Abstract
Helicobacter mustelae, a gastric pathogen of ferrets, synthesizes a distinct iron-dependent urease in addition to its archetypical nickel-containing enzyme. The iron-urease is oxygen-labile, with the inactive protein exhibiting a methemerythrin-like electronic spectrum. Significantly, incubation of the oxidized protein with dithionite under anaerobic conditions leads to restoration of activity and bleaching of the spectrum. Structural analysis of the oxidized species reveals a dinuclear iron metallocenter bridged by a lysine carbamate, closely resembling the traditional nickel-urease active site. Although the iron-urease is less active than the nickel-enzyme, its activity allows H. mustelae to survive the carnivore’s low-nickel gastric environment.
Keywords: diiron, hydrolase, metalloprotein, isoenzyme , enzyme activation
Urease, a urea-hydrolyzing enzyme, is a virulence factor associated with gastric ulceration by Helicobacter pylori, infection-induced urinary stones, and other disease states (1). Furthermore, the plant enzyme is essential for seed germination and is toxic to some fungi and insects (2). Of historical interest, urease from jack bean (Canavalia ensiformis) seeds was the first enzyme to be crystallized (3) and the first protein shown to utilize nickel ions for its catalytic function (4). Crystal structures of ureases from Klebsiella aerogenes (5), Bacillus pasteurii (6), H. pylori (7), and jack bean (8) reveal a highly conserved active site architecture consisting of two nickel ions bridged by a lysine carbamate. Assembly of this metallocenter requires a complex maturation machinery involving several accessory proteins (9). In this study we describe the purification, properties, and structure of an oxygen-labile, iron-dependent urease from a pathogen of ferrets; i.e., a naturally occurring nickel-independent form of this enzyme.
Helicobacter mustelae is a microaerophilic gastric pathogen of the ferret (Mustela putorius furo). This microbe and its mammalian host represent a useful model for H. pylori infection of humans (10, 11). The ferret-associated microorganism produces high levels of urease activity in order to neutralize the acidic gastric contents, much like the pathogen of humans (12, 13). H. pylori cells contain a single genetic cluster (ureABIEFGH) encoding two urease structural subunits, a proton-gated urea permease, and four urease accessory proteins (Fig. S1), whereas H. mustelae contains both this cluster and a second set of urease structural genes (ureA2B2) located ∼106 base pairs downstream and not associated with any apparent maturation factors (14, 15). UreA2 and UreB2 share 57.4% and 69.5% identity to their UreA and UreB counterparts (Fig. S2), where the latter subunits are known to form an active nickel-containing enzyme (16). In contrast to ureABIEFGH which is induced by the addition of nickel ions, transcription of ureA2B2 is up-regulated by iron and down-regulated by nickel ions due to repression via NikR, a nickel-responsive transcriptional regulator (14). Helicobacter felis and Helicobacter acinonychis, infectious agents of cats and big cats, also possess this genetic arrangement (14).
Results
H. mustelae UreA2B2 Is an Oxygen-Labile Urease.
A mutant strain of H. mustelae lacking nikR and ureB constitutively produces UreA2B2 and exhibits low levels of urease activity (14) (see Fig. S3, Table S1). All strains producing UreAB yielded high levels of urease activity that were stable to cell lysis, but the low urease activity of the nikR ureB mutant was lost upon aerobic cell disruption. Significantly, the urease activity of the nikR ureB cells increased under anaerobic conditions and the lysate activity was stable when kept anaerobic (Table S1). Although the mutant strain produces only about 10% of the urease activity of the wild-type strain, it survives acidic shock conditions when provided with urea—consistent with UreA2B2 supporting H. mustelae growth in gastric tissue (14). Furthermore the UreA2B2 activity is independent of UreG or HypB, proteins known to be essential for maturation of the Helicobacter nickel-containing urease (17), suggesting a different activation mechanism (14). In sum, these results suggest that UreA2B2 is a unique oxygen-labile urease that does not require typical accessory proteins for maturation.
Effects of Medium Supplementation with Metal Ions on UreA2B2 Activity.
To investigate the effects of various metal ions on UreA2B2 urease activity, we examined recombinant cells because H. mustelae cell growth was limited to blood agar plates. Thus, we cloned and expressed ureA2B2 in Escherichia coli, grew the cells in Lennox Broth (LB), observed urease activity even in the absence of urease accessory proteins, demonstrated that supplementation with ferrous ions had negligible effects on activity, and found that addition of nickel or zinc led to diminutions of activity (Fig. S4 A and C). The various perturbations to the medium did not affect UreA2B2 production levels in cell extracts (Fig. S4 B and D). These results demonstrate that nickel or zinc additions to culture media do not increase UreA2B2 activity.
