Abstract
During immune responses, neutrophils must integrate survival and death signals from multiple sources to regulate their lifespan. Signals that activate either the Bcl-2- or death receptor-regulated apoptosis pathways can provide powerful stimuli for neutrophils to undergo cell death, but whether they act cooperatively in parallel or directly cross-talk in neutrophils is not known. Previous studies suggested that Bcl-2 family proteins are not required for Fas-induced cell death in neutrophils, but did not examine whether they could modulate its rapid onset. By monitoring the rate of change in neutrophil viability associated with activation of the Fas-triggered death receptor pathway using real-time cell imaging, we show that the Bcl-2-related proteins Bid, Bax, and Bak accelerate neutrophil apoptosis but are not essential for cell death. Increased Bcl-2 or Mcl-1 expression prevents efficient induction of apoptosis by Fas stimulation indicating that the Bcl-2-regulated apoptosis pathway can directly interfere with Fas-triggered apoptosis. Fas has been shown to initiate NFκB activation and gene transcription in cell lines, however gene transcription is not altered in Fas-activated Bid−/− neutrophils, indicating that apoptosis occurs independently of gene transcription in neutrophils. The specification of kinetics of neutrophil apoptosis by Bid impacts on the magnitude of neutrophil IL-1β production, implicating a functional role for the Bcl-2-regulated pathway in controlling neutrophil responses to FasL. These data demonstrate that the intrinsic apoptosis pathway directly controls the kinetics of Fas-triggered apoptosis in neutrophils.
Keywords: inflammation, Fas ligand, type I cell, type II cell, caspase 1-independent
It has long been recognized that lack of neutrophils (neutropenia) is correlated with severe infection (1) and that mutations that impair neutrophil recruitment to infected tissues are detrimental to the host (2). More recently, a deviation in the programmed neutrophil lifespan during infection has been recognized as detrimental, with abnormally accelerated apoptosis of neutrophils exacerbating infection (3). As such, the regulation of neutrophil lifespan is a critical determinant of a circumscribed neutrophilic inflammatory response (4). During homeostatic conditions, ∼100 billion neutrophils are produced daily in humans, and they display a constant, short, lifespan following exit from the bone marrow into the peripheral circulation (5). Without activation, neutrophils exit the circulation and are removed by reticulo-endothelial cells in the spleen, liver, lung, and bone marrow (6). Within an inflammatory milieu, neutrophil lifespan is directly affected by activation and exposure to extracellular regulators (4).
The death receptor apoptotic pathway operates in multiple cell types when cell-surface death receptors, such as Fas (also called CD95 or APO-1) or tumor necrosis factor (TNF) receptor-1, bind their cognate ligands (FasL or TNF). Fas stimulation causes formation of a death-inducing signaling complex (DISC) comprising Fas, Fas-associated protein with Death Domain (FADD), and procaspase 8 (7). The formation of DISC leads to the activation of caspase-8, initially through conformational change and then by auto-proteolysis. Activated caspase-8 can proteolytically activate the effector caspase-3 and caspase-7, thereby triggering apoptosis directly (type I cells), but can also cleave the proapoptotic BH3-only Bcl-2 family member Bid to amplify caspase activation and apoptosis signaling by engaging the intrinsic apoptotic pathway (type II cells; ref. 8). In lymphocytes (type I cells), activation of Fas can drive apoptosis via caspase-8, caspase-3, and caspase-7, independently of tBid induction of Bak/Bax-mediated mitochondrial outer membrane permeabilization (MOMP; ref. 9). In hepatocytes or pancreatic β cells (type II cells), FasL-induced cell death requires amplification of the apoptosis signal via tBid and Bak/Bax-mediated MOMP to overcome X-linked inhibitor of apoptosis (XIAP)-mediated inhibition of effector caspase activity (9). However, the physiological relevance of Bid in regulating Fas signaling is unclear in primary myeloid cells. To date, there are no in vivo or ex vivo studies in primary hematopoietic cells that provide evidence for direct cross-talk between suppressors of the intrinsic apoptotic pathway, such as Bcl-2 and Mcl-1, and the Fas-induced death receptor pathway (10, 11). Although it is established that both intrinsic and death receptor apoptosis signaling pathways can act in parallel to remove activated T cells, preventing chronic inflammation and autoimmunity (12–14), little is known about the response of neutrophils to the cooperative action of these apoptosis pathways. In this study, we examined the potential role of direct functional cross-talk by the intrinsic apoptosis pathway to control the kinetics of neutrophil apoptosis initiated by death receptor activation.
Results
Quantitative Live-Cell Imaging Assay to Study Neutrophil Viability.
FasL induces apoptosis of wild-type neutrophils within 2–3 h (Fig. 1A, Fig. S1, and Movie S1), necessitating the development of assays capable of monitoring rapid changes in cell viability. A high-throughput live-cell imaging assay proved suitable, allowing reproducible quantification of the kinetic changes in neutrophil viability occurring in tissue culture. As Movie S1 demonstrates, neutrophils produce apoptotic blebs immediately before exposing phosphatidylserine and binding of Annexin V, and then become propidium iodide (PI) positive within minutes of binding Annexin V. The brevity of transition of neutrophils from an apoptotic Annexin V+PI− state to a dead Annexin V+PI+ state enabled use of PI as a sensitive measure of neutrophil viability, either on a single-cell basis or as an average intensity of the image field (Fig. S1B). This approach generated data that were highly reproducible between different areas within the same well, and also between identically treated samples in different wells.
