Abstract
Type 2 diabetes is often associated with high blood cholesterol. Here, we investigated the effect of cholesterol loading on MIN6 cells derived from pancreatic β cells. Exposure of MIN6 cells to cholesterol-induced apoptosis in time- and dose-dependent manner. Treatment with methyl-β-cyclodextrin that removes cholesterol from plasma membrane prevented the cells from cholesterol-induced apoptosis. Western blot analysis revealed that the levels of phosphorylated-p38 mitogen-activated protein kinase (P-p38 MAPK) and c-Jun N-terminal kinases (P-JNK) were significantly increased after the cholesterol loading, suggesting that the stress-activated protein kinase signaling was stimulated. A specific p38 inhibitor rescued MIN6 cells from cholesterol-induced apoptosis, while JNK inhibitor failed, suggesting the importance of activation of p38 MAPK signaling in response to cholesterol. The expression of Bip and CHOP, the endoplasmic reticulum (ER) stress markers, remained unaffected, indicating that the ER stress may not be involved in the cytotoxicity of cholesterol on the ΜΙΝ6 cells. The intracellular concentration of reactive oxygen species measured by use of 2′,7′-dichlorofluorescin diacetate was significantly increased after cholesterol loading, demonstrating the induced apoptosis was mediated through oxidative stress. Addition of reduced form of glutathione in the medium rescued MIN6 cells from apoptosis induced by cholesterol loading. Taken together, these results demonstrate that the free cholesterol loading can induce apoptosis of MIN6 cells mediated by oxidative stress and the activation of p38 MAPK signaling.
Electronic supplementary material
The online version of this article (doi:10.1007/s12192-011-0265-7) contains supplementary material, which is available to authorized users.
Keywords: Cholesterol, MIN6, Apoptosis, Oxidative stress
Introduction
Type 2 diabetes (T2D) is characterized by an inability of the endocrine pancreas to secrete sufficient insulin to meet the metabolic demands associated with insulin resistance and obesity. The final common pathway in the pathogenesis of T2D, however, is the failure of β cells in the pancreas, which occurs when they can no longer sustain insulin hypersecretion in the setting of insulin resistance. The decreased cell mass and increased apoptosis of β cells were observed in T2D patients (Prentki and Nolan 2006). The reasons for β cell failure in T2D are not known.
The growing evidences demonstrated hyperlipidemia in obesity and type 2 diabetes is characterized by high levels of free fatty acids, low-density lipoprotein (LDL), triglyceride, and cholesterol (Prentki and Nolan 2006). It is of note that rat and human β cells express high-affinity LDL receptors leading to lipid accumulation in β cells (Cnop et al. 2002; Grupping et al. 1997). It was also reported LDL causes rat β-cell death (Cnop et al. 2002). However, how LDL exerts toxic effect on β cells is still not fully understood. LDL belongs to the lipoprotein family, which mainly transports cholesterol from the liver and small intestine to other peripheral tissues. While elevated levels of plasma cholesterol are a common feature of patients with T2D, the potential role of cholesterol in lipotoxic disease of islets has only recently been explored. For example, ATP-binding cassette transporter, subfamily A, member 1 (ABCA1) is a cellular cholesterol transporter. It was reported that the absence of ABCA1 in β cells results in decreased exocytosis of insulin, and also results in decreased cholesterol efflux and accumulation of cholesterol in the plasma membrane (Brunham et al. 2007; de Souza et al. 2010). These studies indicate that cholesterol is an important factor in mediating the toxic effect of LDL on β cells. In this study, we utilized MIN6 cell line to examine the effect of cholesterol loading on β cells. MIN6 cell line derived from a transgenic mouse expressing the large T-antigen of SV40 in pancreatic β cells exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets therefore is widely used in the experiments for diabetic research (Ishihara et al. 1993)
We used the cholesterol/methyl β-cyclodextrin complexes to deliver cholesterol to ΜΙΝ6 to mimic the increase of endogenous cholesterol induced by elevated plasma LDL, avoiding the influences from other component of LDL. The most recent studies also utilized similar cholesterol/cyclodextrin (CD) complexes and demonstrated that excessive cholesterol uptake by β cells may contribute to apoptosis partially due to mitochondrial dysfunction, although they did not give detailed molecular mechanism (Zhao et al. 2010). It was demonstrated that elevated cholesterol content in lipoproteins, as seen in hypercholesterolemia, favors the activation of the stress-activated phosphorylated-p38 mitogen-activated protein kinase (p38-MAPK) pathway in cells from the vessel wall (Dobreva et al. 2005). Our present study demonstrates that elevation of intracellular cholesterol directly induces apoptosis of pancreatic β cells through the generation of intracellular reactive oxygen species (ROS) and activation of phosphorylated-p38 mitogen-activated protein kinase (p38 MAPK) signaling.
Materials and methods
Reagents
250× Cholesterol lipid concentrate (CLC) was purchased from GIBCO (Grand Island, NY, USA). It consists of methyl-β-CD and cholesterol in an optimized ratio containing the cholesterol about 7 mM. It was used for cholesterol loading to MIN6 at 1:250–1:50 dilutions. Methyl-β-CD was obtained from Sigma and used to deplete cholesterol from MIN6 cells at 0.125%. SB20358, SP600125 and reduced form of glutathione (GSH) were obtained from Beyotime Institute of Biotechnology (Shanghai, China). The specificity of SB203580 as p38 inhibitor and SP600125 as c-Jun N-terminal kinases (JNK) inhibitor was demonstrated in the previous studies (Bennett et al. 2001; Cuenda et al. 1995; Saklatvala et al. 1996; Ward et al. 1997).
Cell line
MIN6 cells were kindly donated from Prof. J. Miyazaki (University of Osaka, Osaka, Japan). MIN6 cells were grown in Dulbecco's modified Eagle's medium (25 mmol/l glucose) equilibrated with 5% CO2 and 95% air at 37 C. The medium was supplemented with 10% fetal bovine serum, 50 mg/l streptomycin, and 75 mg/1 penicillin sulfate. MIN6 cells used in the present study were harvested at passages 16–23. When indicated, the cells were incubated with diluted CLC at different time intervals. The sterol contents in the whole cell extracts were determined by an enzymatic cholesterol assay kit. When MIN6 cells were incubated with 250× diluted CLC and 2 mM reduced glutathione (cho./GSH), they were incubated for 24 h.
