Abstract
Angiogenesis is a critical step during cancer progression. The vascular endothelial growth factor (VEGF) is a major stimulator for angiogenesis and is predominantly contributed by cancer cells in tumors. Inhibition of the VEGF signaling pathway has shown promising therapeutic benefits for cancer patients, but adaptive tumor responses are often observed, indicating the need for further understanding of VEGF regulation. We report that a novel G protein-coupled receptor, GPR56, inhibits VEGF production from the melanoma cell lines and impedes melanoma angiogenesis and growth, through the serine threonine proline-rich (STP) segment in its N-terminus and a signaling pathway involving protein kinase Cα. We also present evidence that the two fragments of GPR56, which are generated by autocatalyzed cleavage, played distinct roles in regulating VEGF production and melanoma progression. Finally, consistent with its suppressive roles in melanoma progression, the expression levels of GPR56 are inversely correlated with the malignancy of melanomas in human subjects. We propose that components of the GPR56-mediated signal pathway may serve as new targets for anti-angiogenic treatment of melanoma.
Keywords: GPR56, adhesion GPCR, VEGF, angiogenesis, melanoma
Introduction
Angiogenesis is a process of nascent blood vessel formation (1) and is critical for tumor growth and metastasis (2). When tumors reach ~1 mm in diameter, hypoxia develops and induces the secretion of vascular growth factor A (VEGFA, or VEGF) from cancer cells (3). VEGF recruits endothelial cells and stimulates new blood vessel formation (4) to ensure sufficient oxygen and nutrient supply for the proliferation of cancer cells. Inhibiting this process reduces tumor sizes and has been proposed as a non-conventional therapy for cancer treatment (5, 6). Various angiogenesis inhibitors have been developed and many of them show promising tumor inhibitory effects (7). However, adaptive responses to single anti-angiogenic therapy, manifested as increased invasion and metastasis of cancer cells, have been observed both experimentally and clinically (8–10). The mechanisms for this adaptive response are not clear, but its occurrence strongly argues for combinations of anti-angiogenic regimens to effectively treat cancer.
VEGF is a potent growth factor for angiogenesis and is a common target for anti-angiogenesis inhibition (11). It binds to the VEGF receptors (VEGFRs) on endothelial cells and promotes their proliferation and migration during angiogenesis. The main source of VEGF in tumors is cancer cells and its expression is tightly regulated at both transcriptional and posttranscriptional levels (12). Under hypoxic conditions, VEGF mRNA is induced by the hypoxia-inducible factor alpha (HIF-1alpha) and subsequently regulated by alternative splicing. Four main isoforms of VEGF have been identified: VEGF121, VEGF165, VEGF189, and VEGF206, with VEGF165 being the most abundantly expressed. All VEGF isoforms except VEGF121 could associate with extracellular matrix (ECM). The ECM-bound VEGF can be released and activated by heparinases or matrix metalloproteinases, providing an additional layer of regulation for VEGF activity in vivo (13). A less well-characterized mechanism of VEGF regulation is secretion. VEGF contains a signal peptide (14) and presumably is secreted through conventional vesicle trafficking process, from ER to Golgi, and to plasma membrane. In mast cells (15) and neutrophils (16), for example, VEGF is stored in secretory granules, and its release is stimulated by the activation of protein kinase C (PKC).
We report here that VEGF production from melanoma cells is regulated by an atypical G protein-coupled receptor (GPCR), GPR56. GPR56 belongs to the family of adhesion GPCRs, a newly identified family of class B GPCRs implicated in both cell adhesion and G protein-coupled signaling (17). The adhesion GPCRs are highly conserved and their importance in development and diseases has been increasingly recognized (17–22), but their regulatory mechanisms remain poorly understood. Adhesion GPCRs share a GPCR proteolytic site (GPS), through which the extracellular stalks are separated from the transmembrane domains by autocatalytic cleavage. The cleaved fragments could still associate with each other non-covalently and form a heterodimeric complex. This cleavage in adhesion GPCRs is required for their proper functions, since mutations in GPS motifs resulted in failure of the receptors to localize on cell surface (23, 24).
Our previous work showed that GPR56 inhibited melanoma growth and metastasis from MC-1 cells in a xenograft model (25). We present evidence here that, depending on the presence or absence of a serine threonine proline-rich (STP) segment in its N-terminus, GPR56 differentially regulates VEGF production, angiogenesis, and melanoma growth, via a signaling pathway involving PKCα. We propose that GPR56 exists in different activation states in melanoma cells, which are modulated by its STP segment and induce opposing outcomes on angiogenesis and melanoma progression. Specific targeting of tumor-promoting activity of GPR56 may thus serve as a new strategy for angiogenesis inhibition and melanoma treatment.
Materials and Methods
Cell lines
The MC-1, SM, and MA-1 cells were derived from the human melanoma cell line, A375 (ATCC #CRL-1619) (25, 26), and maintained in Dulbecco’s Modified Eagle Medium (DMEM) with 10% FBS and 4 mM glutamine. WM266-4 cells were obtained from ATCC (CRL-1676) and maintained in Minimal Essential Medium with Earle’s salt, 10% FBS, 0.1 mM non-essential amino acids, 1 mM sodium pyruvate, 1.5 g/L sodium bicarbonate, and 2 mM glutamine. YURIF cells were purchased from the Yale University Cell Core Facility (27) and maintained in Opi-MEM (Invitrogen) with 5% FBS. HEK293 cells were maintained in DMEM with 10% FBS and 2 mM glutamine. All the cell lines were passaged in our laboratory for less than six months after receipt.
Mice
NOD-SCID mice (NOD.CB17-Prkdcscid/J) and NSG mice (NOD.Cg-Prkdcscid Il2rgtm1Wjl/SzJ) were purchased from the Jackson Laboratory (Bar Harbor, Maine). Gpr56−/− mice (Genentech Inc., CA) were maintained through crossbreedings between Gpr56+/− mice. All the mice were housed in the animal facility at the University of Rochester Medical Center or Massachusetts Institute of Technology, in accordance to the animal care guidelines from the Division of Laboratory Animal Medicine at University of Rochester Medical Center or MIT.
ELISA for VEGF Quantification
Cells were seeded at a density of 1.0 × 105 cells per well in 24-well plates and cultured in serum-free medium for three days. Media were collected every 24 hours for VEGF-specific ELISA analyses, following the instructions from the manufacturer (R&D Systems).
To test the effects of PKC on VEGF production, MC-1 cells were incubated with the PKC inhibitors, chelerythrine chloride and Ro-31-8425 (EMD4Biosciences, NJ), or the PKC activator, phorbol myristate acetate (PMA; Sigma, MO), for 48 hours in serum-free medium. The media of the treated cells were subjected to ELISA. To examine the effects of PKCα on VEGF production, MC-1(ΔSTP-GPR56) and MC-1(GPRC) cells were transiently transfected with the construct expressing a dominant negative (DN) mutant of PKCα, or vector control (Addgene). Media were collected 48 hours after transfection for ELISA analyses. To assess whether the DN- PKCα sequesters PMA-induced VEGF production, MC-1(GPR56) cells transiently expressing DN-PKCα or vector control were subjected to PMA (10 µg/ml) stimulation. Media were collected 48 hours after stimulation for ELISA analyses,
Analyses of Activation of PKC Isoforms
Cell fractionation to assess activated PKC isoforms was performed as previously reported (28). Briefly, cells were lysed in isotonic buffer (20 mM Tris–HCl (pH 7.5, 150 mM NaCl, 10 mM EDTA, 5 mM EGTA, 20 mM sodium fluoride, 5 mM sodium pyrophosphate, 1 mM sodium vanadate, 1 µM okadaic acid and a cocktail of protease inhibitor). Membrane fractions were collected after two rounds of centrifugations and were subjected to western blot analyses, using antibodies against PKCα, PKCβ1, PKCβ2, PKCγ, PKCδ, or PKCµ (1: 1000, Santa Cruz Biotechnology). The rabbit anti-LRP6 antibody (1: 500, Cell Signaling) was used as a loading control. The intensity of each PKC band was measured by AlphaImager software and normalized to the intensity of LRP6 band from the same sample. The ratio of the PKC level in MC-1(GPR56), MC-1(ΔSTP-GPR56), or MC-1(GPRC) cells, over that in MC-1(EV) cells (which was arbitrarily set to 1) was defined as the “Relative Intensity” score.
