Abstract
Diabetes mellitus type 1 (DMT1) is an autoimmune disease characterized by the destruction of insulin-producing cells in the pancreas. Diabetic patients are more susceptible to recurrent and uncontrolled infections, with worse prognoses than in healthy individuals. Macrophages (Mϕs) derived from DMT1 individuals have compromised mounting of inflammatory and immune responses. The mechanisms responsible for these alterations remain unknown. It has been shown that the presence of extra- and intracellular heat shock proteins (hsp) positively modulates immune cell function. Using naive Mϕs derived from non-obese diabetic (NOD) mice, a well-established mouse model for DMT1, we demonstrate that heat shock (HS) as well as treatment with geldanamycin (GA), significantly improves diabetic Mϕ activation, resulting in increased phagocytosis and killing of bacteria. Induction of HS did not affect the aberrant NOD-Mϕ cytokine profile, which is characterized by elevated IL-10 levels and normal tumor necrosis factor alpha. Our observations were consistent at pre-diabetic (normal random blood glucose) and diabetic (random blood glucose greater than 250 mg/dl) stages, suggesting that HS and GA treatment may compensate for intrinsic genetic alterations present in diabetic cells regardless of the stage of the disease. The mechanisms associated to this phenomenon are unknown, but they may likely be associated with the induction of hsp expression, a common factor between HS and GA treatment. Our results may open a new field for non-classical function of hsp and indicate that hsp expression may be used as a part of therapeutic approaches for the treatment of complications associated with DMT1 as well as other autoimmune diseases.
Keywords: Bacteria, Internalization, Diabetes mellitus type 1, Glucose
Introduction
Heat shock protein (hsp) expression constitutes the most primitive mechanism for cellular protection. This function is accomplished due to the proteins’ intracellular chaperone activity and is responsible for the refolding of stress-denatured proteins and stabilization of transcription and translation (Lindquist and Craig 1988; Morimoto 1991; De Maio 1999). More recently, it has been shown that hsp also regulate other cellular activities such as cytoskeleton rearrangement (Han et al. 2000; Kirby et al. 1994; Liao et al. 1995; Dou et al. 2003; Lavoie et al. 1993; Piotrowicz and Levin 1997), endocytosis and phagocytosis (Vega et al. 2010; Vega and De Maio 2005), and activation of immune cells (Vega et al. 2008; Asea et al. 2000, 2002). Especially interesting is the capacity of both extra- and intracellular hsp to regulate the immune system by activation of key cells such as macrophages (Mϕs) and dendritic cells, thereby controlling inflammatory and immune responses (Vega et al. 2010; De Maio 2010; Asea et al. 2000, 2002; Basu et al. 2001). The mechanisms associated with this activation remain unknown, but it has been proposed that they are part of the stress response (SR). Thus, hsp-mediated immune activation may constitute a primary signal to activate and control the inflammatory process resulting in faster destruction of pathogens, clearance of necrotic and apoptotic cells (AC), activation of healing mechanisms, and homeostasis restoration. In agreement with this, induction of hsp in several clinical conditions, such as ischemia/reperfusion, infections, respiratory distress, hemorrhage, and diabetes, has been observed (Donnelly et al. 1992; De Maio 1999; Giffard et al. 2008; Villar et al. 1993). Among these conditions, diabetes and, more specifically, diabetes mellitus type 1 (DMT1) represents an interesting scenario for a potential modulatory effect of hsp, particularly since functional and developmental defects of professional phagocytes have been associated with the initiation and progression of this autoimmune disease (Bagley et al. 2008; Hutchings et al. 1990; Jun et al. 1999; Maree et al. 2005; Gordon and Taylor 2005).
pt?>In the USA alone, an estimated 2 million people have been diagnosed with DMT1, while worldwide, the incidence of this disease continues to increase rapidly (by 2–5% per year). DMT1 is the second most common chronic disease diagnosed in children. The only definitive treatment for DMT1 is a pancreatic transplant, which is only performed in patients who have advanced disease because it is a highly complicated and risky procedure. Similarly, there are no successful strategies for the treatment of the underlying inflammatory process responsible for the destruction of β cells in the pancreas as well as prevention of DMT1 development in susceptible individuals. Aggressive treatment of diabetes and factors contributing to diabetes can delay and possibly prevent the development of serious complications, such as heart disease, kidney failure, nerve damage, and recurrent infections. The current treatment for DMT1, which in the USA alone has reached a cost of $40 billion per year, involves subcutaneous administration of insulin with careful attendance to both exercise and nutrition. It is well accepted that the initiation and the progression of DMT1 is linked to intrinsic dysfunction in professional phagocytes (i.e., Mϕs), which results in a compromised mounting of immune and inflammatory responses (Fan et al. 2004; Shultz et al. 1995; O’Brien et al. 2006; Trudeau et al. 2000; Marée et al. 2008; Mohammad et al. 2006). For example, it has been shown that deficient internalization of AC by Mϕs derived from diabetic individuals is accompanied by decreased production of IL-10 and increased secretion of pro-inflammatory cytokines, generating an unusual pro-inflammatory environment in response to AC (O'Brien et al. 2006; Trudeau et al. 2000; Marée et al. 2008; Mohammad et al. 2006). On the contrary, incubation of DMT1-derived Mϕs with pro-inflammatory stimuli has been shown to produce unusually high levels of PGE2, which leads to down-regulation of cellular immune activation and therefore a decrease in inflammation (Benhamou et al. 1995). Clinically, DMT1 patients are more susceptible to the development of recurrent and more severe infections than non-diabetic individuals (Pozzilli and Leslie 1994). Inflammatory processes in DMT1 patients are the most common cause of glucose imbalance, hospitalization, limb amputation, wound healing delay, and/or potentially fatal consequences. Thus, priority has been given to the development of clinical or pharmacological interventions that improve regulation of inflammation in diabetic patients. In this study, we characterize the inflammatory and immune responses of naive Mϕs derived from non-obese diabetic (NOD) mice, a well-established animal model for DMT1, and the effect of heat shock (HS) and GA treatment on their response to pro-inflammatory stimuli.