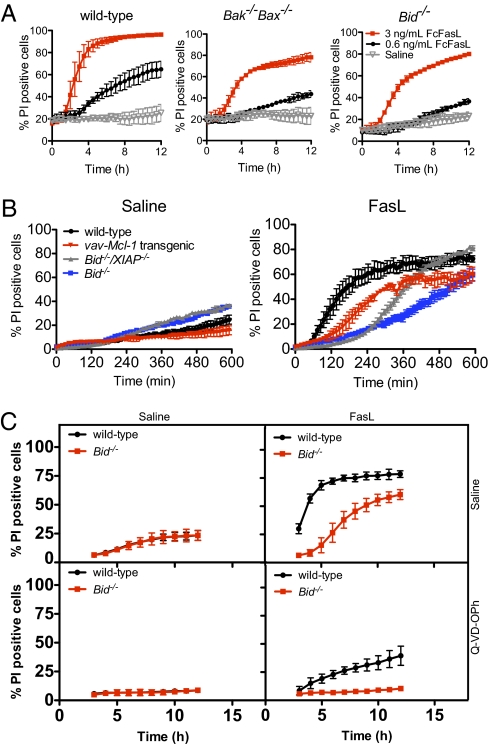
Fig. 1.
Bid, Bak, and Bax accelerate Fas signaling in a dose-dependent manner. (A) Deficiency in both Bak and Bax impairs Fas-induced apoptosis in a dose-dependent manner but does not prevent apoptosis. Neutrophils were incubated with 3 ng/mL or 0.6 ng/mL FcFasL, and cell viability was monitored using PI. Mean ± SEM from two (Bak−/−Bax−/−) or four (Bid−/−) independent neutrophil samples. (B) XIAP does not discriminate between type I and type II signaling in neutrophils. Neutrophils from wild-type, vav-Mcl-1 transgenic, Bid−/− and Bid−/−XIAP−/− mice were stimulated with Flag–FasL crosslinked with anti-Flag antibodies. Data are mean ± SEM from three independent samples. (C) Caspase inhibition completely blocks apoptosis of Bid−/− neutrophils but not wild-type neutrophils. Neutrophils were treated with 10 μM Q-VD-OPh for 15 min before stimulation with 0.6 ng/mL FcFasL. Viability was monitored every hour according to A. Data are mean ± SD from three independent samples.
To exclude the possibility of any potential phototoxic effects of the real-time fluorescent imaging confounding interpretation of the kinetics of neutrophil death, purified neutrophils were also cultured in standard tissue culture conditions and analyzed in parallel for cell viability using visual inspection of eosin-exclusion, FACS-based analysis of Annexin V/PI staining, and a biochemical analysis of caspase-3 and caspase-7 activation in response to FasL. In all experiments, the kinetic profile of neutrophil death in response to FasL appeared indistinguishable from the data generated using the live cell imaging platform. To ensure that we were measuring the specific kinetics of responses to FasL, we compared the kinetics of cell death in neutrophils from wild-type and mutant mice that are defective for Fas expression (Faslpr mice). This analysis showed that FasL readily induced apoptosis in wild-type neutrophils but not in Faslpr neutrophils (Fig. S1B). A biochemical analysis further supported the specificity of the response to the FasL stimulus, with active, cleaved caspase-3 only detected in FasL-treated wild-type neutrophils but not in FasL-treated Faslpr neutrophils (Fig. S1A). FasL-induced death was dose dependent (Fig. S1C) and depended on the source of FasL. Two sources of FasL were tested: a recombinant Flag-tagged FasL crosslinked with anti-Flag antibody, and FcFasL that exists in a hexameric form. The two reagents produced comparable data, although FcFasL displayed greater apoptosis-inducing activity (Fig. S1D), which is consistent with previous reports (15).
Bid and Bak/Bax Accelerate FasL-Induced Neutrophil Apoptosis.
Expression of Bcl-2 family proteins is altered in neutrophils following stimulation by inflammatory cytokines and ligands for Toll-like receptors (16–18). During inflammatory responses, FasL plays key roles in the induction of apoptosis not only in activated T cells but also other hematopoietic cell subsets, including neutrophils (12–14, 19). We hypothesized that the intrinsic and extrinsic apoptosis pathways might cooperate at sites of inflammation and that alteration in the expression or functional capacity of the intrinsic apoptosis pathway would regulate responses to activation of the extrinsic death pathway.