The cell images were obtained by a phase-contrast microscope (IMT-2, Olympus, Tokyo, Japan) equipped with a digital microscope camera (PDMC II, Polaroid, Waltham, MA, USA).
Determination of total sterol contents
Lipid was extracted by the method of Bligh and Dyer (1959). Total sterol contents in the lipid were measured by an enzymatic cholesterol assay kit (Roche Diagnostics, Mannheim, Germany), which determines the levels of 3β-hydroxysterols.
Analysis of adherent cell number and apoptosis assay
The number of adherent cells after cholesterol loading was analyzed by removing the detached cells by washing the plate once with the medium. The adherent cells were counted after trypsinization and collection by centrifugation.
Apoptosis of MIN6 cells were assessed by immunocytochemistry of active caspase-3 and the terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate-biotin nick end labeling (TUNEL) method using an in situ apoptosis kit (Takara, Otsu, Japan). Procedures for immunocytochemical analysis were described previously (Lu et al. 2006, 2008). In brief, after fixation and blocking, the cells were incubated with the first antibody against rabbit active caspase-3 (Sigma-Aldrich, St. Louis, MO, USA) followed by incubation with anti-rabbit IgG antibody conjugated with Alexa Fluor 488 (Molecular Probes, Eugene, OR, USA). For the detection of Alexa fluor-568 fluorescence, the main beam splitter for excitation, the secondary beam splitter for emission, and barrier filter were 568, 570, and 585 nm long pass, respectively. Several images were captured with the same set of optical parameters. The densitometric analysis was performed using a Multi-Gauge software in LAS-1000 (Fuji Film).
Determination of insulin secretion
MIN6s were exposed to 250× diluted CLC for 24 h in the growth medium. After washing with Krebs–Ringer buffer (KRB) containing 3 mmol/l of glucose, the cells were incubated with the KRB buffer for 1 h and then transferred to the medium containing 20 mmol/l of glucose. At the end of the 1-h stimulation period, the amount of insulin released in the spent medium was determined by ELISA insulin kit (obtained from R & D System Inc. Minneapolis, MN, USA), according to the manufacturer’s instructions.
Western blot analysis
Procedures for preparation of whole cell lysates and Western blot analysis were described previously (Lu et al. 2006, 2008). In brief, whole cell lysates (30 μg/lane) were separated by 10% SDS-PAGE, and transferred onto polyvinylidene difluoride membrane (Amersham Pharmacia, Piscataway, NJ, USA). The blots were probed with the first antibodies as described below, followed by incubation with horseradish peroxidase-conjugated anti-rabbit or mouse IgG antibody. Rabbit anti-phospho-p38 MAPK (T180/Y182), anti-phospho-Akt (S347), and anti-phospho-JNK (T183/Y185) were purchased from Cell Signaling (Beverly, MA, USA). Mouse monoclonal anti-binding immunoglobulin protein (Bip)/GRP78 antibody was from BD Biosciences (Bedford, MA, USA). Rabbit anti-C/EBP homologous protein (CHOP; GADD153) was from Santa Cruz (CA, USA). Rabbit anti-actin and anti-cleaved caspase-3 antibody was from Sigma-Aldrich. The proteins were visualized using enhanced chemiluminescence reagents (Pierce, Rockford, IL, USA). The images of the blotted membranes were obtained by an LAS-1000 lumino-image analyzer (Fuji Film, Tokyo, Japan). Densitometric analysis was performed with the same instrument.
Measurement of cellular ROS production
Intracellular ROS were measured by a fluorescent dye technique (Kozaki et al. 2007). MIN6 cells were cultured on glass cover slips and were treated for 30 min with 20 μM 2′,7′-dichlorofluorescin diacetate (H2DCFDA, Molecular Probes) in phosphate buffered saline. The cover slips were fixed and mounted. For the detection of fluorescence of 2′,7′-dichlorofluorescein, the main beam splitter for excitation, the secondary beam splitter for emission, and barrier filter were 488, 570, and 505 nm long pass, respectively. Several images were captured with the same set of optical parameters. The densitometric analysis was performed using a Multi-Gauge software in LAS-1000 (Fuji Film).
Statistical analysis
Statistical analysis was performed with ANOVA followed by Bonferroni’s multiple t test, and a p value <0.05 was considered statistically significant.
Results
Cholesterol loading induces apoptosis of MIN6
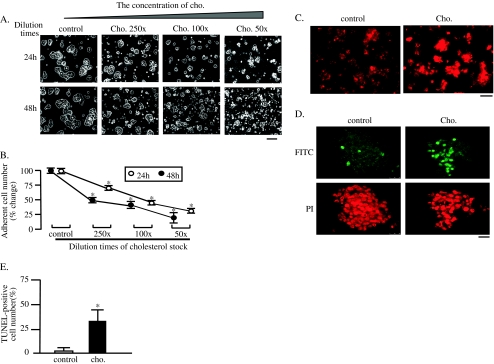
250× CCLC used in the present study is protein-free water-soluble cholesterol/methyl-β-CD complexes, using the CD as a vehicle (Gorfien et al. 2000). The concentration of CD in the CLC is very low (about 10−4%), enabling us to load cholesterol to MIN6 cells without inducing significant cell toxicity. As shown in supplemental Figs. 1a, b, loading of CD up to 0.001% did not have any effect on adherent cell number and morphology. The CLC was diluted 50, 100, and 250 times by the growth medium and loaded to MIN6 cells. As shown in Fig. 1a, many MIN6 cells were detached within 24 h after cholesterol loading in a dose-dependent manner; a significant decrease being observed at 250 times dilution of the CLC. On the other hand, most of cholesterol-unloaded cells (control) remained adherent. The number of detached cells after cholesterol loading for 48 h was significantly higher than that after 24-h loading of cholesterol stock. These data demonstrated that cholesterol loading induces deleterious effect on cell adherence of MIN6 cells in dose- and time-dependent manners. Statistical analysis demonstrated that decrease in the adherent cell number induced by cholesterol loading were significant (Fig. 1b). The 250 times dilution of the CLC was used in the subsequent experiments.
Fig. 1.