Results and discussion
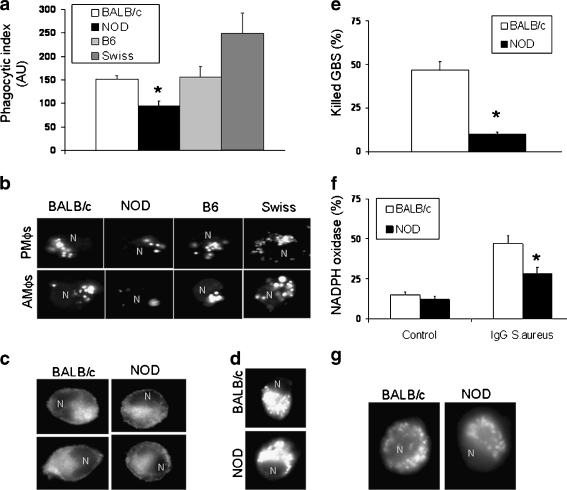
Mϕs derived from NOD mice have decreased phagocytic and killing activities in comparison with cells derived from non-diabetic strains Professional phagocytes, such as Mϕs, are widely distributed cells. Genetic and environmental factors that affect their responses have been linked to the pathogenesis of DMT1, although the exact mechanisms remain unknown (Fan et al. 2004; Shultz et al. 1995). It has been suggested that deficient internalization and/or clearance of AC by Mϕs may be linked to the development of autoimmunity (O’Brien et al. 2006; Trudeau et al. 2000; Marée et al. 2008; Mohammad et al. 2006). Both AC and pathogens are internalized by phagocytosis, although the cell surface receptors involved in these processes are different. Therefore, we hypothesize that the clinically reported high incidence of infections in diabetic patients is due to deficient clearance of pathogens, secondary to an impaired inflammatory response. To evaluate this possibility, we compared bacteria (Staphylococcus aureus and Escherichia coli) phagocytic capacity of two populations of naive Mϕs, alveolar (AMϕs) and peritoneal (PMϕs) Mϕs, derived from female (8 weeks old) non-obese diabetic (NOD/ShiLtJ) mice with cells isolated from non-diabetic strains (BALB/c, C57BL6 [B6] and Swiss mice). All mice were purchased from Jackson Laboratories (Bar Harbor, ME, USA) and kept at the UCSD animal facility for a week before performing experiments. The major component of diabetes susceptibility in NOD/ShiLtJ (NOD) mice is the unique MHC haplotype, H2g7 = Kd-Aad-Abg7-Enull-Db, that is linked to defects in antigen presenting processes, T-cell regulation, cytokine production, and wound healing (Fan et al. 2004). In brief, Mϕs were harvested by lavage and incubated in the presence of Fluorescein isothiocyanate (FITC)-conjugated IgG-opsonized S. aureus as previously described (Vega and De Maio 2005). Phagocytosis was quantified by fluorometry (Fig. 1a) or visualized by fluorescent microscopy (Fig. 1b). We observed that both AMϕs and PMϕs derived from NOD mice internalized significantly lower amounts of fluorescent IgG-opsonized S. aureus when compared with cells isolated from non-diabetic strains (Fig. 1a, b). It is important to remark that at 8 weeks of age, NOD female mice are at the pre-diabetic stage characterized by normal random blood glucose levels (≥250 mg/dl), with a body weight that does not differ from that observed in non-diabetic animals. On the contrary, these animals have altered glucose tolerance as well as insulinemia (Amrani et al. 1998). Spontaneous onset of DMT1 had been observed in 80% of NOD female mice (≥12 weeks old), which is characterized by elevated random blood glucose levels (≤250 mg/dl). Thus, our observations showed that Mϕs derived from female diabetic NOD mice (14 weeks old) also had a lower phagocytic capacity in comparison with cells isolated from BALB/c mice (35 and 101 AU, respectively), suggesting that decreased phagocytosis in NOD cells is independent of the blood glucose levels or the stage of the disease. Lower phagocytic capacity by NOD-derived cells was observed independently of the nature of the ligand or the presence or absence of opsonins. NOD-reduced phagocytosis was not due to a diminished expression of the FcγRs on the cell surface (Fig. 1c) or to a decreased binding capacity, suggesting that processes such as phagosome formation and/or maturation may be responsible for this NOD phenotype. Interestingly, we observed no differences in endocytosis of transferrin (clathrin-mediated endocytosis) (Fig. 1d) or cholera toxin (lipid-mediated endocytosis) (data not shown), strongly indicating the presence of specific alterations in the activation of the NOD phagocytic signal transduction pathway. Diminished phagocytosis of NOD-derived Mϕs is linked to a 5.3-fold lower killing capacity of live group B Streptococcus (GBS) (Fig. 1e) and a deficient production of superoxide anion by the NADPH oxidase (Fig. 1f). No difference in the amount of lysosomes (measured by Lysotraker-red staining) were observed between NOD and non-diabetic Mϕs (Fig. 1g), although we cannot discard the existence of differences in enzymatic activities that may be responsible for this diminished killing. In summary, our data shows that Mϕs derived from NOD mice have decreased (compromised) phagocytic and killing responses. These alterations may be responsible for the increased susceptibility to recurrent and more prolonged infections in diabetic patients. Similarly, it has been shown that NOD mice required more time to resolve experimentally induced inflammatory processes and infections (Rich and Lee 2005; Bouma et al. 2005). The precise mechanism(s) involved in this depressed response remains unclear. Maree et al. (2005, 2008) have suggested that a combination between a low rate of engulfment (phagosome formation) and diminished particle digestion are responsible for the decreased clearance of AC by diabetic Mϕs. Fan et al. (2006) linked deficient activation of small Rho GTPases, such as CdC42 and Rac, to altered cytoskeleton rearrangements in major murine models of spontaneous autoimmunity. In addition to these alterations, NOD mice show an impaired recruitment of professional phagocytes in response to local inflammation (Bouma et al. 2005), which is associated with a deficient production of local chemokines and an increased secretion of IL-10. Moreover, it has been shown that neutrophils derived from diabetic patients have decreased bactericidal activity, resulting in further delays in pathogen destruction and, therefore, delays in the tissue repair and wound healing processes (Delamaire et al. 1997; Naghibi et al. 1987).