To test this hypothesis, we examined the role of the proapoptotic proteins Bid, Bak and Bax in Fas-induced signaling in neutrophils. Upon treatment with FasL alone, Bid−/− or Bak−/−Bax−/− neutrophils were less sensitive than wild-type neutrophils and died more slowly, but still more rapidly than the saline-treated controls (Fig. 1 A and B). The resistance of Bid−/− and Bak−/−Bax−/− neutrophils to FasL could be largely overcome by higher concentrations of FasL (Fig. 1A), although kinetic differences remained. This indicates that the Bid-mediated, and Bak/Bax-dependent activation of the intrinsic apoptotic pathway is required for efficient induction of apoptosis by FasL in neutrophils. Loss of XIAP, a caspase inhibitor that can discriminate between type I and type II FasL-induced apoptosis signaling (9), accelerated FasL-induced apoptosis in the absence of Bid but not to the same rate of apoptosis as the wild-type neutrophil population (Fig. 1B). To examine the dependence of apoptosis on caspase activity, we analyzed the response of wild-type and Bid−/− neutrophils to FasL after incubating with Q-VD-OPh, a broad spectrum caspase inhibitor (20). As shown in Fig. 1C, caspase inhibition completely blocks the apoptosis of Bid−/− neutrophils and results in near complete inhibition of apoptosis of wild-type neutrophils. Although it is possible that Bid regulates caspase-independent apoptosis in neutrophils, it is more likely that Q-VD-OPh fails to block all caspase activity induced by the FcFasL signal. No difference in the kinetics of apoptosis was found between Bim-deficient neutrophils compared with wild-type neutrophils treated with FasL (Fig. S2).
Bcl-2 and Mcl-1 Delay FasL-Induced Neutrophil Apoptosis.
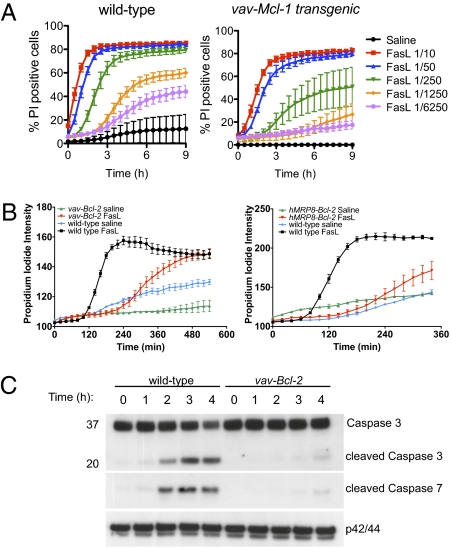
The delay in Fas-induced apoptosis in Bid−/− and Bak−/−Bax−/− neutrophils suggested that prosurvival proteins such as Bcl-2 and Mcl-1 may also interfere with the kinetics of Fas-induced apoptosis. To examine the role of Bcl-2 and Mcl-1 in directly regulating Fas-mediated signal transduction pathways, we analyzed the kinetics of FasL-induced death of purified bone marrow neutrophils from vav-Mcl-1, vav-Bcl-2, and myeloid-restricted hMRP8-Bcl-2 transgenic mice. Neutrophils with increased expression of Mcl-1 (Figs. 1B and 2A, Movie S2) or Bcl-2 (Fig. 2) showed delayed responses to FasL stimulation. FasL did induce apoptosis of Mcl-1 or Bcl-2 overexpressing neutrophils but at considerably later timepoints than wild-type neutrophils, demonstrating that suppressors of the intrinsic apoptotic pathway regulate the kinetics of FasL-induced neutrophil apoptosis. Consistent with the dose dependence of FasL sensitivity of Bid−/− and Bak−/−Bax−/− neutrophils, the resistance of vav-Mcl-1 transgenic neutrophils could be largely overcome by higher concentrations of FasL. A biochemical analysis of signaling pathways downstream of Fas supported the live cell imaging data. Neither caspase-3 nor caspase-7 were activated at early times in FasL-treated neutrophils overexpressing Bcl-2 (Fig. 2C). The level of Fas expression on the surface of wild-type and Bcl-2 transgenic neutrophils was comparable (Fig. S3). These data indicate that increased Mcl-1 or Bcl-2 expression may extend the survival of neutrophils that had encountered FasL in vivo.
Fig. 2.
Overexpression of Mcl-1 or Bcl-2 inhibits FasL-induced apoptosis of neutrophils. (A) Mcl-1 over-expression inhibits FasL-induced apoptosis of neutrophils. Neutrophils were incubated with different concentrations of FcFasL for the indicated time. Viability was monitored using PI and the proportion of dead cells was determined using MetaMorph. Images were collected every 30 min. Data are mean ± SEM from four independent samples. (B) The average PI intensity of wild-type, vav-Bcl-2, or hMRP8-Bcl-2 transgenic neutrophils was used to monitor the viability of the population with time. Mean ± SD of duplicate or triplicate fields of view are shown. (C) Immunoblot analysis of wild-type and vav-Bcl-2 neutrophils stimulated with Flag-FasL crosslinked with anti-Flag antibodies. Antibodies specific to (total) caspase 3 and cleaved caspase 7 were used to monitor activation of the Fas apoptosis pathway. Immunoblotting for ERK1/2 is shown as a loading control. Data are representative of at least three independent experiments.
ABT-737 Sensitizes Bcl-2 Transgenic Neutrophils to FasL.
