Abstract
N-Methylimidazolium chloride is found to catalyze a coupling reaction between monophosphates and activated phosphorous-nitrogen intermediates such as a phosphorimidazolide and phosphoromorpholidate to form biologically important unsymmetrical pyrophosphate diesters. The catalyst is much more active, cheaper, and less explosive than 1H-tetrazole, known as the best catalyst for the pyrophosphate formation over a decade. The mild and neutral reaction conditions are compatible with allylic pyrophosphate formation in Lipid I syntheisis. 31P NMR experiments suggest that the catalyst acts not only as an acid but also as a nucleophile to form cationic and electrophilic phosphor-N-methylimidazolide intermediates in the pyrophosphate formation.
Keywords: N-Methylimidazolium chloride, Pyrophosphate formation, Phosphorimidazolide, Phosphoromorpholidate, Lipid I, NDP-sugar donors
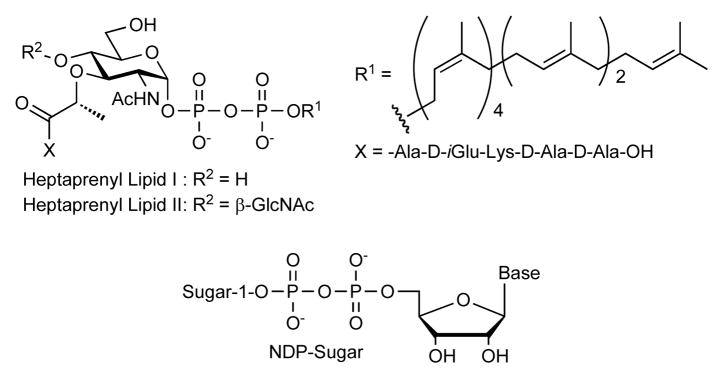
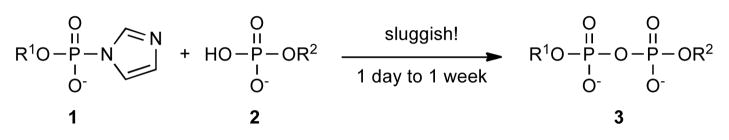
Attachment of a sugar to a protein or another sugar chain gives the parent biomolecules diverse functions. Enzymes that catalyze formation of glycosidic bonds to other molecules are known as glycosyltransferases (Gtfs), and they typically use glycosyl donors containing a nucleoside diphosphate (NDP) or lipid pyrophosphate (LPP) on the anomeric carbon (Figure 1).1 Synthetic methods to make NDP- and LPP-sugar donors are required to elucidate glycosyltransferase function and to synthesize natural and unnatural oligosaccharides chemoenzymatically.2 Our laboratory has been searching for efficient methods to make bacterial LPP-sugars such as Lipid I and Lipid II for studying enzymes involved in cell envelope biosynthesis.3 Although synthetic routes to these molecules have been reported, we have not been satisfied with the existing approaches.3 x, 4, 5 Here we describe an improved method to make pyrophosphate bonds that is compatible with the formation of both NDP- and LPP-sugars.
Figure 1.
Chemical strategies to make NDP- and LPP-sugars involve condensation of two phosphates, which requires activation of one of them.2 Phosphoromorpholidates6 and phosphorimidazolides7 are usually employed as activated intermediates in these condensations. Since nucleoside 5′-monophosphoromorpholidates are commercially available, phosphoromorpholidate intermediates are commonly used to make NDP-sugars, and 1H-tetrazole is often used to catalyze the condensation reactions.8 Because phosphorimidazolides are more reactive that phosphoromorpholidates they are preferred for making LPP-sugars.3a–c, 4, 5 They can be readily prepared in situ using excess carbonyl diimidazole (CDI) followed by a methanol quench. These intermediates are sufficiently stable that neither the corresponding symmetrical pyrophosphate nor the methyl phosphodiester forms during activation and quenching. Unfortunately, the desired unsymmetrical pyrophosphate products also form very slowly (Scheme 1). Divalent metal ions such as Zn2+, Mg2+, Mn2+, Cd2+, and Sn2+ have been reported to promote pyrophosphate bond formation,9 but can promote decomposition of allylic lipid pyrophosphates,10 which are both acid- and nucleophile-sensitive.
Scheme 1.
