Abstract
FK506 binding protein 51 (FKBP51, also called FKBP5) belongs to a family of immunophilins, FK506 binding proteins (FKBPs). FKBP family members are targets for drugs such as rapamycin. Although FKBP51 shares characteristics with other FKBPs, it also has unique features, especially the role in its regulation of important signaling pathways such as the AKT kinase/protein kinase B pathway. In this review, we will focus on the function of FKBP51 as a scaffolding protein in the regulation of AKT activation and, in turn, its role in tumorigenesis and response to chemotherapy.
Keywords: AKT, AKT phosphorylation, FKBP51, Scaffolding protein, chemoresistance, chemosensitivity
Introduction
Despite the development of targeted therapy, chemotherapy remains the front line therapy for a wide variety of cancers. However, resistance to chemotherapy is a major hurdle for successful cancer therapy. Individuals have different genetic backgrouds that contribute to variation in response to chemotherapy. In addition, tumors could develop resistance to a specific drug or classes of drugs through different mechanisms. Therefore, understanding mechanisms responsible for chemoresistance will help develop strategies to sensitize cancer cells to chemotherapy. A hallmark of cellular transformation is the aberrant promotion of cell survival pathways by stimuli activating oncogenic pathways and/or evading apoptotic pathways [1]. AKT (also known as PKB) is a critical survival kinase that plays an important role in tumorogenesis and chemoresistance. AKT activity is tightly regulated at many levels, including a balance between phosphorylation and dephosphorylation status at two sites, Ser473 and Thr308. Several proteins have been studied extensively with regard to their roles in regulation of the phosporylation of these two sites [2, 3]. Recent evidence indicates that the immunophilin FKBP51 can regulate AKT phosphorylation through a scaffolding mechanism and, as a result, can influence response to a variety of antineoplastic agents [4]. This finding adds another layer of regulation of AKT activity. Therefore, FKBP51 could be a future biomarker for tumor development and chemoresistance.
FKBP51
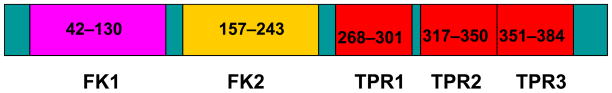
FK506 binding protein 51 (FKBP51, also called FKBP5) is a 51-kDa FK506 binding protein that is a member of a family of immunophilins, FK506 binding proteins (FKBPs). Human FKBP51 was first cloned from a HeLa cell cDNA library in 1995 [5]. FKBP51 contains two consecutive FKBP domains and a three-unit repeat of the TPR domain (Figure 1). The first FKBP domain (FK1) of FKBP51 shares 48% sequence identity to the FK domain of FKBP12 and has measurable PPIase activity, with a binding pocket for FK506 and rapamycin. The second FKBP domain (FK2) is also structurally similar to the FKBP domain of FKBP12, despite having only 26% sequence identity. However, FK2 lacks measurable PPIase activity. Presumably, FK2 resulted from an FK domain duplication event, but subsequently lost its PPIase activity. However, it appears to have gained protein interaction ability. There appear to be cooperative protein–protein interactions involving TPR and FK2 rather than the active PPIase FK1. The FKBP51 structure provides important initial insights into the relative orientations of the FK1, FK2, and TPR domains, motifs that are important for protein interaction and/or drug ligand binding [6]. One of the major functions of FKBP51 is its involvement in the modulation of steroid receptor function, including progesterone, androgen, and glucocorticoid receptors, by forming a complex with the heat shock proteins Hsp90/Hsp70 [6–9]. However, recent evidence suggested that FKBP51 has functions extending beyond its role in steroid receptor signaling to include tumorigenesis and chemoresistance [10, 11]. One recent discovery has focused our attention on the role of FKBP51 in the regulation of an important pathway, the AKT kinase/protein kinase B pathway [4, 12].
Figure 1.
Structure of FKBP51 with its major domains that are critical for its function and binding to other proteins.