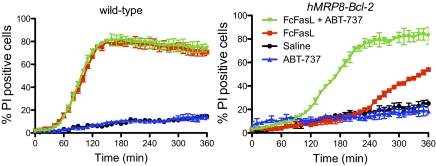
To confirm that the effects of Bcl-2 overexpression on FasL-induced apoptosis were specific to changes in Bcl-2 expression, we examined the effects of the BH3 mimetic, ABT-737, which binds Bcl-2, Bcl-xL, and Bcl-w, but not Mcl-1 (21, 22). Inhibition of Bcl-2 with ABT-737 markedly accelerated the death of FasL-treated hMRP8-Bcl-2 transgenic neutrophils but had no effect in wild-type neutrophils, which are more dependent upon Mcl-1 (ref. 21; Fig. 3 and Movie S3). These data demonstrate that amplification of apoptosis signaling through engagement of the intrinsic apoptotic pathway plays a critical role in the early phases of FasL-induced killing in neutrophils and suggest that ABT-737 may synergise with FasL to induce the apoptosis of Bcl-2 overexpressing cells in vivo.
Fig. 3.
The BH3 mimetic ABT-737, which inhibits Bcl-2, Bcl-xL and Bcl-w but not Mcl-1 or A1, synergizes with FasL to induce apoptosis of Bcl-2 overexpressing neutrophils but not wild-type neutrophils. Neutrophils from wild-type and hMRP8-bcl-2 transgenic mice were incubated with ABT-737, FcFasL or a combination of ABT-737 and FcFasL. Staining with PI was used to monitor cell viability. Mean ± SD of duplicate or triplicate fields of view are shown. Data are representative of at least three independent experiments.
FasL Does Not Induce Gene Expression in Neutrophils.
Activation of death receptors can activate not only caspase-dependent apoptosis but also certain nonapoptotic signaling pathways, some of which appear to be caspase-dependent and some of which appear to be caspase-independent (23). Some of the caspase-independent effects of death receptor signaling are reported to be mediated by receptor (TNFRSF)-interacting serine-threonine kinase 1 (RIPK1), which induces caspase-independent necrotic cell death and has been proposed to also activate NF-κB transcription factors (23, 24). The delineation of signaling pathways downstream of the Fas death receptor has been a complex problem due to an inability to extricate the nonapoptotic signaling pathways from the potent apoptosis-inducing effects of FasL. Using Bid-deficient neutrophils that exhibit delayed apoptosis when stimulated with FasL, we examined the effects of FasL on signal transduction and gene expression using microarray. FasL induced rapid apoptosis of wild-type neutrophils and resulted in the differential gene expression of only a limited number of genes (Fig. 4 A and B). In contrast, no genes were differentially induced in Bid-deficient neutrophils stimulated with FasL (Fig. 4 A and B). Consistently, cycloheximide treatment of neutrophils does not impair Fas-induced apoptosis (Fig. S4). These data indicate that FasL-induced apoptosis can occur independently of gene transcription in neutrophils.
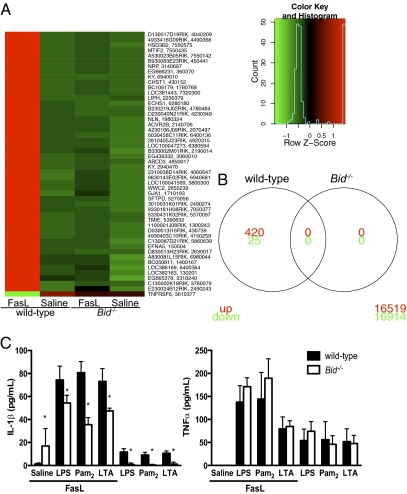
Fig. 4.
FasL does not induce gene transcription in the absence of Bid. Neutrophils from wild-type or Bid−/− mice were stimulated with FcFasL for 1 h. (A) Heatmap showing differentially expressed probes in FasL-stimulated wild-type neutrophils but not in FasL-stimulated Bid−/− neutrophils. (B) Venn diagram demonstrating the number of genes with significant (false discovery rate < 0.1) increases or decreases in expression in wild-type but not Bid−/− neutrophils following FasL stimulation. Numbers outside the circles show the number of probes included in the analysis but not differentially expressed. (C) IL-1β production is reduced in Bid−/− neutrophils after FasL stimulation. Purified bone marrow neutrophils were primed for 4 h with 10 ng/mL LPS, 100 ng/mL Pam2CSK4 or 10 μg/mL lipotechoic acid (LTA) before stimulation with FcFasL for 9 h. Supernatant was analyzed for IL-1β production by ELISA. *P < 0.05, wild-type versus Bid−/−. Data are mean ± SD shown from six independent samples.
To explore functional roles for Bid regulation of Fas-induced apoptosis in neutrophils, we examined Fas-induced IL-1β production, which is dependent on caspase activity but independent of caspase-1 (25). We primed wild-type and Bid−/− neutrophils with the Toll-like receptor (TLR) ligands LPS, Pam2CSK4, or lipotechoic acid and then induced processing of pro-IL-1β by stimulating the cells with FcFasL. As shown in Fig. 4C, IL-1β production was reduced in Bid−/− neutrophils compared with wild-type neutrophils despite normal levels of TNFα production, indicating that the initial response to TLR activation was normal but subsequent Fas-induced IL-1β production was dependent on Bid. The data also demonstrate that TNFα production is enhanced by FcFasL, but that this is independent of Bid. Taken together, the data suggest that the processing of pro-IL-1β to bioactive IL-1β is regulated by Bid and that this occurs independently of gene transcription.