One approach to the synthesis of Lipid I and Lipid II used 1H-tetrazole as a Brönsted acid catalyst to promote condensation of a a lipid phosphate and a sugar phosphorimidazolide, but the reaction still took 4–7 days.4 Skein et al. have shown that 1H-tetrazole is not effective for activation of the phosphorimidazolide.9a Herein we report the use of N-methylimidazolium chloride (NMI·HCl) as a catalyst for the phosphorimidazolide coupling reaction in Lipid I synthesis. The catalyst is much more active, cheaper, and less explosive than 1H-tetrazole. The reaction conditions are mild, neutral, and compatible with the allylic pyrophosphate bond formation. We show that this method also works well for NMP-morpholidate coupling reactions with a sugar 1-phosphate. In addition, we report a new synthetic route to make Lipid I that involves introduction of the peptide after pyrophosphate bond formation. The peptide is the variable portion in naturally occurring Lipid I and II substrates,11 and our new approach allows more flexibility in the synthesis of substrates containing different oligopeptides for mechanistic investigations of peptidoglycan biosynthetic enzymes.
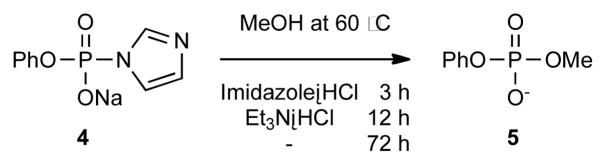
In the condensation of two phosphates counter ions play important roles, but little attention has been paid to them. In fact, in chemical schemes the counter cations of activated phosphate 1 and phosphate monoester 2 are often omitted (Scheme 1). Trialkylammonium salts of 1 and 2 are frequently used for pyrophosphate formation because they increase the solubility of both 1 and 2 in organic solvents; they also increase the nucleophilicity of 2. However, the trialkyammonium salts are not acidic enough (aqueous pKEt3NH+ 10.8) to effectively protonate 1, as required for P-N bond dissociation, which may account for sluggish reactivity in the formation of pyrophosphate bonds. Cramer et al. showed that methanolysis of sodium phosphorimidazolide 4 is much faster in the presence of imidazolium chloride (pKimidazole·H+ 7.0) than triethylammonium chloride (Scheme 2).7b We wondered whether adding a salt such as imidazolium or pyridinium chloride, which comprise a weaker base than a trialkylamine and a stronger acid than a phosphate, to a mixture of 1 and 2 would lead to counter ion exchange to produce more a electrophilic imidazolium or pyridinium phosphorimidazolide 1 in situ.
Scheme 2.
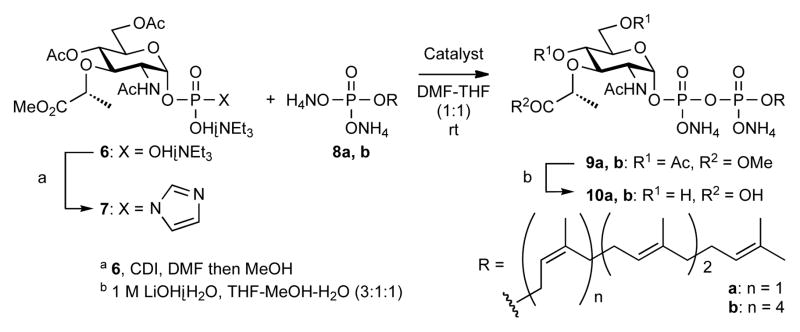
Phosphorimidazolide 7, prepared from the parent triethylammonium phosphate 612 and CDI, was used to make the pyrophosphates of polyprenyl monophosphate diammonium salts 8a and 8b in anhydrous THF-DMF (1:1) solvent (Scheme 3).13 The complete conversion of tetraprenyl phosphate 8a to the corresponding pyrophosphate 9a took one week in the absence of catalyst (Table 1, Entry 1). Addition of weaker amine hydrochlorides (Entries 2, 3, 4, and 7) promoted the reaction much more effectively than triethylamine, which did not appear to accelerate coupling. N-methylimidazolium chloride (NMI·HCl, pKBH+ 7.1) was more effective than 1H-imidazole chloride (Im·HCl, pKBH+ 7.0) (Entry 4 vs. 5) and comparable to pyridinium chloride (Py·HCl, pKBH+ 5.2) (Entries 5 vs. 7). Use of trifluoromethanesulfonic acid salts (NMI·HOTf and Py·HOTf) slightly reduced the yields of pyrophosphate 10a (Entry 5 vs. 6, 7 vs. 8). While 1H-tetrazole accelerated pyrophosphate formation with phosphorimidazolide 7, the reaction was slower and the yield reduced compared with NMI·HCl and Py·HCl (Entry 5, 7 vs. 9). NMI·HCl transformed the longer lipid phosphpate, heptaprenyl 8b, into the corresponding pyrophosphate 9b in good yield within half a day (Entry 10).