AKT kinase/protein kinase B pathway
The Akt gene is the cellular homology of the v-akt oncogene transduced by AKT8, an acute transforming retrovirus in mice [13]. Since the cloning and characterization of three human AKT isoforms, many exciting breakthroughs have elucidated the AKT regulation and downstream signaling pathway of AKT [2, 3]. All three AKT isoforms (AKT1, 2 and 3) share similar structures, including an N-terminal regulatory domain; a pleckstrin homology (PH) domain; a hinge region connecting the PH domain to a kinase domain with serine/threonine specificity; and a C-terminal region necessary for kinase activity [14]. The AKT kinase/protein kinase B pathway is constitutively activated in many types of cancer and, thus, plays a major role in tumorigenesis. This pathway is an attractive therapeutic target in cancer because it triggers many biological events, from cell growth and proliferation to survival and migration, which contribute to the initiation and maintenance of cancer [15]. Because of the importance of this pathway, AKT activity is tightly regulated (Figure 2). Normally, AKT is activated in a growth-factor-dependent manner, with the most important upstream activators of AKT being the class I phosphoinositide 3-kinases (PI3Ks), which generate the lipid second messenger phosphatidylinositol-3,4,5-trisphosphate (PIP3). PIP3 binds directly to the PH domain of AKT, thereby recruiting AKT to the plasma membrane where it is activated [2]. Full activation of AKT requires phosphorylation at Thr308 within the activation loop of the kinase domain and at Ser473 in a hydrophobic motif just C-terminal to the kinase domain. Thr308 is phosphorylated by PDK1, a kinase that also has a PH domain directing PIP3 binding, while Ser473 is phosphorylated by mTOR complex 2 (mTORC2) [16–18]. Although Thr308 is essential for AKT kinase activity and Ser473 is not, Ser473 plays a key role in stabilizing and maintaining Thr308 phosphorylation, thus the overall strength and duration of AKT signaling [19]. AKT activity is also negatively regulated by lipids or protein phosphatases. PTEN is a lipid phosphatase that dephosphorylates PIP3, thereby inhibiting AKT activation.[20] Recently, two protein phosphatases have been shown to dephosphorylate AKT and negatively regulate AKT activity. PP2A holoenzymes dephosphorylate Ser308 [21], while PHLPP1 and PHLPP2 specifically dephosphorylate Ser473 [22–24]. Therefore, the balance between the actions of kinases and phosphatases determines AKT activity. Misregulation of the AKT pathway can result in AKT constitutive activation and cancer predisposition. Mechanisms known to be involved in the misregulation of this pathway include constitutive activation of growth factor receptors (EGFR, HER2, c-Met) and other common oncogene products like fusion proteins (BCR-Abl), leading to constitutive activation of PI3K; PI3K mutations; loss of tumor suppressor PTEN function; and amplification or aberrant activation of AKT [15, 25–27].
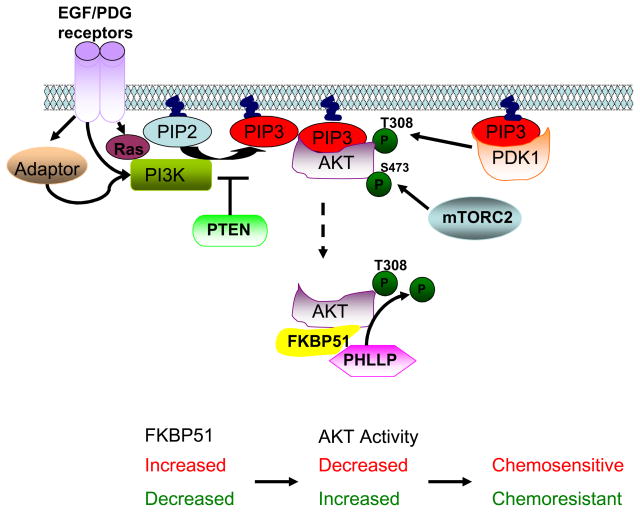
Figure 2. Model of FKBP51 affecting AKT phosphorylation and AKT activation.
One of the early events in AKT activation is the recruitment of PIP3 to the cellular membrane. PI3K regulates PIP3 levels by converting PIP2 to PIP3, and this process is inhibited by PTEN. PI3K is activated by growth factor signaling through both Ras and receptor kinase signaling. AKT becomes fully activated by phosphorylation at two sites, S473 and T308. Phosphorylation of S473 is primarily mediated by mTORC2 and this site is dephosphorylated by PHLLP phosphatase. FKBP51 functions as a scaffolding protein to bring PHLLP closer to the AKT 473 site and to facilitate the dephosphorylation of S473, which in turn down regulates AKT signaling. Through their influence on AKT phosphorylation, FKBP51 cellular levels affect sensitivity to chemotherapy. Lower FKBP51 resulted in AKT activation and resistance to chemotherapy, while increased FKBP51 levels reduced AKT activity and increased chemosensitivity.
