Abstract
Our previous study showed that pretreatment with 5-hydroxymethyl-2-furfural (5-HMF) led to protection against hypoxic injury via a p-ERK-mediated pathway in vitro. Whether the protection of 5-HMF against hypoxia is effective in vivo is unknown. The present study is aimed to verify the role of 5-HMF in acute hypobaric hypoxia using Kunming mice as an in vivo model and further investigate the underlying mechanisms. Mice pretreated with or without 5-HMF for 1 h were exposed to acute hypobaric hypoxic condition for 6 h and then the survival time, the survival rate, the permeability of blood–brain barrier (BBB), the histological analysis in hippocampus and cortex, and the phosphorylation level of mitogen-activated protein kinases (ERK, JNK, and p38) were investigated. The results showed that 5-HMF significantly increased the survival time and the survival rate of mice. Accordingly, pretreatment with 5-HMF markedly attenuated acute hypobaric hypoxia-induced permeability of BBB (P < 0.01). In addition, the cellular damage extent of the hippocampus and the cortex induced by hypoxia for 6 h was also attenuated by pretreatment with 5-HMF, especially in the hippocampus CA1 region. Furthermore, the activation of ERK rather than JNK and p38 was involved in the protection of 5-HMF against acute hypobaric hypoxia. In summary, 5-HMF enhanced the survival capability of mice and decreased acute hypoxic damage to the brain, which may be associated with the effects on BBB and p-ERK.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-011-0264-8) contains supplementary material, which is available to authorized users.
Keywords: 5-HMF, Hypobaric hypoxia, Brain injury, BBB
Introduction
Acute hypobaric hypoxia is one of the main causes of acute mountain sickness (AMS), high-altitude cerebral edema (HACE), and high-altitude pulmonary edema (HAPE). Acute mountain sickness (AMS) develops within a few hours after arriving at high altitude and the principal symptoms of AMS are headache, anorexia, nausea, vomiting, and malaise (Gallagher and Hackett 2004; Natah et al. 2009). HACE is considered to be the end stage of severe AMS and has been suggested to be an osmotic cell swelling and vasogenic edema, raising the possibility that acute hypoxia may increase blood–brain barrier (BBB) permeability (Roach and Hackett 2001). Prevention for adaptation to high-altitude environment includes slow ascent, appropriate descent, and drug therapy (Wright et al. 2008; Tissot van Patot et al. 2009). Among them, drug therapy is considered to be the main prevention measure. It is urgent to develop effective drugs to avoid acute hypobaric hypoxia injury.
As we know, hypoxic response occurs through a number of mechanisms. Mitogen-activated protein kinase (MAPK) family members function as critical mediators of signal transduction pathways in regulating cell death and survival (Johnson and Lapadat 2002). MAPKs include extracellular signal regulated kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 MAPK. They are activated by phosphorylation in response to many kinds of stress (Davis 2000; Zhang et al. 2007). Among them, the roles of JNK and p38 MAPK in cell death and survival are controversial (Ono and Han 2000; Lin 2003; Weerackody et al. 2009). The activation of ERK can mediate two apparently opposing processes from cell proliferation to cell death (Mebratu and Tesfaigzi 2009; Cagnol and Chambard 2010). There is now increasing evidence that MAPKs have dual effects in hypoxia and some compounds that influence ERK can function as protective agents (Muslin 2008; Ohori 2008; Montagut and Settleman 2009). Therefore, MAPKs might play a pivotal role in hypoxia.
In previous study, we found that 5-hydroxymethyl-2-furfural (5-HMF) could prevent damage from hypoxia in vitro. Pretreatment with 5-HMF markedly attenuated hypoxia-induced cell necrosis and apoptosis. And the protective role was closely associated with the level of p-ERK protein (Li et al. 2010). 5-HMF is a common major product of carbohydrate metabolism. Its molecular formula is C3H6O3. It exists abundantly in a lot of plants and foods which contain carbohydrate (Murkovic and Bornik 2007; Wu 2009). Accumulated studies have suggested that 5-HMF has favorable biological effects such as anti-oxidant activity, inhibiting sickling of red blood cells, and ameliorate hemorheology (Abdulmalik et al. 2005; Lin et al. 2008; Li et al. 2009). Whether 5-HMF may protect against acute hypoxic injury in vivo is still not clear. In this study, our goal was to investigate whether 5-HMF may protect mice against brain injury induced by hypobaric hypoxia and to further focus on MAPK pathway to explore the possible mechanisms involved.
Materials and methods
Materials
5-HMF (Sigma, USA); hematoxylin and eosin (HE) kits (Santa Cruz, USA); Evans blue dye (Sigma, USA); p-ERK and ERK (Santa Cruz, USA); p-JNK, JNK, and p-38 antibody (Cell Signal Technology, USA); and p-p38 and β-actin antibody (Sigma, USA) were used in this study.