Cholesterol loading induces apoptosis of MIN6. CLC was diluted as indicated and loaded to MIN6 cells at intervals. The cell images were obtained with a phase-contrast microscope at 24 and 48 h after the exposure. Bar 25 μm (a). The number of adherent cells were counted after removing the detached cells and trypsinization, and expressed as percentage of the number of cholesterol-non-loaded control cells (b). Mean ± SD (n = 3). *p < 0.05 vs. the levels of control. At 24 h after exposure to cholesterol, the cells cultured on the cover slips were fixed and subjected to immunocytochemistry with the first antibody against rabbit active caspase-3 and the second rabbit IgG conjugated with Alexa fluor 568. Representative images are shown. Bar 25 μm (c). DNA fragmentation was also analyzed by the TUNEL method at 24 h after cholesterol exposure. Representative images are shown. Bar 25 μm (d). The ratio of TUNEL-positive to adherent cells is shown in graphical form. Mean ± SD (n = 5). *p < 0.05 vs. the level of control (e)
To determine whether the cell death of MIN6s was because of apoptosis, both cholesterol-loaded and -nonloaded cells cultured on the cover slips were subjected to the immunocytochemistry using the antibody against the active caspase-3. Caspase-3 is synthesized as inactive pro-enzyme that is processed in cells undergoing apoptosis by self-proteolysis and/or cleavage by other upstream proteases (e.g., caspases 8, 9, and 10). The detection of active caspase-3 is widely used in many cell lines undergoing apoptosis (Jakob et al. 2008; Resendes et al. 2004). As shown in Fig. 1c, cells with intense fluorescent signals were significantly more in the cholesterol-loaded group than those in control group. This result demonstrated that cholesterol loading induces apoptosis of MIN6 cells. This was again confirmed by TUNEL staining (Fig. 1d). Only few TUNEL-positive cells were detected in control cells, whereas about 40% of the adherent cholesterol-loaded cells were positively stained at 24 h. This increase in the number of TUNEL-positive cells was significant after cholesterol load (Fig. 1e).
It was also reported that the insulin secretion was impaired by cholesterol accumulation in MIN6 cells (Hao et al. 2007). We thus examined the effect of cholesterol loading on the insulin secretion in MIN6 cells. As shown in supplemental Fig. 2, the insulin secretion in response to 20 mM glucose in cholesterol-loaded cells was much less than that in control cells, suggesting that the glucose-stimulated insulin secretion was also significantly impaired by cholesterol-loading in β cells.
Cholesterol depletion prevents MIN6 from apoptosis induced by cholesterol loading
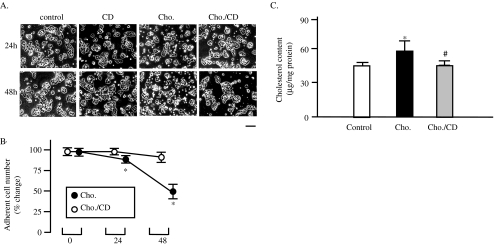
Methyl-β-CD is a specific cholesterol-binding agent that selectively extracts the unesterified cholesterol from plasma membrane (Gustavsson et al. 1999). To confirm the apoptosis of MIN6s because of cholesterol accumulation on plasma membrane, the experiment using CD was performed. As shown in Fig. 2a, many of cholesterol-loaded MIN6 cells were detached at 24 and 48 h after cholesterol exposure (Fig. 2a, cho.). The treatment with 0.125% of CD in cholesterol-loaded cells, however, prevented cells from detachment induced by cholesterol loading (Fig. 2a, CD/cho.). Treatment of the control cells with 0.125% of CD did not affect the viability of cells attachment or cell morphology, indicating the minimal cytotoxicity of CD used (Fig. 2a, CD). The difference in adherent cell numbers between cholesterol-loaded and CD/cholesterol-loaded loading cells was significant (Fig. 2b).
Fig. 2.
CD reverses cytotoxicity of cholesterol in MIN6. MIN6 cells were exposed to 250× diluted CLC (cho.) or 0.125% CD (CD) alone, or diluted CLC and CD together (cho./CD). The cell images were obtained with a phase-contrast microscope at 24 and 48 h after the exposure. Bar 25 μm (a). The numbers of adherent cells were expressed as percentage of the number of control cells (b). Mean ± SD (n = 3). *p < 0.05 vs. the levels of cholesterol loaded-cells (cho.). MIN6 cells were exposed to cholesterol with or without CD for 24 h, and then sterol contents in the whole cell extracts were determined by an enzymatic cholesterol assay kit (c). n = 3, mean ± SD; *p < 0.05 vs. the levels of contro; #p < 0.05 vs. the levels of cholesterol-loaded cells (cho.)
The effects of cholesterol loading and CD treatment on total sterol contents in MIN6s were studied (Fig. 2c). The treatment of cholesterol-loaded cells with CD restored the cellular cholesterol contents in MIN6 cells.
These data thus strongly suggested the viability of MIN6s was sensitive to the cholesterol accumulation in plasma membrane.