UreA2B2 Lacks Nickel and Contains Iron.
To directly assess the metal content of UreA2B2, the protein was purified both aerobically and anaerobically from nikR ureB H. mustelae cells collected off plates and also aerobically from recombinant E. coli cells grown in broth culture. The purified proteins (see Fig. S5) were examined for metal contents by inductively coupled plasma-atomic emission spectroscopy and for iron by 1,10-phenanthroline assays (Table 1).
Table 1.
Kinetic properties and metal contents of purified H. mustelae ureases
| Protein | Source | Purification conditions | Vmax [U (mg protein)-1] | Km (mM) | Metals per heterodimer* |
| UreA2B2 | H. mustelae nikR ureB | Aerobic (O2) | NA† (10.7 ± 0.4) | NA† | ICP: 2.3 Fe, 0.2 Zn; PA: 2.01 ± 0.05 Fe |
| UreA2B2 | H. mustelae nikR ureB | EDTA, O2 | NA† | NA† | ICP: 2.3 Fe, 0.2 Zn |
| UreA2B2 | H. mustelae nikR ureB | BP‡, O2 | NA† | NA† | ICP: 1.9 Fe, 0.1 Zn |
| UreA2B2 | H. mustelae nikR ureB | Anaerobic | 14.0 ± 0.2 | 1.6 ± 0.1 | ICP: 1.1 Fe, 0.7 Zn; PA: 1.05 ± 0.07 Fe |
| UreA2B2 | E. coli pEC015 | O2 | NA†(9.8 ± 0.5) | NA† | ICP: 1.0 Fe, < 0.1 Zn; PA: 1.13 ± 0.12 Fe |
| UreA2B2 | E. coli pEC015 | EDTA, βME, O2 | NA† | NA† | ICP: 0.8 Fe, < 0.1 Zn; PA: 0.89 ± 0.02 Fe |
| UreAB | H. mustelae wild-type | EDTA, βME, O2 | 390 ± 6 | 1.7 ± 0.1 | ICP: 0.6 Ni, 0.2 Fe, < 0.1 Zn |
| UreAB | H. mustelae nikR ureB2 | EDTA, βME, O2 | 840 ± 20 | 2.3 ± 0.2 | ICP: 1.1 Ni |
*Measured by ICP-AES (ICP) or based on reactivity with 1,10-phenanthroline (PA, average of triplicate experiments ± standard deviation).
†Not active; numbers in parentheses are specific activities of samples after reductive activation for 80 min.
‡Treated with 2,2-bipyridyl and dithionite prior to aerobic purification.
As purified aerobically from H. mustelae, UreA2B2 contains ∼2 equivalents of iron per heterodimer, along with very small amounts of zinc and no detectable nickel. The iron content was not greatly affected by treatment with EDTA or 2,2-bipyridyl (BP), indicating that the metal is tightly bound and chelator inaccessible—as is the case for nickel-containing ureases. When UreA2B2 was purified from H. mustelae under anaerobic conditions in the absence of chelator, both iron and zinc (1.1 and 0.7 equivalents, respectively) were found. A similar sample of anaerobically isolated enzyme (containing 1.3 equivalents of iron and 0.42 equivalents of zinc) treated with EDTA resulted in less zinc content (to 0.26 equivalents) while not affecting the iron content or the activity. Thus, zinc content does not correlate with enzyme activity. The anaerobic protein samples consistently bound less iron than aerobic samples, compatible with lower binding affinity for ferrous ions compared to the more highly charged ferric ions.
Aerobic purification of UreA2B2 from E. coli yielded a sample with only ∼1 equivalent of iron regardless of the presence or absence of EDTA and 2-mercaptoethanol (βME). Trace amounts of zinc and no nickel were detected. This result is consistent with UreA2B2 spontaneously acquiring some active site iron regardless of the host or with E. coli utilizing an endogenous iron incorporation system that partially compensates for unidentified maturation proteins in H. mustelae. In summary, these results demonstrate that regardless of the host UreA2B2 contains iron and small amounts of zinc while lacking nickel.