Western Blots
Cells were lysed in RIPA buffer and separated on a 12% SDS-polyacrylamide gel for western blot analyses using the goat anti-VEGF antibody (0.2 mg/ml, R&D systems, MN), sheep anti-GPRN (0.4 mg/ml, R&D systems), rabbit anti-GPRC (1– 300), FcGPRN (1 mg/ml), mouse anti-TG2 (1: 100, Abcam, MA), or mouse anti-GAPDH (1: 1000) antibodies, followed by detection by HRP-conjugated secondary antibodies and enhanced chemiluminescence detection system (Pelkin Elmer, MA).
Tumor Studies
5 × 105 of melanoma cells were injected subcutaneously into NOD-SCID, or NSG mice. Tumor were harvested and weighted five weeks after injections. To study tumor angiogenesis, frozen sections of tumors were stained with rat anti-PECAM antibody (1:5, BD Pharmingen, CA), or mouse anti-human vimentin antibody (1:50, Leica Microsystems, IL), and rabbit anti-GPRC antibody (1: 300), followed by detection with Alexa 488 or Alexa 594 donkey anti-rabbit, anti-mouse, or anti-rat secondary antibodies (1: 400, Invitrogen). Images were captured by the SPOT software and processed with Adobe Photoshop. The numbers of blood vessels in two to five randomly chosen fields on each tumor section, or GPR56-positive and GPR56-negative areas on each GPR56-expressing tumor section, were counted.
Results
GPR56 inhibits angiogenesis in melanoma
We previously reported that GPR56 inhibited melanoma growth and metastasis in xenograft models (25). Angiogenesis is a critical process during tumor progression (2). To determine whether GPR56 inhibits melanoma progression by regulating angiogenesis, tumor sections from MC-1 cells expressing GPR56 or vector control were sectioned and stained with an antibody against the platelet and endothelial cell adhesion molecule-1 (PECAM-1). Significantly fewer blood vessels were observed on sections from MC-1(GPR) cells than the controls (Figure 1A and B), suggesting that GPR56 inhibits angiogenesis in melanomas.
Figure 1. GPR56 inhibits angiogenesis in melanomas.
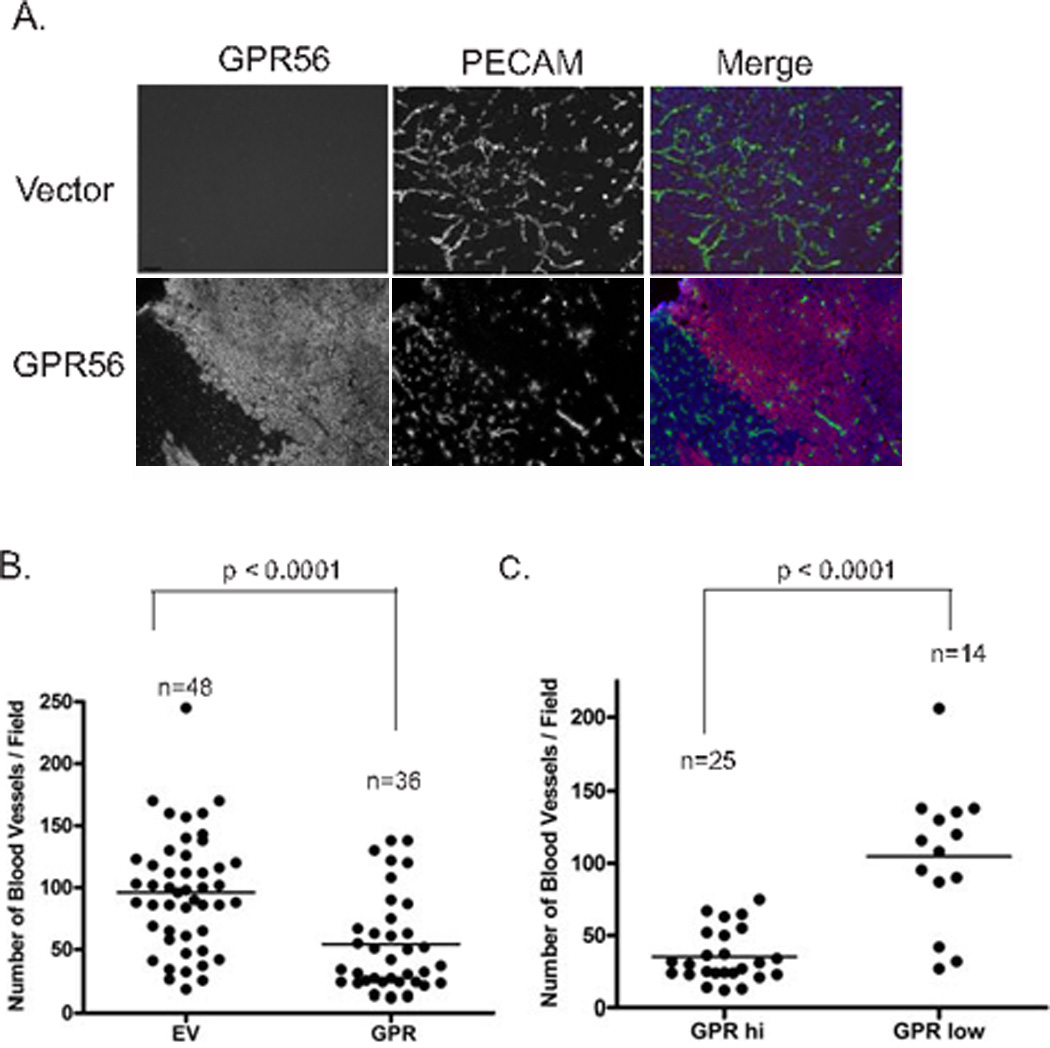
A. Expression of GPR56 resulted in reduction in angiogenesis. Tumor sections from melanoma cells expressing wild-type GPR56 or vector control were stained with anti-GPRC antibody (red) and anti-PECAM antibody (green).
B. Quantification of blood vessel density in GPR56-expressing tumors and vector control.
C. Quantifications of blood vessel density in areas of the same GPR56-expressing tumors.
We frequently observed loss of GPR56 expression in some areas of tumors from MC-1(GPR) cells, probably due to the growth advantage of cells that express low levels of ectopic GPR56. We postulated that these areas would contain more blood vessels than the adjacent areas that retain GPR56 expression. To test this, MC-1(GPR56) tumor sections were co-stained with the anti-GPR56 antibody (anti-GPRC) and the anti-PECAM antibody, or an antibody against the human-specific vimentin (Figure 1A and Supplementary Figure S1). GPR56-low and–high areas that contain tumor cells but not mouse stroma were selected. Quantification of blood vessels in these areas revealed an inverse relationship between expression levels of GPR56 and blood vessel density (Figure 1A and C), arguing strongly that ectopic expression of GPR56 in MC-1 cells inhibits melanoma angiogenesis.