Discussion
Here we demonstrate direct regulation of the extrinsic death receptor pathway by the Bcl-2 family regulated pathway in neutrophils, via Mcl-1, Bcl-2, and the caspase-8-mediated activation of the proapoptotic BH3-only protein Bid (10). These data illustrate three key concepts in cell death pathways in neutrophils: (i) Bid, Bak, and Bax accelerate Fas-induced neutrophil apoptosis but are not absolutely required for apoptosis to occur; (ii) Mcl-1 and Bcl-2 inhibit Fas-induced neutrophil apoptosis but this can be largely overcome by a strong Fas signal; and (iii) Fas-induced signaling does not contribute to changes in gene expression in neutrophils. We therefore propose that neutrophils cannot be classified as either type I or type II cells, given that apoptosis occurs independently of Bid in FasL-stimulated lymphocytes (type I cells), and that Bid is critically important for Fas-induced apoptosis of hepatocytes (type II cells). Furthermore, although the loss of XIAP can restore the sensitivity of Bid−/− hepatocytes to Fas-induced apoptosis, this is only partially the case in Bid−/−XIAP−/− neutrophils (9). The data suggest that other processes, possibly involving cIAP1 and cIAP2, contribute to caspase inhibition in neutrophils, and that this inhibition can be overcome with a strong Fas signal.
We speculate that prosurvival Bcl-2 family members such as Mcl-1 and Bcl-2 protect neutrophils in an inflammatory environment rich in death signals such as FasL. FasL triggers the release of proinflammatory IL-1β from neutrophils via a caspase-dependent process that is independent of caspase-1 (25), and Bcl-2 and Mcl-1 indirectly interfere with this processing by delaying FasL-induced apoptosis and caspase activation. The delay in Fas-induced neutrophil apoptosis may also promote a chronic inflammatory response by allowing the accumulation of neutrophils during a resolution phase of inflammation involving FasL-expressing T cells.
These effects on neutrophil survival are very likely to have clinical relevance. Perturbations in neutrophil lifespan have been associated with acute lung injury (26), systemic inflammatory response syndrome (SIRS; ref. 27), bronchiolitis obliterans organizing pneumonia, acute respiratory distress syndrome (ARDS; ref. 28), and pathogenic infections including influenza, Streptococcus pneumoniae, respiratory syncytial virus, herpes simplex virus, and human cytomegalovirus (4, 29–33). The inflammatory milieu is rich in extracellular signals that are known to increase expression of prosurvival Bcl-2 family proteins. Stimulation of neutrophils with Toll-like receptor (TLR) ligands, such as LPS, or inflammatory cytokines, such as G-CSF and GM-CSF, induces Mcl-1 and A1, reduces the expression of proapoptotic proteins such as Bax, and increases Bim expression (16, 18, 34). Increased levels of G-CSF and GM-CSF have been found in broncho-alveolar lavage cell supernatants from patients with neutrophilic lung inflammation, such as cystic fibrosis, idiopathic fibrosis, pneumonia, and acute allergic alveolitis, but not in individuals without neutrophilic lung inflammation (18). Both Bcl-2 and Mcl-1 are expressed in bone marrow neutrophils and can promote their survival (11, 34–37). Neutrophils isolated from septic patients express high levels of Bcl-2 as well as Bim (34). Further work will be required to examine potential roles for Bcl-2 and Mcl-1 in the regulation of extrinsic death pathways in vivo during infection and in patients with myeloid proliferative disorders, hypereosinophilic syndromes, and autoimmune disorders such as systemic lupus erythematosus (38, 39). Such studies will help define the biochemical and cellular mechanisms that govern both the functional activation of neutrophils at a site of inflammation and the subsequent cell death and disposal of neutrophils to understand how distinct pathways, such as pathogen recognition, cytokine signal transduction, and cell death pathways, integrate to drive acute and chronic inflammatory disease.
Materials and Methods
Mice.
C57BL/6, vav-Bcl-2 transgenic (40), hMRP8 Bcl-2 transgenic (41), vav-Mcl-1 transgenic (42), Bid−/− (43), ubiquitin-GFP (44), Bid−/−XIAP−/− (9), and XIAP−/− (45) mice were bred at the Walter and Eliza Hall Institute. All transgenic or gene targeted mice were generated on an inbred C57BL/6 background (for the latter using C57BL/6-derived ES cells) or were backcrossed with C57BL/6 mice for >10 generations, except for hMRP8-Bcl-2 transgenic mice, which were a mixed FVB/N and C57BL/6 background. All experiments were carried out in accordance with institute animal ethics guidelines and approval.
Bone Marrow Chimeras.