Stress-related cell signaling is activated under cholesterol loading on MIN6 cells
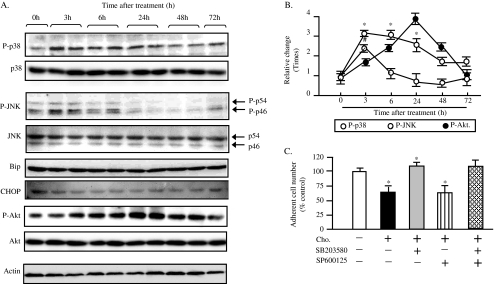
Hypercholesterolemia is the major risk factor for atherosclerosis. Free cholesterol accumulation induces apoptosis of macrophage through endoplasmic reticulum (ER) stress or oxidative stress (Feng et al. 2003; Yao and Tabas 2001). The cholesterol loading also induces apoptosis in the neuronal cells (Inoue et al. 1999). Cells respond to various stresses by an upregulation of stress-activated protein kinases (SAPKs) such as JNK and p38 MAPK (Johnson and Lapadat 2002). SAPKs are a family of signal transduction proteins that are activated under a diverse set of environmental cellular stresses such as oxidative stress and ER stress (Xu et al. 2005). The prolonged activation of JNK, p38, and its subsequent phosphorylation of various transcription factors have been implicated in the initiation of the apoptotic cascade (Xia et al. 1995). Taken together, it is possible that apoptosis in β cell induced by cholesterol loading could be mediated by the activation of stress–SAPKs pathway. In addition, there are several evidences showing β cell damage and insulin resistance appear to be at least partially triggered by inflammatory, oxidative, and endoplasmic reticulum stress-induced pathways including the MAPK signaling cascade (Wellen and Hotamisligil 2005). To explore possible involvement of SAPKs in apoptosis of MIN6s induced by cholesterol loading, we determined the phosphorylation status of these kinases by Western blot analysis. Phosphorylation of T180/Y182 and T183/Y185 are required for the activation of p38 MAPK and JNK, respectively (Fleming et al. 2000; Raingeaud et al. 1995; Tobiume et al. 2002). We thus used the antibodies against P-p38 MAPK (T180/Y182) and P-JNK (T183/Y185) to examine the activation of p38 and JNK. The results are shown in Fig. 3a, exposure of MIN6 to cholesterol activated p38 MAPK and JNK (P-p38, P-JNK in Figs. 3a, b) within 3 h, and the activation of p38 MAPK persisted for 24 h, while the levels of total p38 MAPK and JNK remained unchanged (p-38, JNK in Fig. 3a). When analyzed quantitatively, the degrees of phosphorylation of these kinases in cholesterol-loaded MIN6 cells at indicated time points were significantly higher than those in control. The sustained activation might trigger apoptosis of cholesterol-loaded MIN6 cells because it is reported that the amplified stress response of SAPKs induces growth arrest and cell death (Fleming et al. 2000; Marshall 1995).
Fig. 3.
SAPKs are activated by cholesterol loading to MIN6. Whole cell lysates were prepared at 0, 3, 6,24, 48, and 72 h after the exposure to 250 times diluted CLC, and subjected to Western blot analysis using antibodies against phospho-T180/Y182 p38 MAPK (P-p38), total p38 MAPK (p38), phospho-T183/Y185 JNK (P-JNK, P-p54,P-p46), total JNK (JNK, p54,P46), Bip, CHOP, phospho-S347 Akt(P-Akt), total Akt(Akt) and actin. The representative images are shown in a. The quantitative results are shown in b. The phospho-kinase levels were normalized by the actin levels, and are expressed as relative changes of the level at 0 time point. Mean ± SD (n = 3). *, #p < 0.05 vs. the 0 point. The cells were loaded by the cholesterol alone or by the combination of cholesterol and SAPK inhibitors as indicated manner for 24 h. SB203580, 10 μM; SP600125, 10 μM. The number of adherent cells were then counted after removing the detached cells and trypsinization, and expressed as percentage of the number of cholesterol-nonloaded control cells (c). Mean ± SD (n = 3). *p < 0.05 vs. the levels of control
To further identify the significance of activation of p38 and JNK signaling in β cells exposed to cholesterol, we investigated the effect of SAPK inhibitors in rescuing cells from apoptosis induced by cholesterol loading. Indeed, activation of the MAPK JNK represents a central signal transduction event promoting peripheral insulin resistance, suppressing insulin production and secretion, and increasing apoptosis of islet cells (Hirosumi et al. 2002; Kaneto et al. 2004). However, the role of the p38 MAPKs (which are closely related to JNK) in these processes remains poorly understood. Our results were shown in Fig. 3c, the adherent cell number of cholesterol loading cells was significantly decreased, compared to that of control. The addition of SB205830, a specific p38 chemical inhibitor, to the cholesterol-loading cells significantly increased the adherent cell number when compared to that of cholesterol-loaded cells. The treatment of specific JNK inhibitor SP600125, however, did not have significant effect on rescuing cells from cholesterol-induced cell death. The combination of SB205830 and SP600125 showed the similar adherent cell number as SB205830. This data strongly demonstrated that the p38 MAPK signaling plays a key role in cholesterol-induced apoptosis in β cells. This is similar to the previous report demonstrating that cholesterol, as the main component of LDL, activates stress-activated p38 MAPK signaling in cells from the vessel wall, an event that might contribute to the development of atherosclerosis (Dobreva et al. 2005).
We next investigated the possible contribution of ER stress after cholesterol loading. ER stress is characterized by increased level of ER stress markers such as CHOP and immunoglobulin heavy-chain Bip, a major ER chaperone. Western blot analysis revealed that expressions of both Bip and CHOP in the cholesterol-loaded cells were not increased significantly, compared to those in control. This result suggests ER stress pathway was not activated during the cholesterol loading-induced apoptosis of MIN6 cells (Fig. 3a, Bip and CHOP).
Cholesterol is the main component of lipid raft/caveolae, which functions as a platform of cell signaling proteins including insulin-Akt-Bad signaling, an important cascade in maintaining cell survival. It was reported that endogenous cholesterol depletion by serum depletion could destroy caveolae structure and induce apoptosis of mouse embryonic fibroblast prepared from DHCR24−/− mice (Lu et al. 2006). We thus examined the change in active and phosphorylated Akt (P-Akt) after cholesterol loading in MIN6s. The Western blot analysis showed that level of P-Akt was significantly increased within 6 h and reached to a peak at 24 h after exposure to cholesterol, followed by a decrease after 48 h and recovered to the initial level at 72 h after cholesterol loading, while the total Akt remained unchanged (Figs. 3a, b; p-Akt and Akt).
Taken together, these data from Western blotting suggested that the cholesterol loading in MIN6 cells activates stress-related p38 MAPK signaling as well as survival-related Akt signaling within the early time after cholesterol exposure. The transient activation of Akt might be caused by increased formation of lipid raft/caveolae by the cholesterol overload. Since the activation of Akt was suppressed when cholesterol loading was continued, the sustained activation of SAPKs might contribute to the apoptosis by cholesterol loading. These data suggested there might be crosstalk between SAPKs signaling and Akt cascade.