Expression of the C-terminal fragment of GPR56 induces angiogenesis and melanoma growth
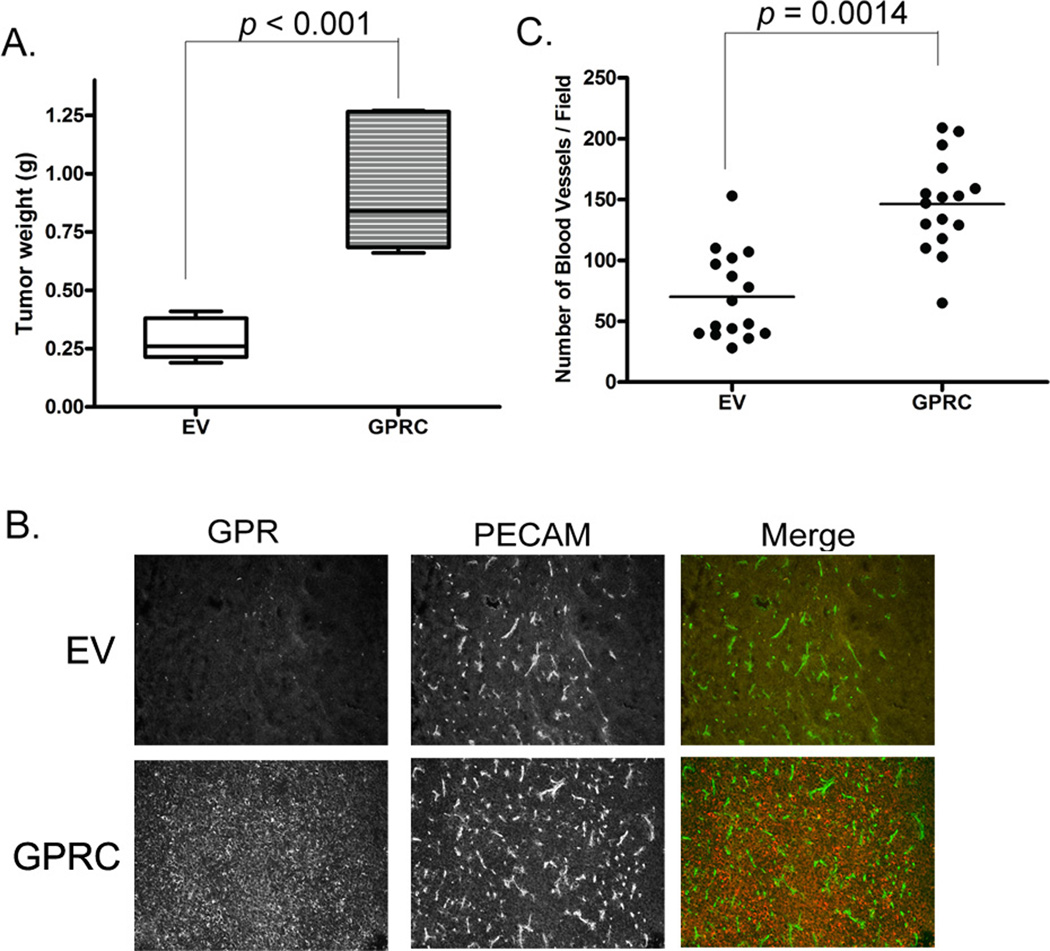
Like many members of the adhesion GPCR family, mature GPR56 receptor is cleaved into two fragments at the junction between its N-terminus (GPRN) and C-terminal transmembrane domains (GPRC) (25). The GPRN and GPRC fragments still associate with each other after cleavage, but significant portions of them remain unbound. We hypothesized that the GPRN signals through GPRC to inhibit melanoma growth and angiogenesis and deletion of GPRN in GPR56 should abolish this inhibition. To test this, the GPRC fragment was over-expressed in MC-1 cells. This expression did not alter cell proliferation significantly in vitro (Supplementary Figure S2), but, in vivo, it not only failed to inhibit melanoma growth, but dramatically induced melanoma growth and angiogenesis (Figure 2A–C), in direct contrast with the inhibition observed from full-length GPR56. These results indicated a potential antagonistic relationship between the GPRN and GPRC fragments in MC-1 cells.
Figure 2. Ectopic expression of GPRC fragment induces tumor angiogenesis and growth in MC-1 cells.
A. Expression of GPRC in MC-1 cells significantly enhanced tumor growth.
B. Expression of GPRC induced tumor angiogenesis. Tumor sections from MC-1(EV) and MC-1(GPRC) cells were stained with the anti-PECAM antibody (green) and the anti-GPRC (red).
C. Blood vessel densities were significantly higher in tumors from the MC-1(GPRC) cells than those from the MC-1(EV) cells. **: p < 0.001, Student’s t-test.
The STP segment in GPR56 binds to TG2 and its deletion in GPR56 led to enhanced angiogenesis and tumor growth
The antagonistic relationship of GPRN and GPRC fragments implicated from the above finding led us to speculate that a factor might bind to GPRN and modulate the stimulatory or inhibitory effects of GPRC. We previously reported that tissue transglutaminse, TG2, binds to GPRN (25). To test whether TG2 might mediate the inhibitory function of GPRC, we mapped the region in GPR56 that binds to TG2. GPRN fragments of various lengths were expressed and purified as human Fc fusion proteins (Supplementary Figure S3A and B) and their binding to TG2 was tested on overlay assays (25). A serine, threonine, proline-rich (STP) segment of around 70 aa was shown to be both necessary and sufficient for binding with TG2 (Supplementary Figure S3C and D).
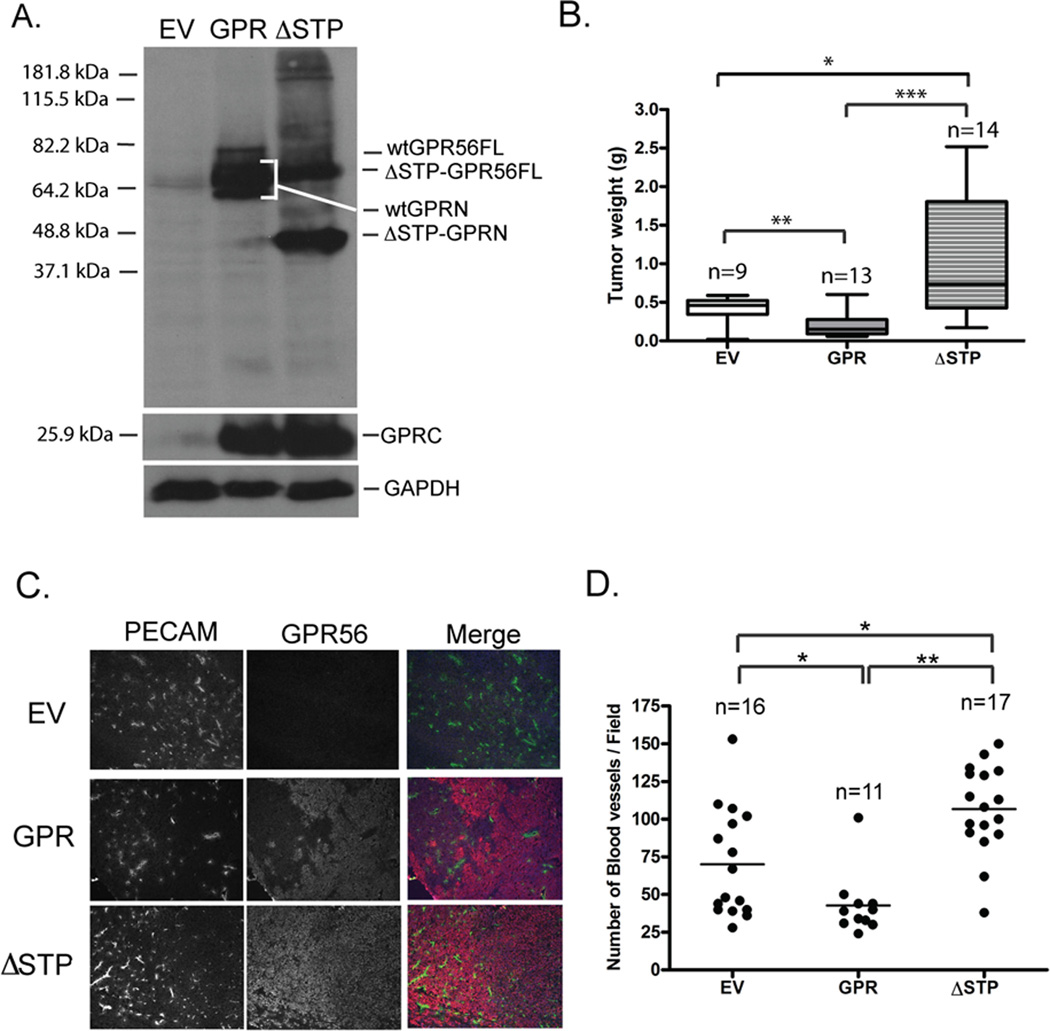
We reasoned that, if TG2 mediates the suppressive roles of GPR56 in melanoma progression, deletion of the STP motif in GPR56 would reverse the inhibition by GPR56. To test this hypothesis, ΔSTP-GPR56 was expressed in MC-1 cells (MC-1(ΔSTP-GPR56)), processed into a C-terminal fragment of ~25 kDa and an N-terminal fragment of ~45 kDa (Figure 3A), and localized on cell surface as the full-length GPR56 (Supplementary Figure S4). The expression of ΔSTP-GPR56 did not perturb MC-1 cell proliferation significantly in vitro (Supplementary Figure S2), but led to increased tumor growth and blood vessel densities in vivo (Figure 3B, C and D), similar to GPRC but in contrast to the full-length receptor, indicating that the STP segment is required for the inhibitory effect of GPR56 on melanoma angiogenesis and growth.