For comparison, the oxygen-stable UreAB was isolated from wild-type and nikR ureB2 H. mustelae (Fig. S5). In contrast to the iron-dominated metal content of UreA2B2, UreAB purified from wild-type and nikR ureB2 H. mustelae contained 0.6 and 1.1 equivalents of nickel, respectively (Table 1). NikR represses transcription of genes encoding nickel import proteins in H. mustelae and E. coli (18, 19); thus, the nikR ureB2 mutant likely contains higher cellular levels of nickel compared to wild-type cells, thereby accounting for the ∼2-fold increase in nickel content (and specific activity, see below) of UreAB in this strain.
Kinetic Properties of UreA2B2.
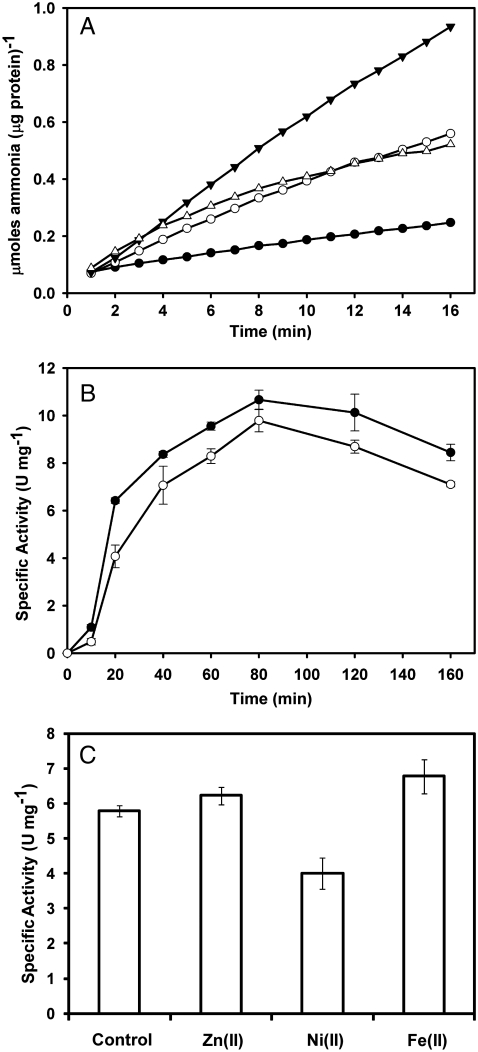
UreA2B2 purified anaerobically from nikR ureB H. mustelae was active [Vmax of ∼14 U (mg protein)-1], whereas all samples of this protein purified aerobically lacked activity (Table 1). Progress curves generated using anaerobically prepared UreA2B2 demonstrated inhibition by acetohydroxamic acid, a common inhibitor of nickel-containing ureases, and twofold enhancement of activity by 1 mM EDTA, consistent with tight binding of the active site iron and chelation of an inhibitory metal ion (Fig. 1A). Metal ion inhibition of ureases is well established (20), and the inclusion of NiCl2 in the assay led to a time-dependent decrease of activity. Exposure to air (∼20 min) abolished the activity of purified enzyme, confirming its oxygen lability. Of great importance, subjecting aerobic UreA2B2 (which contains only trace levels of zinc) to gas exchange and dithionite treatment led to its reactivation (Fig. 1B) and the reactivated H. mustelae- and E. coli-derived proteins possessed activities approaching that of the enzyme isolated using strictly anaerobic purification procedures (Table 1). The inclusion of one equivalent of nickel or zinc in the reductive reactivation buffer containing E. coli-derived protein did not have a marked effect on the resultant specific activity (Fig. 1C); iron may have stimulated modestly and nickel may have slightly inhibited the process. These results further establish that neither nickel nor zinc is required for UreA2B2 activity.
Fig. 1.
Activity of purified UreA2B2. (A) Progress curves for UreA2B2 purified anaerobically from H. mustelae when assayed in anaerobic buffer containing 50 mM urea (empty circle) or further supplemented with 3 mM acetohydroxamic acid (filled circles), 1 mM EDTA (solid upside down triangle), or 100 μM NiCl2 (empty triangle). Data points represent the average of duplicate experiments. (B) Activation kinetics of oxidized UreA2B2 by dithionite. Inactive samples (∼10 μM heterodimer) purified aerobically from H. mustelae (filled circles) and E. coli (open circles) were subjected to vacuum/argon cycling, supplemented with ∼100 μM dithionite, and incubated at ambient temperature. At the indicated time points, samples were assayed anaerobically in buffer containing 50 mM urea. Data points represent the average of triplicate experiments ± standard deviation. (C) Specific activity after reductive activation of E. coli-derived protein in the presence of metal ions. Protein (∼10 μM) was incubated with 1 mM dithionite and one equivalent of FeSO4, NiCl2, or ZnCl2 for 90 min before aliquots were assayed. Bars represent the average of triplicate experiments ± standard deviation.