Oxidative stress contributes to cholesterol-induced apoptosis of MIN6
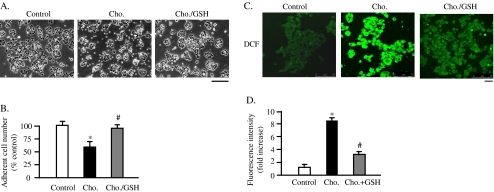
Several reports have shown a link between high dietary cholesterol and/or fat exposure to oxidative stress in animal model. These studies showed an upregulation of ROS production, lipid peroxidation, and oxidized nucleotides and proteins, reflecting the increased oxidative stress in response to high-cholesterol or high-fat diet (Zhang et al. 2005; Montilla et al. 2006). To study the possible effect of cholesterol accumulation on ROS generation in MIN6 cells, the effect of reduced form of GSH on protection of β cells from cholesterol-induced apoptosis was investigated. As shown in Figs. 4a, b, the adherent cell number of cholesterol/GSH group was significantly increased compared to that of cholesterol-loaded cells. This suggested that the excess ROS generation after cholesterol loading might contribute to the apoptosis. To confirm this possibility further, the intracellular ROS levels were measured after exposure to cholesterol, utilizing a cell permeable indicator for ROS, H2DCFDA. As shown in Figs. 4c, d, confocal microscopic images revealed that cholesterol loading markedly increased the fluorescent intensity in most of MIN6 cells while much weaker signal was observed in control, as well as in cholesterol/GSH group. This effect of cholesterol loading persisted for 12 hs. This is consistent with the previous report that free cholesterol loading induces the generation of ROS after 12 h of cholesterol exposure in macrophage (Hung et al. 2006). The generation of excess ROS due to overproduction will damage DNA, protein, and membrane lipids (Orrenius et al. 2007). In addition to such direct effects on cell components, ROS excess has been demonstrated to affect the intracellular signaling cascades that involve SAPKs, resulting in apoptosis (Johnson and Lapadat 2002).
Fig. 4.
Cholesterol loading induces the excess generation of ROS. The MIN6 cells were exposed to cholesterol alone (cho.) or the combination of cholesterol and 2 mM of reduced glutathione (cho./GSH) for 24 h. The cell images were obtained with a phase-contrast microscope at 24 h after the exposure. Bar 25 μm (a). The numbers of adherent cells were expressed as percentage of the number of control cells (b). Mean ± SD (n = 3). *p < 0.05 vs. the levels of cholesterol loaded-cells (cho.). The cells were subjected to the incubation with 20 μM H2DCFDA, for 30 min at 37°C. The cells were then fixed and mounted. The cell images were obtained with confocal laser microscope. Bar 30 μm (c). Fluorescent intensities of DCF (2′,7′ dichlorofluorescein) are expressed as fold increases of the of control level (d). Mean ± SD (n = 20). *p < 0.05 vs. the levels of control; #p < 0.05 vs. the levels of cholesterol-loaded cells (cho.)
Discussion
The present study for the first time demonstrated that the intracellular cholesterol accumulation induces apoptosis of MIN6 pancreatic β cells (Figs. 1–2). It has been reported that MAPK cascades play an important role in regulation of cell viability, proliferation, senescence, and death. In mammals, there are three MAPKs: extracellular signal-regulated kinase (ERK), p38 MAPK, and JNK (Orrenius et al. 2007). Whereas ERK mediates proliferative stimuli, p38 MAPK and JNK are mainly involved in signal transduction for stress responses and apoptosis especially when cells are under stress such as oxidative stress and ER stress. These kinases are thus called SAPKs. To clarify the molecular basis of cholesterol-induced MIN6s apoptosis, we first examined activation of SAPK cascades (Figs. 3a, b). p38 MAPK and JNK in cholesterol-loaded MIN6s were rapidly and persistently activated, and the activation lasted at least for 3 h. It is known that transient and persistent activation of SAPKs results in different cell fate (Marshall 1995). Early/transient activation of p38 MAPK/JNK is associated with cell survival, while late/sustained activation leads to apoptosis. Therefore, the apoptosis of MIN6s upon cholesterol exposure was likely to be caused by the persistent activation of p38 MAPK and JNK. The experiments utilizing SAPK inhibitors further demonstrated that the p38 MAPK, other than JNK signaling plays an important role in the apoptosis of β cells loaded with free cholesterol (Fig. 3c). p38 MAPK signaling was recently found to be important for the insulin secretion and for survival of pancreatic cells by regulating protein kinase D (Sumara et al. 2009). They found that p38δnull mice are protected against high-fat feeding-induced insulin resistance and oxidative stress-mediated cell failure. Interestingly, we also observed the similar results showing that cholesterol loading impaired the insulin secretion of β cells (Supplemental Figure 2). Our data confirmed the significance of p38 MAPK signaling in both insulin secretion and cell survival of pancreatic β cells at the cellular level.
We next investigated the possible stresses induced by cholesterol loading in MIN6s. The expression of Bip and CHOP, two ER stress markers revealed no significant changes after cholesterol loading (Fig. 3a). The experiment using H2DCFDA, however, clearly demonstrated that cholesterol loading induced ROS generation in MIN6 cells. Consistent with this result, GSH rescued β cells from cholesterol-induced apoptosis by reducing intracellular ROS (Fig. 4). These data suggested that the excess generation of ROS by cholesterol loading induces the persistent activation of the p38 MAPK signaling. Taken together, it was demonstrated that apoptosis of MIN6 cells induced by cholesterol loading is mediated through oxidative stress pathway.
A previous study has reported that free cholesterol accumulation could induce ER stress-mediated apoptosis in macrophage (Feng et al. 2003). The authors used combination of an inhibitor of acyl-CoA: cholesterol acyltransferase (ACAT), 58035 and U18666A, an inhibitor of cholesterol trafficking to ER. It was demonstrated that the cholesterol accumulation in ER depletes calcium stores of ER, resulting in the ER stress and apoptosis. However, the other groups demonstrated that the plasma membrane is the site of free cholesterol accumulation induced by ACAT inhibitor and this triggers cell death by apoptosis (Kellner-Weibel et al. 1999). The CLC used in the present study is protein-free, water-soluble cholesterol/CD complexes. This kind of cholesterol-loaded CD is made of the combination of more cholesterol and much less CD so that it can deliver the cholesterol into plasma membrane directly and increase the cholesterol content in the plasma membrane. The plasma membrane thus should be the site of cholesterol accumulation by the CLC-mediated delivery in the present study. These data together suggested that there are different sites of cholesterol accumulation in cells under the different experimental conditions, which may result in the different mechanism of cholesterol-induced cytotoxicity.