Fig. 1.
NOD-derived Mϕs displayed decreased phagocytic and killing activities. Female NOD/ShiLtJ mice (8 weeks old) were at the pre-diabetic stage, measured as normal random blood glucose levels (≥250 mg/ml). No differences were observed in body weights between the strains. Peritoneal (PMϕs) and alveolar (AMϕs) macrophages were isolated by peritoneal or alveolar lavage and cultured for 16 h in RMPI1640 media (glucose level, 2,000 mg/L) supplemented with 10% FBS. Phagocytic assays were performed as described in Vega and De Maio (2005). In brief, cells (5 × 105 cells/well) were pre-incubated (30 min) with serum-free (SF) medium before the addition of FITC IgG-opsonized Staphylococcus aureus suspension (70 μg/ml, 60 min). Non-internalized bacteria were removed by washing with acidic PBS (pH 5.0). a Quantification of phagocytosis by fluorometry. Results were rectified by the number of live cells measured as MTT and expressed as arbitrary units (AU). n = 5 mice in each group, experiments were performed in triplicate. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Newman-Keuls test. *p < 0.01 with respect to non-diabetic strains. b Visualization of internalized FITC IgG-opsonized Staphylococcus aureus. Phagocytic assay was performed as described above then cells were fixed in 4% PFA, and visualization of internalized particles was performed using a fluorescent microscope. A representative experiment is presented. c Non-permeabilized cell surface immunostaining of FcγRs using a commercial FITC-conjugated antibody. d Endocytosis of AlexaFluor594-conjugated transferrin was performed as described by Vega et al. (2010). In brief, Mϕs (5 × 105 cells/well) were pre-incubated in SF medium (30 min) before adding AF594-transferrin (50 μg/ml, 30 min). Internalized signal was visualized by fluorescent microscopy. A representative experiment is presented. e Killing assay was performed by incubating (120 min at 37°C) PMϕs in the presence of live GBS with a multiplicity of infection (MOI) of 1 (one Mϕ per one GBS). Quantification of surviving bacteria was performed as described by Crotty Alexander et al. (2010), and results are expressed as percentage of killed bacteria. f Quantification of NADPH oxidase activation. The percentage of NADPH oxidase activity was estimated by incubating PMϕs with NBT in the presence or the absence of DPI, an inhibitor of NADPH oxidase as described previously (Vega and De Maio 2005). FITC IgG-opsonized Staphylococcus aureus (70 μg/ml) were incubated for 1.5 h after NBT incubation and NBT oxidation by superoxide anion was monitored at 540 nm and normalized by the protein content in each sample. The percentage of NADPH activation was calculated by subtracting the NBT plus DPI value from the NBT value. Results are expressed as the activity of NADPH oxidase (percentage of total superoxide anion production). n = 5 mice in each group, experiments were performed in triplicate. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Newman–Keuls test. *p < 0.01 with respect to non-diabetic strain. g Visualization of lysosomes labeled with Lysotraker-red (Invitrogen) in PMϕs derived from BALB/c and NOD mice. A representative experiment is presented
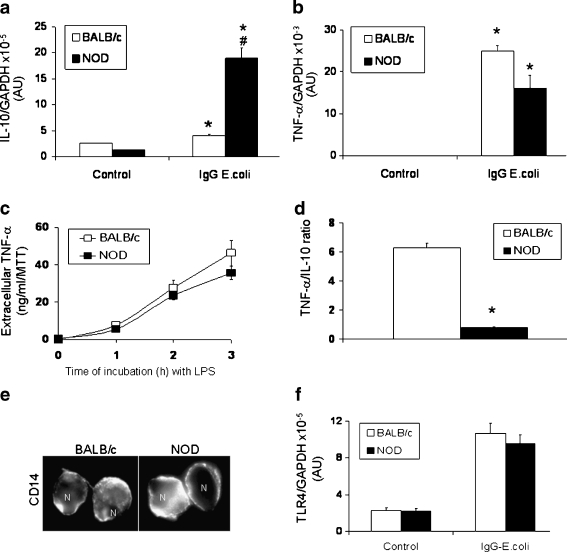
NOD-derived Mϕs express significantly higher levels of IL-10 in response to pro-inflammatory stimuli Previous reports have shown that in diabetic individuals there is a defective clearance of AC by Mϕs (Trudeau et al. 2000; Mathis et al. 2001). Moreover, in Mϕs derived from NOD mice, it has been reported that, together with the impaired phagocytosis of AC (O’Brien et al. 2006), an abnormal production of IL-12 (Rainbow et al. 2008), IL-21 (King et al. 2004), and IL-1β (Bouma et al. 2005) also occurs. It is important to remark that quantitative and kinetic balance of pro- and anti-inflammatory mediators contributes to restoring immunological homeostasis and determines the final outcome of biological processes (Brown et al. 2003). Thus, we investigated potential alterations in cytokine production by NOD Mϕs in response to pro-inflammatory stimuli such as bacteria particles or lipopolysaccharide (LPS). In brief, PMϕs derived from NOD and non-diabetic (BALB/c) mice were incubated in the presence and absence of LPS or FITC IgG-opsonized E. coli, and production of cytokines was evaluated by qRT-PCR and ELISA (Fig. 2). We observed that non-stimulated cells (control) have similar and extremely low levels of IL-10 and tumor necrosis factor alpha (TNF-α). FITC IgG-opsonized E. coli activation of NOD-derived PMϕs resulted in a significantly higher induction of IL-10 when compared to BALB/c-derived cells (Fig. 2a). Similar results were observed when PMϕs were incubated with LPS (100 ng/ml, 2 h) or zymozan (200 μg/ml, 2 h) (data not shown), suggesting that increased production of IL-10 is a common phenomenon after activation of CD14/TLR4 (by LPS), FcγR (by IgG-opsonized E. coli) or TLR2/TLR6 (by zymozan) pathways. No differences in the levels of these receptors (Figs. 1c and 2e, f) were observed between NOD and BALB/c derived cells, suggesting that another mechanism is responsible for the larger production of IL-10. Interestingly, activation of Mϕs with these pro-inflammatory ligands resulted in similar induction of TNF-α (Fig. 2b, c). Thus, calculated