PLoS ONE. 2008;3:e3045. doi: 10.1371/journal.pone.0003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Syed Z, Leal WS. Mosquitoes smell and avoid the insect repellent DEET. Proc Natl Acad Sci USA. 2008;105:13598–13603. doi: 10.1073/pnas.0805312105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hill SR, Hansson BS, Ignell R. Characterization of antennal trichoid sensilla from female southern house mosquito, Culex quinquefasciatus Say. Chem Senses. 2009;34:231–252. doi: 10.1093/chemse/bjn080. [DOI] [PubMed] [Google Scholar]
- 141.Du YJ, Millar JG. Electroantennogram and oviposition bioassay responses of Culex quinquefasciatus and Culex tarsalis (Diptera: Culicidae) to chemicals in odors from Bermuda grass infusions. J Med Entomol. 1999;36:158–166. doi: 10.1093/jmedent/36.2.158. [DOI] [PubMed] [Google Scholar]
- 142.Olagbemiro TO, Birkett MA, Mordue Luntz AJ, Pickett JA. Laboratory and field responses of the mosquito, Culex quinquefasciatus, to plant-derived Culex spp. oviposition pheromone and the oviposition cue skatole. J Chem Ecol. 2004;30:965–976. doi: 10.1023/b:joec.0000028461.86243.19. [DOI] [PubMed] [Google Scholar]
- 143.Ishida Y, Cornel AJ, Leal WS. Identification and cloning of a female antenna-specific odorant-binding protein in the mosquito Culex quinquefasciatus. J Chem Ecol. 2002;28:867–871. doi: 10.1023/a:1015253214273. [DOI] [PubMed] [Google Scholar]
- 144.Pelletier J, Leal WS. Genome analysis and expression patterns of odorant-binding proteins from the Southern House mosquito Culex pipiens quinquefasciatus. PLoS One. 2009;4:e6237. doi: 10.1371/journal.pone.0006237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu XZ, et al. (1)H, (15)N, and (13)C chemical shift assignments of the mosquito odorant binding protein-1 (CquiOBP1) bound to the mosquito oviposition pheromone. Biomol NMR Assign. 2009;3:195–197. doi: 10.1007/s12104-009-9173-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Xia YF, Zwiebel LJ. Identification and characterization of an odorant receptor from the West Nile virus mosquito, Culex quinquefasciatus. Insect Biochem Mol Biol. 2006;36:169–176. doi: 10.1016/j.ibmb.2005.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pelletier J, Hughes DT, Luetje CW, Leal WS. An odorant receptor from the southern house mosquito Culex pipiens quinquefasciatus sensitive to oviposition attractants. PLoS One. 2010;5:e10090. doi: 10.1371/journal.pone.0010090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hughes DT, Pelletier J, Luetje CW, Leal WS. Odorant receptor from the southern house mosquito narrowly tuned to the oviposition attractant skatole. J Chem Ecol. 2010;36:797–800. doi: 10.1007/s10886-010-9828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Klowden MJ. Endogenous regulation of the attraction of Aedes aegypti mosquitoes. J Am Mosq Control Assoc. 1994;10:326–332. [PubMed] [Google Scholar]
- 150.Reiter P, Amador MA, Colon N. Enhancement of the CDC ovitrap with hay infusions for daily monitoring of Aedes aegypti populations. J Am Mosq Control Assoc. 1991;7:52–55. [PubMed] [Google Scholar]
- 151.Bohbot J, et al. Molecular characterization of the Aedes aegypti odorant receptor gene family. Insect Mol Biol. 2007;16:525–537. doi: 10.1111/j.1365-2583.2007.00748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zhou JJ, He XL, Pickett JA, Field LM. Identification of odorant-binding proteins of the yellow fever mosquito Aedes aegypti: Genome annotation and comparative analyses. Insect Mol Biol. 2008;17:147–163. doi: 10.1111/j.1365-2583.2007.00789.x. [DOI] [PubMed] [Google Scholar]
- 153.Melo ACA, Rützler M, Pitts RJ, Zwiebel LJ. Identification of a chemosensory receptor from the yellow fever mosquito, Aedes aegypti, that is highly conserved and expressed in olfactory and gustatory organs. Chem Senses. 2004;29:403–410. doi: 10.1093/chemse/bjh041. [DOI] [PubMed] [Google Scholar]
- 154.Bohbot J, Vogt RG. Antennal expressed genes of the yellow fever mosquito (Aedes aegypti L.); characterization of odorant-binding protein 10 and takeout. Insect Biochem Mol Biol. 2005;35:961–979. doi: 10.1016/j.ibmb.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 155.Ishida Y, et al. Intriguing olfactory proteins from the yellow fever mosquito, Aedes aegypti. Naturwissenschaften. 2004;91:426–431. doi: 10.1007/s00114-004-0551-7. [DOI] [PubMed] [Google Scholar]
- 156.Leite NR, et al. Structure of an odorant-binding protein from the mosquito Aedes aegypti suggests a binding pocket covered by a pH-sensitive “Lid.”. PLoS One. 2009;4:e8006. doi: 10.1371/journal.pone.0008006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Li S, et al. Multiple functions of an odorant-binding protein in the mosquito Aedes aegypti. Biochem Biophys Res Commun. 2008;372:464–468. doi: 10.1016/j.bbrc.2008.05.064. [DOI] [PubMed] [Google Scholar]
