Abstract
Due to their adjuvant effect and their ability to chaperone tumor-associated peptides, heat shock proteins constitute a potent alarm signal for the immune system and can lead to activation of anti-tumor T-cell immunity. Radiofrequency ablation has been reported to induce heat shock protein expression especially that of heat shock protein 70 in sublethally damaged tumor cells. In this study, we evaluated the release of heat shock protein 70 into the serum of cancer-bearing patients directly after radiofrequency ablation. Sera of 22 patients undergoing radiofrequency ablation for the treatment of primary and secondary malignancies of the liver, kidney, and lung, as well as control sera of 20 patients undergoing diagnostic liver biopsy were analyzed using a manufactured heat shock protein 70 ELISA. A significant increase in serum levels of heat shock protein 70 was detectable in the patient cohort 1 day after radiofrequency ablation. More than a twofold increase was observed in nine out of 22 patients, which tended to correlate with favorable clinical outcome. No patient of the control group revealed a comparable increase. Radiofrequency ablation can lead to a release of heat shock protein 70 into the serum, which is transiently detectable 1 day after treatment. Elevated heat shock protein 70 serum levels may constitute a biomarker for favorable clinical outcome.
Keywords: Radiofrequency ablation, Heat shock protein, HSP-70, Soluble HSP
Introduction
Heat shock proteins (HSP) constitute a highly conserved group of proteins involved in many vital chaperoning processes such as protein folding, transport, or antigenic presentation. HSP are abundantly expressed intracellularly, but have also been described at the cytoplasmic membrane of tumor or virally infected cells (Bolhassani and Rafati 2008). They are also found in the intercellular space under various physiological situations (Abe et al. 2004; Nakajima et al. 2009; Njemini et al. 2003; Rea et al. 2001), including cell necrosis (Basu et al. 2000). One of the main functions of intracellular HSP is tissue protection against otherwise lethal injuries by induction of protective mechanisms including inhibition of apoptosis (Garrido et al. 2006). Moreover, extracellular HSP are thought to be a major danger signal for the immune system and indeed, binding of HSP-70 leads to activation and maturation of dendritic cells (den Brok et al. 2006; Dromi et al. 2009). As a consequence, HSP are involved in various immunological processes including anti-cancer immune responses (Calderwood et al. 2007; Srivastava 1994). During infection, prokaryotic HSP constitute a major antigen recognized by the host immune system (Burnie et al. 2006; Mustafa et al. 1993). Moreover, due to their ability to chaperone antigenic peptides, host-derived HSP are involved in antigen-specific immunity (Blachere et al. 1993, 1997) and HSP preparations derived from tumor or virally infected cells induce antigen-specific immunity in animal models (Arnold et al. 1995; Srivastava 2002). The pathways involved in HSP-related immunogenicity essentially include cross-presentation of antigenic peptides on membrane MHC class I molecules expressed on antigen presenting cells to CD8+ effector cells, but also direct loading of HSP-derived peptides onto MHC class II for the activation of CD4+ helper T cells (Calderwood et al. 2005; Figueiredo et al. 2009). Thus, because HSP combine adjuvant and antigen-specific signals, they play a key role in activating the immune system, a peculiar property that could be harnessed in anti-cancer therapy (Figueiredo et al. 2009; Testori et al. 2008).
During radiofrequency (RF) ablation, alternating current leads to frictional heating and to a local increase in temperature above 60°C resulting in immediate cell death, essentially by coagulative necrosis. This process actively continues several days to weeks after the treatment itself (Clasen et al. 2008; Pereira 2007). In the tissue adjacent to the necrotic area, the temperature is lower and creates cellular stress conditions. Inside this so-called transition zone, cellular metabolism still occurs (Frich et al. 2006). Animal studies showed that HSP expression in tumor cells increases shortly after RF ablation (Yang et al. 2004). Moreover, immunochemistry analyses revealed that HSP-70 is expressed in the transition zone surrounding coagulated liver tissue up to 5 days post-RF ablation (Rai et al. 2005; Schueller et al. 2004a). Finally, increased expression of HSP-70 has been reported in human liver biopsy material obtained 24 h after RF ablation (Schueller et al. 2004b).
The aim of our study was to evaluate whether soluble HSP-70 can be detected in the patient’s serum shortly after RF ablation, and whether HSP-70 serum levels correlate with patient characteristics and clinical parameters.
Materials and methods
Patient characteristics
This study was approved by the institutional ethics committee of the University of Tuebingen, Germany. All patients gave their written informed consent before entering the study. Twenty-two patients (Pat) who were treated at the institution of the authors between 2003 and 2005 using percutaneous RF ablation for primary or secondary malignancies of liver, lung, and kidney were included. Clinical characteristics are summarized in Table 1. The cohort comprised 16 men and six women, with a mean age of 63 years (range: 28–81 years) at the time of their first RF ablation. Twelve patients suffered from colorectal carcinoma and four from hepatocellular carcinoma. The remaining patients were treated for manifestations of renal cell carcinoma (n = 2), cholangiocellular carcinoma (n = 1), neuroendocrineous carcinoma of the pancreas (n = 1), carcinoma of the adrenal gland (n = 1), and choroidal melanoma (n = 1). Eighteen patients were treated for lesions in the liver, two for kidney tumors, one suffered from lung metastases, and one had both lung and liver metastases. During the study, 13 patients received one RF application, eight subjects had two consecutive ablations, and one was treated three times during a 4-month period. In addition to RF, patients received the standard therapy regimens according to clinical guidelines.
Table 1.