Anaerobic UreA2B2 exhibited a Km (∼2 mM) similar to that for UreAB; however, UreAB had much greater activity [Vmax of ∼390 and 840 U (mg protein)-1], respectively, from the wild-type or nikR ureB2 mutant H. mustelae cells). For comparison, urease from H. mustelae cells grown on 5% sheep blood and Trypticase soy agar [a medium with more nickel (21)] exhibited a specific activity of 1,560 U mg-1 and a urea Km of 0.45 mM (16).
Analysis of the UreA2B2 Metallocenter.
To probe the electronic properties of the UreA2B2 metallocenter, UV-visible absorption spectroscopy was carried out on aerobic and anaerobic samples. Wavelength scans of the aerobically purified protein indicated a small and broad absorption at ∼500 nm, with more prominent shoulders at ∼380 nm and ∼320 nm (Fig. 2A). These features are reminiscent of the methemerythrin spectrum attributed to a μ-oxo bridged diferric metallocenter (22). Addition of sodium dithionite to oxidized UreA2B2 under anaerobic conditions caused bleaching of the spectrum, in parallel to the restoration of activity, consistent with direct reduction of the active site ferric ions (Fig. 2B). Exposure of the reduced sample to oxygen led to the redevelopment of the chromophore.
Fig. 2.
Electronic absorption spectra of UreA2B2. (A) Inactive UreA2B2 (100 μM) was purified aerobically from E. coli and its spectrum obtained in buffer containing 200 mM Tris-HCl, pH 7.4. (B) Dithionite reduction of UreA2B2 under anaerobic conditions. The protein sample shown in A was gas exchanged by vacuum/argon cycling, supplemented with degassed 0.5 mM dithionite, and incubated at ambient temperature for 60 min, with spectra recorded every 10 min. Only the 350–600 nm region is shown as dithionite yields intense absorption features below 350 nm.
Additional evidence that the UreA2B2-associated iron is associated with a homodinuclear center was derived from electron paramagnetic resonance (EPR) studies. In particular, neither the oxidized nor the reduced sample exhibited an EPR spectrum in the standard perpendicular mode. For an oxidized dinuclear center [i.e., Fe(III)/Fe(III)], antiferromagnetic coupling yields an overall S = 0 accounting for lack of signal. For reduced [Fe(II)/Fe(II)] species, the ferrous ions often possess a signal with a high g-value in parallel mode that may not be visible in perpendicular mode. A lack of signal for both redox states would not be expected for a mononuclear iron site or for an iron-zinc heterodinuclear site, thus ruling out significant levels of these species.
Alignment of UreA2B2 with other urease sequences (Fig. S2) indicated the presence of a Lys at position 218 in UreB2, comparable to the Lys that forms a carbamate which serves as a ligand bridging the two metal ions in nickel-containing ureases. Recombinant E. coli cells expressing mutated ureA2B2, where this residue was substituted with Ala, Arg, or Glu, lacked urease activity, consistent with Lys218 playing a critical role at the active site. A homology model of UreA2B2, created using the structure of H. pylori urease as a template, identified a unique Cys residue near the active site that initially was suspected of being important for metal ion specificity. Substitution of this residue by Ala, as found in H. pylori and most other urease sequences, had only modest effects (85% activity retained) in whole E. coli cells grown in LB when compared to recombinant cultures containing wild-type UreA2B2; hence, we conclude that Cys245 is not required for proper metallocenter assembly.
Crystal Structure of UreA2B2.
To identify potential functionally relevant differences between the active sites of the UreA2B2 iron-containing active site in comparison to that of nickel ureases, the aerobically isolated protein was crystallized and the structure elucidated at 3.0 Å resolution (Table S2). As expected, the tertiary structure matches that of other ureases, and the quaternary structure matches that of H. pylori urease (7), being a superstructure of four UreA2B2 trimers arranged as a hollow ball with tetrahedral symmetry (Fig. 3A). The active sites open to the outer surface of the sphere, with access partially occluded by the 317 to 333 loop. This active site flap adopts a different conformation in various urease crystal forms, and has high flexibility in all cases (Fig 3A).