Animals and groups
Healthy male Kunming mice weighing 25 ± 2 g were randomly divided into normoxia and hypoxia groups. In the hypoxia groups, 100 mg/kg 5-HMF or vehicle (saline water) were administered intraperitoneally 1 h before the onset of the hypoxic exposure. The normoxia groups were simultaneously under normoxia with 100 mg/kg 5-HMF or vehicle. We chose a dose of 100 mg/kg, in which 5-HMF could show an obviously protective effect against hypoxic injury after 1 h of administration (Abdulmalik et al. 2005). The animal protocol was approved by the University Institution Animal Care and Use Committee of Capital Medical University and was consistent with the NIH Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23).
Hypobaric hypoxia
Conscious mice were exposed to hypoxic condition in a decompression chamber (Guizhou Fenglei, China). Humidity in the chamber was maintained at 40–50% and temperature at 23–25°C. Mice were acclimatized in the chambers in room air for 30 min to 1 h before experiments. Two altitudes were selected based on trials and previous reports (Zhang et al. 2004, 2009) to create hypoxic conditions in the chambers: (1) 9,500 m (5.2% O2) at a velocity of approximately 45 m/s for lethal acute hypobaric hypoxia, under which untreated mice died in a relatively uniform time period, and (2) the altitude of 8,300 m (7.0% O2) at a velocity of approximately 20 m/s for sublethal acute hypobaric hypoxia, as it was severe enough to induce typical hypoxic organ injuries yet allowed the mice to survive so that hypoxia-induced changes in permeability of BBB, histological pathology, and the level of protein could be analyzed (Zhang et al. 2009).
Determination of the tolerance to lethal acute hypobaric hypoxia
Survival under lethal acute hypobaric hypoxia was examined in control mice and mice pretreated with 5-HMF (100 mg/kg). Mice in the control group were given the same dose of vehicle. The dose volume was 5 ml/kg. Control mice and mice pretreated with 5-HMF were intraperitoneally injected with vehicle or 100 mg/kg 5-HMF, respectively, 1 h before the onset of the lethal acute hypoxic exposure for 15 min. The above two groups were then exposed to simulated acute hypobaric hypoxia of 9,500 m. The time of death was measured after achieving the altitude and was defined as the time of the last breath by an investigator who was blindfolded. The survival rate (%) was defined as the number of surviving mice divided by the number of total mice.
Evans blue dye extravasation method
Permeability of the BBB was quantified using the modified Evans blue extravasation method as described previously (Kaya et al. 2004). Using this method, we quantified the concentration of albumin–Evans blue dye in brain homogenates.
For this assay, after exposure to sublethal acute hypoxia for 6 h, mice were anesthetized by an injection of sodium pentobarbital (60 mg/kg, i.p.) with five mice in each group. Briefly, Evans blue (1% in saline; 5 ml/kg) was intravenously administered 30 min before mice were euthanized. Perfusion was then performed through the left ventricle with saline to remove intravascular Evans blue until the fluid from the right atrium became colorless. The brain was quickly harvested, weighed, and homogenized in 2.5 ml of 0.1 M phosphate-buffered saline (pH 7.2). To precipitate the protein, 2.5 ml of 50% trichloroacetic acid was added, followed by vortex mixing for 2 min. The supernatant was cooled to 10°C and centrifuged at 12,000 rpm for 30 min. The Evans blue concentration was measured with a spectrophotometer at 610 nm and converted using a standardized curve to quantify the amount of Evans blue per brain weight (ng/mg).
Histological analysis
After exposure to sublethal acute hypoxia for 6 h, animals were immediately anesthetized by an injection of sodium pentobarbital (60 mg/kg, i.p.) and were then perfused with ice-cold phosphate-buffered saline followed by 4% paraformaldehyde via the left ventricle of the heart. The whole brain was removed and postfixed in 4% paraformaldehyde. For histological analysis with hematoxylin and eosin (HE) staining, consecutive coronal sections of 4 μm in thickness were prepared using paraffin sectioning technique. The hippocampus CA1 and the cortex above it were examined, and cellular damage was evaluated in each hemisphere.
Whole-tissue homogenate preparation
To determine the phosphorylation and protein levels of MAPKs, the whole cortex and hippocampus were extracted. Total protein extract buffer (RIPA buffer—50 mM Tris pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS), which contained protease inhibitors and phosphatase inhibitors, was added to each sample on ice for 30 min, and then samples were centrifuged for 20 min at 14,000 rpm. Protein concentration was determined by the Bradford protein assay, and the samples were stored at −80°C.
SDS–PAGE and western blot analysis
The phosphorylation and protein expression levels of MAPKs were analyzed by western blot. Briefly, 100 μg of protein/lane was loaded in 10% SDS–PAGE gel. Then, the proteins were transferred onto the nitrocellulose membranes (Bio-Rad, USA), 60 V for 2 h. The transferred proteins on the nitrocellulose membranes were washed for 10 min with 0.1% TBST followed by the blocking solution with 5% nonfat milk in TBST for 2 h.
First, the blocked membranes were incubated with p-ERK, p-p38, and p-JNK antibodies (1:1,000, 1:1,000, and 1:1,000 dilutions) overnight at 4°C, followed by incubation for 2 h with the secondary antibody (1:1,000 dilution). The membranes were washed three times (each for 10 min) in TBST after the incubation with the primary or secondary antibodies. Finally, the presence of the target proteins was revealed by a chemiluminescence assay.