TNF-α/IL-10 ratios were significantly lower (7.8-fold) in NOD-derived Mϕs, suggesting a strong anti-inflammatory component in response to pro-inflammatory stimuli (Fig. 2d). It is important to remark that other non-diabetic strains, such as Swiss and DBA, have similar responses to those observed in BALB/c Mϕs. These data demonstrate abnormal regulation of the IL-10 pathway in NOD-derived Mϕs, which results in a large expression of this cytokine in response to pro-inflammatory stimuli. The mechanisms that control this unusual expression of IL-10 remain unknown and require further investigation. Excessive or mistimed IL-10 synthesis is linked to a down-regulation of the inflammatory response to the extent that pathogens escape immune control, resulting in chronic non-healing or recurrent infections. For example, transgenic mice that constitutively over-express IL-10 are highly susceptible to infections (Groux et al 1999; Feng et al. 2002), and elevated levels of IL-10 observed in several autoimmune diseases are linked to the occurrence of recurrent infections (Hedrich and Bream 2010; Ishida et al. 1994; Sanjabi et al. 2009). Although the association between IL-10 and autoimmunity is well documented, the mechanisms that explain how elevated levels of this cytokine promote autoimmunity are not fully understood. Years of investigation have suggested that the effects of IL-10 on B cell function play a critical role in the onset of autoimmunity by stabilizing auto-reactive clones, prolonging B cell survival, differentiation, proliferation, and antibody production (Lalani et al. 1997; Moore et al. 2001; te Velde et al. 1992; Levy and Brouet 1994; Llorente et al. 1995).
Fig. 2.
NOD-derived Mϕs respond to pro-inflammatory stimuli with a strong induction of IL-10. PMϕs (2.5 × 105 cells/well) derived from NOD or BALB/c mice (female, 8 weeks old) were pre-incubated in SF medium (30 min) and then IgG-opsonized Escherichia coli particles (70 μg/ml) were added (60 min). Total RNA was isolated using Trizol. cDNA was produced using random primers, and levels of IL-10 (a) and TNF-α (b) were measured by qRT-PCR. Results are expressed as number of copies of each cytokine messenger rectified by number of copies of GAPDH levels. c Detection of TNF-α in the extracellular medium by ELISA. PMϕs (5 × 106 cells/well) were incubated in complete medium in the presence of LPS (100 ng/ml) for 1, 2, and 3 h. Extracellular medium was collected. TNF-α levels were measured and rectified by MTT values in each well. d Calculated TNF-α/IL-10 ratios in response to IgG-conjugated Escherichia coli particles incubation. These ratios were calculated from data presented in a and b. e CD14 immunostaining using non-permeabilized PMϕs derived from NOD and BALB/c mice. Immunostaining was performed as described in Vega and De Maio (2003). f TLR4 expression measured by qRT-PCR. n = 5 mice in each group, experiments were performed in triplicate. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Newman–Keuls test. *p < 0.01, with respect to control BALB/c. #p < 0.01, with respect to control stimulated BALB/c
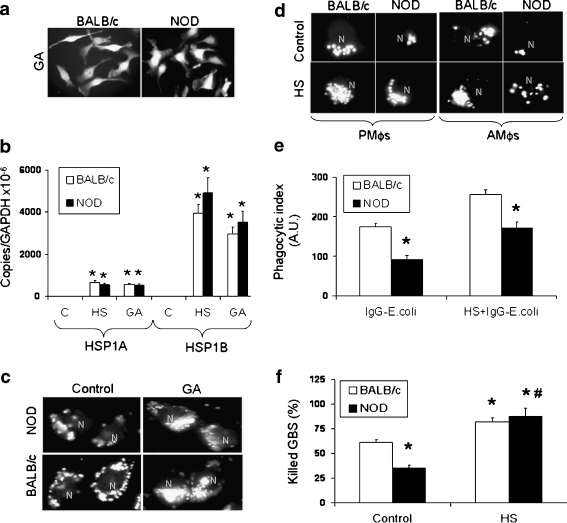
Expression of hsp reverts the NOD-phenotype by improving phagocytosis and killing activities We have previously reported that induction of hsp results in increased phagocytosis by J774 cells, which depends on the synthesis of new proteins (Vega and De Maio 2005). The mechanisms responsible for this increase remain unknown, although our investigations strongly suggest a critical role of Hsp70 (Vega et al. 2010). Thus, we decided to evaluate if the activation of the heat shock response (HSR) may compensate for the genetic or epigenetic alterations associated with the NOD phenotype. To accomplish this possibility, AMϕs and PMϕs derived from NOD and BALB/c mice were submitted to heat shock (HS) (42°C, 90 min) or incubated with geldanamycin (GA, 1 μg/ml), a specific Hsp90 inhibitor, as previously described (Vega and De Maio 2005). GA treatment activates the HSR by disrupting the Hsp90/heat shock factor-1 (HSF-1) complex. Then, trimerarized HSF-1 translocates to the nucleus and initiates hsp gene transcription. We observed that NOD and BALB/c derived Mϕs express comparable levels of Hsp70 in response to GA treatment (Fig. 3a) or to HS (Fig. 3b). Although both HSP1A and HSP1B are strongly up-regulated in comparison with control (resting) cells, a significantly higher induction of HSP1B was reported in response to HS as well as GA treatment (Fig. 3b). HSP1B expression has been implicated in the increased survival of FcγR-activated Mϕs (Smith et al. 2010) and its expression regulates the induction of pro- (IL-1β) and anti- (IL-10) inflammatory cytokines. Our data show that treatment with GA (Fig. 3c) as well as HS/recovery (Fig. 3d) results in significant increases in phagocytosis of FITC-conjugated IgG-opsonized S. aureus by both NOD and BALB/c derived Mϕs. HS-mediated phagocytic enhancement in non-diabetic cells is larger than that observed in NOD-Mϕs (Fig. 3e). Although it is important to remark that activation of HSR in heat-shocked NOD-Mϕs resulted in phagocytic indexes that are similar to those observed in non-heat-shocked (control) non-diabetic cells exposed to bacteria particles (Fig. 3e). These data demonstrate that NOD-Mϕs