Fig. 3.
Structure and unique features of dinuclear Fe-containing H. mustelae UreA2B2. (A) Molecular surface of the UreA2B2 dodecameric superstructure. One (UreA2B2)3 trimer is rainbow-colored from blue to red, indicating increasing chain mobility as measured by B factors, with one UreA2B2 unit shown as a ribbon with the active site irons as brown spheres. The three active site flaps are visible as red-orange patches and, for the ribbon representation, a gap is due to disorder of four residues that were not modeled. (B) An unbiased 2Fo-Fc electron density map of the metallocenter, generated from a model before the metals or metal ligands were built, is compared with the K. aerogenes urease structure. Nickels (teal spheres), side chains not included in the phasing model (green carbons and labeled), and other local protein atoms (white carbons) are shown, and the electron density is contoured at two levels (0.3 and 1.3 e/Å3). (C) Active site region highlighting (in cyan) residues differing between UreA2B2-type ureases and other ureases. The molecular surface is shown except for the active site flap (red ribbon) and irons (brown spheres). The view is as for the molecule shown as a ribbon in (A).
At this resolution, the ligands to the iron atoms are arranged in an indistinguishable manner from their equivalents in the nickel ureases (Fig. 3B). Some additional electron density near the Fe1 atom is similar in placement and shape to the density seen for acetohydroxamic acid bound to H. pylori urease (PDB entry 1E9Y) (7). With acetate present in the mother liquor of our crystals, this density may represent acetate binding to the active site, but because an unambiguous identification cannot be made we have not modeled this density. Comparison of B factors of the metal ions with those of ligating residues indicates that the metal sites are fully occupied, consistent with preferential crystallization of the metallated form of the enzyme.
Discussion
The biochemical and crystallographic data described here demonstrate that H. mustelae UreA2B2 is a dinuclear ferrous ion-dependent urease; i.e., a distinct nickel-independent form of the enzyme. Oxidation by O2 likely leads to a μ-oxo bridged diferric inactive species that can be chemically reduced to restore activity. The presence of two types of urease in H. mustelae, H. felis (23), and H. acinonychis may represent an evolutionary adaptation to their niches, as previously hypothesized on the basis of the differential regulation of their urease gene clusters (14). Helicobacter species require urease activity to colonize the host gastric tissue; however, carnivores have diets rich in iron and low in nickel content (24) so infection by these pathogens would be hindered if they were limited to possessing only nickel ureases. The capacity to produce either a nickel- or iron-urease allows these microbes to colonize their hosts regardless of their host’s diets.
The basis of the distinct metal specificities for the two ureases in H. mustelae remains obscure. The immediate environment around the iron urease active site is nearly identical to that in nickel ureases, and so we considered the broader context of the whole protein chains, which reveals 92 residues that are conserved in UreA2B2 sequences and lacking in nickel ureases (Fig. S2). Notably, mapping these UreA2B2-distinct residues onto the urease structure (Fig. S6) revealed a prominent cluster that encircles the entrance to the active site (Fig. 3C). This finding leads us to the hypothesis that the difference in metal discrimination occurs during the metal loading process.
An interesting question is whether a conventional nickel-dependent urease could function with iron. The apoprotein from K. aerogenes can be produced in E. coli either by leaving out an accessory gene in the transformed operon or by growing the cells in the absence of nickel, and its activation has been well studied (25). Roughly (∼15%) of the K. aerogenes urease apoprotein is activated by incubation in bicarbonate-containing buffer with 100 μM nickel [yielding ∼400 U (mg protein)-1], less activity is obtained with manganese and cobalt [7 and 9 U (mg protein)-1], and no activity is obtained for copper or zinc. Our efforts to activate the conventional K. aerogenes urease apoprotein in the same conditions with ferrous rather than nickel ions led to variable levels of highly oxygen-labile activity, with the maximum observed activity for the iron-substituted enzyme of 9.0 ± 1.4 U (mg protein)-1. Thus, under artificial conditions low levels of iron-dependent urease activity can be obtained in a conventional urease.