Summary of patient characteristics and HSP-70 results
| RF ablation patient group | Liver biopsy patient group | |||
|---|---|---|---|---|
| Patients | n = 22 | Patients | n = 20 | |
| Male | n = 16 | Male | n = 13 | |
| Female | n = 6 | Female | n = 7 | |
| Mean agea | 63 years (range: 28–81 years) | p = 0.002 | Mean agea | 50 years (range: 20–70 years) |
| RF ablations | n = 32 (1–3 sessions per patient) | |||
| 1 session | n = 13 | |||
| 2 sessions | n = 8 | |||
| 3 sessions | n = 1 | |||
| Treated organ | Biopsy organ | |||
| Liver | n = 18 | Liver | n = 20 | |
| Kidney | n = 2 | |||
| Lung | n = 1 | |||
| Lung and liver | n = 1 | |||
| Primary diagnosisb | Primary diagnosisb | |||
| CRC | n = 12 | Chronic viral hepatitis | n = 6 | |
| HCC | n = 4 | HCC | n = 6 | |
| RCC | n = 2 | Hepatic metastases | n = 2 | |
| CCC | n = 1 | PBC | n = 2 | |
| NCP | n = 1 | PSC | n = 1 | |
| AGC | n = 1 | Steatohepatitis | n = 1 | |
| CM | n = 1 | Ethylic liver cirrhosis | n = 1 | |
| Graft rejection after LTX | n = 1 | |||
| HSP-70 | HSP-70 | |||
| Mean HSP-70 serum pre-RF (day 0) | 4.6 ng/ml | Mean HSP-70 serum pre-biopsy (day 0) | <1.0 ng/ml | |
| SD 3.0 | ||||
| Mean HSP-70 serum post-RF (day 1) | 7.3 ng/ml | p = 0.001 | Mean HSP-70 serum post-biopsy (day 1) | <1.0 ng/ml |
| SD 4.6 | ||||
| Mean HSP-70 serum first follow-up post-RF | 3.4 ng/ml | p = 0.004 | ||
| SD 2.0 | ||||
aAge at first RF ablation or at biopsy
bCRC colorectal carcinoma, HCC hepatocellular carcinoma, RCC renal cell carcinoma, CCC cholangiocellular carcinoma, NCP neuroendocrineous carcinoma of the pancreas, AGC adrenal gland carcinoma, CM choroidal melanoma, PSC primary sclerosing cholangitis, PBC primary biliary cirrhosis, LTX liver transplantation
Follow-up visits (including magnetic resonance imaging (MRI) examinations) were scheduled 4 weeks after RF ablation followed by regular controls at 2–3 month intervals. The patients preferably received MRI scans for follow-up. Study follow-up was terminated 3 years after the first RF ablation of the last patient included in the study. By the end of the follow-up, seven patients had died and ten patients revealed a stable disease including one individual (Pat XXI) who developed a new tumor entity (lung cancer) unrelated to the RF diagnosis. Five other patients suffered from tumor progression.
Twenty patients (13 men and seven women with a mean age of 50 years, range 20–70 years) who underwent diagnostic liver biopsy were also investigated to assess possible HSP release in stress situations due to liver intervention corresponding to percutaneous placement of an RF applicator (Table 1). This control group was intentionally matched to the RF group according to the interventional procedure. However, it has to be noted that patients in the control group were significantly younger than patients in the RF group (63 vs. 50 years, p = 0.002). Biopsy indications were B and C chronic viral hepatitis (n = 6), hepatocellular carcinoma (n = 6), primary biliary cirrhosis (n = 2), metastases (n = 2), primary sclerosing cholangitis (n = 1), steatohepatitis (n = 1), ethylic liver cirrhosis (n = 1), and rejection after liver transplantation (n = 1). The supportive medication (local anesthesia) was similar to that received by patients included in the experimental group.
Radiofrequency ablation
The 22 patients received a total of 32 RF ablation sessions (one to three treatment sessions) with the ablation of 47 lesions (one to five ablated lesions per session). For all procedures, we used an impedance-controlled monopolar ablation system with MR-compatible electrodes (Tyco Healthcare, Burlington, MA, USA) and a monopolar ablation system with an umbrella electrode (LeVeen, Boston Scientific, USA). The supportive treatment was similar for all patients, including midazolam and pethidine for analgosedation as per the clinical standard doses depending on the patient’s needs. Local anesthesia was performed with lidocaine 1%. No other treatment-related medication was applied.
The tumor volume, extent of the tumor lesions, size of the coagulative necrosis after RF ablation and dimensions of the ablated non-malignant tissue were assessed using CT scans or MR imaging. Volumes were calculated using the formula π/6 × [length] × [depth] × [width].
Serum preparation and HSP-70 measurements
Blood samples were obtained at consecutive time-points for each patient. The first sample was collected on the day of treatment before RF ablation (day 0) and the second the next morning (day 1). In the case of Pat IV, serum obtained before the second RF ablation was collected 1 day before the intervention. A third sample was generally taken during the first regular follow-up visit 1 month after RF ablation; additional samples were obtained during control visits at 2 to 3-month intervals (not shown). In one case, an additional serum sample was taken on day 3 after RF (Pat XXII). Altogether, for 29 of the 32 sessions, 58 serum samples from two consecutive days pre- and post-RF ablation were available and 34 sera were obtained at later time points. Sera from patients of the control group were obtained at day 0 just before the biopsy and at day 1, using a procedure similar to that used for patients of the RF ablation group. Blood samples were allowed to coagulate for 4 h at room temperature and serum was collected after two consecutive centrifugation steps at 760 g and 4°C. Sera were stored at −80°C and thawed at 4°C before testing.
Detection of inducible HSP-70 (HSP70A1A; Kampinga et al. 2009) was performed with an HSP-70 ELISA kit (EKS-700, StressGen Biotechnologies Corporation, Victoria, Canada), according to the manufacturer’s instructions. Fluorescence quantification was done at 450 nm including a correction wave length of 540 nm using a SPECTRA-MAX 340 ELISA reader (Molecular Devices Corporation, Sunnyvale, CA, USA). All serum samples were diluted 1:2 and measured in duplicates, whereby all sera obtained from each patient were assessed in the same experiment. Mean HSP-70 serum levels were calculated using a standard curve obtained by serial dilution of recombinant HSP-70 protein provided with each kit and included on each individual plate. Test sensitivity had been determined by the manufacturer as being 540 pg/ml. Since the dilution factor was 1:2, we estimated the detection threshold of our test to be approximately 1.0 ng HSP-70/ml serum.