Scheme 3.
Table 1.
Effect of Catalysts on Pyrophsophate Formation via Scheme 3
| Entry | 8 | Catalyst (4 eq) | pKa (H2O) | Time (d) | Yield of 10 (%) |
|---|---|---|---|---|---|
| 1 | 8a | - | - | 7 | 69 |
| 2 | 8a | Et3N·HCl | 10.8 | 7 | 71 |
| 3 | 8a | DMAP·HCl | 9.2 | 1 | 69 |
| 4 | 8a | Imidazole·HCl | 7.0 | 1 | 75 |
| 5 | 8a | N-Mehylimidazole HCl | 7.1 | 0.5 | 77 |
| 6 | 8a | N-Mehylimidazole HOTf | 7.1 | 0.5 | 72 |
| 7 | 8a | Pyridine·HCl | 5.2 | 0.5 | 75 |
| 8 | 8a | Pyridine·HOTf | 5.2 | 0.5 | 68 |
| 9 | 8a | Tetrazole | 4.9 | 1 | 63 |
| 10 | 8b | N-Mehylimidazole HCl | 7.1 | 0.5 | 54 |
Yields were determined after removal of protecting groups.
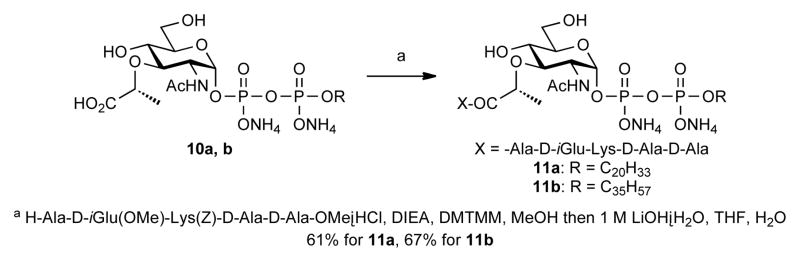
The MurNAc polyprenyl pyrophosphates 10a, b were used to make the corresponding Lipid I analogs via a new route in which the protected pentapeptide was coupled following formation of the diphosphate (Scheme 4). DMTMM14 was the most effective reagent for condensation between 10a, b and a small excess of protected pentapeptide.15 Subsequent hydrolysis afforded tetra- and heptaprenyl Lipid I 11a, b in good yields. Both can be quantitatively converted to the corresponding Lipid II analogs chemoenzymatically for studies of peptidoglycan biosynthetic enzymes and their inhibitors.3b
Scheme 4.
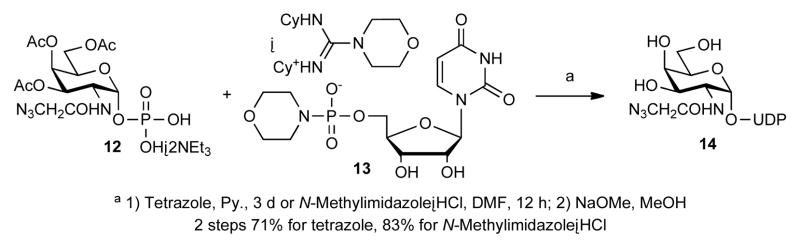
We also investigated the use of the NMI·HCl catalyst for NDP-sugar synthesis. Khorana’s morpholidate method, which involves coupling commercially available nucleoside 5′-monophosphoromorpholidate 4-morpholine-N,N′-dicyclohexyl-carboxamidine salts, is the most widely used approach for NDP-sugar synthesis.6 These NMP-morpholidate intermediates have a poor leaving group (pKmorpholine·H+ 8.4) and a basic counter cation (pKguanidine·H+ 11.9). Condensation of sugar 1-phosphate 1216 and UMP-morpholidate 13 took three days even in the presence of tetrazole, long considered the best catalyst for these reactions (Scheme 5).8, 17 In contrast, our NMI·HCl catalyst in DMF greatly improved the reaction rate (12 h) and yield,18 giving the desired UDP-GalNAz 14 product after removal of the acetate protecting groups.