Urease thus joins a small group of enzymes for which homologous sequences are known to bind alternative metals for carrying out the same catalytic function. Examples of such enzymes include the Mo-, V-, and Fe-nitrogenases (26), Mn- and Fe-superoxide dismutases (27), di-Fe, Mn-Fe, and di-Mn class I ribonucleotide reductases (28), and Zn- vs. Ni-glyoxylases (29). Whereas metallocenter assembly for the different nitrogenases makes use of distinct sets of accessory proteins, the basis of metal specificity in superoxide dismutases is less clear. Ribonucleotide reductases are of interest because formation of active enzyme occurs spontaneously with O2 for the di-ferrous protein, whereas activation of the di-Mn protein requires hydroperoxyl anion from a reduced flavoprotein. A possible close parallel to urease is glyoxylase, for which high resolution structures of various metallated proteins indicate differences in coordination for active (octahedral) vs. inactive (five-coordinate) sites. This finding demonstrates that very subtle changes in metallocenter structure can have profound effects on activity, a situation likely to be relevant to Fe- and Ni-ureases.
Materials and Methods
Bacterial Strains and Growth.
The H. mustelae strains used in this study were previously described (14) and include wild-type cells and nikR ureB (a constitutive producer of UreA2B2), nikR ureB2 (a constitutive producer of UreAB), nikR ureB ureB2 (a urease-negative control), and ureB2 (a nickel-responsive producer of UreAB) mutants. Cells were grown on blood agar plates [Columbia blood agar base (Oxoid) with 7% defibrinated horse blood (HEMA Resources) and DENT selective supplement (Oxoid)] after inoculating with cells from frozen stock. Plates were incubated for ∼72 h at 37 °C in Gas-Pak anaerobic systems (Becton, Dickinson and Company) using CampyGen (Oxoid) sachets to maintain a microaerobic environment. Studies involving the heterologous production of UreA2B2 in E. coli were carried out by using BL21-Gold (Stratagene) transformed with pEC015 or its site-directed variants (see SI Text) and grown in LB (Fisher Scientific) at 37 °C with shaking.
Urease and Protein Assays.
Urease activity was determined by quantifying the ammonia released during urea degradation by the formation of indophenol which was monitored at 625 nm (30). One unit of activity is defined as the amount of enzyme necessary to degrade 1 μmol of urea per min at 37 °C. Standard assay buffer consisted of 50 mM Hepes, pH 7.8, and 50 mM urea unless otherwise indicated. Kinetic constants were determined by fitting data with a standard Michaelis Menten equation using SigmaPlot software (Systat Software, Inc.). Measurements of activities for whole cells, disrupted cells, and cell-free extracts are described in the SI Text. Protein concentrations were determined by using the Bio-Rad Protein Assay with bovine serum albumin as the standard. Molecular masses of 86,949 Da for UreA2B2, and 86,344 Da for UreAB were used to calculate protein concentrations.
Isolation and Metal Content of Ureases.
UreA2B2 was purified from H. mustelae both aerobically and anaerobically and from E. coli aerobically, while UreAB was purified aerobically from H. mustelae (see SI Text). Metal analysis included inductively coupled plasma-atomic emission spectroscopy (ICP-AES) and use of the Fe-specific chromogenic chelator 1,10-phenanthroline (see SI Text).
In Vitro Reactivation of Aerobically-Purified UreA2B2.
UreA2B2 purified aerobically from H. mustelae or E. coli was degassed and transferred into an anaerobic Coy chamber. Protein samples (∼10 μM, 20 mM Tris-HCl, pH 7.4, 300 mM NaCl) were mixed with ∼0.1 mM sodium dithionite and incubated at ambient temperature for the times indicated. Aliquots of the reaction mixtures were assayed for urease activity under anoxic conditions. To assess the effect of added metal ions on the reactivation, E. coli-derived protein (∼10 μM in 20 mM Tris-HCl, pH 7.4, containing 300 mM NaCl) was mixed with 1 mM sodium dithionite and 10 μM FeSO4, NiCl2, or ZnCl2 for 90 min at ambient temperature before aliquots of the mixtures were assayed for urease activity.
Absorption Spectroscopy.
The electronic spectra of UreA2B2 samples were monitored by using a Shimadzu UV-2401PC spectrophotometer at room temperature. Samples in sealed serum vials were made anaerobic by repeated (> 10) vacuum/argon cycles on a Schlenk line before being transferred to anaerobic cuvettes using gas-tight syringes (Hamilton). Degassed sodium dithionite solution was anaerobically transferred into the anoxic protein solution, incubated at room temperature for up to 60 min, and spectra were recorded every 10 min. The protein concentration was diluted by less than 1% due to the addition of dithionite.
EPR Spectroscopy.