Statistical analyses
Mean HSP-70 serum levels were calculated for all patients with detectable serum HSP-70 levels (RF ablation group for day 0, n = 26; for day 1, n = 26; and for the first regular follow-up visit, n = 23). Differences in RF HSP-70 serum levels were tested using a two-sided Student’s t test and p values lower than 0.05 were considered significant. Paired and unpaired t tests were used for comparing pre- and post-RF HSP-70 levels in the RF-treated patients and HSP-70 levels of the control versus RF groups, respectively. T tests were performed for all patients with detectable HSP-70 in the serum (comparison pre- and post-RF, n = 24 patients; comparison first regular follow-up visit and pre-RF, n = 22 patients).
Correlations between HSP-70 levels and patients’ clinical and laboratory parameters were tested by performing regression analyses and determining R2 values. R2 values higher than 0.95 were considered significant.
Results
HSP-70 release in patients treated with RF ablation
Table 2 shows patient individual HSP-70 measurements pre-RF (day 0 for all but one patient) and at day 1, as well as the ratio day 1/pre-RF. Five patients had no detectable HSP-70 before or directly after RF (<1 ng/ml). In the other cases, mean level of HSP-70 was 4.6 ng/ml before (SD 3.0, range 1.1–12.8 ng/ml) and 7.3 ng/ml after RF treatment (SD 4.6, range 1.3–16.4 ng/ml). Altogether, the serum level of HSP-70 in the whole group was significantly higher 1 day after treatment than before treatment (paired t test, p = 0.001, see Table 1), with a mean ratio pre- versus post-RF of 1.7. An increase in the HSP-70 level of more than twofold was observed in a large proportion of the patients (41%, n = 9 patients) and of RF interventions (42%, n = 10 from 24 sessions). The strongest induction was observed in patients II and XX, with a 3.1-fold increase at day 1. In two patients (III and XVII), HSP-70 was detectable in the serum only after treatment.
Table 2.
Patients’ results of the HSP-70 ELISA and clinical course
| HSP-70 [ng/ml] | HSP-70 ratio | HSP-70 [ng/ml] | Clinical course | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pat. # | Ablation | Pre-RFa | Day 1 | Day 1/pre-RF | Day 3 | Day 11 | 1 Mb | Follow up (M) | Outcome |
| I | #2 | 2.5 | 3.4 | 1.4 | 1.4 | 45 | Septicemia, PD | ||
| II | #1 | 2.5 | 7.8 | 3.1 | 4.6 | 13 | Death | ||
| #2 | 4.6 | 9.3 | 2.0 | 5.2 | |||||
| #3 | 5.2 | 8.6 | 1.7 | 3.8 | |||||
| III | #2 | <1.0 | 1.3 | n.d. | <1.0 | 32 | PD | ||
| IV | #1 | <1.0 | <1.0 | n.d. | 1.1 | 49 | SD | ||
| #2 | 1.1 | 1.5 | 1.4 | <1.0 | |||||
| V | #1 | 1.2 | <1.0 | n.d. | <1.0 | 10 | Death | ||
| VI | #1 | 4.5 | 11.5 | 2.6 | 2.0 | 30 | Death | ||
| VII | #1 | 12.8 | 12.5 | 1.0 | 7.7 | 22 | Death | ||
| VIII | #1 | 6.7 | 15.7 | 2.3 | 2.2 | 37 | SD | ||
| IX | #1 | 3.3 | 4.7 | 1.4 | 2.4 | 47 | PD | ||
| #2 | 4.2 | 3.6 | 0.9 | 1.4 | |||||
| X | #1 | 3.8 | 2.9 | 0.8 | 2.9 | 39 | Death | ||
| XI | #1 | 2.2 | 2.6 | 1.2 | n.a. | 37 | SD | ||
| XII | #1 | 1.3 | 2.6 | 2.0 | 1.3 | 10 | PD | ||
| XIII | #1 | 4.3 | 6.2 | 1.4 | 2.5 | 44 | SD | ||
| #2 | 3.7 | 9.9 | 2.7 | 5.1 | |||||
| XIV | #1 | 4.5 | 11.1 | 2.5 | 3.4 | 44 | SD | ||
| XV | #1 | 12.2 | 11.3 | 0.9 | 7.3 | 37 | SD | ||
| XVI | #1 | 6.9 | 7.0 | 1.0 | 2.2 | 26 | SD | ||
| #2 | 5.4 | 12.8 | 2.4 | 4.1 | |||||
| XVII | #1 | <1.0 | 1.4 | n.d. | 1.7 | n.a. | 3 | Death | |
| XVIII | #1 | 1.9 | <1.0 | n.d. | 1.1 | 40 | SD | ||
| XIX | #1 | 2.3 | 2.4 | 1.0 | 2.1 | 38 | PD | ||
| XX | #1 | 4.1 | 13.0 | 3.1 | 4.7 | 40 | SD | ||
| #2 | 8.3 | 5.8 | 0.7 | 7.7 | |||||
| XXI | #1 | 2.9 | 5.3 | 1.8 | 2.2 | 18 | New tumor entity | ||
| XXII | #1 | 7.7 | 16.4 | 2.1 | 7.7 | n.a. | 25 | Death | |
Numbers in italic indicate day 0 value of subsequent RF ablation
M month(s), n.d. not determinable, n.a. data not available, PD progressive disease, SD stable disease
aPre-RF = day 0 for all patients except for Pat IV#2 (day -1)
bHSP-70 serum levels 1 M after RF ablation, except for Pat II #1 (2 M) and #3 (3 M), and Pat IV #2 (5 M)
We then analyzed 34 serum samples obtained during follow-up control visits (Table 2 and data not shown, time points between 2 and 8 months after RF ablation). In most patients, HSP-70 serum levels had returned to reduced, often lower than baseline, levels 1–5 months after treatment (mean 3.4 ng/ml, p = 0.004, Table 1). Afterwards, HSP-70 measurements remained generally stable. In two cases, serum samples were collected 1–2 weeks following intervention and showed HSP-70 levels similar to those found before or at day 1 after intervention (7.7 ng/ml on day 0 and 7.7 ng/ml on day 3 for Pat XXII, see also Fig. 1; <1, 1.4, and 1.7 ng/ml, for Pat XVII at days 0, 1, and 11, respectively).