In the present study, we measured the concentration of intracellular cholesterol in MIN6 cells after 250× cho./CD complexes (CLC). If we use the μmol/4 × 107 cells as the unit, we can compare the concentrations with those in human platelets measured by other researchers (Tada et al. 2010). Our present experiments showed that the intracellular cholesterol levels are 8 μmol/4 × 107 cells in control, 9.6 μmol/4 × 107 cells in CLC-loaded cells. These data were similar to those obtained from the platelets prepared from healthy control and diabetic patients (11.9 and 15.6 μmol/4 × 107 cells, respectively). The data obtained from mice strongly demonstrated only elevated plasma total cholesterol level rather than free fatty acid leads to increased islet cholesterol (Hao et al. 2007; Tada et al. 2010). In addition, in the West of Scotland Coronary Prevention Study trial, plasma LDL cholesterol was lowered by 26% with pravastatin; and over the following 6 years, the risk of developing T2D was reduced by 30% (Freeman et al. 2001). Taken together, we believe that the elevated LDL concentration in plasma should also increase the content of cholesterol in islet β cells in human T2D patients.
A question arises whether the effect of cholesterol loading is specific for pancreatic β cells. We previously tested the cholesterol loading on the different cell types and found cholesterol has opposing effect on different cell types. The same extent of cholesterol loading induces the cell death not only in MIN6, but also in HEK 293 cells and in mouse embryonic fibroblasts, while it stimulates proliferation of mouse neuroblastoma cell line N2A cells (unpublished data). As shown in the present study, cholesterol loading in MIN6s activates stress-related SAPKs as well as survival-related Akt signaling within the early time after exposure to cholesterol. The transient activation of Akt might be caused by increased formation of lipid raft/caveolae by the cholesterol overload. There is a cross-talk between SAPKs stress signaling and Akt survival pathway under the condition of cellular cholesterol accumulation. Which signaling is dominant seems to be tissue/cell-type specific.
How cholesterol in the plasma membrane induces oxidative stress is still unclear. Emerging evidence indicates that cholesterol-rich microdomains of plasma membrane, lipid rafts and/or caveolae may be critically involved in distal redox-sensitive signaling events and ultimate cell fate. Zhang et al. elegantly demonstrated that death receptor ligands and apoptotic factors stimulate lipid raft clustering, which results in aggregation and activation of NAD(P)H oxidase and consequent endothelial dysfunction (Zhang et al. 2006). Hence, lipid rafts are implicated in both growth and death signaling through NAD(P)H oxidase-generated ROS. It has been reported that cholesterol accumulation in the plasma membrane promotes formation of membrane lipid raft (Gaus et al. 2003). Taken together, it is suggested that cholesterol loading-induced apoptosis of MIN6 cells might be mediated through promoted formation of lipid raft, resulting in the activation of NAD(P)H oxidase and generation of ROS, which activates SAPKs, JNK, and p38.
In conclusion, the present study demonstrated that cholesterol accumulation in the plasma membrane induces apoptosis of MIN6 through generation of ROS and activation of p38 MAPK signaling pathway. The cytotoxicity of cholesterol has been recognized most in the macrophage and in vascular endothelial cells. Our finding provides direct evidence at the cellular level that hypercholesterolemia under diabetic conditions impairs not only cardiovascular but also pancreatic β cell functions. This indicates that prevention of hypercholesterolemia could be useful in treating T2D.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Low doses of CD does not induce cytotoxicity in MIN6 cells. The MIN6 cells were exposed to CD with indicated concentrations for 24 h. The cell images were obtained with a phase-contrast microscope at 24 h after the exposure. Bar 25 μm (Supplemental Figure 1a). The numbers of adherent cells were expressed as percentage of the number of control cells (Supplemental Figure 1b). Mean ± SD (n = 3). *p < 0.05 vs. the levels of control (PDF 342 kb)
The cholesterol loading impairs glucose-induced insulin secretion. MIN6 cells were exposed to cholesterol for 24 h in the growth medium. After washing with KRB buffer containing 3 mmol/l of glucose, the cells were incubated with KRB buffer for 1 h and then transferred to the medium containing 20 mmol/l of glucose. At the end of the 1-h stimulation period, the amount of insulin release in the spent medium was determined by ELISA insulin kit, according to the manufacturer instructions (PDF 342 kb)
MIN6 cells were exposed to 250 times diluted CLC in the growth medium for 24 h. The cells then were harvested and counted. The total lipids were extracted from cells and subjected to the intracellular cholesterol measurement. Mean ± SD (n = 3). *p < 0.05 vs. the levels of control (PDF 342 kb)
Acknowledgment
This work was supported by grants from National Natural Science Foundation of China to QC (No.30800575) and Natural Science Foundation of Liaoning Provincial Education Board (No. 2009A304).
Footnotes
X Lu and J Liu contributed equally to this work.
Contributor Information
Xiuli Lu, Email: luxiulidr@gmail.com.
Bing Gao, Email: gaobing_cn@yahoo.com.cn.