For hematopoietic reconstitution experiments, congenic C57BL/6.SJL (Ptprca Pep3b (Ly5.1)) mice were reconstituted with 106 C57BL/6 (Ptprcb Pep3a (Ly5.2)) fetal liver cells from Bak−/−Bax−/− E14 embryos or from 5 × 106 bone marrow cells from either Bim−/−Rag1−/−−, Rag1−/−− or wild-type mice after two 5.5-Gy doses of irradiation given 3 h apart (46).
Neutrophil Purification.
Bone marrow neutrophils were prepared as described (47). Neutrophils were resuspended in phenol red-free DMEM/10% FCS for imaging studies. The purity of neutrophil preparations was routinely >98% as assessed by cytology following May–Grünwald Giemsa staining.
Apoptosis Assays.
Neutrophils were stimulated with supernatant from FcFasL-transfected 293T cells (provided by Pascal Schneider and Jurg Tschopp) or with 100 ng/mL Flag–FasL (Axxora) crosslinked with anti-Flag antibody (M2, Sigma-Aldrich). A total of 250 ng of Flag–FasL was crosslinked with 250 ng of anti-Flag antibody.
Preparation of Cell Lysates and Immunoblotting.
Lysates were prepared as described (47). Neutrophil lysates were analyzed using antibodies to cleaved caspase-7 (Millipore), cleaved caspase-3 (Cell Signaling), and total caspase-3 (Cell Signaling). Antibodies specific to human Bcl-2 and Fas were generated at the Walter and Eliza Hall Institute.
Live Cell Imaging and Quantitative Analysis.
A total of 105 neutrophils were loaded into each well and viability monitored with Annexin V-Alexa Fluor 488 (Invitrogen) and 2 μg/mL PI (Sigma). For some experiments, neutrophils were labeled with 160 nM Cell Tracker Green (Invitrogen). Images were collected every 2–60 min using a Nikon Biostation IM-Q, a BD Pathway 855 Bioimager or Zeiss Axiovert microscope at 37 °C/10% CO2. Quantitative analysis of neutrophil viability was performed using Image J or a custom made MetaMorph (v7.2.0, Molecular Devices) journal suite incorporating the count nuclei function to segment the cell nuclei and Integrated Morphometry Analysis to select and measure the nuclei of interest (Metamorph journal suite available on request). ABT-737 was used at 1 μM (Abbott Laboratories). Q-VD-OPh was used at 10 μM (SM Biochemicals).
Microarray Analysis.
Purified bone marrow neutrophils were pooled from nine Bid-deficient or seven wild-type mice and then stimulated for 1 h with FcFasL or saline. Total RNA was prepared using TRIzol (Invitrogen). RNA was hybridized to Illumina WG-6 Mouse Version 2 BeadChips. Data analysis was carried out using limma software. Expression values were neqc normalized (48). Probes were filtered if they failed to achieve a detection P value of 0.95 on at least two arrays. Differential expression was assessed using linear modeling and empirical Bayes moderated t statistics (49). Array quality weights were estimated to allow for variations in RNA quantity (50).
Cytokine ELISA.
A total of 3 × 105 neutrophils were stimulated for 4 h with 10 ng/mL LPS (Axxora), 100 ng/mL Pam2CSK4 (Invivogen), or 10 μg/mL lipotechoic acid (Invivogen) before stimulation for 9 h with a 1/1,000 dilution of FcFasL to induce IL-1β release. Soluble IL-1β and TNFα were measured by ELISA (eBioscience).
Statistics.
Unless otherwise specified, data are presented as mean ± SD or SEM. Comparisons were performed using a Student t test.
Supplementary Material
Acknowledgments
This research was supported by National Health and Medical Research Council (NHMRC) Grants 637367, 461219, and 461221; NHMRC Independent Research Institutes Support Scheme Grant 361646; Victorian State Government Operational Infrastructure Support Grant and National Institutes of Health Grants CA022556 and CA43540; Leukemia and Lymphoma Society SCOR Grant 7413; Carden Fellowship Fund of the Cancer Council, Victoria (D.M.); NHMRC CDA 575531 (to B.A.C.), Australian Research Council QEII DP1094854 (B.A.C.); NHMRC Practitioner Fellowship 637309 (to A.W.R.); and a Victorian Cancer Agency Fellowship (to A.W.R.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110358108/-/DCSupplemental.