To analyze the protein levels of ERK, p38, JNK, and actin, the levels were determined at the same time with the same protocol, and individual bands were quantified. Those membranes were respectively stripped using buffer containing 62.5 mM Tris–Cl (pH 6.7), 100 mM 2-mercaptoethanol, and 2% SDS for 20 min at 55°C following a period of constant shaking. Next, they were reprobed with primary antibody against ERK, p38, JNK, and actin at a 1:1,000, 1:1,000, 1:1,000, and 1:10,000 dilutions overnight at 4°C. The values of the relative optical density of each band corresponding to p-ERK, p-p38, and p-JNK were normalized to the value of ERK, p38, and JNK, respectively, to demonstrate protein levels.
Statistical analysis
Data were expressed as mean ± SD and assessed with one-way ANOVA followed by SPSS software. Comparisons between normoxic group and hypoxic group or hypoxic group and group pretreated with 5-HMF under hypoxia were made with unpaired Student’s t test. Differences between groups were considered to be significant at P <0.05.
Results
Effect of 5-HMF on tolerance to lethally acute hypoxia
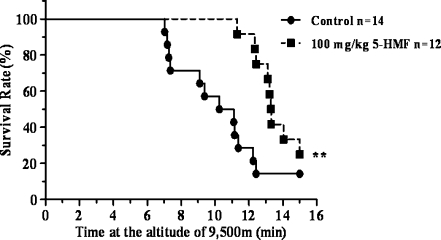
Hypobaric hypoxia is a specific physical environment occurring at high elevations. A high-altitude environment was replicated using a decompression chamber. After mice were exposed to 9,500 m of acute hypobaric hypoxia, survival rate and survival time of the mice were simultaneously recorded within 15 min. The survival rate of mice decreased to 14.3% after exposure to hypoxia for 15 min, which indicated that the extent of acute hypobaric hypoxia was lethal to most of the mice. Nevertheless, as shown in Fig. 1, 5-HMF improved the survival rate over two times (the survival rate increased by 33.3%). Compared to the hypoxia control, the survival time of mice pretreated with 100 mg/kg 5-HMF increased more than 3 min (13.8 ± 1.5 min vs. 10.5 ± 2.8 min, P < 0.01). Thus, these data suggest that a potential protective mechanism can be induced by 5-HMF and render the mice more resistance to hypoxia.
Fig. 1.
Kaplan–Meier survival plots of conscious mice exposed to lethal acute hypobaric hypoxia after 100 mg/kg 5-HMF administration. Mice were exposed to simulated acute hypobaric hypoxia at 9,500 m (lethal hypoxia) at a velocity of about 45 m/s at 21°C in a decompression chamber after 1 h of 5-HMF administration. Mice were monitored for survival for 15 min immediately after exposure to acute hypobaric hypoxia. Control mice received vehicle alone 1 h before being exposed to acute hypobaric hypoxia. **p < 0.01, versus control. Control group: n = 14; 100 mg/kg 5-HMF group: n = 12
Effect of 5-HMF on blood–brain barrier permeability
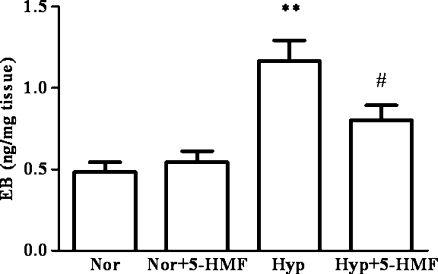
Cellular swelling in brain edema is together with BBB under pathological conditions. Here, BBB permeability and the effect of 5-HMF on it were evaluated by Evans blue dye extravasation method. Under normoxia, 5-HMF did not affect the permeability of the BBB; however, after hypoxia, there was an obvious increase in the concentration of Evans blue in brain extracts (Fig. 2, the concentration of 1.2 ± 0.3 ng/mg vs. 0.5 ± 0.1 ng/mg, P < 0.01), which indicated a high BBB permeability and a severe injury in the brain. The group pretreated with 5-HMF showed an obvious decrease in the concentration of Evans blue in comparison with the hypoxia control (0.8 ± 0.2 ng/mg vs. 1.2 ± 0.3 ng/mg, P < 0.05). This finding provides an evidence that acute hypobaric hypoxia can increase the permeability of BBB, and this deterioration in BBB permeability can be partly blocked by pretreatment with 5-HMF, which might be involved in the protective role of 5-HMF against hypoxic injury.
Fig. 2.