References
- Bennett BL, Sasaki DT, Murray BW, O'Leary EC, Sakata ST, Xu W, Leisten JC, Motiwala A, Pierce S, Satoh Y, Bhagwat SS, Manning AM, Anderson DW. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc Natl Acad Sci U S A. 2001;98:13681–13686. doi: 10.1073/pnas.251194298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BLIGH EG DYER WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Brunham LR, Kruit JK, Pape TD, Timmins JM, Reuwer AQ, Vasanji Z, Marsh BJ, Rodrigues B, Johnson JD, Parks JS, Verchere CB, Hayden MR. Beta-cell ABCA1 influences insulin secretion, glucose homeostasis and response to thiazolidinedione treatment. Nat Med. 2007;13:340–347. doi: 10.1038/nm1546. [DOI] [PubMed] [Google Scholar]
- Cnop M, Hannaert JC, Grupping AY, Pipeleers DG. Low density lipoprotein can cause death of islet beta-cells by its cellular uptake and oxidative modification. Endocrinology. 2002;143:3449–3453. doi: 10.1210/en.2002-220273. [DOI] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-F. [DOI] [PubMed] [Google Scholar]
- Souza JC, Oliveira CA, Carneiro EM, Boschero AC, Oliveira HC. Cholesterol toxicity in pancreatic islets from LDL receptor-deficient mice. Diabetologia. 2010;53:2461–2. doi: 10.1007/s00125-010-1868-8. [DOI] [PubMed] [Google Scholar]
- Dobreva I, Zschornig O, Waeber G, James RW, Widmann C. Cholesterol is the major component of native lipoproteins activating the p38 mitogen-activated protein kinases. Biol Chem. 2005;386:909–918. doi: 10.1515/BC.2005.106. [DOI] [PubMed] [Google Scholar]
- Feng B, Yao PM, Li Y, Devlin CM, Zhang D, Harding HP, Sweeney M, Rong JX, Kuriakose G, Fisher EA, Marks AR, Ron D, Tabas I. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat Cell Biol. 2003;5:781–792. doi: 10.1038/ncb1035. [DOI] [PubMed] [Google Scholar]
- Fleming Y, Armstrong CG, Morrice N, Paterson A, Goedert M, Cohen P. Synergistic activation of stress-activated protein kinase 1/c-Jun N-terminal kinase (SAPK1/JNK) isoforms by mitogen-activated protein kinase kinase 4 (MKK4) and MKK7. Biochem J. 2000;352(Pt 1):145–154. doi: 10.1042/0264-6021:3520145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DJ, Norrie J, Sattar N, Neely RD, Cobbe SM, Ford I, Isles C, Lorimer AR, Macfarlane PW, McKillop JH, Packard CJ, Shepherd J, Gaw A. Pravastatin and the development of diabetes mellitus: evidence for a protective treatment effect in the West of Scotland Coronary Prevention Study. Circulation. 2001;103:357–362. doi: 10.1161/01.cir.103.3.357. [DOI] [PubMed] [Google Scholar]
- Gaus K, Gratton E, Keble EP, Jones NS. Visualizing lipid structure and raft domains in living cells with two-photon microscopy. Proc Natl Acad Sci USA. 2003;26:15554–15559. doi: 10.1073/pnas.2534386100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorfien S, Paul B, Walowitz J, Keem R, Biddle W, Jayme D. Growth of NS0 cells in protein-free, chemically defined medium. Biotechnol Prog. 2000;16:682–687. doi: 10.1021/bp000109a. [DOI] [PubMed] [Google Scholar]
- Grupping AY, Cnop M, Schravendijk CF, Hannaert JC, Berkel TJ, Pipeleers DG. Low density lipoprotein binding and uptake by human and rat islet beta cells. Endocrinology. 1997;138:4064–4068. doi: 10.1210/en.138.10.4064. [DOI] [PubMed] [Google Scholar]
- Gustavsson J, Parpal S, Karlsson M, Ramsing C, Thorn H, Borg M, Lindroth M, Peterson KH, Magnusson KE, Stralfors P. Localization of the insulin receptor in caveolae of adipocyte plasma membrane. FASEB J. 1999;13:1961–1971. [PubMed] [Google Scholar]
- Hao M, Head WS, Gunawardana SC, Hasty AH, Piston DW. Direct effect of cholesterol on insulin secretion: a novel mechanism for pancreatic beta-cell dysfunction. Diabetes. 2007;56:2328–2338. doi: 10.2337/db07-0056. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Hung YC, Hong MY, Huang GS. Cholesterol loading augments oxidative stress in macrophages. FEBS Lett. 2006;580:849–861. doi: 10.1016/j.febslet.2005.12.102. [DOI] [PubMed] [Google Scholar]
- Inoue K, Kubota S, Seyama Y. Cholestanol induces apoptosis of cerebellar neuronal cells. Biochem Biophys Res Commun. 1999;256:198–203. doi: 10.1006/bbrc.1998.9497. [DOI] [PubMed] [Google Scholar]
- Ishihara H, Asano T, Tsukuda K, Katagiri H, Inukai K, Anai M, Kikuchi M, Yazaki Y, Miyazaki JI, Oka Y. Pancreatic beta cell line MIN6 exhibits characteristics of glucose metabolism and glucose-stimulated insulin secretion similar to those of normal islets. Diabetologia. 1993;36:1139–1145. doi: 10.1007/BF00401058. [DOI] [PubMed] [Google Scholar]
- Jakob S, Corazza N, Diamantis E, Kappeler A, Brunner T. Detection of apoptosis in vivo using antibodies against caspase-induced neo-epitopes. Methods. 2008;44:255–261. doi: 10.1016/j.ymeth.2007.11.004. [DOI] [PubMed] [Google Scholar]
- Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- Kaneto H, Nakatani Y, Miyatsuka T, Kawamori D, Matsuoka TA, Matsuhisa M, Kajimoto Y, Ichijo H, Yamasaki Y, Hori M. Possible novel therapy for diabetes with cell-permeable JNK-inhibitory peptide. Nat Med. 2004;10:1128–1132. doi: 10.1038/nm1111. [DOI] [PubMed] [Google Scholar]
- Kellner-Weibel G, Geng YJ, Rothblat GH. Cytotoxic cholesterol is generated by the hydrolysis of cytoplasmic cholesteryl ester and transported to the plasma membrane. Atherosclerosis. 1999;146:309–319. doi: 10.1016/S0021-9150(99)00155-0. [DOI] [PubMed] [Google Scholar]
- Kozaki Y, Kambe F, Hayashi Y, Ohmori S, Seo H, Kumazawa T, Mizumura K. Molecular cloning of prostaglandin EP3 receptors from canine sensory ganglia and their facilitatory action on bradykinin-induced mobilization of intracellular calcium. J Neurochem. 2007;100:1636–1647. doi: 10.1111/j.1471-4159.2006.04320.x. [DOI] [PubMed] [Google Scholar]
- Lu X, Kambe F, Cao X, Yoshida T, Ohmori S, Murakami K, Kaji T, Ishii T, Zadworny D, Seo H. DHCR24-knockout embryonic fibroblasts are susceptible to serum withdrawal-induced apoptosis because of dysfunction of caveolae and insulin-Akt-Bad signaling. Endocrinology. 2006;147:3123–3132. doi: 10.1210/en.2005-1426. [DOI] [PubMed] [Google Scholar]