References
- 1.Bodey GP, Buckley M, Sathe YS, Freireich EJ. Quantitative relationships between circulating leukocytes and infection in patients with acute leukemia. Ann Intern Med. 1966;64:328–340. doi: 10.7326/0003-4819-64-2-328. [DOI] [PubMed] [Google Scholar]
- 2.Crowley CA, et al. An inherited abnormality of neutrophil adhesion. Its genetic transmission and its association with a missing protein. N Engl J Med. 1980;302:1163–1168. doi: 10.1056/NEJM198005223022102. [DOI] [PubMed] [Google Scholar]
- 3.Navarini AA, et al. Innate immune-induced depletion of bone marrow neutrophils aggravates systemic bacterial infections. Proc Natl Acad Sci USA. 2009;106:7107–7112. doi: 10.1073/pnas.0901162106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elbim C, Katsikis PD, Estaquier J. Neutrophil apoptosis during viral infections. Open Virol J. 2009;3:52–59. doi: 10.2174/1874357900903010052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price TH, Lee MY, Dale DC, Finch CA. Neutrophil kinetics in chronic neutropenia. Blood. 1979;54:581–594. [PubMed] [Google Scholar]
- 6.Furze RC, Rankin SM. The role of the bone marrow in neutrophil clearance under homeostatic conditions in the mouse. Faseb J. 2008;22:3111–3119. doi: 10.1096/fj.08-109876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kischkel FC, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- 9.Jost PJ, et al. XIAP discriminates between type I and type II FAS-induced apoptosis. Nature. 2009;460:1035–1039. doi: 10.1038/nature08229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- 11.Villunger A, Scott C, Bouillet P, Strasser A. Essential role for the BH3-only protein Bim but redundant roles for Bax, Bcl-2, and Bcl-w in the control of granulocyte survival. Blood. 2003;101:2393–2400. doi: 10.1182/blood-2002-07-2132. [DOI] [PubMed] [Google Scholar]
- 12.Hughes PD, et al. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutcheson J, et al. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–217. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Weant AE, et al. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 15.Holler N, et al. Two adjacent trimeric Fas ligands are required for Fas signaling and formation of a death-inducing signaling complex. Mol Cell Biol. 2003;23:1428–1440. doi: 10.1128/MCB.23.4.1428-1440.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.François S, et al. Inhibition of neutrophil apoptosis by TLR agonists in whole blood: involvement of the phosphoinositide 3-kinase/Akt and NF-kappaB signaling pathways, leading to increased levels of Mcl-1, A1, and phosphorylated Bad. J Immunol. 2005;174:3633–3642. doi: 10.4049/jimmunol.174.6.3633. [DOI] [PubMed] [Google Scholar]
- 17.Bauer A, Kirschnek S, Häcker G. Inhibition of apoptosis can be accompanied by increased Bim levels in T lymphocytes and neutrophil granulocytes. Cell Death Differ. 2007;14:1714–1716. doi: 10.1038/sj.cdd.4402185. [DOI] [PubMed] [Google Scholar]
- 18.Dibbert B, et al. Cytokine-mediated Bax deficiency and consequent delayed neutrophil apoptosis: a general mechanism to accumulate effector cells in inflammation. Proc Natl Acad Sci USA. 1999;96:13330–13335. doi: 10.1073/pnas.96.23.13330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Renshaw SA, et al. Inflammatory neutrophils retain susceptibility to apoptosis mediated via the Fas death receptor. J Leukoc Biol. 2000;67:662–668. doi: 10.1002/jlb.67.5.662. [DOI] [PubMed] [Google Scholar]
- 20.Chauvier D, Ankri S, Charriaut-Marlangue C, Casimir R, Jacotot E. Broad-spectrum caspase inhibitors: from myth to reality? Cell Death Differ. 2007;14:387–391. doi: 10.1038/sj.cdd.4402044. [DOI] [PubMed] [Google Scholar]
- 21.van Delft MF, et al. The BH3 mimetic ABT-737 targets selective Bcl-2 proteins and efficiently induces apoptosis via Bak/Bax if Mcl-1 is neutralized. Cancer Cell. 2006;10:389–399. doi: 10.1016/j.ccr.2006.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oltersdorf T, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 23.Holler N, et al. Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat Immunol. 2000;1:489–495. doi: 10.1038/82732. [DOI] [PubMed] [Google Scholar]
- 24.Kreuz S, et al. NFkappaB activation by Fas is mediated through FADD, caspase-8, and RIP and is inhibited by FLIP. J Cell Biol. 2004;166:369–380. doi: 10.1083/jcb.200401036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miwa K, et al. Caspase 1-independent IL-1beta release and inflammation induced by the apoptosis inducer Fas ligand. Nat Med. 1998;4:1287–1292. doi: 10.1038/3276. [DOI] [PubMed] [Google Scholar]
- 26.Matute-Bello G, Martin TR. Science review: apoptosis in acute lung injury. Crit Care. 2003;7:355–358. doi: 10.1186/cc1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Paunel-Görgülü A, et al. Mcl-1-mediated impairment of the intrinsic apoptosis pathway in circulating neutrophils from critically ill patients can be overcome by Fas stimulation. J Immunol. 2009;183:6198–6206. doi: 10.4049/jimmunol.0901264. [DOI] [PubMed] [Google Scholar]