Scheme 5.
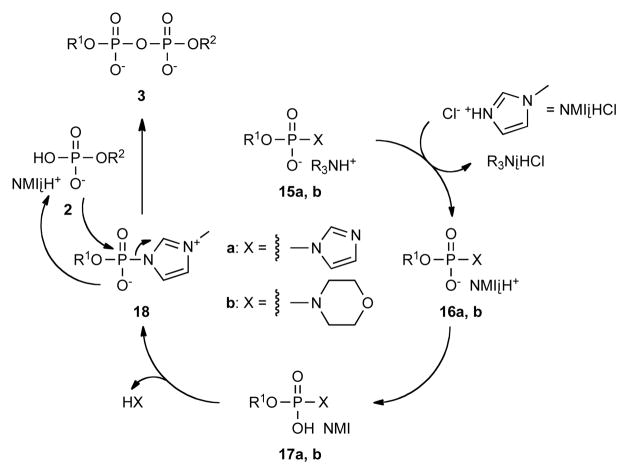
The difference between Im·HCl and NMI·HCl as catalysts suggests that these compounds act not only acids but also as nucleophiles in pyrophosphate formation as shown in Scheme 6. Electronically neutral phosphorimidazolide and phosphoromorpholidate 15a, b could be substituted by N-methylimidazole to form a cationic phosphor-N-methylimidazolide intermediate 18, which would be more susceptible to nucleophilic displacement with a monophosphate anion 2 to give pyrophosphate 3. Consistent with the formation of a new, cationic intermediate, we observe the appearance of downfield-shifted 31P resonances in the 31P-NMR spectra of phosphorimidazolide 7 and UMP-morpholidate 13 after the addition of NMI-HCl.19 In the case of 13, the downfield 31P resonance that appeared upon addition of excess NMI-HCl had a chemical shift similar to that of authentic TFA-protected UMP-N-methylimidazolide (−10.6 vs. −10.9 ppm).20 Although phosphor-N-methylimidazolide is a very reactive intermediate, its preparation requires multiple steps including treatment with acidic trifluoroacetic anhydride, during which care must be given to its moisture sensitivity. In contrast, adding NMI-HCl as a catalyst to less reactive intermediates allows simple manipulation and ready availability of starting materials while being compatible with acid-sensitive functional groups.
Scheme 6.
N-Methylimidazolium chloride was found to be superior in activity, cost, and safety to 1H-tetrazole, long considered the best catalyst for pyrophosphate bond formation. Our new method combines availability and stability of phosphorimidazolide and phosphoromorpholidate with high reactivity of phosphor-N-methylimidazolide. The mild and neutral reaction conditions are compatible with allylic pyrophosphate formation in Lipid I.
Supplementary Material
Acknowledgments
This research was supported by the National Institutes of Health (R01 GM076710 and R01 GM066174).