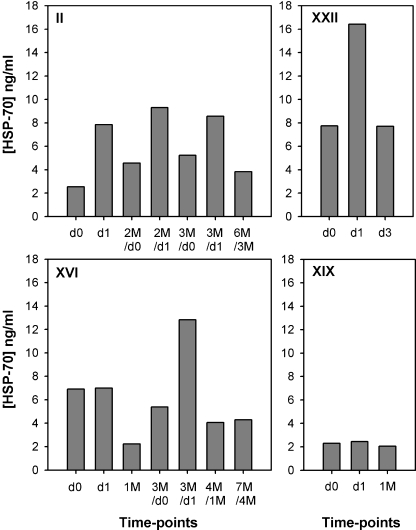
Fig. 1.
HSP-70 serum levels measured by ELISA in four patients. Patients II and XVI received three and two RF treatments, respectively, patients XXII and XIX only one. Time points of serum collection are indicated. D day, M month
Figure 1 exemplifies the results obtained from four characteristic patients. In Pat II, a strong increase in HSP-70 serum concentration was observed after the first two sessions of RF ablation (3.1-and 2.0-fold increase, respectively), whereas a weaker, but still above average change occurred after the third procedure (fold increase, 1.7). Pat XVI had a high HSP-70 baseline serum level (day 0, 6.9 ng/ml) which remained unchanged after the first ablation (day 1, 7.0 ng/ml). However, this level was significantly increased after the second treatment 3 months later (day 0, 5.4 ng/ml; day 1, 12.8 ng/ml) and had returned to baseline values 1 and 4 months thereafter (4.1 and 4.3 ng/ml). In Pat XXII, the HSP-70 level was clearly enhanced 1 day after RF (fold increase, 2.1) but had returned to its baseline value 3 days later (day 0, 7.7 ng/ml; day 1, 16.4 ng/ml, and day 3, 7.7 ng/ml). In contrast to these donors, no change in the HSP-70 levels was observed in Pat XIX.
Altogether, we found that HSP-70 is released into the blood of patients undergoing tumor ablation, with a more than twofold increase in serum levels in a subgroup of patients. Our observations suggest that the HSP-70 serum level peaks approximately 1 day after treatment and return to lower levels several days thereafter.
HSP-70 serum level in patients receiving liver biopsy
We then considered whether these changes in HSP-70 serum levels were specific for RF ablation or reflected physiological stress. For this, we analyzed 40 serum samples from 20 patients who underwent liver biopsy for diagnostic purposes. In this group, HSP-70 could not be detected in the serum either before or after RF ablation (Table 1).
Of note, two patients in this group presented with conditions which could have led to an increased HSP-70 blood level. One of those suffered from rapid tumor progression resulting in an increase in bilirubinemia, cholestasis, and cholangitis on the day of the liver biopsy which necessitated an endoscopic retrograde cholangiopancreaticography and systemic therapy. This should be regarded as an additional stress factor. The second patient suffered from cytomegalovirus encephalitis with systemic signs of active inflammation at the time of biopsy. Despite these inflammatory conditions, no HSP-70 could be detected in these two patients (<1 ng/ml).
Importantly, diagnostic biopsy did not lead to a detectable systemic release of HSP-70 in the patient’s serum suggesting that the HSP-70 production observed in patients receiving RF is specific for this treatment and not due to stress factors associated with general clinical intervention.
HSP-70 serum levels, patients’ characteristics, and laboratory findings
Since the amount of HSP-70 in the serum has been reported to be influenced by several physiological conditions, we next analyzed whether HSP-70 serum levels could be associated with patients characteristics or with clinical parameters in the two investigated cohorts. When available, we analyzed total cholesterol (RF group, n = 10; control group, n = 14 patients), blood glucose (RF group, n = 13; control group, n = 11 patients), as well as body weight (RF group, n = 22; control group, n = 17 patients), height (RF group, n = 18; control group, n = 15 patients) and body mass index (RF group, n = 18; control group, n = 15 patients). A tendency to higher, albeit not significant, total cholesterol was observed for the RF group as compared to the patients undergoing biopsy (mean total cholesterol: 190 and 170 mg/dl for the RF and biopsy group, respectively, p = 0.3). All other parameters were not significantly different between the two patient groups (data not shown). Moreover, no correlation could be observed between any of these parameters and HSP-70 serum levels (data not shown).
HSP-70 serum level and clinical data
We then determined the extent to which the release of HSP-70 in the serum possibly correlates with the volume of the tumor lesions, the volume of RF-induced necrosis and the estimated volume of the ablated non-malignant tissue. The mean volume of the tumor lesions was 9.23 cm³ (range 0.11–37.5 cm³). The mean volume of total ablated tissue was 36.3 cm³ (range 7.95–104.38 cm³). Therefore, the calculated mean volume of ablated non-malignant tissue was 27.1 cm3, leading to a tumor: non-malignant tissue ratio in the ablated lesions of 1:3. We found no correlation between the changes in HSP-70 concentration (i.e., ratio: day 1/day 0) in the pre- and post-RF patient’s sera and these clinical values (R2 < 0.1 for all three parameters, data not shown).