- 28.Lopez AD, Avasarala S, Grewal S, Murali AK, London L. Differential role of the Fas/Fas ligand apoptotic pathway in inflammation and lung fibrosis associated with reovirus 1/L-induced bronchiolitis obliterans organizing pneumonia and acute respiratory distress syndrome. J Immunol. 2009;183:8244–8257. doi: 10.4049/jimmunol.0901958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colamussi ML, White MR, Crouch E, Hartshorn KL. Influenza A virus accelerates neutrophil apoptosis and markedly potentiates apoptotic effects of bacteria. Blood. 1999;93:2395–2403. [PubMed] [Google Scholar]
- 30.Engelich G, White M, Hartshorn KL. Neutrophil survival is markedly reduced by incubation with influenza virus and Streptococcus pneumoniae: role of respiratory burst. J Leukoc Biol. 2001;69:50–56. [PubMed] [Google Scholar]
- 31.Saez-Lopez C, et al. Immediate-early antigen expression and modulation of apoptosis after in vitro infection of polymorphonuclear leukocytes by human cytomegalovirus. Microbes Infect. 2005;7:1139–1149. doi: 10.1016/j.micinf.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Engelich G, White M, Hartshorn KL. Role of the respiratory burst in co-operative reduction in neutrophil survival by influenza A virus and Escherichia coli. J Med Microbiol. 2002;51:484–490. doi: 10.1099/0022-1317-51-6-484. [DOI] [PubMed] [Google Scholar]
- 33.Lindemans CA, et al. Respiratory syncytial virus inhibits granulocyte apoptosis through a phosphatidylinositol 3-kinase and NF-kappaB-dependent mechanism. J Immunol. 2006;176:5529–5537. doi: 10.4049/jimmunol.176.9.5529. [DOI] [PubMed] [Google Scholar]
- 34.Andina N, Conus S, Schneider EM, Fey MF, Simon HU. Induction of Bim limits cytokine-mediated prolonged survival of neutrophils. Cell Death Differ. 2009;16:1248–1255. doi: 10.1038/cdd.2009.50. [DOI] [PubMed] [Google Scholar]
- 35.Dzhagalov I, St John A, He YW. The antiapoptotic protein Mcl-1 is essential for the survival of neutrophils but not macrophages. Blood. 2007;109:1620–1626. doi: 10.1182/blood-2006-03-013771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steimer DA, et al. Selective roles for antiapoptotic MCL-1 during granulocyte development and macrophage effector function. Blood. 2009;113:2805–2815. doi: 10.1182/blood-2008-05-159145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Villunger A, O'Reilly LA, Holler N, Adams J, Strasser A. Fas ligand, Bcl-2, granulocyte colony-stimulating factor, and p38 mitogen-activated protein kinase: Regulators of distinct cell death and survival pathways in granulocytes. J Exp Med. 2000;192:647–658. doi: 10.1084/jem.192.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren Y, et al. Increased apoptotic neutrophils and macrophages and impaired macrophage phagocytic clearance of apoptotic neutrophils in systemic lupus erythematosus. Arthritis Rheum. 2003;48:2888–2897. doi: 10.1002/art.11237. [DOI] [PubMed] [Google Scholar]
- 39.Midgley A, McLaren Z, Moots RJ, Edwards SW, Beresford MW. The role of neutrophil apoptosis in juvenile-onset systemic lupus erythematosus. Arthritis Rheum. 2009;60:2390–2401. doi: 10.1002/art.24634. [DOI] [PubMed] [Google Scholar]
- 40.Ogilvy S, et al. Constitutive Bcl-2 expression throughout the hematopoietic compartment affects multiple lineages and enhances progenitor cell survival. Proc Natl Acad Sci USA. 1999;96:14943–14948. doi: 10.1073/pnas.96.26.14943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagasse E, Weissman IL. bcl-2 inhibits apoptosis of neutrophils but not their engulfment by macrophages. J Exp Med. 1994;179:1047–1052. doi: 10.1084/jem.179.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell KJ, et al. Elevated Mcl-1 perturbs lymphopoiesis, promotes transformation of hematopoietic stem/progenitor cells, and enhances drug resistance. Blood. 2010;116:3197–3207. doi: 10.1182/blood-2010-04-281071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kaufmann T, et al. The BH3-only protein bid is dispensable for DNA damage- and replicative stress-induced apoptosis or cell-cycle arrest. Cell. 2007;129:423–433. doi: 10.1016/j.cell.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 44.Schaefer BC, Schaefer ML, Kappler JW, Marrack P, Kedl RM. Observation of antigen-dependent CD8+ T-cell/ dendritic cell interactions in vivo. Cell Immunol. 2001;214:110–122. doi: 10.1006/cimm.2001.1895. [DOI] [PubMed] [Google Scholar]
- 45.Harlin H, Reffey SB, Duckett CS, Lindsten T, Thompson CB. Characterization of XIAP-deficient mice. Mol Cell Biol. 2001;21:3604–3608. doi: 10.1128/MCB.21.10.3604-3608.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bouillet P, et al. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. doi: 10.1126/science.286.5445.1735. [DOI] [PubMed] [Google Scholar]
- 47.Croker BA, et al. Neutrophils require SHP1 to regulate IL-1β production and prevent inflammatory skin disease. J Immunol. 2011;186:1131–1139. doi: 10.4049/jimmunol.1002702. [DOI] [PubMed] [Google Scholar]
- 48.Shi W, Oshlack A, Smyth GK. Optimizing the noise versus bias trade-off for Illumina whole genome expression BeadChips. Nucleic Acids Res. 2010;38:e204. doi: 10.1093/nar/gkq871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article 3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 50.Ritchie ME, et al. Empirical array quality weights in the analysis of microarray data. BMC Bioinformatics. 2006;7:261. doi: 10.1186/1471-2105-7-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.