FKBP51 negatively regulates the AKT pathway
Scaffolding proteins have been shown to play an important role in signal transduction. We have recently identified the immunophilin FKBP51 (also known as FKBP5) as a scaffolding protein that can enhance PHLPP-AKT interaction and facilitate PHLPP-mediated dephosphorylation of AKT-Ser473 (Figure 2) [4]. The FKBP51 C-terminal containing the TPR domain binds to the phospatase, PHLPP, while the N-terminal containing FKBP domain is responsible for AKT binding. Deletion of the N terminal or C-terminal significantly impairs the interaction between AKT and PHLPP. AKT has three isoforms (AKT1, 2 and 3) and PHLPP has two isoforms (PHLPP1 and 2) [3, 14, 22, 23]. It is known that PHLPP1 specifically regulates AKT 2 and 3, and PHLPP2 regulates AKT1 and 3 [23]. However, overexpression or downregulation of FKBP51 alters the interactions between both PHLPP isoforms with their corresponding AKT isoforms, suggesting that FKBP51 facilitates isoform-specific interaction between AKT and PHLPP. The FKBP activity is not required for the interaction. The functional consequence of this interaction results in the regulation of level of AKT phosphorylation and AKT activity. Downregulation of FKBP51 results in decreased PHLPP-AKT interaction and increased AKT phosphorylation at the Ser473 site. Therefore, FKBP51 may also function as a tumor suppressor in the AKT signaling pathway, similar to PTEN. Indeed, FKBP51 levels are low or lost in pancreatic cancer cell lines and patient samples of pancreatic cancer, correlating with increased AKT Ser473 phosphorylation and downstream genes of the AKT pathway such as phosphorylated FOXO1 and GSK3β. In addition, the downregulation of FKBP51 was also observed in breast cancer cell lines. However, whether FKBP51 is downregulated in other types of cancers and whether changes in FKBP51 levels correlate with AKT phosphorylation in other type of cancers remain to be determined. In addition, whether FKBP51 truly functions as a tumor suppressor remains to be determined. Further studies using FKBP51-knock out mice should reveal the role of FKBP51 in tumor suppression. In addition to driving tumor growth, AKT hyperactivation also renders tumors resistant to chemotherapy [28]. Through its effects on AKT phosphorylation, FKBP51 regulates sensitivity to a variety of anti-neoplastic drugs, including gemcitabine, AraC, and taxol. High levels of FKBP51 lead to decreased AKT phosphorylation and increased chemosensitivity, whereas low levels of FKBP51 result in increased AKT phosphorylation and decreased chemosensitivity. Pancreatic cancer cells expressing low level of FKBP51 are sensitized to AraC once they overexpress FKBP51 [4]. These facts make FKBP51 a potentially important biomarker for sensitivity to chemotherapy, and variation in FKBP51 levels might determine patients’ response to chemotherapy.
Future directions and conclusions
The identification of the scaffolding function of FKBP51 in the regulation of the AKT pathway has introduced a new component contributing to the complex molecular mechanisms of AKT activation. However, several questions are raised based on these observations. How is FKBP51 regulated, and are there any specific signals that affect the putative scaffolding function? Is FKBP51 a tumor suppressor? This will requires a future mouse model to confirm. With regard to downstream events, are there any downstream signals more dependent on Ser 473 activation? Obviously, the level of FKBP51 is another major determinant of the activation of this pathway. Based on studies using lymphoblastoid cell lines from 300 individuals, large variations in FKBP51 expression were observed and those variations correlated with cellular sensitivity to gemcitabine and AraC [29]. This observation also raised the question of whether variation in common genetic variation or somatic mutations might be responsible for variation in FKBP51 levels. Future studies with the application of Next Generation DNA sequencing methods would help reveal the potential influence of common or rare genetic variation on the regulation of FKBP51 levels.