Effect of 5-HMF on BBB permeability under acute hypobaric hypoxia using Evans blue in mice. The animals were exposed to normoxia or simulated sublethally acute hypobaric hypoxia of 8,300 m in a decompression chamber for 6 h after 1 h of 100 mg/kg 5-HMF or vehicle administration. The Evans blue extravasation assay was used to evaluate the BBB permeability and it was measured as described in the “Materials and methods” section. The hypoxia group demonstrated a significantly higher concentration of Evans blue in brain tissue than the control group. 5-HMF treatment decreased Evans blue extravasation in hypoxia-stressed group. The Evans blue extravasation is converted using a standardized curve to quantify the amount of Evans blue per brain weight (ng/mg). All data were expressed as means ± SD, n = 5; **p < 0.01, versus normoxia control; #p < 0.05, versus hypoxia control. Nor normoxia, Hyp hypoxia, 5-HMF 100 mg/kg 5-HMF
Histological analysis in hippocampus and cortex
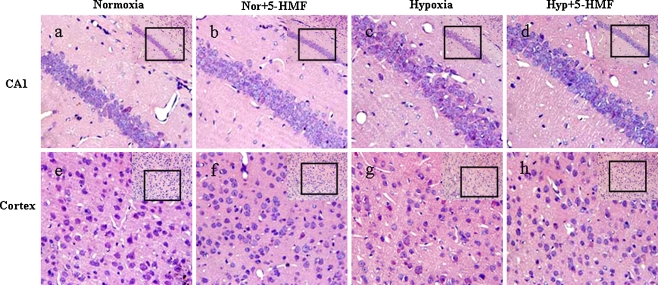
In brain areas, the hippocampal CA1 and cortex are particularly vulnerable to insults associated with lower oxygen supply. We examined the extent that hippocampal CA1 and cortical neurons were affected by acute exposure to sublethal hypobaric hypoxia (8,300 m, 6 h) and evaluated the effect of 5-HMF on it by hematoxylin and eosin (HE) staining.
In normoxia group, pyramidal neurons in CA1 were laid regularly and intensively in three to four layers and presented round and clear nucleoli (Fig. 3a). In contrast, widespread damage was evident in the hypoxia group (Fig. 3c). Pyramidal neurons either irregularly arranged and presented a shrunken appearance with minimal cytoplasm or had disappeared. 5-HMF did not block the insult, but reduced the effect of insult (Fig. 3d).
Fig. 3.
Prevention of acute hypobaric hypoxia-induced cellular damage or death in cortex and hippocampus by 100 mg/kg 5-HMF pretreatment 1 h before exposure to sublethally acute hypobaric hypoxia. The animals were exposed to normoxia or simulated sublethally acute hypobaric hypoxia of 8,300 m in a decompression chamber for 6 h after 1 h of 100 mg/kg 5-HMF or vehicle administration. Representative HE stains (n = 5) in the CA1 region of hippocampus (a–d) and the cortex (e–h) with low magnification insets in the upper right corners in the different conditions are shown. The box indicates the source of the high magnification image. a, e Normoxia control; b, f 5-HMF administration in normoxia; c, g hypoxia control; d, h 5-HMF administration in hypoxia. Nor normoxia, Hyp hypoxia, 5-HMF 100 mg/kg 5-HMF
Similarly, compared to the neurons in cortex in normoxia group (Fig. 3e), the nerve cells in the cortex from hypoxia group were loosely distributed and some cellular nucleus were shrunken, weakly stained, and even disappeared, which indicated that those neurons were extensively deteriorated or dying (Fig. 3g). This result was consistent with the previous studies about the hypobaric hypoxic injury in brain (Maiti et al. 2008). However, pretreatment of 100 mg/kg 5-HMF attenuated the deterioration of the nerve cells, which were less shrunken and showed a relatively normal appearance, consistent with the situation in hippocampus (Fig. 3h). In addition, almost all of the nerve cells appeared normal in cortex and hippocampal CA1 subfields of mice pretreated with 100 mg/kg 5-HMF under normoxia (Fig. 3b, f).
Effect of 100 mg/kg 5-HMF on MAPKs levels
MAPKs have been shown previously to play a pivotal role in adaptive process to hypoxic stress. But it is unclear whether MAPKs were also associated with the process of 5-HMF protection against acute hypobaric hypoxia-induced brain injury. Subsequently, we measured the levels of several classic MAPKs (ERK, JNK, and p38) in cortex and hippocampus by western blotting.
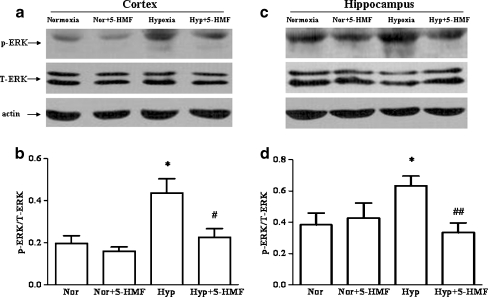
To determine the phosphorylation levels of ERK, the ratio of p-ERK to T-ERK in the cortex and hippocampus of mice were quantified with primary antibodies against p-ERK and T-ERK, respectively. Typical results in Fig. 4a, c and the quantitative analysis in Fig. 4b, d indicated that ERK phosphorylation levels increased (P < 0.05) in the cortex and hippocampus of mice from hypoxia group in comparison to the normoxia group. Interestingly, compared with the hypoxic groups, the immunoblots revealed a decrease in the level of p-ERK protein of the 5-HMF pretreated group under hypoxia. This result was consistent with our previous study in vitro (Li et al. 2010). The above data indicated that 5-HMF decreased ERK phosphorylation levels, which might be involved in the protective role of 5-HMF against acute hypobaric hypoxic injury.
Fig. 4.