Figure 3. Deletion of the STP segment in GPR56 led to significantly enhanced tumor growth and angiogenesis.
A. Western blot analyses of the expression of ΔSTP-GPR56 in MC-1 cells.
B. Expression of ΔSTP-GPR56 led to enhanced tumor growth. *: p < 0.05; **: p < 0.01; ***: p < 0.001. Student’s t-test.
C. Expression of ΔSTP-GPR56 led to enhanced tumor angiogenesis. Tumor sections were stained with the anti-PECAM antibody (green) and the anti-GPRC antibody (red).
D. Blood vessel density on sections stained with anti-PECAM antibody was quantified as described in the legend for Figure 2. *: p < 0.05; **: p < 0.01; ***: p < 0.001. Student’s t-test.
ΔSTP-GPR56 induces VEGF production in melanoma cell lines
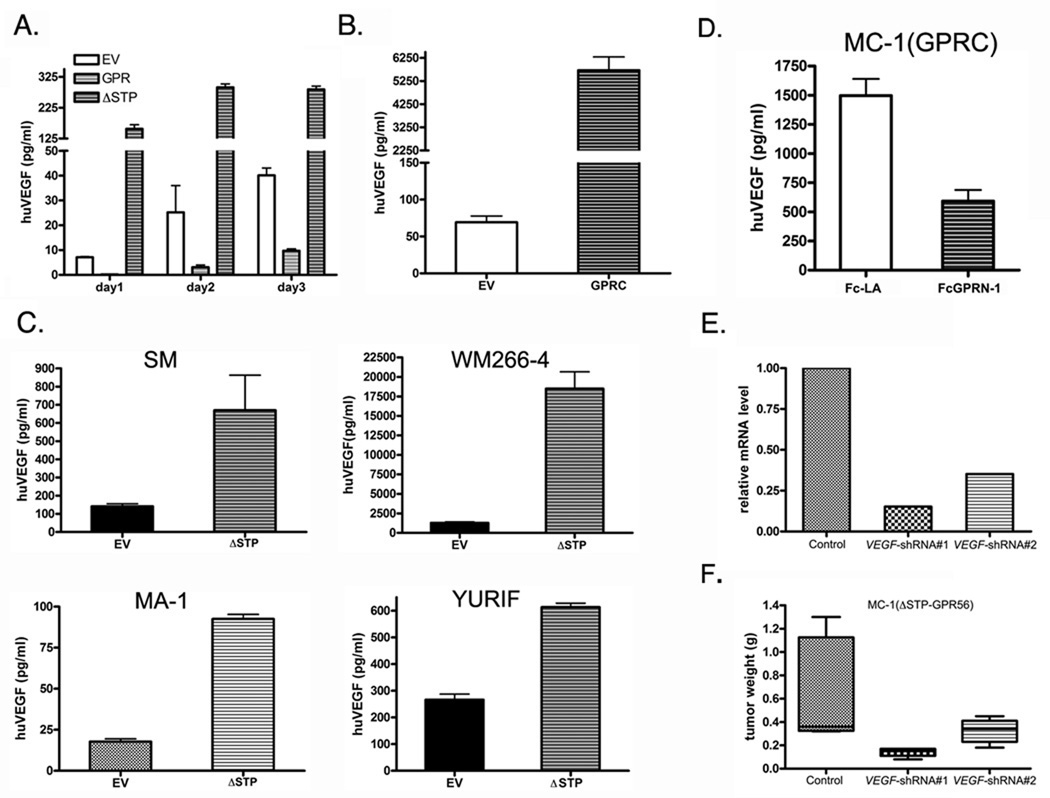
To examine whether GPR56 affects tumor angiogenesis by modulating VEGF production, the concentrations of VEGF in the media from the MC-1(EV), MC-1(GPR), and MC-1(ΔSTP-GPR56) cells were determined by ELISA. VEGF was lower in the media of MC-1(GPR) cells than that of MC-1(EV) cells (Figure 4A), but dramatically elevated in the media from MC-1 (ΔSTP-GPR56) and MC-1(GPRC) cells (Figure 4A and B), suggesting that GPR56 inhibits, but ΔSTP-GPR56 or GPRC promotes, the production of VEGF by MC-1 cells. Consistent with these in vitro observations, the level of VEGF in the circulation of mice bearing MC-1(ΔSTP-GPR56) cells or MC-1(GPRC) tumors was higher than controls, and the reverse was observed for MC-1(GPR56) tumors (Supplementary Figure S5). To examine whether GPR56 regulates VEGF production in melanoma cells other than MC-1 cells, ΔSTP-GPR56 was expressed in four additional human melanoma cell lines, SM, WM266-4, MA-1, and YURIF. Its expression induced VEGF production in all of them (Figure 4C), suggesting that regulation of VEGF production by GPR56 might be a shared mechanism in malignant melanoma.
Figure 4.
GPR56 regulates melanoma progression by inhibiting VEGF production through the STP segment.
A. Expression of GPR56 significantly inhibited production of VEGF in the media of MC-1 cells, but expression of ΔSTP-GPR56 enhanced it.
B. Expression of GPRC in MC-1 cells significantly increased production of VEGF.
C. Expression of ΔSTP-GPR56 induced VEGF production in multiple melanoma cell lines.
D. FcGPRN-1 protein inhibited VEGF production from MC-1(GPRC) cells. FcLA protein was used as a control.
E. VEGF mRNA was knocked down by shRNAs in MC-1(ΔSTP-GPR56) cells.
F. Knocking down of VEGF in MC-1(ΔSTP-GPR56) cells led to a reduction in tumor growth.
The above opposing roles of GPRC and full-length GPR56 on VEGF production predicted an antagonistic relationship between GPRN and GPRC in melanoma cells. To directly test this, purified FcGPRN fusion protein (shown as FcGPRN-1 in Figure S3) was added onto MC-1(GPRC) cells and resulted in a significant reduction of VEGF production relative to controls (Figure 4D), suggesting strongly that GPRN inhibits the activity of GPRC during VEGF production of melanoma cells. Since GPRC could be detected in both “GPRN-bound” and “GPRN-free” states in melanoma cell lysates (25), depleting the whole GPR56 molecule would abolish both and, depending on the ratio of the two states, may result in no net change or cell-specific changes of VEGF production in melanoma cells. Consistent with this, we did not observe significant changes of VEGF production from MA-1 cells expressing GPR56 shRNAs (Supplementary Figure S6).