- Lu X, Kambe F, Cao X, Kozaki Y, Kaji T, Ishii T, Seo H. 3beta-Hydroxysteroid-delta24 reductase is a hydrogen peroxide scavenger, protecting cells from oxidative stress-induced apoptosis. Endocrinology. 2008;149:3267–3273. doi: 10.1210/en.2008-0024. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Montilla P, Espejo I, Munoz MC, Bujalance I, Munoz-Castaneda JR, Tunez I. Protective effect of red wine on oxidative stress and antioxidant enzyme activities in the brain and kidney induced by feeding high cholesterol in rats. Clin Nutr. 2006;25:146–153. doi: 10.1016/j.clnu.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Gogvadze V, Zhivotovsky B. Mitochondrial oxidative stress: implications for cell death. Annu Rev Pharmacol Toxicol. 2007;47:143–183. doi: 10.1146/annurev.pharmtox.47.120505.105122. [DOI] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ. Islet beta cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingeaud J, Gupta S, Rogers JS, Dickens M, Han J, Ulevitch RJ, Davis RJ. Pro-inflammatory cytokines and environmental stress cause p38 mitogen-activated protein kinase activation by dual phosphorylation on tyrosine and threonine. J Biol Chem. 1995;270:7420–7426. doi: 10.1074/jbc.270.13.7420. [DOI] [PubMed] [Google Scholar]
- Resendes AR, Majo N, Segales J, Espadamala J, Mateu E, Chianini F, Nofrarias M, Domingo M. Apoptosis in normal lymphoid organs from healthy normal, conventional pigs at different ages detected by TUNEL and cleaved caspase-3 immunohistochemistry in paraffin-embedded tissues. Vet Immunol Immunopathol. 2004;99:203–213. doi: 10.1016/j.vetimm.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Saklatvala J, Rawlinson L, Waller RJ, Sarsfield S, Lee JC, Morton LF, Barnes MJ, Farndale RW. Role for p38 mitogen-activated protein kinase in platelet aggregation caused by collagen or a thromboxane analogue. J Biol Chem. 1996;271:6586–6589. doi: 10.1074/jbc.271.12.6586. [DOI] [PubMed] [Google Scholar]
- Sumara G, Formentini I, Collins S, Sumara I, Windak R, Bodenmiller B, Ramracheya R, Caille D, Jiang H, Platt KA, Meda P, Aebersold R, Rorsman P, Ricci R. Regulation of PKD by the MAPK p38delta in insulin secretion and glucose homeostasis. Cell. 2009;136:235–248. doi: 10.1016/j.cell.2008.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada S, Kitanaka A, Kubota Y, Ito M, Taminato T. Automated assay for determining cellular cholesterol using a random access chemistry analyser. Ann Clin Biochem. 2010;47:168–170. doi: 10.1258/acb.2010.009204. [DOI] [PubMed] [Google Scholar]
- Tobiume K, Saitoh M, Ichijo H. Activation of apoptosis signal-regulating kinase 1 by the stress-induced activating phosphorylation of pre-formed oligomer. J Cell Physiol. 2002;191:95–104. doi: 10.1002/jcp.10080. [DOI] [PubMed] [Google Scholar]
- Ward SG, Parry RV, Matthews J, O'Neill L. A p38 MAP kinase inhibitor SB203580 inhibits CD28-dependent T cell proliferation and IL-2 production. Biochem Soc Trans. 1997;25:304S. doi: 10.1042/bst025304s. [DOI] [PubMed] [Google Scholar]
- Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115:1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME. Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science. 1995;270:1326–1331. doi: 10.1126/science.270.5240.1326. [DOI] [PubMed] [Google Scholar]
- Xu C, Bailly-Maitre B, Reed JC. Endoplasmic reticulum stress: cell life and death decisions. J Clin Invest. 2005;115:2656–2664. doi: 10.1172/JCI26373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao PM, Tabas I. Free cholesterol loading of macrophages is associated with widespread mitochondrial dysfunction and activation of the mitochondrial apoptosis pathway. J Biol Chem. 2001;276:42468–42476. doi: 10.1074/jbc.M101419200. [DOI] [PubMed] [Google Scholar]
- Zhang X, Dong F, Ren J, Driscoll MJ, Culver B. High dietary fat induces NADPH oxidase-associated oxidative stress and inflammation in rat cerebral cortex. Exp Neurol. 2005;191:318–325. doi: 10.1016/j.expneurol.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Zhang AY, Yi F, Zhang G, Gulbins E, Li PL. Lipid raft clustering and redox signaling platform formation in coronary arterial endothelial cells. Hypertension. 2006;47:74–80. doi: 10.1161/10.1161/01.HYP.0000196727.53300.62. [DOI] [PubMed] [Google Scholar]
- Zhao YF, Wang L, Lee S, Sun Q, Tuo Y, Wang Y, Pei J, Chen C. Cholesterol induces mitochondrial dysfunction and apoptosis in mouse pancreatic beta-cell line MIN6 cells. Endocr. 2010;37:76–82. doi: 10.1007/s12020-009-9275-y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Low doses of CD does not induce cytotoxicity in MIN6 cells. The MIN6 cells were exposed to CD with indicated concentrations for 24 h. The cell images were obtained with a phase-contrast microscope at 24 h after the exposure. Bar 25 μm (Supplemental Figure 1a). The numbers of adherent cells were expressed as percentage of the number of control cells (Supplemental Figure 1b). Mean ± SD (n = 3). *p < 0.05 vs. the levels of control (PDF 342 kb)
The cholesterol loading impairs glucose-induced insulin secretion. MIN6 cells were exposed to cholesterol for 24 h in the growth medium. After washing with KRB buffer containing 3 mmol/l of glucose, the cells were incubated with KRB buffer for 1 h and then transferred to the medium containing 20 mmol/l of glucose. At the end of the 1-h stimulation period, the amount of insulin release in the spent medium was determined by ELISA insulin kit, according to the manufacturer instructions (PDF 342 kb)
MIN6 cells were exposed to 250 times diluted CLC in the growth medium for 24 h. The cells then were harvested and counted. The total lipids were extracted from cells and subjected to the intracellular cholesterol measurement. Mean ± SD (n = 3). *p < 0.05 vs. the levels of control (PDF 342 kb)