Finally, we followed the clinical course of the disease after RF intervention. Patients were monitored for a mean time period of 30 months (range 3–49 months) after the first RF ablation (Table 2). Interestingly, six of the nine patients (67%) who showed at least a twofold increase of serum HSP-70 directly after RF ablation were still alive at follow-up time, all but one with stable disease (only Pat XII had a tumor progression). Three patients had died, the shortest follow-up time being 13 months for Pat II.
Two additional patients who did not present with HSP-70 serum levels above the detection limit before RF ablation had detectable HSP-70 in the serum 1 day after RF ablation. One of these patients had progressive disease at the end of follow-up (Pat III); the other died after 3 months due to rapid tumor progression (Pat XVII).
In striking contrast, eight of 13 patients (61%) without detectable increase of HSP-70 serum levels suffered from progressive disease (in contrast to one out of nine—i.e., 11%—in the other group). Four patients had stable disease for more than 3 years; one patient developed a new tumor entity unrelated to the initial diagnosis (Pat XXI).
Since HSP-70 can chaperone antigenic peptides from the cell of origin, this observation raised the question whether the source of HSP-70 could influence the clinical course. In the case of local recurrence (n = 9) at the site of previous ablation, it is likely that malignant tissue was left untreated or sublethally damaged in the transition zone. HSP-70 from these cells may carry tumor-associated peptides capable of inducing a specific anti-tumor immune response, whereas HSP-70 from a transition zone located in non-malignant tissue should carry “silent” self-antigen-derived peptides. We estimated the size of this transition zone by calculating the surface of the necrotic area assuming a spherical form of the necrosis. No correlation could be found between the extent of HSP release and the size of this transitional zone (R2 = 0.0187, not shown). In summary, no correlation between HSP-70 release, tumor size, volume of ablated tissue and local recurrence could be observed in this small patient cohort. Although the patient cohort was small, an interesting observation was that clinical outcome was found to be better in the group with significant HSP-70 release than in the group without detectable increase of HSP-70 serum levels.
Discussion
Radiofrequency ablation is being applied increasingly for the treatment of various tumors including hepatocellular carcinoma (HCC), renal cell carcinomas, and metastatic hepatic and pulmonary lesions (Pereira 2007) and leads to favorable responses and survival rates comparable to those of patients undergoing surgical resection (Gillams and Lees 2008). Besides local tumor control, multiple observations indicate that RF ablation enhances anti-tumor immune responses in vivo. An induction of anti-tumor immunity after RF application has been observed, e.g., partial protection against tumor re-challenge can be achieved by treating melanoma-bearing mice with RF ablation and by adoptive transfer of splenocytes from these mice into RF-naïve animals (den Brok et al. 2004). On the cellular level, activation and maturation of antigen-loaded dendritic cells in the bone marrow and in the periphery was demonstrated (den Brok et al. 2006; Dromi et al. 2009), and T lymphocytes obtained from hepatoma-bearing rabbits after RF application did proliferate in vitro in the presence of autologous tumor lysate (Wissniowski et al. 2003). In patients, increased IFN-γ production by peripheral T cells was observed upon stimulation with autologous HCC tumor lysate and allogenic tumor-cell lines (Zerbini et al. 2006). We also recently described that specific stimulation of antibodies and T cells recognizing tumor-associated antigens can occur after RF ablation in cancer patients (Widenmeyer et al. 2010). One mechanism responsible for such effect of RF ablation on immune effectors could be the in situ expression of danger signals, including HSP (Rai et al. 2005; Schueller et al. 2004a; Schueller et al. 2004b; Yang et al. 2004). In particular, due to its dual function as danger signal and antigen-chaperone, HSP-70 plays a pivotal role in activating cells of the innate and adaptive immunity (Chen et al. 2009; Figueiredo et al. 2009; Qiao et al. 2008).
We investigated the release of HSP-70 into the serum of patients after RF ablation. Baseline levels of HSP-70 were generally higher (mean, 4.9 ng/ml) in this group than in the patients receiving biopsies (<1 ng/ml). Studies using a manufactured ELISA test generally report a significant variation of HSP-70 serum level in healthy donors ranging from less than 1 up to 14 ng/ml (Abe et al. 2004; Adewoye et al. 2005; Fehrenbach et al. 2005; Genth-Zotz et al. 2004; Molvarec et al. 2007; Njemini et al. 2003; Walsh et al. 2001) and thus comparable to the levels that we found. Several physiological conditions may influence these levels, such as ageing (Rea et al. 2001), pregnancy (Molvarec et al. 2007) or physical exercise (Fehrenbach et al. 2005; Walsh et al. 2001). Additionally, pathologic situations such as acute infection or heart failure have been shown to increase the level of HSP-70 measured in the blood (Genth-Zotz et al. 2004; Njemini et al. 2003). One report describes a modest elevation of HSP-70 in the serum of patients with localized prostate carcinoma (mean: 0.8 ng/ml) as compared to a control group of healthy donors (mean: 0.5 ng/ml; Abe et al. 2004). We could not observe any correlation between HSP-70 serum levels and the patients’ characteristics or laboratory and clinical parameters tested. Although obtained in a relatively small cohort, our data suggest that ageing patients with advanced tumors may have elevated serum HSP-70 levels, possibly reflecting ongoing inflammation associated with tumor burden.
Interestingly, we observed a more than twofold increase in the serum HSP-70 levels in a large subgroup of patients (nine of 22 tested) approximately 24 h following RF application. This increase (up to more than threefold) was transient. Results obtained for one patient on day 3 suggest that the peak of HSP in the blood occurs in the few hours after thermal treatment. Although further investigations will be necessary to pinpoint this time window, our results are in accordance with previous findings showing maximal in situ HSP-70 transcription at 10 h (Yang et al. 2004) and protein expression at 24 h (Schueller et al. 2004b). Other inflammation-related parameters such as, e.g., cytokines TNF-α or IL-6 could also be evaluated directly after RF treatment.