Effect of 5-HMF pretreatment on the level of p-ERK protein. The animals were exposed to normoxia or simulated sublethally acute hypobaric hypoxia of 8,300 m in a decompression chamber for 6 h after 1 h of 100 mg/kg 5-HMF or vehicle administration. Whole tissue extracts from cortex and hippocampus were analyzed with western blotting. Representative results showed that the levels of p-ERK, not T-ERK, increased in the cortex (a) and hippocampus (c) of mice in response to hypoxic exposure. And 5-HMF treatment decreased the levels of p-ERK in hypoxia-stressed group. Quantitative analysis (b, d) verified an increase of p-ERK in the cortex and hippocampus of mice under hypoxia and an decrease of p-ERK in the 5-HMF treated hypoxia group. All data were expressed as means ± SD, n = 4; *p < 0.05, versus normoxia control; #p < 0.05, ##p < 0.01, versus hypoxia control. Nor normoxia, Hyp hypoxia, 5-HMF 100 mg/kg 5-HMF, p-ERK phosphorylated ERK, T-ERK total protein of ERK
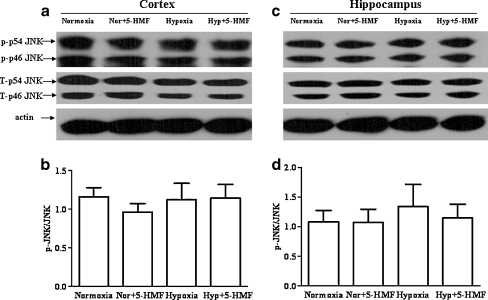
Furthermore, the phosphorylation levels of JNK and p38 in the cortex and hippocampus of mice were quantified by western blot respectively. As shown in Figs. 5 and 6, we did not find significant changes in these protein expression levels in the cortex and hippocampus following acute hypobaric hypoxia, although the phosphorylational levels of JNK and p38 in the hippocampus showed a trend of raise. In addition, we noticed that compared with the hypoxia group, 5-HMF did not change the levels of p-JNK or p-p38 both in the cortex and in the hippocampus after the mice were exposed to acute hypobaric hypoxia. These results suggest that JNK and p38 pathways might not be involved in the protection of 5-HMF against hypoxia.
Fig. 5.
Effect of 5-HMF pretreatment on the level of p-JNK protein. The animals were exposed to normoxia or simulated sublethally acute hypobaric hypoxia of 8,300 m in a decompression chamber for 6 h after 1 h of 100 mg/kg 5-HMF or vehicle administration. Whole-tissue extracts from cortex and hippocampus were analyzed with western blotting. Representative results and quantitative analysis showed that the levels of p-JNK did not change significantly in the cortex (a, b) and hippocampus (c, d) of mice under hypoxia. And 5-HMF treatment did not change the levels of p-JNK in hypoxia-stressed group. All data were expressed as means ± SD, n = 4. Nor normoxia, Hyp hypoxia, 5-HMF 100 mg/kg 5-HMF, p-p38 phosphorylated JNK, T-p38 total protein of JNK
Fig. 6.
Effect of 5-HMF pretreatment on the level of p-p38 protein. The animals were exposed to normoxia or simulated sublethally acute hypobaric hypoxia of 8,300 m in a decompression chamber for 6 h after 1 h of 100 mg/kg 5-HMF or vehicle administration. Whole-tissue extracts were analyzed with western blotting. Representative results and quantitative analysis showed that the levels of p-p38 did not change significantly in the cortex (a, b) and hippocampus (c, d) of mice under hypoxia. And 5-HMF treatment did not change the levels of p-p38 in hypoxia-stressed group. All data were expressed as means ± SD, n = 4. Nor normoxia, Hyp hypoxia, 5-HMF 100 mg/kg 5-HMF, p-p38 phosphorylated p38, T-p38 total protein of p38
Discussion
High altitude is a special living environment in which hypobaric hypoxia severely threatens human health. When there is failure of adaptation to the environment, the human body will generate maladaptive responses that lead to various forms of acute altitude illness, such as AMS, HACE, HAPE, etc. (Stream et al. 2009; Wilson et al. 2009). Higher BBB permeability is thought to be the main reason that leads to HACE (Yarnell et al. 2000). Preventive measures to inhibit the higher BBB permeability will attenuate the altitude sickness in plateau. Drug development would be an effective means, but there is still lack of extensive studies in this field. Therefore, it is very important to find a critical molecule involved in protecting against acute hypobaric hypoxic injury.
5-HMF, the final product of carbohydrate metabolism, has been reported to exist abundantly in a lot of plants and foods which have carbohydrate (Murkovic and Bornik 2007; Wu 2009). Some studies have reported the role of 5-HMF in anti-oxidation (Choi et al. 2007; Li et al. 2009). The role of 5-HMF in anti-hypoxia was not known until we found the protective role against hypoxia in vitro (Li et al. 2010). Can 5-HMF protect against hypoxia in vivo? What is the underlying mechanism? In the present study, we have focused on demonstrating the protective effects of 5-HMF against acute hypobaric hypoxia in mice and the possible molecular mechanisms underlying the effects.