The level of VEGF produced from MC-1(ΔSTP-GPR56) cells was increased relative to controls, demonstrating that the STP segment in GPR56 is essential for its inhibitory role on VEGF production. The STP segment binds to TG2, therefore TG2 might participate in the inhibition of VEGF production by GPR56. To test this, TG2 mRNA was knocked down in MC-1 cells by shRNAs (Supplementary Figure S7A). Although a minimal increase of VEGF was observed in the MC-1(TG2-shRNA) cells relative to the controls (Supplementary Figure S7B), this increase was much smaller than the massive induction of VEGF observed in MC-1(ΔSTP-GPR56) cells, indicating that TG2 might not mediate the inhibitory effects of GPR56 on VEGF production.
We next explored the molecular mechanism by which GPR56 might regulate VEGF production in MC-1 cells. We did not observe significant difference in VEGF mRNA levels among the MC-1(EV), MC-1(GPR), and MC-1(GPRC) cells; the level of VEGF mRNA in MC-1(ΔSTP-GPR56) cells was slightly higher than those in the other two cell lines, but the magnitude of increase (~2 fold) could not account for the large increase of VEGF levels in the conditioned medium (> 20 fold) (Supplementary Figure S8A). Similarly, no significant difference in intracellular VEGF levels was observed between the MC-1(EV) and MC-1(GPR) cells, and in MC-1(ΔSTP-GPR56) cells, the amount of intracellular VEGF was reduced relative to the other two cell lines (Supplementary Figure S8B). We also did not observe alterations in alternative splicing or ECM retention of VEGF in MC-1 cells expressing GPR56 or ΔSTP-GPR56 (data not shown). These data collectively imply that GPR56 might not regulate VEGF production at the levels of mRNA or protein synthesis, but instead might inhibit its secretion/release from melanoma cells.
VEGF Secreted from MC-1(EV), MC-1(GPR56), and MC-1(ΔSTP-GPR56) cells contribute to angiogenesis and tumor growth
VEGF is a major stimulator of angiogenesis during development and diseases. In tumors, it is mainly secreted by cancer cells (30) and its level correlates with poor prognosis of cancer (31). To test whether VEGF secreted from MC-1 cells contribute to the angiogenesis and tumor progression regulated by GPR56, VEGF mRNA was knocked down in MC-1(ΔSTP-GPR56) cells by shRNAs (Figure 4E). These MC-1(ΔSTP-GPR56+VEGF-shRNA) cells were injected subcutaneously into mice. Tumor growth from these cells was significantly impaired compared with controls (Figure 4F), demonstrating that VEGF produced from MC-1(ΔSTP-GPR56) cells directly contributes to their tumor progression. Consistent with this, conditioned media from MC-1(ΔSTP-GPR56) cells accelerated the wound closure of endothelial monolayer in a VEGF-dependent manner in scratch assays (Supplementary Figure S9A and B).
GPR56 regulates VEGF production through protein kinase Cα (PKCα)
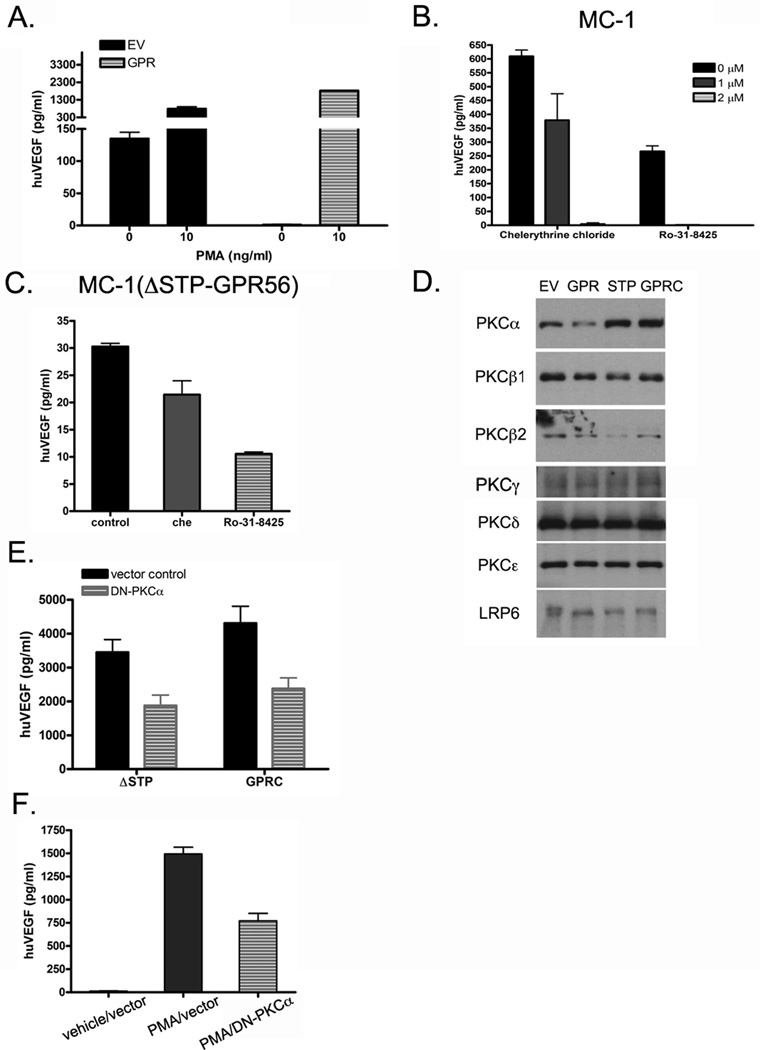
PKC activation has been shown to induce VEGF release from specific granules in multiple cell types (16, 32, 33). To test whether GPR56 also regulates VEGF secretion in melanoma cells through PKC, phorbol myristate acetate (PMA), a potent PKC activator, was added to MC-1(GPR) cells and shown to abrogate the inhibition of VEGF secretion by GPR56 (Figure 5A). Conversely, administration of PKC inhibitors, chelerythrine chloride and Ro-31-8425, resulted in significant reduction of VEGF secretion in MC-1 and MC-1(ΔSTP-GPR56) cells (Figure 5B and C), suggesting that GPR56 regulates VEGF production from MC-1 cells via PKC.
Figure 5. GPR56 and ΔSTP-GPR56 regulate VEGF secretion through PKCα.
A: Activation of PKC by PMA rescued the inhibition of VEGF secretion by GPR56.
B. Inhibition of PKC activities led to a reduction in VEGF secretion from MC-1 cells.
C. Inhibition of PKC activities led to a reduction of VEGF secretion from MC-1(ΔSTP-GPR56) cells. Serum starved MC-1(ΔSTP-GPR56) cells were incubated with PKC inhibitors for 4 hours before the media were collected for ELISA analyses.
D. PKCα was reduced in the particular fractions from MC-1(GPR) cells, but increased in those of MC-1(ΔSTP-GPR) and MC-1(GPRC) cells, relative to MC-1(EV) cells. LRP6 was used as a loading control.
E. Expression of dominant negative (DN) PKCα in MC-1(ΔSTP-GPR) or MC-1(GPRC) cells led to reduced VEGF production in the medium.