subjected to HS display similar phagocytic capacity to that observed in non-diabetic (control) cells. This HS-mediated improvement may result in significant improvements in the mounting of the inflammatory response in vivo in diabetic individuals. Moreover, HS also resulted in a significant increase in the killing capacity of PMϕs derived from NOD mice, to levels that did not differ from those reached by BALB/c-derived heat-shocked PMϕs (Fig. 3f). The mechanisms associated with these observations are under investigation. Although we must highlight that HS did not prevent the NOD-associated increased expression of IL-10 or revert the abnormally low production of superoxide anion by the NADPH oxidase in response to pro-inflammatory ligands, suggesting that activation of other pathways are responsible for the modification of NOD phenotype by HS. Unpublished data from our laboratory strongly suggests that HS improves the killing capacity of PMϕs by up-regulating the expression of antimicrobial peptides and iNOS (Vega and Crotty Alexander, unpublished data). The mechanisms by which hsp expression modulates the inflammatory response of professional phagocytes may also be related to the control of vesicle trafficking and/or cytoskeleton rearrangements. Previous reports have shown F-actin formation after HS (Vega and De Maio 2005; Collier and Schlesinger 1986; Han et al. 2000) as well as a close association of Hsp70 with actin-filaments, microtubules, tubulin, and intermediate filaments (Kirby et al. 1994; Liao et al. 1995; Dou et al. 2003). Small hsp, in particular Hsp27, have also been implicated in the regulation of the cytoskeleton by participating in F-actin formation around plasma membrane rufflings and pinocytosis (Lavoie et al. 1993) and by inducing stabilization of actin filaments (Piotrowicz and Levin 1997). Another potential explanation may be related to the capacity of hsp, more specifically Hsp70, to interact with membranes (Vega et al. 2008; Arispe et al. 2002, 2004; Multhoff et al. 1995), which may provide a modulatory effect over key processes such as vesicle formation, fusion, and/or recycling, as well as membrane fluidity. These changes may control phagosome movements, assembling of protein complexes (i.e., receptors) on the membrane as well as targeting key proteins to required cellular compartments. Finally, we cannot discard the contribution of the classic cytoprotective role of hsp, as these proteins may play a critical role during Mϕ activation by stabilizing cell structures (i.e., phagosomes and lysosomes) and therefore allowing clearance and destruction of pathogens and/or damaged cells (Morimoto 1991; Xu and Wick 1996), as well as protecting key newly synthesized pro- and anti-inflammatory polypeptides from damage.
Fig. 3.
Increased phagocytosis and bactericidal activity are observed in NOD-derived Mϕs after hsp expression. Heat shock (HS) and treatment with GA were performed as described by Vega and De Maio (2005) in Mϕs-derived from female BALB/c and NOD female mice (8 weeks old). a Naive PMϕs (5 × 105 cells/slide) were isolated as previously described in Fig. 1 and incubated with GA (1 μg/ml, 6 h). Cells were fixed in 4% PFA and permeabilized with cold acetone. Immunostaining for Hsp70 was performed using a polyclonal commercial antibody. No Hsp70 expression was detected by immunostaining when control cells (untreated with GA) were used. b PMϕs (5 × 105 cells) were subjected to HS (42°C, 90 min) in SF medium and allowed to recover at 37°C for 3 h. Total mRNA was isolated as described in Fig. 2 and qRT-PCR was performed using commercial probes for mouse HSP1A and HSP1B. Phagocytic assay was performed using PMϕs and AMϕs as described in Fig. 1 using FITC-conjugated IgG-opsonized Staphylococcus aureus particles. Cells were either incubated in the presence or absence of GA (1 μg/ml, 3 h) (c) or subjected or not to HS (d) and then visualization of internalized FITC-conjugated IgG-opsonized Staphylococcus aureus was performed by fluorescent microscopy. (e) Phagocytic indexes were measured by fluorometry as described in Fig. 1 using PMϕs derived from NOD and BALB/c mice (f) PMϕs were subjected to HS and allowed to recover for 24 h, and then cells were incubated with live GBS at an MOI of 1 (3 h at 37°C). Surviving bacteria colonies were enumerated as described in Fig. 1. Results are expressed as percentage of killed bacteria. n = 5 mice in each group, experiments were performed in triplicate. Statistical analysis was performed by one-way analysis of variance (ANOVA) followed by Newman–Keuls test. *p < 0.01 with respect to BALB/c control (non-heat shocked) cells. #p < 0.01 with respect to NOD control (non-heat shocked) cells
Conclusions
Our data show the capacity of the HSR to up-regulate phagocytosis and killing activities in both diabetic and non-diabetic cells. We propose that these increases are part of the stress response, whose final goal is to eliminate necrotic cells, cell debris, and infectious agents with minimal tissue damage. In DMT1 individuals, as well as other patients with compromised function of professional phagocytes, activation of HSR may compensate for genetic or epigenetic dysfunction in professional phagocytes resulting in significant improvements in their response to injury or to infections.