Although liver biopsy and RF ablation procedures are comparable in specific modalities of action and supportive treatment, no patient undergoing biopsy showed a detectable change in serum HSP-70. We therefore concluded that percutaneous RF ablation can specifically induce a systemic release of HSP-70 into the circulation, most probably related to the induction of coagulative necrosis. This was especially observed in a subgroup of patients and prompted us to analyze whether this could be related to the intensity of the RF treatment. However, we did not find any correlation between the increase of HSP-70 serum levels and the coagulation extent. Furthermore, neither the volume of the primary site or histological type of the tumor nor the estimated volume of the ablated non-malignant tissue had any apparent impact on the amount of released HSP-70. Since it has been reported that HSP are expressed on the cells in the transition zone (Rai et al. 2005) or on a small rim immediately adjacent to the final margin of coagulation (Solazzo et al. 2010), we estimated the size of this transition zone by calculating the surface of the necrotic area assuming a spherical form of the necrosis, but here again, no correlation could be found. Schueller et al. studied the relation between the energy applied, the tissue necrosis, and the in situ expression of HSP-70 and HSP-90. In this model, athymic nude rats were transplanted with a human HCC cell line and subsequently treated with RF ablation. Only a slight statistically significant correlation was observed between the energy applied and tumor necrosis. A tendency for the expression of heat shock proteins to increase according to the extent of tumor necrosis and the RF energy was also noted (Schueller et al. 2004a).
Since HSP-70 has been reported to be differentially expressed in various tumors (Abe et al. 2004; Chen et al. 2009; Nakajima et al. 2009), inter-individual variations in the amount of released HSP-70 may be expected and could explain our findings. Evaluation of a larger cohort may enable the identification of important parameters relating to HSP-70 release.
The most striking observation in our study, although not significant in this small patient cohort, was the difference in tumor progression and survival time between the patients who showed an increase in HSP-70 serum concentrations and those who did not. Since RF ablation has been shown to induce anti-tumor immune responses, it is tempting to hypothesize that the release of HSP-70 in the serum might lead to potent activation of tumor-specific immune responses directed against chaperoned antigenic peptides, thus resulting in a better clinical outcome. Since HSP-70 can chaperone antigenic peptides from the cells from which it originated, it might carry tumor-specific antigens when arising from sublethally damaged malignant tissue. In vitro experiments have shown that the HSP released into supernatants after hyperthermia carry peptides from tumor cells (Ito et al. 2006). Our results indeed suggest a protective function of HSP-70 release after tumor cell destruction, rather than supporting previous findings reporting tumorigenic properties of HSP overexpression in tumor cells (Garrido et al. 2006).
The controlled necrosis induced by a locally placed surgical instrument inside the tumors—even more when applicated with fluid—could lead to optimal release of HSP and endogenous anti-tumor immunization. RF ablation represents the ideal tool to induce this controlled necrosis since it can be performed under imaging guidance and allows not only a precise assessment of the extension of the necrosis, but also the application of fluid into the tumor. Precise definition of the technical conditions leading to optimal release of tumor cell content may establish this technique as a novel module in cancer immunotherapy (Srivastava 2003). Although the results are still preliminary, our study provides the possibility of testing in a large patient cohort whether measurement of HSP-70 serum levels could be used as a relevant biomarker for RF ablation efficacy.
Acknowledgments
We wish to thank Lynne Yakes for editorial assistance as well as Hansjoerg Schild for helpful discussion.
Footnotes
Sebastian P. Haen and Cécile Gouttefangeas contributed equally to this work.
This work was supported by a grant from the University of Tuebingen, Fortüne project No. 1530-0-0 and from third-party funding donated by Hölle and Hüttner AG.
Sebastian P. Haen is supported by the Deutsche José Carreras Leukemia Foundation.
No authors have any commercial interest in this report.
References
- Abe M, Manola JB, Oh WK, Parslow DL, George DJ, Austin CL, Kantoff PW. Plasma levels of heat shock protein 70 in patients with prostate cancer: a potential biomarker for prostate cancer. Clin Prostate Cancer. 2004;3:49–53. doi: 10.3816/cgc.2004.n.013. [DOI] [PubMed] [Google Scholar]
- Adewoye AH, Klings ES, Farber HW, Palaima E, Bausero MA, McMahon L, Odhiambo A, Surinder S, Yoder M, Steinberg MH, Asea A. Sickle cell vaso-occlusive crisis induces the release of circulating serum heat shock protein-70. Am J Hematol. 2005;78:240–242. doi: 10.1002/ajh.20292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold D, Faath S, Rammensee H, Schild H. Cross-priming of minor histocompatibility antigen-specific cytotoxic T cells upon immunization with the heat shock protein gp96. J Exp Med. 1995;182:885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kappa B pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- Blachere NE, Udono H, Janetzki S, Li Z, Heike M, Srivastava PK. Heat shock protein vaccines against cancer. J Immunother Emphasis Tumor Immunol. 1993;14:352–356. doi: 10.1097/00002371-199311000-00016. [DOI] [PubMed] [Google Scholar]