The environment of hypobaric hypoxia in high altitude was mimicked as described before. Our results indicated that 5-HMF could increase both the survival time and the survival rate of mice under hypobaric hypoxia, thus it confirmed the protective effects of 5-HMF against hypoxia. Altitude-induced hypoxia can induce various changes which leads to pathological increases of cerebral blood flow, changes of permeability in BBB, and cerebral edema (Zengren et al. 1999; Yuan et al. 2008; Natah et al. 2009). The protective effect of 5-HMF on mice might be related to changes of permeability in BBB and lessening of brain injury.
Cellular swelling in brain injury is together with BBB under pathological conditions (Beaumont et al. 2000; Fischer et al. 2002). Interestingly, our results indicated that acute hypobaric hypoxia for 6 h increased Evans blue dye extravasation and 5-HMF obviously attenuated the extravasation, which indicated that 5-HMF protected the integrity of BBB under acute hypobaric hypoxia. Furthermore, we found that the nerve cells in cortex and hippocampus scattered and presented a shrunken appearance or had disappeared after acute hypobaric hypoxia. And 100 mg/kg of 5-HMF reduced the cellular damage partly and made them arrange more closely. These findings strongly suggested that 5-HMF exerted a protective effect against acute hypobaric hypoxia-induced brain edema and protected BBB from the injury caused by acute hypobaric hypoxia.
We confirmed that 5-HMF protected against brain injury induced by acute hypobaric hypoxia; further investigation was performed with focuses on the possible molecular mechanisms involved in the protective effects of 5-HMF. As noted, MAPKs are early responders to hypoxic conditions among numerous signal molecules (Kim et al. 2009). MAPKs are serine/threonine kinases that regulate various cellular responses such as proliferation, differentiation, and apoptosis (Cross et al. 2000; Pearson et al. 2001). In this study, we tested the responses of three subfamily members of MAPK (ERK, JNK, and p38) to acute hypobaric hypoxia and the effects of 5-HMF on them in the cortex and hippocampus of mice. The results showed that it was ERK rather than JNK and p38 that was activated by acute hypobaric hypoxia and 5-HMF inhibited the activation. ERK, an extracellular signal regulated kinase, is a key molecule responsible for damage/survival under hypoxia (Robinson and Cobb 1997; Johnson and Lapadat 2002). Phosphorylation of ERK is activated in response to hypoxia and the effect changes with different conditions, depending on the stimulus and its persistence time (Singh et al. 2007; Werlen et al. 2003). A recent study demonstrated that inhibition of p-ERK could protect hippocampal cells of mice from death (Park et al. 2008). Consistent with this study, our study showed that 5-HMF reduced the p-ERK activated by hypoxia and exerted the protection against hypoxic injury in the cortex and hippocampus of mice. All together, these findings suggested that 5-HMF might protect against brain injury induced by acute hypobaric hypoxia via an ERK-mediated pathway.
In conclusion, this is the first time to be demonstrated that 5-HMF can prevent damage from acute hypobaric hypoxia-induced brain injury. Although the mechanisms are not fully elucidated, the protective role of 5-HMF is closely associated with the maintenance of integrity of BBB, attenuating the cerebral damage and inhibiting the level of p-ERK. Further studies may be necessary to better understand the protective role of 5-HMF in response to hypoxia.
Electronic supplementary material
Below is the link to the electronic supplementary material.
(JPEG 78 kb)
Acknowledgments
We would like to thank Dr. Ping Liang, the executive editor of Progress in Natural Science and Dr. Ying Wu in Institute of Basic Medical Sciences, for their critical reading of this manuscript. This work was supported by the National Basic Research Program of China (2006CB504100), and the grants of the Natural Sciences Foundation of China (30831160514, 81071066, and 81000856).
Abbreviations
- 5-HMF
5-Hydroxymethyl-2-furfural
- MAPK
Mitogen-activated protein kinases
- BBB
Blood–brain barrier
- HE
Hematoxylin and eosin
- ERK
Extracellular signal regulated kinases
- JNK
c-Jun N-terminal kinases
- AMS
Acute mountain sickness
- HACE
High-altitude cerebral edema
- HAPE
High-altitude pulmonary edema
Footnotes
Ming-Ming Li and Li-Ying Wu contributed equally to this work.
Contributor Information
Ling-Ling Zhu, Phone: +86-682-10077, FAX: +86-10-68213039, Email: linglingzhu@hotmail.com.
Ming Fan, Phone: +86-10-68214026, FAX: +86-10-68213039, Email: fanming@nic.bmi.ac.cn.