Acknowledgments
Authors would like to thank Dr. Antonio De Maio (UCSD) and Dr. Victor Nizet (UCSD) for their support and mentoring. We also thank Molly Wofford for editing this manuscript. This work was supported by an University of California Academic Senate research grant (SUR127H) and by Dr. Crotty Alexander’s Biomedical Research Fellowship from The Hartwell Foundation.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s12192-011-0273-7
References
- Amrani A, Durant S, Throsby M, Coulaud J, Dardenne M, Homo-Delarche F. Glucose homeostasis in the nonobese diabetic mouse at the prediabetic stage. Endocrinology. 1998;139(3):1115–1124. doi: 10.1210/en.139.3.1115. [DOI] [PubMed] [Google Scholar]
- Arispe N, Doh M, Maio A. Lipid interaction differentiates the constitutive and stress-induced heat shock proteins Hsc70 and Hsp70. Cell Stress Chaperones. 2002;7(4):330–338. doi: 10.1379/1466-1268(2002)007<0330:LIDTCA>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arispe N, Doh M, Simakova O, Kurganov B, Maio A. Hsc70 and Hsp70 interact with phosphatidylserine on the surface of PC12 cells resulting in a decrease of viability. FASEB J. 2004;18(14):1636–1645. doi: 10.1096/fj.04-2088com. [DOI] [PubMed] [Google Scholar]
- Asea A, Kabingu E, Stevenson MA, Calderwood SK. HSP70 peptidembearing and peptide-negative preparations act as chaperokines. Cell Stress Chaperones. 2000;5(5):425–431. doi: 10.1379/1466-1268(2000)005<0425:HPBAPN>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asea A, Rehli M, Kabingu E, Boch JA, Bare O, Auron PE, Stevenson MA, Calderwood SK. Novel signal transduction pathway utilized by extracellular HSP70: role of toll-like receptor (TLR) 2 and TLR4. J Biol Chem. 2002;277(17):15028–15034. doi: 10.1074/jbc.M200497200. [DOI] [PubMed] [Google Scholar]
- Bagley J, Tian C, Iacomini J. Prevention of type 1diabetes in NOD mice by genetic engineering of hematopoietic stem cells. Methods Mol Biol. 2008;433:277–285. doi: 10.1007/978-1-59745-237-3_17. [DOI] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14(3):303–313. doi: 10.1016/S1074-7613(01)00111-X. [DOI] [PubMed] [Google Scholar]
- Benhamou PY, Mullen Y, Clare-Salzler M, Sangkharat A, Benhamou C, Shevlin L, Go VL. Essential fatty acid deficiency prevents autoimmune diabetes in nonobese diabetic mice through a positive impact on antigen-presenting cells and Th2 lymphocytes. Pancreas. 1995;11(1):26–37. doi: 10.1097/00006676-199507000-00003. [DOI] [PubMed] [Google Scholar]
- Bouma G, Nikolic T, Coppens JM, Helden-Meeuwsen CG, Leenen PJ, Drexhage HA, Sozzani S, Versnel MA. NOD mice have a severely impaired ability to recruit leukocytes into sites of inflammation. Eur J Immunol. 2005;35(1):225–235. doi: 10.1002/eji.200425513. [DOI] [PubMed] [Google Scholar]
- Brown JR, Goldblatt D, Buddle J, Morton L, Thrasher AJ. Diminished production of anti-inflammatory mediators during neutrophil apoptosis and macrophage phagocytosis in chronic granulomatous disease (CGD) J Leukoc Biol. 2003;73:591–599. doi: 10.1189/jlb.1202599. [DOI] [PubMed] [Google Scholar]
- Collier NC, Schlesinger MJ. The dynamic state of heat shock proteins in chicken embryo fibroblasts. J Cell Biol. 1986;103:1495–1507. doi: 10.1083/jcb.103.4.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty-Alexander LE, Maisey HC, Timmer AM, Rooijakkers SHM, Gallo RL, Köckritz-Blickwede M, Nizet V. M1T1 group A streptococcal pili promote epithelial colonization but diminish systemic virulence through neutrophil extracellular entrapment. J Mol Med. 2010;88:371–881. doi: 10.1007/s00109-009-0566-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maio A. Heat shock proteins: facts, thoughts, and dreams. Shock. 1999;11(1):1–12. doi: 10.1097/00024382-199901000-00001. [DOI] [PubMed] [Google Scholar]
- Maio A. Extracellular heat shock proteins, cellular export vesicles, and the stress observation system: a form of communication during injury, infection, and cell damage: it is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones. 2010;101(2):294–301. doi: 10.1007/s12192-010-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamaire M, Maugendre D, Moreno M, Goff MC, Allannic H, Genetet B. Impaired leucocyte functions in diabetic patients. Diabet Med. 1997;14(1):29–34. doi: 10.1002/(SICI)1096-9136(199701)14:1<29::AID-DIA300>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Donnelly TJ, Sievers RE, Vissern FL, Welch WJ, Wolfe CL. Heat shock protein induction in rat hearts. A role for improved myocardial salvage after ischemia and reperfusion? Circulation. 1992;85(2):769–778. doi: 10.1161/01.cir.85.2.769. [DOI] [PubMed] [Google Scholar]
- Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H. Chaperones increase association of protein with microtubules. Proc Natl Acad Sci USA. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan H, Longacre A, Meng F, Patel V, Hsiao K, Koh JS, Levine JS. Cytokine dysregulation induced by apoptotic cells is a shared characteristic of macrophages from nonobese diabetic and systemic lupus erythematosus-prone mice. J Immunol. 2004;172(8):4834–4843. doi: 10.4049/jimmunol.172.8.4834. [DOI] [PubMed] [Google Scholar]