In summary, the identification of the novel scaffolding function of FKBP51 in regulating the AKT pathway suggests that FKBP51 could be a potential biomarker for predicting both tumorigenesis and chemoresistance. Obviously, future clinical trials to assess this hypothesis are warranted. In addition, many newly developed inhibitors targeting signaling molecules in this pathway, including mTOR inhibitors, inhibitors of PI3K and AKT have already demonstrated clinical efficacy on different tumors [30–33]. Therefore, whether the addition of inhibitors of the PI3K-AKT pathway would help reverse chemoresistance for patients with low FKBP51 levels in tumors needs to be tested both in vitro, in animal models as well as in clinical trial. Obviously, tumors are highly mutated in many of the genes within this pathway. How mutations in other genes within this pathway might influence the effect of FKBP51 on response to therapy also needs to be explored. If indeed, levels of FKBP51 show effects on treatment outcomes in vivo, it could be used as a biomarker in combinations with other markers to stratify patients into different treatment regimen. Given the importance of the AKT pathway in many disease pathophysiology process, such as insulin response and diabetes [34–36], it would be also interesting to determine whether individual variation in FKBP51levels might affect insulin response or other AKT related disease processes.
Acknowledgments
K22 CA130828, R01 CA138461, U19 GM61388 (The Pharmacogenetics Research Network), ASPET-Astellas Award and a PhRMA Foundation “Center of Excellence in Clinical Pharmacology” Award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1**.Hanahan Douglas W, Robert A. Hallmarks of Cancer: The Next Generation. Cell. 2011;144(5) doi: 10.1016/j.cell.2011.02.013. This is the most recent review of cancer biology by Weinberg, which gives a very thorough overview of the development that occurred during the last ten years in cancer research. [DOI] [PubMed] [Google Scholar]
- 2.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 3.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129(7):1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4**.Pei H, et al. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16(3):259–66. doi: 10.1016/j.ccr.2009.07.016. This is the first report of the role of FKBP51 in regulation of AKT pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baughman G, et al. FKBP51, a novel T-cell-specific immunophilin capable of calcineurin inhibition. Mol Cell Biol. 1995;15(8):4395–402. doi: 10.1128/mcb.15.8.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sinars CR, et al. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 2003;100(3):868–73. doi: 10.1073/pnas.0231020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jinwal UK, et al. The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules. J Neurosci. 30(2):591–9. doi: 10.1523/JNEUROSCI.4815-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni L, et al. FKBP51 promotes assembly of the Hsp90 chaperone complex and regulates androgen receptor signaling in prostate cancer cells. Mol Cell Biol. 30(5):1243–53. doi: 10.1128/MCB.01891-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang CB, et al. FKBP family proteins: immunophilins with versatile biological functions. Neurosignals. 2008;16(4):318–25. doi: 10.1159/000123041. [DOI] [PubMed] [Google Scholar]
- 10.Romano S, et al. FK506 binding proteins as targets in anticancer therapy. Anticancer Agents Med Chem. 10(9):651–6. doi: 10.2174/187152010794479816. [DOI] [PubMed] [Google Scholar]
- 11*.Li L, Lou Z, Wang L. The role of FKBP5 in cancer aetiology and chemoresistance. Br J Cancer. 104(1):19–23. doi: 10.1038/sj.bjc.6606014. This review summarized in an easily understandable way of the current knowledge about FKBP51 in cancer aetiology and treatment. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pei H, Lou Z, Wang L. Emerging role of FKBP51 in AKT kinase/protein kinase B signaling. Cell Cycle. 9(1):6–7. doi: 10.4161/cc.9.1.10290. [DOI] [PubMed] [Google Scholar]