References
- Abdulmalik O, Safo MK, Chen Q, Yang J, Brugnara C, Ohene-Frempong K, Abraham DJ, Asakura T. 5-Hydroxymethyl-2-furfural modifies intracellular sickle haemoglobin and inhibits sickling of red blood cells. Br J Haematol. 2005;128:552–561. doi: 10.1111/j.1365-2141.2004.05332.x. [DOI] [PubMed] [Google Scholar]
- Beaumont A, Marmarou A, Hayasaki K, Barzo P, Fatouros P, Corwin F, Marmarou C, Dunbar J. The permissive nature of blood brain barrier (BBB) opening in edema formation following traumatic brain injury. Acta Neurochir Suppl. 2000;76:125–129. doi: 10.1007/978-3-7091-6346-7_26. [DOI] [PubMed] [Google Scholar]
- Cagnol S, Chambard JC. ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- Choi CW, Jung HA, Kang SS, Choi JS. Antioxidant constituents and a new triterpenoid glycoside from Flos Lonicerae. Arch Pharm Res. 2007;30:1–7. doi: 10.1007/BF02977770. [DOI] [PubMed] [Google Scholar]
- Cross TG, Scheel-Toellner D, Henriquez NV, Deacon E, Salmon M, Lord JM. Serine/threonine protein kinases and apoptosis. Exp Cell Res. 2000;256:34–41. doi: 10.1006/excr.2000.4836. [DOI] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/S0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Fischer S, Wobben M, Marti HH, Renz D, Schaper W. Hypoxia-induced hyperpermeability in brain microvessel endothelial cells involves VEGF-mediated changes in the expression of zonula occludens-1. Microvasc Res. 2002;63:70–80. doi: 10.1006/mvre.2001.2367. [DOI] [PubMed] [Google Scholar]
- Gallagher SA, Hackett PH. High-altitude illness. Emerg Med Clin North Am. 2004;22:329–355. doi: 10.1016/j.emc.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kaya M, Gulturk S, Elmas I, Kalayci R, Arican N, Kocyildiz ZC, Kucuk M, Yorulmaz H, Sivas A. The effects of magnesium sulfate on blood-brain barrier disruption caused by intracarotid injection of hyperosmolar mannitol in rats. Life Sci. 2004;76:201–212. doi: 10.1016/j.lfs.2004.07.012. [DOI] [PubMed] [Google Scholar]
- Kim JY, Kim YJ, Lee S, Park JH. The critical role of ERK in death resistance and invasiveness of hypoxia-selected glioblastoma cells. BMC Cancer. 2009;9:27. doi: 10.1186/1471-2407-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YX, Li Y, Qian ZJ, Kim MM, Kim SK. In vitro antioxidant activity of 5-HMF isolated from marine red alga Laurencia undulata in free-radical-mediated oxidative systems. J Microbiol Biotechnol. 2009;19:1319–1327. doi: 10.4014/jmb.0901.00004. [DOI] [PubMed] [Google Scholar]
- Li MM, Wu LY, Zhao T, Xiong L, Huang X, Liu ZH, Fan XL, Xiao CR, Gao Y, Ma YB, Chen JJ, Zhu LL, Fan M. The protective role of 5-HMF against hypoxic injury. Cell Stress Chaperones. 2010 doi: 10.1007/s12192-010-0238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin A. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays. 2003;25:17–24. doi: 10.1002/bies.10204. [DOI] [PubMed] [Google Scholar]
- Lin AS, Qian K, Usami Y, Lin L, Itokawa H, Hsu C, Morris-Natschke SL, Lee KH. 5-Hydroxymethyl-2-furfural, a clinical trials agent for sickle cell anemia, and its mono/di-glucosides from classically processed steamed Rehmanniae Radix. J Nat Med. 2008;62:164–167. doi: 10.1007/s11418-007-0206-z. [DOI] [PubMed] [Google Scholar]
- Maiti P, Singh SB, Mallick B, Muthuraju S, Ilavazhagan G. High altitude memory impairment is due to neuronal apoptosis in hippocampus, cortex and striatum. J Chem Neuroanat. 2008;36:227–238. doi: 10.1016/j.jchemneu.2008.07.003. [DOI] [PubMed] [Google Scholar]
- Mebratu Y, Tesfaigzi Y. How ERK1/2 activation controls cell proliferation and cell death: is subcellular localization the answer? Cell Cycle. 2009;8:1168–1175. doi: 10.4161/cc.8.8.8147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagut C, Settleman J. Targeting the RAF–MEK–ERK pathway in cancer therapy. Cancer Lett. 2009;283:125–134. doi: 10.1016/j.canlet.2009.01.022. [DOI] [PubMed] [Google Scholar]
- Murkovic M, Bornik MA. Formation of 5-hydroxymethyl-2-furfural (HMF) and 5-hydroxymethyl-2-furoic acid during roasting of coffee. Mol Nutr Food Res. 2007;51:390–394. doi: 10.1002/mnfr.200600251. [DOI] [PubMed] [Google Scholar]
- Muslin AJ. MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin Sci Lond. 2008;115:203–218. doi: 10.1042/CS20070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natah SS, Srinivasan S, Pittman Q, Zhao Z, Dunn JF. Effects of acute hypoxia and hyperthermia on the permeability of the blood–brain barrier in adult rats. J Appl Physiol. 2009;107:1348–1356. doi: 10.1152/japplphysiol.91484.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohori M. ERK inhibitors as a potential new therapy for rheumatoid arthritis. Drug News Perspect. 2008;21:245–250. doi: 10.1358/dnp.2008.21.5.1219006. [DOI] [PubMed] [Google Scholar]