- Fan H, Patel VA, Longacre A, Levine JS. Abnormal regulation of the cytoskeletal regulator Rho typifies macrophages of the major murine models of spontaneous autoimmunity. J Leukoc Biol. 2006;79(1):155–165. doi: 10.1189/jlb.0705408. [DOI] [PubMed] [Google Scholar]
- Feng CG, Kullberg MC, Jankovic D, Cheever AW, Caspar P, Coffman RL. Transgenic mice expressing human interleukin-10 in the antigen-presenting cell compartment show increased susceptibility to infection with Mycobacterium avium associated with decreased macrophage effector function and apoptosis. Infect Immun. 2002;70:6672–6679. doi: 10.1128/IAI.70.12.6672-6679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giffard RG, Han RQ, Emery JF, Duan M, Pittet JF. Regulation of apoptotic and inflammatory cell signaling in cerebral ischemia: the complex roles of heat shock protein 70. Anesthesiology. 2008;109(2):339–348. doi: 10.1097/ALN.0b013e31817f4ce0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- Groux H, Cottrez F, Rouleau M, Mauze S, Antonenko S, Hurst S. A transgenic model to analyze the immunoregulatory role of IL-10 secreted by antigen-presenting cells. J Immunol. 1999;162:1723–1729. [PubMed] [Google Scholar]
- Han SI, Ha KS, Kang KI, Kim HD, Kang HS. Heat shock-induced actin polymerization, SAPK/JNK activation, and heat-shock protein expression are mediated by genistein-sensitive tyrosine kinase(s) in K562 cells. Cell Biol Int. 2000;24:447–457. doi: 10.1006/cbir.2000.0512. [DOI] [PubMed] [Google Scholar]
- Hedrich CM, Bream JH. Cell type-specific regulation of IL-10 expression in inflammation and disease. Immunol Res. 2010;47(1–3):185–206. doi: 10.1007/s12026-009-8150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchings P, Rosen H, O'Reilly L, Simpson E, Gordon S, Cooke A. Transfer of diabetes in mice prevented by blockade of adhesion-promoting receptor on macrophages. Nature. 1990;348(6302):639–642. doi: 10.1038/348639a0. [DOI] [PubMed] [Google Scholar]
- Ishida H, Muchamuel T, Sakaguchi S, Andrade S, Menon S, Howard M. Continuous administration of anti-interleukin 10 antibodies delays onset of autoimmunity in NZB/WF1 mice. J Exp Med. 1994;179(1):305–310. doi: 10.1084/jem.179.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun HS, Yoon CS, Zbytnuik L, Rooijen N, Yoon JW. The role of macrophages in T cell-mediated autoimmune diabetes in nonobese-diabetic mice. J Exp Med. 1999;189(2):347–358. doi: 10.1084/jem.189.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117(2):265–277. doi: 10.1016/S0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- Kirby BA, Merril CR, Ghanbari H, Wallace WC. Heat shock proteins protect against stress-related phosphorylation of tau in neuronal PC12 cells that have acquired thermotolerance. J Neurosci. 1994;14:5687–5693. doi: 10.1523/JNEUROSCI.14-09-05687.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalani I, Bhol K, Ahmed AR. Interleukin-10: biology, role in inflammation and autoimmunity. Ann Allergy Asthma Immunol. 1997;79:469–483. doi: 10.1016/S1081-1206(10)63052-9. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock: HSP27 stabilization of the microfilament organization. J Biol Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- Levy Y, Brouet JC. Interleukin-10 prevents spontaneous death of germinal center B cells by induction of the bcl-2 protein. J Clin Invest. 1994;93:424–428. doi: 10.1172/JCI116977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao J, Lowthert LA, Ghori N, Omary MB. The 70-kDa heat shock proteins associate with glandular intermediate filaments in an ATP-dependent manner. J Biol Chem. 1995;270:915–922. doi: 10.1074/jbc.270.2.915. [DOI] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Llorente L, Zou W, Levy Y. Role of interleukin 10 in the B lymphocyte hyperactivity and autoantibody production of human systemic lupus erythematosus. J Exp Med. 1995;181:839–844. doi: 10.1084/jem.181.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maree AF, Komba M, Dyck C, Labecki M, Finegood DT, Edelstein-Keshet L. Quantifying macrophage defects in type 1 diabetes. J Theor Biol. 2005;233(4):533–551. doi: 10.1016/j.jtbi.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Marée AF, Komba M, Finegood DT, Edelstein-Keshet L. A quantitative comparison of rates of phagocytosis and digestion of apoptotic cells by macrophages from normal (BALB/c) and diabetes-prone (NOD) mice. J Appl Physiol. 2008;104(1):157–169. doi: 10.1152/japplphysiol.00514.2007. [DOI] [PubMed] [Google Scholar]
- Mathis D, Vence L, Benoist C. Beta-cell death during progression to diabetes. Nature. 2001;414(6865):792–798. doi: 10.1038/414792a. [DOI] [PubMed] [Google Scholar]
- Mohammad MK, Morran M, Slotterbeck B, Leaman DW, Sun Y, Grafenstein H, Hong SC, McInerney MF. Dysregulated Toll-like receptor expression and signaling in bone marrow-derived macrophages at the onset of diabetes in the non-obese diabetic mouse. Int Immunol. 2006;18(7):1101–1113. doi: 10.1093/intimm/dxl045. [DOI] [PubMed] [Google Scholar]