F. Expression of DN-PKCα inhibited PMA-induced production of VEGF in MC-1(GPR56) cells.
PKCs are represented by as many as ten different isoforms (34). Except for the two atypical PKCs, all other eight isoforms (PKCα, PKCβ1, PKCβ2, PKCγ, PKCδ, PKCε, PKCη, PKCθ) are sensitive to PMA activation and could be involved in stimulating VEGF secretion. To examine which isoform might be regulated by GPR56, we investigated their activities in MC-1 cells expressing full-length GPR56, ΔSTP-GPR56, GPRC, and vector control. Because activated PKC typically translocates from cytosol to membranes (35), its level in the particular fraction of cell lysates directly correlates with its activation state. Consequently, the particular fractions of MC-1(EV), MC-1(GPR), MC-1(ΔSTP-GPR), and MC-1(GPRC) cells were collected and the levels of PKCα, PKCβ1, PKCβ2, PKCγ, PKCε, and PKCε in each fraction were determined by western blotting analyses using isoform-specific antibodies. We found that the level of PKCα was reduced in the particular fractions of MC-1(GPR) cells and increased in those of MC-1(ΔSTP-GPR) and MC-1(GPRC) cells (Figure 5D and Supplementary Figure S10), suggesting that PKCα is inhibited by full-length GPR56 and activated by ΔSTP-GPR56 and GPRC, and thus may mediate the regulation of VEGF production by these receptors. Consistent with this, expression of a dominant negative mutant of PKCα led to a significant reduction of VEGF secretion in MC-1(ΔSTP-GPR) and MC-1(GPRC) cells as well as the PMA-induced VEGF secretion from MC-1(GPR56) cells (Figure 5E and F). The level of PKCβ2 was decreased in the particular fractions of MC-1(GPR) cells, but did not show any increase in those of MC-1(ΔSTP-GPR) and MC-1(GPRC) cells, thus unlikely contribute directly to the VEGF production regulated by GPR56 and its derivatives. None of the remaining PKC isoforms tested showed significant differential regulation by GPR56 (Figure 5D and Supplementary Figure S10).
Expression levels of GPR56 are inversely correlated with the progression of human melanomas
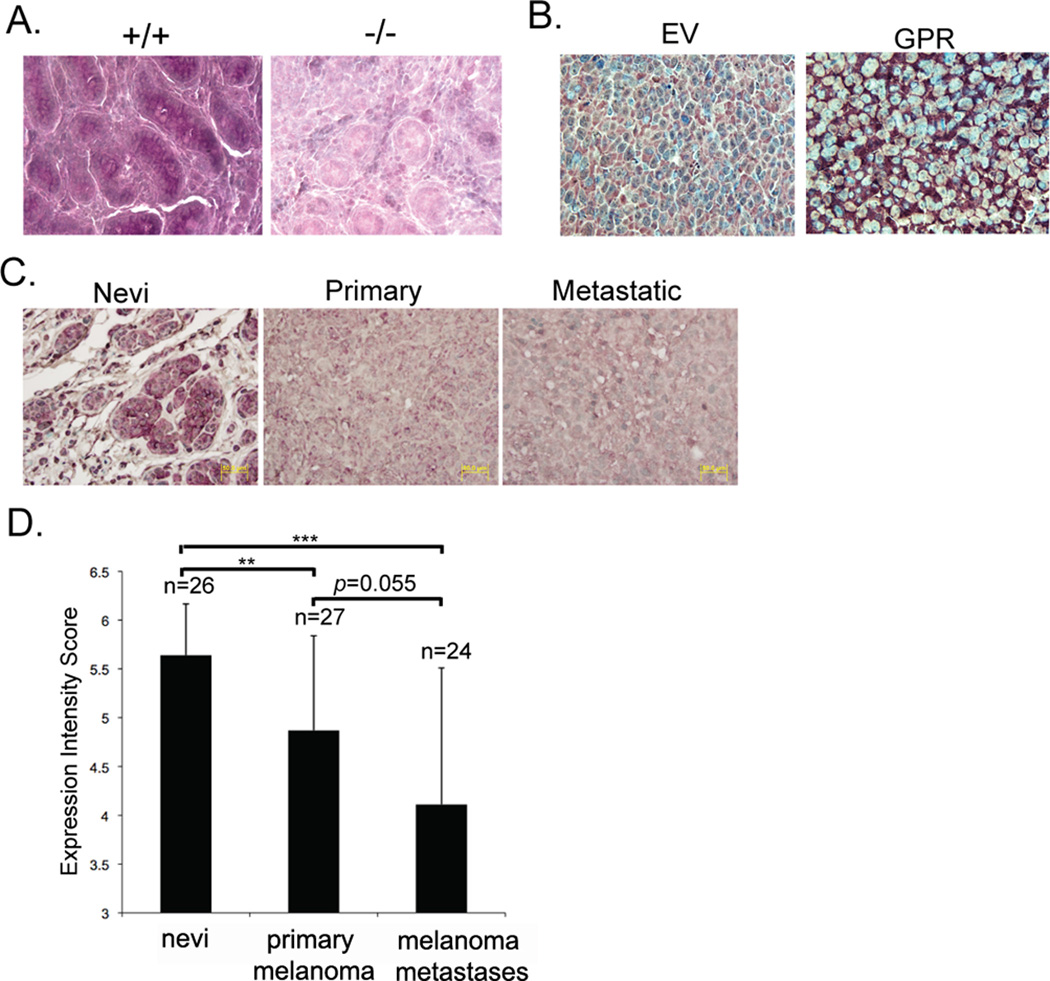
To investigate expression of GPR56 in melanocytic lesions, we analyzed the expression pattern of GPR56 on human tissue microarrays (TMAs) of nevi, primary melanomas, and metastatic melanomas by immunohistochemistry using an antibody raised against the STP segment of GPR56. The antibody specifically recognized GPR56 on formalin-fixed sections from mouse tissues and human melanoma xenografts (Figure 6A and B). Expression intensities of GPR56 on the TMAs were determined by one of us (G.S.) and decreased significantly as melanoma progresses from nevi to primary melanomas, and appeared to follow this trend of decline (although not with statistical significance) in metastatic melanomas (Figure 6C and D).
Figure 6. The expression levels of GPR56 are inversely correlated with melanoma progression.
A, B. Immunohistochemical analyses on sections from wild-type or Gpr56−/− testes (A), or tumors from MC-1 cells that express human GPR56 (B). The anti-GPRN antibody specifically recognized GPR56 on these sections.
C. Immunohistochemical analyses on melanoma tissue microarrays using the anti-GPRN antibody.
D. Expression intensity scores of GPR56 on melanoma tissue microarrays. **: p < 0.001; ***: p < 0.0001.
Discussion
Malignant melanoma is a devastating disease with significant resistance to current therapies (36). Recently, anti-angiogenic inhibitors have shown promising therapeutic potentials in melanoma (7, 37). A common target for anti-angiogenic therapy is VEGF. Several VEGF inhibitors have been pursued clinically and some of them have been approved by FDA (38). However, relapse was frequently observed in VEGF inhibitor-treated patients (6, 7), partly attributable to incomplete inhibition of VEGF activity. Most of the VEGF inhibitors block the downstream effects of VEGF, after its secretion and release from cells, either by neutralizing diffusible VEGF or inhibiting the signaling pathway it stimulates. Since the supply of VEGF is not affected, continuous administration of inhibitors is required to sequester its activity and frequently some VEGF escapes the inhibition, by its retention in extracellular matrix (ECM), for example (39). This problem may be alleviated by reducing production and/or secretion of bioactive VEGF at source, thus increasing the efficacy of anti-angiogenic effects of VEGF inhibitors.