E. coli-derived UreA2B2 (100 μM) in 20 mM Tris-HCl, pH 7.4, with 300 mM NaCl was degassed by several vacuum/argon cycles and transferred into an anaerobic chamber (∼2.5% H2 and balance N2). One aliquot was transferred directly into an EPR tube whereas a second sample (230 μL) was treated with sodium dithionite (2.3 μL of 50 mM, final concentration of ∼0.5 mM) at ambient temperature. After 20 min the tubes were sealed with septa, removed from the chamber, and frozen in liquid N2. The samples were examined by EPR spectroscopy at 4.3 K using a Bruker E300X spectrometer operating at X-band and equipped with an Oxford Instruments liquid helium flow system with a CF-935 cryostat and an ITC-503 temperature controller.
UreA2B2 Structural Characterization.
Crystallization, data collection, structure solution, and refinement are described in SI Text. Two models were deposited in the Protein Databank as entries 3QGA (3.0 Å, wild-type) and 3QGK (3.0 Å, wild-type, no solvent), respectively.
Supplementary Material
Acknowledgments.
We thank Jeroen Stoof and Arnoud van Vliet for strains and advice, Callia Palioca for crystallizing the protein, Art Robbins for contributions to the structure solution, Thomas Casey for EPR analysis, and Eric Hegg for use of his anaerobic chamber. This work was supported by the National Institutes of Health (GM083136 to P.A.K. and DK04586 to R.P.H.), with additional funding (to E.L.C.) by the Hugh and Rosenberg Fellowships.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The atomic coordinates have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3QGA, 3QGK).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106915108/-/DCSupplemental.
References
- 1.Mobley HLT, Hausinger RP. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989;53:85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Witte C-P. Urea metabolism in plants. Plant Sci. 2011;180:431–438. doi: 10.1016/j.plantsci.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 3.Sumner JB. The isolation and crystallization of the enzyme urease. J Biol Chem. 1926;69:435–441. [Google Scholar]
- 4.Dixon NE, Gazzola C, Blakeley RL, Zerner B. Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel? J Am Chem Soc. 1975;97:4131–4133. doi: 10.1021/ja00847a045. [DOI] [PubMed] [Google Scholar]
- 5.Jabri E, Carr MB, Hausinger RP, Karplus PA. The crystal structure of urease from Klebsiella aerogenes. Science. 1995;268:998–1004. [PubMed] [Google Scholar]
- 6.Benini S, et al. A new proposal for urease mechanism based on the crystal structures of the native and inhibited enzyme from Bacillus pasteurii: why urea hydrolysis costs two nickels. Structure. 1999;7:205–216. doi: 10.1016/S0969-2126(99)80026-4. [DOI] [PubMed] [Google Scholar]
- 7.Ha N-C, et al. Supramolecular assembly and acid resistance of Helicobacter pylori urease. Nat Struct Biol. 2001;8:505–509. doi: 10.1038/88563. [DOI] [PubMed] [Google Scholar]
- 8.Balasubramanian A, Ponnuraj K. Crystal structure of the first plant urease from jack bean: 83 years of journey from its first crystal to molecular structure. J Mol Biol. 2010;400:274–283. doi: 10.1016/j.jmb.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Carter EL, Flugga N, Boer JL, Mulrooney SB, Hausinger RP. Interplay of metal ions and urease. Metallomics. 2009;1:207–221. doi: 10.1039/b903311d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox JG, et al. Helicobacter mustelae-associated gastritis in ferrets: an animal model of Helicobacter pylori gastritis in humans. Gastroenterology. 1990;99:352–361. doi: 10.1016/0016-5085(90)91016-y. [DOI] [PubMed] [Google Scholar]
- 11.Solnick JV, Schauer DB. Emergence of diverse Helicobacter species in the pathogenesis of gastric and enterohepatic diseases. Clin Microbiol Rev. 2001;14:59–97. doi: 10.1128/CMR.14.1.59-97.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sachs G, Weeks DL, Melchers K, Scott DR. The gastric biology of Helicobacter pylori. Annu Rev Physiol. 2003;65:349–369. doi: 10.1146/annurev.physiol.65.092101.142156. [DOI] [PubMed] [Google Scholar]