- Moore KW, Waal Malefy TR, Coffman RL. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Morimoto RI. Heat shock: the role of transient inducible responses in cell damage, transformation, and differentiation. Cancer Cells. 1991;3(8):295–301. [PubMed] [Google Scholar]
- Multhoff G, Botzler C, Wiesnet M, Müller E, Meier T, Wilmanns W, Issels RD. A stress-inducible 72-kDa heat-shock protein (HSP72) is expressed on the surface of human tumor cells, but not on normal cells. Int J Cancer. 1995;61(2):272–279. doi: 10.1002/ijc.2910610222. [DOI] [PubMed] [Google Scholar]
- Naghibi M, Smith RP, Baltch AL, Gates SA, Wu DH, Hammer MC, Michelsen PB. The effect of diabetes mellitus on chemotactic and bactericidal activity of human polymorphonuclear leukocytes. Diabetes Res Clin Pract. 1987;4(1):27–35. doi: 10.1016/S0168-8227(87)80030-X. [DOI] [PubMed] [Google Scholar]
- O'Brien BA, Geng X, Orteu CH, Huang Y, Ghoreishi M, Zhang Y, Bush JA, Li G, Finegood DT, Dutz JP. A deficiency in the in vivo clearance of apoptotic cells is a feature of the NOD mouse. J Autoimmun. 2006;26(2):104–115. doi: 10.1016/j.jaut.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Piotrowicz RS, Levin EG. Basolateral membrane-associated 27-kDa heat shock protein and microfilament polymerization. J Biol Chem. 1997;272:25920–25927. doi: 10.1074/jbc.272.41.25920. [DOI] [PubMed] [Google Scholar]
- Pozzilli P, Leslie RD. Infections and diabetes: mechanisms and prospects for prevention. Diabet Med. 1994;11(10):935–941. doi: 10.1111/j.1464-5491.1994.tb00250.x. [DOI] [PubMed] [Google Scholar]
- Rainbow DB, Esposito L, Howlett SK, Hunter KM, Todd JA, Peterson LB, Wicker LS. Commonality in the genetic control of Type 1 diabetes in humans and NOD mice: variants of genes in the IL-2 pathway are associated with autoimmune diabetes in both species. Biochem Soc Trans. 2008;36(Pt 3):312–315. doi: 10.1042/BST0360312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich J, Lee JC. The pathogenesis of Staphylococcus aureus infection in the diabetic NOD mouse. Diabetes. 2005;54(10):2904–2910. doi: 10.2337/diabetes.54.10.2904. [DOI] [PubMed] [Google Scholar]
- Sanjabi S, Zenewicz LA, Kamanaka M, Flavell RA. Anti-inflammatory and pro-inflammatory roles of TGF-beta, IL-10, and IL-22 in immunity and autoimmunity. Curr Opin Pharmacol. 2009;9(4):447–453. doi: 10.1016/j.coph.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shultz LD, Schweitzer PA, Christianson SW, Gott B, Schweitzer IB, Tennent B, McKenna S, Mobraaten L, Rajan TV, Greiner DL. Multiple defects in innate and adaptive immunologic function in NOD/LtSz-scid mice. J Immunol. 1995;154(1):180–191. [PubMed] [Google Scholar]
- Smith KJ, Twal WO, Soodavar F, Virella G, Lopes-Virella MF, Hammad SM. Heat shock protein 70B' (HSP70B') expression and release in response to human oxidized low density lipoprotein immune complexes in macrophages. J Biol Chem. 2010;285(21):15985–15993. doi: 10.1074/jbc.M110.113605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velde AA, Waal Malefijt R, Huijbens RJ. IL-10 stimulates monocyte Fc gamma R surface expression and cytotoxic activity. Distinct regulation of antibody-dependent cellular cytotoxicity by IFN-gamma, IL-4, and IL-10. J Immunol. 1992;149:4048–4052. [PubMed] [Google Scholar]
- Trudeau JD, Dutz JP, Arany E, Hill DJ, Fieldus WE, Finegood DT. Neonatal beta-cell apoptosis: a trigger for autoimmune diabetes? Diabetes. 2000;49(1):1–7. doi: 10.2337/diabetes.49.1.1. [DOI] [PubMed] [Google Scholar]
- Vega VL, Maio A. Geldanamycin treatment ameliorates the response to LPS in murine macrophages by decreasing CD14 surface expression. Mol Biol Cell. 2003;14(2):764–773. doi: 10.1091/mbc.E02-08-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega VL, Maio A. Increase in phagocytosis after geldanamycin treatment or heat shock: role of heat shock proteins. J Immunol. 2005;175(8):5280–5287. doi: 10.4049/jimmunol.175.8.5280. [DOI] [PubMed] [Google Scholar]
- Vega VL, Rodríguez-Silva M, Frey T, Gehrmann M, Diaz JC, Steinem C, Multhoff G, Arispe N, Maio A. Hsp70 translocates into the plasma membrane after stress and is released into the extracellular environment in a membrane-associated form that activates macrophages. J Immunol. 2008;180(6):4299–4307. doi: 10.4049/jimmunol.180.6.4299. [DOI] [PubMed] [Google Scholar]
- Vega VL, Charles W, Maio A. A new feature of the stress response: increase in endocytosis mediated by Hsp70. Cell Stress Chaperones. 2010;15(5):517–527. doi: 10.1007/s12192-009-0165-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villar J, Edelson JD, Post M, Mullen JB, Slutsky AS. Induction of heat stress proteins is associated with decreased mortality in an animal model of acute lung injury. Am Rev Respir Dis. 1993;147(1):177–181. doi: 10.1164/ajrccm/147.1.177. [DOI] [PubMed] [Google Scholar]
- Xu Q, Wick G. The role of heat shock proteins in protection and pathophysiology of the arterial wall. Mol Med Today. 1996;2(9):372–379. doi: 10.1016/S1357-4310(96)10034-4. [DOI] [PubMed] [Google Scholar]