- Blachere NE, Li Z, Chandawarkar RY, Suto R, Jaikaria NS, Basu S, Udono H, Srivastava PK. Heat shock protein-peptide complexes, reconstituted in vitro, elicit peptide-specific cytotoxic T lymphocyte response and tumor immunity. J Exp Med. 1997;186:1315–1322. doi: 10.1084/jem.186.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolhassani A, Rafati S. Heat-shock proteins as powerful weapons in vaccine development. Expert Rev Vaccin. 2008;7:1185–1199. doi: 10.1586/14760584.7.8.1185. [DOI] [PubMed] [Google Scholar]
- Burnie JP, Carter TL, Hodgetts SJ, Matthews RC. Fungal heat-shock proteins in human disease. FEMS Microbiol Rev. 2006;30:53–88. doi: 10.1111/j.1574-6976.2005.00001.x. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Theriault JR, Gong J. Message in a bottle: role of the 70-kDa heat shock protein family in anti-tumor immunity. Eur J Immunol. 2005;35:2518–2527. doi: 10.1002/eji.200535002. [DOI] [PubMed] [Google Scholar]
- Calderwood SK, Mambula SS, Gray PJ., Jr Extracellular heat shock proteins in cell signaling and immunity. Ann NY Acad Sci. 2007;1113:28–39. doi: 10.1196/annals.1391.019. [DOI] [PubMed] [Google Scholar]
- Chen T, Guo J, Han C, Yang M, Cao X. Heat shock protein 70, released from heat-stressed tumor cells, initiates antitumor immunity by inducing tumor cell chemokine production and activating dendritic cells via TLR4 pathway. J Immunol. 2009;182:1449–1459. doi: 10.4049/jimmunol.182.3.1449. [DOI] [PubMed] [Google Scholar]
- Clasen S, Krober SM, Kosan B, Aebert H, Fend F, Bomches A, Claussen CD, Pereira PL. Pathomorphologic evaluation of pulmonary radiofrequency ablation: proof of cell death is characterized by DNA fragmentation and apoptotic bodies. Cancer. 2008;113:3121–3129. doi: 10.1002/cncr.23882. [DOI] [PubMed] [Google Scholar]
- Brok MH, Sutmuller RP, Voort R, Bennink EJ, Figdor CG, Ruers TJ, Adema GJ. In situ tumor ablation creates an antigen source for the generation of antitumor immunity. Cancer Res. 2004;64:4024–4029. doi: 10.1158/0008-5472.CAN-03-3949. [DOI] [PubMed] [Google Scholar]
- Brok MH, Sutmuller RP, Nierkens S, Bennink EJ, Frielink C, Toonen LW, Boerman OC, Figdor CG, Ruers TJ, Adema GJ. Efficient loading of dendritic cells following cryo and radiofrequency ablation in combination with immune modulation induces anti-tumour immunity. Br J Cancer. 2006;95:896–905. doi: 10.1038/sj.bjc.6603341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dromi SA, Walsh MP, Herby S, Traughber B, Xie J, Sharma KV, Sekhar KP, Luk A, Liewehr DJ, Dreher MR, Fry TJ, Wood BJ. Radiofrequency ablation induces antigen-presenting cell infiltration and amplification of weak tumor-induced immunity. Radiology. 2009;251:58–66. doi: 10.1148/radiol.2511072175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehrenbach E, Niess AM, Voelker K, Northoff H, Mooren FC. Exercise intensity and duration affect blood soluble HSP72. Int J Sports Med. 2005;26:552–557. doi: 10.1055/s-2004-830334. [DOI] [PubMed] [Google Scholar]
- Figueiredo C, Wittmann M, Wang D, Dressel R, Seltsam A, Blasczyk R, Eiz-Vesper B. Heat shock protein 70 (HSP70) induces cytotoxicity of T-helper cells. Blood. 2009;113:3008–3016. doi: 10.1182/blood-2008-06-162727. [DOI] [PubMed] [Google Scholar]
- Frich L, Bjornland K, Pettersen S, Clausen OP, Gladhaug IP. Increased activity of matrix metalloproteinase 2 and 9 after hepatic radiofrequency ablation. J Surg Res. 2006;135:297–304. doi: 10.1016/j.jss.2006.05.010. [DOI] [PubMed] [Google Scholar]
- Garrido C, Brunet M, Didelot C, Zermati Y, Schmitt E, Kroemer G. Heat shock proteins 27 and 70: anti-apoptotic proteins with tumorigenic properties. Cell Cycle. 2006;5:2592–2601. doi: 10.4161/cc.5.22.3448. [DOI] [PubMed] [Google Scholar]
- Genth-Zotz S, Bolger AP, Kalra PR, Haehling S, Doehner W, Coats AJ, Volk HD, Anker SD. Heat shock protein 70 in patients with chronic heart failure: relation to disease severity and survival. Int J Cardiol. 2004;96:397–401. doi: 10.1016/j.ijcard.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Gillams AR, Lees WR. Five-year survival following radiofrequency ablation of small, solitary, hepatic colorectal metastases. J Vasc Interv Radiol. 2008;19:712–717. doi: 10.1016/j.jvir.2008.01.016. [DOI] [PubMed] [Google Scholar]
- Ito A, Honda H, Kobayashi T. Cancer immunotherapy based on intracellular hyperthermia using magnetite nanoparticles: a novel concept of “heat-controlled necrosis” with heat shock protein expression. Cancer Immunol Immunother. 2006;55:320–328. doi: 10.1007/s00262-005-0049-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kampinga HH, Hageman J, Vos MJ, Kubota H, Tanguay RM, Bruford EA, Cheetham ME, Chen B, Hightower LE (2009) Guidelines for the nomenclature of the human heat shock proteins. Cell Stress Chaperones 14:105–111 [DOI] [PMC free article] [PubMed]