- Ono K, Han J. The p38 signal transduction pathway: activation and function. Cell Signal. 2000;12:1–13. doi: 10.1016/S0898-6568(99)00071-6. [DOI] [PubMed] [Google Scholar]
- Park HJ, Kim HJ, Ra J, Zheng LT, Yim SV, Chung JH. Protective effect of topiramate on kainic acid-induced cell death in mice hippocampus. Epilepsia. 2008;49:163–167. doi: 10.1111/j.1528-1167.2007.01308.x. [DOI] [PubMed] [Google Scholar]
- Pearson G, Robinson F, Beers Gibson T, Xu BE, Karandikar M, Berman K, Cobb MH. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr Rev. 2001;22:153–183. doi: 10.1210/er.22.2.153. [DOI] [PubMed] [Google Scholar]
- Roach RC, Hackett PH. Frontiers of hypoxia research: acute mountain sickness. J Exp Biol. 2001;204:3161–3170. doi: 10.1242/jeb.204.18.3161. [DOI] [PubMed] [Google Scholar]
- Robinson MJ, Cobb MH. Mitogen-activated protein kinase pathways. Curr Opin Cell Biol. 1997;9:180–186. doi: 10.1016/S0955-0674(97)80061-0. [DOI] [PubMed] [Google Scholar]
- Singh S, Upadhyay AK, Ajay AK, Bhat MK. p53 regulates ERK activation in carboplatin induced apoptosis in cervical carcinoma: a novel target of p53 in apoptosis. FEBS Lett. 2007;581:289–295. doi: 10.1016/j.febslet.2006.12.035. [DOI] [PubMed] [Google Scholar]
- Stream JO, Luks AM, Grissom CK. Lung disease at high altitude. Expert Rev Respir Med. 2009;3:635–650. doi: 10.1586/ers.09.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissot van Patot MC, Keyes LE, Leadbetter G, Hackett PH. Ginkgo biloba for prevention of acute mountain sickness: does it work? High Alt Med Biol. 2009;10:33–43. doi: 10.1089/ham.2008.1085. [DOI] [PubMed] [Google Scholar]
- Weerackody RP, Welsh DJ, Wadsworth RM, Peacock AJ. Inhibition of p38 MAPK reverses hypoxia-induced pulmonary artery endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1312–H1320. doi: 10.1152/ajpheart.00977.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werlen G, Hausmann B, Naeher D, Palmer E. Signaling life and death in the thymus: timing is everything. Science. 2003;299:1859–1863. doi: 10.1126/science.1067833. [DOI] [PubMed] [Google Scholar]
- Wilson MH, Newman S, Imray CH. The cerebral effects of ascent to high altitudes. Lancet Neurol. 2009;8:175–191. doi: 10.1016/S1474-4422(09)70014-6. [DOI] [PubMed] [Google Scholar]
- Wright A, Brearey S, Imray C. High hopes at high altitudes: pharmacotherapy for acute mountain sickness and high-altitude cerebral and pulmonary oedema. Expert Opin Pharmacother. 2008;9:119–127. doi: 10.1517/14656566.9.1.119. [DOI] [PubMed] [Google Scholar]
- Wu CD. Grape products and oral health. J Nutr. 2009;139:1818S–1823S. doi: 10.3945/jn.109.107854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarnell PR, Heit J, Hackett PH. High-altitude cerebral edema (HACE): the Denver/Front Range experience. Semin Neurol. 2000;20:209–217. doi: 10.1055/s-2000-9830. [DOI] [PubMed] [Google Scholar]
- Yuan F, Guo Z, Xu Y, Wang X, Bu HM, Zhong N, Zhang Y, Zhou ZN. Comparison of the effects of chronic intermittent hypobaric hypoxia and continuous hypobaric hypoxia on hemodynamics in rats. Sheng Li Xue Bao. 2008;60:687–694. [PubMed] [Google Scholar]
- Zengren Y, Jiaying L, Fengzhi L, Peihua Y, Youmei L, Fangren S. Effect of acute hypoxia and hypoxic acclimation on hemorheological behavior in rats with frostbite. Clin Hemorheol Microcirc. 1999;20:189–195. [PubMed] [Google Scholar]
- Zhang SX, Miller JJ, Gozal D, Wang Y. Whole-body hypoxic preconditioning protects mice against acute hypoxia by improving lung function. J Appl Physiol. 2004;96:392–397. doi: 10.1152/japplphysiol.00829.2003. [DOI] [PubMed] [Google Scholar]
- Zhang N, Gao G, Bu X, Han S, Fang L, Li J. Neuron-specific phosphorylation of c-Jun N-terminal kinase increased in the brain of hypoxic preconditioned mice. Neurosci Lett. 2007;423:219–224. doi: 10.1016/j.neulet.2007.07.028. [DOI] [PubMed] [Google Scholar]
- Zhang K, Zhao T, Huang X, Liu ZH, Xiong L, Li MM, Wu LY, Zhao YQ, Zhu LL, Fan M. Preinduction of HSP70 promotes hypoxic tolerance and facilitates acclimatization to acute hypobaric hypoxia in mouse brain. Cell Stress Chaperones. 2009;14:407–415. doi: 10.1007/s12192-008-0094-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
(JPEG 78 kb)