- 13.Suerbaum S, Michetti P. Helicobacter pylori Infection. New Engl J Med. 2002;347:1175–1186. doi: 10.1056/NEJMra020542. [DOI] [PubMed] [Google Scholar]
- 14.Stoof J, et al. Inverse nickel-responsive regulation of two urease enzymes in the gastric pathogen Helicobacter mustelae. Environ Microbiol. 2008;10:2586–2597. doi: 10.1111/j.1462-2920.2008.01681.x. [DOI] [PubMed] [Google Scholar]
- 15.O’Toole PW, et al. Comparative genomics and proteomics of Helicobacter mustelae, an ulcerative and carcinogenic gastric pathogen. BMC Genomics. 2010;11:164. doi: 10.1186/1471-2164-11-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dunn BE, Sung C-C, Taylor NS, Fox JG. Purification and characterization of Helicobacter mustelae urease. Infect Immun. 1991;59:3343–3345. doi: 10.1128/iai.59.9.3343-3345.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olson JW, Mehta NS, Maier RJ. Requirement of nickel metabolism proteins HypA and HypB for full activity of both hydrogenase and urease in Helicobacter pylori. Mol Microbiol. 2001;39:176–182. doi: 10.1046/j.1365-2958.2001.02244.x. [DOI] [PubMed] [Google Scholar]
- 18.Rowe JL, Starnes GL, Chivers PT. Complex transcriptional control links NikABCDE-dependent nickel transport with hydrogenase expression in Escherichia coli. J Bacteriol. 2005;187:6317–6323. doi: 10.1128/JB.187.18.6317-6323.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoof J, Kuipers E, van Vliet A. Characterization of NikR-responsive promoters of urease and metal transport genes of Helicobacter mustelae. BioMetals. 2009;23:145–159. doi: 10.1007/s10534-009-9275-7. [DOI] [PubMed] [Google Scholar]
- 20.Pearson MA, Michel LO, Hausinger RP, Karplus PA. Structure of Cys319 variants and acetohydroxamate-inhibited Klebsiella aerogenes urease. Biochemistry. 1997;36:8164–8172. doi: 10.1021/bi970514j. [DOI] [PubMed] [Google Scholar]
- 21.Wolfram L, Bauerfiend P. Activities of urease and nickel uptake of Helicobacter pylori proteins are media- and host-dependent. Helicobacter. 2009;14:264–270. doi: 10.1111/j.1523-5378.2009.00685.x. [DOI] [PubMed] [Google Scholar]
- 22.Kao W-C, et al. Isolation, purification and characterization of hemerythrin from Methylococcus capsulatus (Bath) J Inorg Biochem. 2008;102:1607–1614. doi: 10.1016/j.jinorgbio.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 23.Pot RGJ, et al. UreA2B2: a second urease system in the gastric pathogen Helicobacter felis. FEMS Immunol Med Microbiol. 2007;50:273–279. doi: 10.1111/j.1574-695X.2007.00212.x. [DOI] [PubMed] [Google Scholar]
- 24.Solomons NW, Viteri F, Shuler TR, Nielsen FH. Bioavailability of nickel in man: effects of foods and chemically-defined dietary constituents on the absorption of inorganic nickel. J Nutr. 1982;112:39–50. doi: 10.1093/jn/112.1.39. [DOI] [PubMed] [Google Scholar]
- 25.Yamaguchi K, et al. Characterization of metal-substituted Klebsiella aerogenes urease. J Biol Inorg Chem. 1999;4:468–477. doi: 10.1007/s007750050333. [DOI] [PubMed] [Google Scholar]
- 26.Joerger RD, Bishop PE. Bacterial alternative nitrogen fixation systems. Crit Rev Microbiol. 1988;16:1–14. doi: 10.3109/10408418809104465. [DOI] [PubMed] [Google Scholar]
- 27.Abreu IA, Cabelli DE. Superoxide dismutases—a review of the metal-associated mechanistic variations. Biochim Biophys Acta. 2010;1804:263–274. doi: 10.1016/j.bbapap.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 28.Cortuvo JA, Jr, Stubbe J. Class I ribonucleotide reductases: metallocofactor assembly and repair in vitro and in vivo. Annu Rev Biochem. 2011;80:733–767. doi: 10.1146/annurev-biochem-061408-095817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.He MM, Clugston SL, Honek JF, Matthews BW. Determination of the structure of Escherichia coli glyoxylase I suggests a structural basis for differential metal activation. Biochemistry. 2000;39:8719–8727. doi: 10.1021/bi000856g. [DOI] [PubMed] [Google Scholar]
- 30.Weatherburn MW. Phenol-hypochlorite reaction for determination of ammonia. Anal Chem. 1967;39:971–974. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.