- Molvarec A, Rigo J, Jr, Nagy B, Walentin S, Szalay J, Fust G, Karadi I, Prohaszka Z. Serum heat shock protein 70 levels are decreased in normal human pregnancy. J Reprod Immunol. 2007;74:163–169. doi: 10.1016/j.jri.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Mustafa AS, Lundin KE, Oftung F. Human T cells recognize mycobacterial heat shock proteins in the context of multiple HLA-DR molecules: studies with healthy subjects vaccinated with Mycobacterium bovis BCG and Mycobacterium leprae. Infect Immun. 1993;61:5294–5301. doi: 10.1128/iai.61.12.5294-5301.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima M, Kato H, Miyazaki T, Fukuchi M, Masuda N, Fukai Y, Sohda M, Ahmad F, Kuwano H. Tumor immune systems in esophageal cancer with special reference to heat-shock protein 70 and humoral immunity. Anticancer Res. 2009;29:1595–1606. [PubMed] [Google Scholar]
- Njemini R, Lambert M, Demanet C, Mets T. Elevated serum heat-shock protein 70 levels in patients with acute infection: use of an optimized enzyme-linked immunosorbent assay. Scand J Immunol. 2003;58:664–669. doi: 10.1111/j.1365-3083.2003.01341.x. [DOI] [PubMed] [Google Scholar]
- Pereira PL. Actual role of radiofrequency ablation of liver metastases. Eur Radiol. 2007;17:2062–2070. doi: 10.1007/s00330-007-0587-0. [DOI] [PubMed] [Google Scholar]
- Qiao Y, Liu B, Li Z. Activation of NK cells by extracellular heat shock protein 70 through induction of NKG2D ligands on dendritic cells. Cancer Immun. 2008;8:12. [PMC free article] [PubMed] [Google Scholar]
- Rai R, Richardson C, Flecknell P, Robertson H, Burt A, Manas DM. Study of apoptosis and heat shock protein (HSP) expression in hepatocytes following radiofrequency ablation (RFA) J Surg Res. 2005;129:147–151. doi: 10.1016/j.jss.2005.03.020. [DOI] [PubMed] [Google Scholar]
- Rea IM, McNerlan S, Pockley AG. Serum heat shock protein and anti-heat shock protein antibody levels in aging. Exp Gerontol. 2001;36:341–352. doi: 10.1016/S0531-5565(00)00215-1. [DOI] [PubMed] [Google Scholar]
- Schueller G, Kettenbach J, Sedivy R, Bergmeister H, Stift A, Fried J, Gnant M, Lammer J. Expression of heat shock proteins in human hepatocellular carcinoma after radiofrequency ablation in an animal model. Oncol Rep. 2004;12:495–499. [PubMed] [Google Scholar]
- Schueller G, Kettenbach J, Sedivy R, Stift A, Friedl J, Gnant M, Lammer J. Heat shock protein expression induced by percutaneous radiofrequency ablation of hepatocellular carcinoma in vivo. Int J Oncol. 2004;24:609–613. [PubMed] [Google Scholar]
- Solazzo SA, Ahmed M, Schor-Bardach R, Yang W, Girnun GD, Rahmanuddin S, Levchenko T, Signoretti S, Spitz DR, Torchilin V, Goldberg SN. Liposomal doxorubicin increases radiofrequency ablation-induced tumor destruction by increasing cellular oxidative and nitrative stress and accelerating apoptotic pathways. Radiology. 2010;255:62–74. doi: 10.1148/radiol.09091196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava PK. Heat shock proteins in immune response to cancer: the fourth paradigm. Experientia. 1994;50:1054–1060. doi: 10.1007/BF01923461. [DOI] [PubMed] [Google Scholar]
- Srivastava P. Interaction of heat shock proteins with peptides and antigen presenting cells: chaperoning of the innate and adaptive immune responses. Annu Rev Immunol. 2002;20:395–425. doi: 10.1146/annurev.immunol.20.100301.064801. [DOI] [PubMed] [Google Scholar]
- Srivastava PK. Hypothesis: controlled necrosis as a tool for immunotherapy of human cancer. Cancer Immun. 2003;3:4. [PubMed] [Google Scholar]
- Testori A, Richards J, Whitman E, Mann GB, Lutzky J, Camacho L, Parmiani G, Tosti G, Kirkwood JM, Hoos A, Yuh L, Gupta R, Srivastava PK. Phase III comparison of vitespen, an autologous tumor-derived heat shock protein gp96 peptide complex vaccine, with physician’s choice of treatment for stage IV melanoma: the C-100-21 Study Group. J Clin Oncol. 2008;26:955–962. doi: 10.1200/JCO.2007.11.9941. [DOI] [PubMed] [Google Scholar]
- Walsh RC, Koukoulas I, Garnham A, Moseley PL, Hargreaves M, Febbraio MA. Exercise increases serum Hsp72 in humans. Cell Stress Chaperones. 2001;6:386–393. doi: 10.1379/1466-1268(2001)006<0386:EISHIH>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widenmeyer M, Shebzukhov Y, Haen SP, Schmidt D, Clasen S, Boss A, Kuprash DV, Nedospasov SA, Stenzl A, Aebert H, Wernet D, Stevanovic S, Pereira PL, Rammensee HG, Gouttefangeas C (2010) Analysis of tumor antigen-specific T cells and antibodies in cancer patients treated with radiofrequency ablation. Int J Cancer (in press) [DOI] [PubMed]
- Wissniowski TT, Hansler J, Neureiter D, Frieser M, Schaber S, Esslinger B, Voll R, Strobel D, Hahn EG, Schuppan D. Activation of tumor-specific T lymphocytes by radio-frequency ablation of the VX2 hepatoma in rabbits. Cancer Res. 2003;63:6496–6500. [PubMed] [Google Scholar]
- Yang WL, Nair DG, Makizumi R, Gallos G, Ye X, Sharma RR, Ravikumar TS. Heat shock protein 70 is induced in mouse human colon tumor xenografts after sublethal radiofrequency ablation. Ann Surg Oncol. 2004;11:399–406. doi: 10.1245/ASO.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Zerbini A, Pilli M, Penna A, Pelosi G, Schianchi C, Molinari A, Schivazappa S, Zibera C, Fagnoni FF, Ferrari C, Missale G. Radiofrequency thermal ablation of hepatocellular carcinoma liver nodules can activate and enhance tumor-specific T-cell responses. Cancer Res. 2006;66:1139–1146. doi: 10.1158/0008-5472.CAN-05-2244. [DOI] [PubMed] [Google Scholar]