- 13.Staal SP, Hartley JW, Rowe WP. Isolation of transforming murine leukemia viruses from mice with a high incidence of spontaneous lymphoma. Proc Natl Acad Sci U S A. 1977;74(7):3065–7. doi: 10.1073/pnas.74.7.3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27(50):6473–88. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 15*.Liu P, et al. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8(8):627–44. doi: 10.1038/nrd2926. This review very nicely summarized the PI3K-AKT-mTOR pathway itself and its importance in cancer, as well as current drugs designed to target critical molecules in this pathway. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarbassov DD, et al. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307(5712):1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 17.Milon A, Miyazawa T, Higashijima T. Transferred nuclear Overhauser effect analyses of membrane-bound enkephalin analogues by 1H nuclear magnetic resonance: correlation between activities and membrane-bound conformations. Biochemistry. 1990;29(1):65–75. doi: 10.1021/bi00453a009. [DOI] [PubMed] [Google Scholar]
- 18.Alessi DR, et al. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr Biol. 1997;7(4):261–9. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 19.Yang J, et al. Crystal structure of an activated Akt/protein kinase B ternary complex with GSK3-peptide and AMP-PNP. Nat Struct Biol. 2002;9(12):940–4. doi: 10.1038/nsb870. [DOI] [PubMed] [Google Scholar]
- 20.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: of feedbacks and cross-talks. Oncogene. 2008;27(41):5527–41. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 21.Padmanabhan S, et al. A PP2A regulatory subunit regulates C. elegans insulin/IGF-1 signaling by modulating AKT-1 phosphorylation. Cell. 2009;136(5):939–51. doi: 10.1016/j.cell.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gao T, Furnari F, Newton AC. PHLPP: a phosphatase that directly dephosphorylates Akt, promotes apoptosis, and suppresses tumor growth. Mol Cell. 2005;18(1):13–24. doi: 10.1016/j.molcel.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 23.Brognard J, et al. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25(6):917–31. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 24.Gao T, Brognard J, Newton AC. The phosphatase PHLPP controls the cellular levels of protein kinase C. J Biol Chem. 2008;283(10):6300–11. doi: 10.1074/jbc.M707319200. [DOI] [PubMed] [Google Scholar]
- 25.Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuan TL, Cantley LC. PI3K pathway alterations in cancer: variations on a theme. Oncogene. 2008;27(41):5497–510. doi: 10.1038/onc.2008.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lemmon MA, Schlessinger J. Cell signaling by receptor tyrosine kinases. Cell. 141(7):1117–34. doi: 10.1016/j.cell.2010.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.West KA, Castillo SS, Dennis PA. Activation of the PI3K/Akt pathway and chemotherapeutic resistance. Drug Resist Updat. 2002;5(6):234–48. doi: 10.1016/s1368-7646(02)00120-6. [DOI] [PubMed] [Google Scholar]
- 29.Li LFB, Kalari K, Jenkins G, Batzler A, Safgren S, Hildebrandt M, Ames M, Schaid D, Wang L. Gemcitabine and cytosine arabinoside cytotoxicity:association with lymphoblastoid cell expression. Cancer Res. 2008:7050–8. doi: 10.1158/0008-5472.CAN-08-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bowles DW, Jimeno A. New phosphatidylinositol 3-kinase inhibitors for cancer. Expert Opin Investig Drugs. doi: 10.1517/13543784.2011.562192. [DOI] [PubMed] [Google Scholar]
- 31.Hartford CM, Ratain MJ. Rapamycin: something old, something new, sometimes borrowed and now renewed. Clin Pharmacol Ther. 2007;82(4):381–8. doi: 10.1038/sj.clpt.6100317. [DOI] [PubMed] [Google Scholar]
- 32.Falasca M. PI3K/Akt signalling pathway specific inhibitors: a novel strategy to sensitize cancer cells to anti-cancer drugs. Curr Pharm Des. 16(12):1410–6. doi: 10.2174/138161210791033950. [DOI] [PubMed] [Google Scholar]
- 33.Ghayad SE, Cohen PA. Inhibitors of the PI3K/Akt/mTOR pathway: new hope for breast cancer patients. Recent Pat Anticancer Drug Discov. 5(1):29–57. doi: 10.2174/157489210789702208. [DOI] [PubMed] [Google Scholar]
- 34.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 12(1):21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tian R. Another role for the celebrity: Akt and insulin resistance. Circ Res. 2005;96(2):139–40. doi: 10.1161/01.RES.0000156076.17807.1F. [DOI] [PubMed] [Google Scholar]
- 36.Vasudevan KM, Garraway LA. AKT signaling in physiology and disease. Curr Top Microbiol Immunol. 347:105–33. doi: 10.1007/82_2010_66. [DOI] [PubMed] [Google Scholar]