- 158.Bohbot JD, Dickens JC. Characterization of an enantioselective odorant receptor in the yellow fever mosquito Aedes aegypti. PLoS One. 2009;4:e7032. doi: 10.1371/journal.pone.0007032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Bohbot JD, et al. Conservation of indole responsive odorant receptors in mosquitoes reveals an ancient olfactory trait. Chem Senses. 2011;36:149–160. doi: 10.1093/chemse/bjq105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.World Health Organization . Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control (WHO Press, Geneva, Switzerland) 2009. [PubMed] [Google Scholar]
- 161.Tabachnick WJ. Challenges in predicting climate and environmental effects on vector-borne disease episystems in a changing world. J Exp Biol. 2010;213:946–954. doi: 10.1242/jeb.037564. [DOI] [PubMed] [Google Scholar]
- 162.Jordan AM. Control of tsetse flies (Diptera: Glossinidae) with the aid of attractants. J Am Mosq Control Assoc. 1995;11:249–255. [PubMed] [Google Scholar]
- 163.Ditzen M, Pellegrino M, Vosshall LB. Insect odorant receptors are molecular targets of the insect repellent DEET. Science. 2008;319:1838–1842. doi: 10.1126/science.1153121. [DOI] [PubMed] [Google Scholar]
- 164.Kramer E. Attractivity of pheromone surpassed by time-patterned application of two nonpheromone compounds. J Insect Behav. 1992;5:83–97. [Google Scholar]
- 165.Anholt RRH, Williams TI. The soluble proteome of the Drosophila antenna. Chem Senses. 2010;35:21–30. doi: 10.1093/chemse/bjp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Knols BGJ, et al. Behavioural and electrophysiological responses of the female malaria mosquito Anopheles gambiae (Diptera: Culicidae) to Limburger cheese volatiles. Bull Entomol Res. 1997;87:151–159. [Google Scholar]
- 167.Healy TP, Copland MJW. Human sweat and 2-oxopentanoic acid elicit a landing response from Anopheles gambiae. Med Vet Entomol. 2000;14:195–200. doi: 10.1046/j.1365-2915.2000.00238.x. [DOI] [PubMed] [Google Scholar]
- 168.Smallegange RC, Qiu YT, van Loon JJA, Takken W. Synergism between ammonia, lactic acid and carboxylic acids as kairomones in the host-seeking behaviour of the malaria mosquito Anopheles gambiae sensu stricto (Diptera: Culicidae) Chem Senses. 2005;30:145–152. doi: 10.1093/chemse/bji010. [DOI] [PubMed] [Google Scholar]
- 169.Straw AD, Branson K, Neumann TR, Dickinson MH. Multi-camera real-time three-dimensional tracking of multiple flying animals. J R Soc Interface. 2011;8:395–409. doi: 10.1098/rsif.2010.0230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Lacey ES, Cardé RT. Activation, orientation and landing of female Culex quinquefasciatus in response to carbon dioxide and odour from human feet: 3-D flight analysis in a wind tunnel. Med Vet Entomol. 2011;25:94–103. doi: 10.1111/j.1365-2915.2010.00921.x. [DOI] [PubMed] [Google Scholar]
- 171.Beeuwkes J, Spitzen J, Spoor CW, van Leeuwen JL, Takken W. 3-D flight behaviour of the malaria mosquito Anopheles gambiae s.s. inside an odour plume. Proc Neth Entomol Soc Meet. 2008;19:137–146. [Google Scholar]
- 172.Silbering AF, Galizia CG. Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J Neurosci. 2007;27:11966–11977. doi: 10.1523/JNEUROSCI.3099-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Broome BM, Jayaraman V, Laurent G. Encoding and decoding of overlapping odor sequences. Neuron. 2006;51:467–482. doi: 10.1016/j.neuron.2006.07.018. [DOI] [PubMed] [Google Scholar]
- 174.Riffell JA, Lei H, Christensen TA, Hildebrand JG. Characterization and coding of behaviorally significant odor mixtures. Curr Biol. 2009;19:335–340. doi: 10.1016/j.cub.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ochieng SA, Park KC, Baker TC. Host plant volatiles synergize responses of sex pheromone-specific olfactory receptor neurons in male Helicoverpa zea. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2002;188:325–333. doi: 10.1007/s00359-002-0308-8. [DOI] [PubMed] [Google Scholar]
- 176.Den Otter CJ. Single sensillum responses in male moth Adoxophyes orana (F. v. R.) to female sex-pheromone components and their geometrical isomers. J Comp Physiol. 1977;121:205–222. [Google Scholar]
- 177.Nikonov AA, Leal WS. Peripheral coding of sex pheromone and a behavioral antagonist in the Japanese beetle, Popillia japonica. J Chem Ecol. 2002;28:1075–1089. doi: 10.1023/a:1015274104626. [DOI] [PubMed] [Google Scholar]
- 178.Su CY, Martelli C, Emonet T, Carlson JR. Temporal coding of odor mixtures in an olfactory receptor neuron. Proc Natl Acad Sci USA. 2011;108:5075–5080. doi: 10.1073/pnas.1100369108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Pitts RJ, Zwiebel LJ. Antennal sensilla of two female anopheline sibling species with differing host ranges. Malar J. 2006;5:26. doi: 10.1186/1475-2875-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.de Bruyne M, Clyne PJ, Carlson JR. Odor coding in a model olfactory organ: The Drosophila maxillary palp. J Neurosci. 1999;19:4520–4532. doi: 10.1523/JNEUROSCI.19-11-04520.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]