Footnotes
Experimental details are described in Supplementary Material (PDF).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Thibodeaux CJ, Melancin CE, III, Liu H-W. Angew Chem Int Ed. 2008;47:9814–9859. doi: 10.1002/anie.200801204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wagner GK, Pesnot T, Field RA. Nat Prod Rep. 2009;26:1172–1194. doi: 10.1039/b909621n. [DOI] [PubMed] [Google Scholar]
- 3.(a) Zhang Y, Fechter EJ, Wang TSA, Barrett D, Walker S, Kahne DE. J Am Chem Soc. 2007;129:3080–3081. doi: 10.1021/ja069060g. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ye XY, Lo MC, Brunner L, Walker D, Kahne D, Walker S. J Am Chem Soc. 2001;123:3155–3156. doi: 10.1021/ja010028q. [DOI] [PubMed] [Google Scholar]; (c) Chen L, Men H, Ha S, Ye XY, Brunner L, Hu Y, Walker S. Biochemistry. 2002;41:6824–6833. doi: 10.1021/bi0256678. [DOI] [PubMed] [Google Scholar]; (d) Perlstein DL, Zhang Y, Wang TS, Kahne DE, Walker S. J Am Chem Soc. 2007;129:12674–12675. doi: 10.1021/ja075965y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Wang TSA, Manning SA, Walker S, Kahne D. J Am Chem Soc. 2008;130:14068–14069. doi: 10.1021/ja806016y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Perlstein DL, Wang TSA, Doud EH, Kahne D, Walker S. J Am Chem Soc. 2010;132:48–49. doi: 10.1021/ja909325m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Men H, Park P, Ge M, Walker S. J Am Chem Soc. 1998;120:2484–2485. [Google Scholar]; (h) Ha S, Chang E, Lo MC, Men H, Park P, Ge M, Walker S. J Am Chem Soc. 1999;121:8415–8426. [Google Scholar]; (i) Lo MC, Men H, Branstrom A, Helm J, Yao N, Goldman R, Walker S. J Am Chem Soc. 2000;122:3540–3541. [Google Scholar]; (j) Chen L, Walker D, Sun B, Hu Y, Walker S, Kahne D. Proc Natl Acad Sci. 2003;100:5658–5663. doi: 10.1073/pnas.0931492100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Chen L, Yuan Y, Helm JS, Hu Y, Rew Y, Shin D, Boger DL, Walker S. J Am Chem Soc. 2004;126:7462–7463. doi: 10.1021/ja047879t. [DOI] [PubMed] [Google Scholar]; (l) Fang X, Tiyanont K, Zhang Y, Wanner J, Boger DL, Walker S. Mol BioSyst. 2006;2:69–76. doi: 10.1039/b515328j. [DOI] [PubMed] [Google Scholar]
- 4.(a) VanNieuwenhze MS, Mauldin SC, Zia-Ebrahimi M, Aikins JA, Blaszczak LC. J Am Chem Soc. 2001;123:6983–6988. doi: 10.1021/ja016082o. [DOI] [PubMed] [Google Scholar]; (b) VanNieuwenhze MS, Mauldin SC, Zia-Ebrahimi M, Winger BE, Hornback WJ, Saha SL, Aikins JA, Blaszczak LC. J Am Chem Soc. 2002;124:3656–3660. doi: 10.1021/ja017386d. [DOI] [PubMed] [Google Scholar]; (c) Liu CY, Guo CH, Chang YF, Wang JT, Shih HW, Hsu YF, Chen CW, Chen SK, Wang YC, Cheng TJR, Ma C, Wong CH, Fang JM, Cheng WC. Org Lett. 2010;12:1608–1611. doi: 10.1021/ol100338v. [DOI] [PubMed] [Google Scholar]
- 5.(a) Schwartz B, Markwalder JA, Wang Y. J Am Chem Soc. 2001;123:11638–11643. doi: 10.1021/ja0166848. [DOI] [PubMed] [Google Scholar]; (b) Schwartz B, Markwalder JA, Seitz SP, Wang Y, Stein RL. J Am Chem Soc. 2002;41:12552–12561. doi: 10.1021/bi026205x. [DOI] [PubMed] [Google Scholar]
- 6.(a) Moffatt JG, Khorana HG. J Am Chem Soc. 1961;83:649–658. [Google Scholar]; (b) Roseman S, Distler JJ, Moffatt JG, Khorana HG. J Am Chem Soc. 1961;83:659–663. [Google Scholar]
- 7.(a) Cramer F, Schaller H, Staab HA. Chem Ber. 1961;94:1612–1621. [Google Scholar]; (b) Schaller H, Staab HA, Cramer F. Chem Ber. 1961;94:1621–1633. [Google Scholar]
- 8.Wittmann V, Wong CH. J Org Chem. 1997;62:2144–2147. doi: 10.1021/jo9620066. [DOI] [PubMed] [Google Scholar]