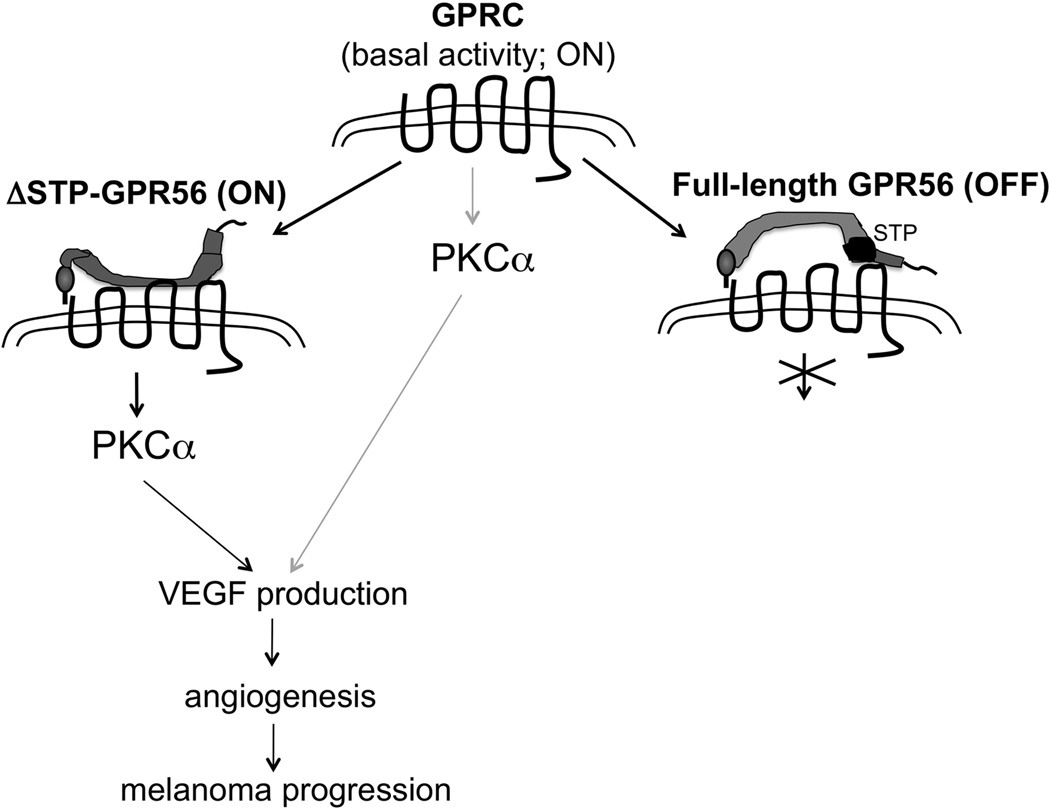
G protein-coupled receptors constitute over 40% of drug targets for treatment of human diseases, but they have not been the main targets for cancer control, mostly due to a lack of understanding of their roles in cancer progression. The involvement of GPCRs on VEGF production in cancer cells has been reported and shown to involve the p38/MAPK pathway (40). We report here that an adhesion GPCR, GPR56, regulates VEGF production and melanoma angiogenesis through a signaling pathway mediated by protein kinase Cα (Figure 7). We also present evidence that the two fragments of GPR56, generated by cleavage upon receptor maturation, could play distinct roles in their regulation of melanoma angiogenesis and progression. While expression of its C-terminal fragment (GPRC) induced VEGF secretion in MC-1 cells, expression of the full-length receptor inhibited it. Most signaling receptors exhibit a certain degree of basal/constitutive activities (41). We postulate that the “free” GPRC might be in such a state and its over-expression led to stimulated VEGF production and melanoma progression (Figure 7). GPRN and ΔSTP-GPRN might inhibit and activate this basal activity of GPRC, respectively, resulting in opposing effects on melanoma progression. Consistent with this notion, our data showed that purified FcGPRN protein was sufficient to inhibit VEGF production from MC-1(GPRC) cells (Figure 4D).
Figure 7. Model of GPR56 function in VEGF production by melanoma cells.
We hypothesize that the free GPRC fragment of GPR56 represents a basal/constitutive “on” state to induce the activation of PKCα, VEGF production, angiogenesis, and melanoma progression. The binding of full-length GPRN switches GPRC to an “off” state and inhibits those processes. The binding of ΔSTP-GPRN, however, activates it and promotes melanoma progression.
ΔSTP-GPR56 promoted VEGF production in multiple melanoma cell lines (Figure 4C), suggesting that the activation mechanisms and resulted functions of GPR56 may be shared among malignant melanomas. Interestingly, alternatively spliced isoforms of GPR56 were reported recently and two of them encode proteins similar to ΔSTP-GPR56 (42). Although the ΔSTP isoforms may function differently in different cell types - they were shown to inhibit the VEGF promoter in HEK293 cells rather than enhancing VEGF production (42), their very existence suggests that perturbing GPR56, by inhibiting its endogenous ΔSTP or activated isoforms, could be used as a strategy to effectively curb angiogenesis and melanoma progression, especially when combined with other VEGF inhibitors. Normal adult tissues tend to express low levels of GPR56 relative to cancerous tissues (43), and Gpr56−/− mice or human patients carrying mutations in the GPR56 gene are viable and grossly healthy, despite defects in brain development (18, 44). Therefore, perturbing GPR56 and its mediated signaling pathway may have few detrimental side effects on cancer patients as long as they do not penetrate the blood-brain barrier.
Whether GPR56 functions similarly in endogenous melanoma progression as in xenografts is yet to be determined. We reported previously that melanoma development in the Ink4a/Arf−/− tyr-HRAS mice did not alter significantly in the absence of GPR56 (43). Perhaps GPR56 plays dual roles in endogenous melanoma progression, as in xenografts, and its complete absence abolishes both and leads to an outcome similar to that in wild-type mice. Alternatively, GPR56 expressed on stromal cells also affect tumor development in endogenous melanomas, which was not assessed in the xenograft model.
The signaling pathways mediated by GPR56, or adhesion GPCRs in general, are poorly understood, despite their increasingly recognized importance in development and diseases (17–22). The results from our study serve as a platform for further investigations in this area. For example, it is not clear whether the inhibition or activation of GPRC imposed by GPRN or ΔSTP-GPRN might involve other interacting proteins. We previously reported that TG2 binds to GPRN (25). However, knockdown of TG2 in MC-1(GPR56) cells by RNAi did not lead to an increase of VEGF production as observed in MC-1(ΔSTP-GPR56) cells, suggesting that the roles of TG2 in GPR56-mediated signaling are not straightforward. It is possible that other factor(s) binds to the STP segment in GPRN and mediates the inactivation of GPRC. The expression or stability of this factor(s) may thus determine the activity of GPR56 in cancer cells. Finally, GPR56 was reported to associate with Gαq (45), consistent with its regulation of PKC activation. Whether this is the case also needs to be investigated.
The apparent separate functions of GPRN and GPRC indicated by our results support the recent proposal that the two subunits of adhesion GPCRs might function as distinct entities (46). It was reported that the two fragments of latrophilin, another adhesion GPCR, could associate with and potentially signal through fragments from other adhesion GPCRs. The GPCR proteolytic site (GPS) is conserved in all adhesion GPCRs and those tested were shown to be cleaved into two fragments (17). Their potential to play distinct functions promises a great complexity of regulation by the adhesion GPCRs in a range of biological processes, including cancer.
Supplementary Material
Acknowledgments
We thank Drs. Hartmut Land and Helene McMurray (University of Rochester) for stimulating discussions.
Financial support: the Ruth L. Kirschstein National Research Service Award (to L.X.), the NIH (HL67424 to A. R. and U54CA126515 to R.O.H.) and the Howard Hughes Medical Institute (to R.O.H.).
Footnotes
Conflicts of Interest: the authors declare no conflict of interest.