- 9.(a) Kadokura M, Wada T, Urashima C, Skein M. Tetrahedron Lett. 1997;38:8359–8362. [Google Scholar]; (b) Khaled A, Piotrowska O, Dominiak K, Augé C. Carbohydr Res. 2008;343:167–178. doi: 10.1016/j.carres.2007.11.009. [DOI] [PubMed] [Google Scholar]; (c) Chu BCF, Orgel LE. Biochim Biophys Acta. 1984;782:103–105. doi: 10.1016/0167-4781(84)90111-8. [DOI] [PubMed] [Google Scholar]; (d) Kanavarioti A, Bernasconi CF, Doodokyan DL, Alberas DJ. J Am Chem Soc. 1989;111:7247–7257. doi: 10.1021/ja00200a053. [DOI] [PubMed] [Google Scholar]; (e) Tsuruta O, Yuasa H, Hashimoto H, Sujino K, Otter A, Li H, Palcic MM. J Org Chem. 2003;68:6400–6406. doi: 10.1021/jo0300035. [DOI] [PubMed] [Google Scholar]; (f) Izumi M, Kaneko S, Yuasa H, Hashimoto H. Org Biomol Chem. 2006;4:681–690. doi: 10.1039/b513897c. [DOI] [PubMed] [Google Scholar]; (g) Shimazu M, Shinozuka K, Sawai H. Tetrahedron Lett. 1990;31:235–238. [Google Scholar]; (h) Sawai H, Wakai H, Shimazu M. Tetrahedron Lett. 1991;32:6905–6906. [Google Scholar]; (i) Lee J, Churchil H, Choi WB, Lynch JE, Roberts FE, Volante RP, Reider PJ. Chem Commun. 1999:729–730. [Google Scholar]; (j) Collier A, Wagner GK. Chem Commun. 2008:178–180. doi: 10.1039/b714379f. [DOI] [PubMed] [Google Scholar]
- 10.Our group employed SnCl2 catalyst for Lipid IV synthesis in reference 3a, but its reproducibility turned out to be poor.
- 11.Bouhss A, Trunkfield AE, Bugg TDH, Mengin-Lecreulx DFEMS. Microbiol Rev. 2008;32:208–233. doi: 10.1111/j.1574-6976.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- 12.Kurosu M, Mahapatra S, Narayanasamy P, Crick DC. Tetrahedron Lett. 2007;48:799–803. [Google Scholar]
- 13.Removal of MeOH and DMF from 7 and counter cation exchange of 8a, b with a trialkylamine are omitted in our method.
- 14.(a) Kunishima M, Kawachi C, Morita J, Terao K, Iwasaki F, Tani S. Tetrahedron. 1999;55:13159–13170. [Google Scholar]; (b) Kunishima M, Kawachi C, Hioki K, Terao K, Tani S. Tetrahedron. 2001;57:1551–1558. [Google Scholar]; (c) Kunishima M, Kitano A, Kawachi C, Watanabe Y, Iguchi S, Hioki K, Tani S. Chem Pharm Bull. 2002;50:549–550. doi: 10.1248/cpb.50.549. [DOI] [PubMed] [Google Scholar]; (d) Mori T, Miyagi M, Suzuki K, Shibasaki M, Saikawa Y, Nakata M. Heterocycles. 2007;72:275–291. [Google Scholar]
- 15.Wong’s group reported that HBTU worked well for a condensation between protecting group-free UDP-MurNAc and protected pentapeptide: Liu H, Sadamoto R, Sears PS, Wong CH. J Am Chem Soc. 2001;123:9616–9617. doi: 10.1021/ja011708w.However, in our case, HBTU and HATU afforded six-membered lactone and lactam at 4-OH and 2-NHAc along with the desired products, respectively.
- 16.(a) Hang HC, Yu C, Pratt MR, Bertozzi CR. J Am Chem Soc. 2004;126:6–7. doi: 10.1021/ja037692m. [DOI] [PubMed] [Google Scholar]; (b) Hang HC, Yu C, Kato DL, Bertozzi CR. Proc Natl Acad Sci USA. 2003;100:14846–14851. doi: 10.1073/pnas.2335201100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.This reaction proceeded faster in pyridine than DMF as reported in the following paper: Moffatt JG, Khorana HG. J Am Chem Soc. 1958;80:3756–3761.
- 18.In contrast to tetrazole catalyst, NMI·HCl promoted the reaction in pyridine as well as DMF.
- 19.In the case of 7, a new resonance at δ −12.4 ppm from a solution of 7 (−11.3 ppm) in d7-DMF was observed in 30 min after addition of NMI·HCl (integral ratio of 7 to the new signal = ca. 5 to 1).
- 20.(a) Bogachev VS. Russ J Bioorg Chem. 1996;22:599–604. [Google Scholar]; (b) Marlow AL, Kiessling LL. Org Lett. 2001;3:2517–2519. doi: 10.1021/ol016170d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.