References
- 1.Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol. 2007;8:464–478. doi: 10.1038/nrm2183. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 3.Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat Rev Cancer. 2003;3:401–410. doi: 10.1038/nrc1093. [DOI] [PubMed] [Google Scholar]
- 4.Semenza GL. Angiogenesis in ischemic and neoplastic disorders. Annu Rev Med. 2003;54:17–28. doi: 10.1146/annurev.med.54.101601.152418. [DOI] [PubMed] [Google Scholar]
- 5.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 6.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–739. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 7.Jain RK. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat Rev Cancer. 2008;8:309–316. doi: 10.1038/nrc2346. [DOI] [PubMed] [Google Scholar]
- 8.Fischer I, Cunliffe CH, Bollo RJ, Raza S, Monoky D, Chiriboga L, et al. High-grade glioma before and after treatment with radiation and Avastin: initial observations. Neuro Oncol. 2008;10:700–708. doi: 10.1215/15228517-2008-042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Norden AD, Young GS, Setayesh K, Muzikansky A, Klufas R, Ross GL, et al. Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology. 2008;70:779–787. doi: 10.1212/01.wnl.0000304121.57857.38. [DOI] [PubMed] [Google Scholar]
- 10.Ebos JM, Lee CR, Cruz-Munoz W, Bjarnason GA, Christensen JG, Kerbel RS. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancopoulos GD, Davis S, Gale NW, Rudge JS, Wiegand SJ, Holash J. Vascular-specific growth factors and blood vessel formation. Nature. 2000;407:242–248. doi: 10.1038/35025215. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara N, Davis-Smyth T. The biology of vascular endothelial growth factor. Endocr Rev. 1997;18:4–25. doi: 10.1210/edrv.18.1.0287. [DOI] [PubMed] [Google Scholar]
- 13.Lee S, Jilani SM, Nikolova GV, Carpizo D, Iruela-Arispe ML. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J Cell Biol. 2005;169:681–691. doi: 10.1083/jcb.200409115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leung DW, Cachianes G, Kuang WJ, Goeddel DV, Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- 15.Grutzkau A, Kruger-Krasagakes S, Baumeister H, Schwarz C, Kogel H, Welker P, et al. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: implications for the biological significance of VEGF206. Mol Biol Cell. 1998;9:875–884. doi: 10.1091/mbc.9.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gaudry M, Bregerie O, Andrieu V, El Benna J, Pocidalo MA, Hakim J. Intracellular pool of vascular endothelial growth factor in human neutrophils. Blood. 1997;90:4153–4161. [PubMed] [Google Scholar]
- 17.Yona S, Lin HH, Siu WO, Gordon S, Stacey M. Adhesion-GPCRs: emerging roles for novel receptors. Trends Biochem Sci. 2008;33:491–500. doi: 10.1016/j.tibs.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 18.Piao X, Hill RS, Bodell A, Chang BS, Basel-Vanagaite L, Straussberg R, et al. G protein-coupled receptor-dependent development of human frontal cortex. Science. 2004;303:2033–2036. doi: 10.1126/science.1092780. [DOI] [PubMed] [Google Scholar]
- 19.Seandel M, James D, Shmelkov SV, Falciatori I, Kim J, Chavala S, et al. Generation of functional multipotent adult stem cells from GPR125+ germline progenitors. Nature. 2007;449:346–350. doi: 10.1038/nature06129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park D, Tosello-Trampont AC, Elliott MR, Lu M, Haney LB, Ma Z, et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature. 2007;450:430–434. doi: 10.1038/nature06329. [DOI] [PubMed] [Google Scholar]
- 21.Monk KR, Naylor SG, Glenn TD, Mercurio S, Perlin JR, Dominguez C, et al. A G protein-coupled receptor is essential for Schwann cells to initiate myelination. Science. 2009;325:1402–1405. doi: 10.1126/science.1173474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhnert F, Mancuso MR, Shamloo A, Wang HT, Choksi V, Florek M, et al. Essential Regulation of CNS Angiogenesis by the Orphan G Protein-Coupled Receptor GPR124. Science. 2010;330:985–989. doi: 10.1126/science.1196554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krasnoperov V, Bittner MA, Holz RW, Chepurny O, Petrenko AG. Structural requirements for alpha-latrotoxin binding and alpha-latrotoxin-stimulated secretion. A study with calcium-independent receptor of alpha-latrotoxin (CIRL) deletion mutants. J Biol Chem. 1999;274:3590–3596. doi: 10.1074/jbc.274.6.3590. [DOI] [PubMed] [Google Scholar]
- 24.Jin Z, Tietjen I, Bu L, Liu-Yesucevitz L, Gaur SK, Walsh CA, et al. Disease-associated mutations affect GPR56 protein trafficking and cell surface expression. Hum Mol Genet. 2007;16:1972–1985. doi: 10.1093/hmg/ddm144. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Begum S, Hearn JD, Hynes RO. GPR56, an atypical G protein-coupled receptor, binds tissue transglutaminase, TG2, and inhibits melanoma tumor growth and metastasis. Proc Natl Acad Sci U S A. 2006;103:9023–9028. doi: 10.1073/pnas.0602681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu L, Shen SS, Hoshida Y, Subramanian A, Ross K, Brunet JP, et al. Gene expression changes in an animal melanoma model correlate with aggressiveness of human melanoma metastases. Mol Cancer Res. 2008;6:760–769. doi: 10.1158/1541-7786.MCR-07-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scott GA, McClelland LA, Fricke AF, Fender A. Plexin C1, a receptor for semaphorin 7a, inactivates cofilin and is a potential tumor suppressor for melanoma progression. J Invest Dermatol. 2009;129:954–963. doi: 10.1038/jid.2008.329. [DOI] [PubMed] [Google Scholar]
- 28.Zhu Y, Lim WG, Tan BJ, Teo TS, Duan W. Identification of an integral plasma membrane pool of protein kinase C in mammalian tissues and cells. Cell Signal. 2005;17:1125–1136. doi: 10.1016/j.cellsig.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Lazova R, Gould Rothberg BE, Rimm D, Scott G. The semaphorin 7A receptor Plexin C1 is lost during melanoma metastasis. Am J Dermatopathol. 2009;31:177–181. doi: 10.1097/DAD.0b013e318196672d. [DOI] [PubMed] [Google Scholar]
- 30.Brown LF, Berse B, Jackman RW, Tognazzi K, Manseau EJ, Senger DR, et al. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993;53:4727–4735. [PubMed] [Google Scholar]
- 31.Graeven U, Fiedler W, Karpinski S, Ergun S, Kilic N, Rodeck U, et al. Melanoma-associated expression of vascular endothelial growth factor and its receptors FLT-1 and KDR. J Cancer Res Clin Oncol. 1999;125:621–629. doi: 10.1007/s004320050325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boesiger J, Tsai M, Maurer M, Yamaguchi M, Brown LF, Claffey KP, et al. Mast cells can secrete vascular permeability factor/ vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of fc epsilon receptor I expression. J Exp Med. 1998;188:1135–1145. doi: 10.1084/jem.188.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Italiano JE, Jr, Richardson JL, Patel-Hett S, Battinelli E, Zaslavsky A, Short S, et al. Angiogenesis is regulated by a novel mechanism: pro- and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood. 2008;111:1227–1233. doi: 10.1182/blood-2007-09-113837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reyland ME. Protein kinase C isoforms: Multi-functional regulators of cell life and death. Front Biosci. 2009;14:2386–2399. doi: 10.2741/3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Newton AC. Protein kinase C: structural and spatial regulation by phosphorylation, cofactors, and macromolecular interactions. Chem Rev. 2001;101:2353–2364. doi: 10.1021/cr0002801. [DOI] [PubMed] [Google Scholar]
- 36.Garbe C, Eigentler TK. Diagnosis and treatment of cutaneous melanoma: state of the art 2006. Melanoma Res. 2007;17:117–127. doi: 10.1097/CMR.0b013e328042bb36. [DOI] [PubMed] [Google Scholar]
- 37.Perez DG, Suman VJ, Fitch TR, Amatruda T, 3rd, Morton RF, Jilani SZ, et al. Phase 2 trial of carboplatin, weekly paclitaxel, and biweekly bevacizumab in patients with unresectable stage IV melanoma: a North Central Cancer Treatment Group study, N047A. Cancer. 2009;115:119–127. doi: 10.1002/cncr.23987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferrara N, Hillan KJ, Gerber HP, Novotny W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat Rev Drug Discov. 2004;3:391–400. doi: 10.1038/nrd1381. [DOI] [PubMed] [Google Scholar]
- 39.Kadenhe-Chiweshe A, Papa J, McCrudden KW, Frischer J, Bae JO, Huang J, et al. Sustained VEGF blockade results in microenvironmental sequestration of VEGF by tumors and persistent VEGF receptor-2 activation. Mol Cancer Res. 2008;6:1–9. doi: 10.1158/1541-7786.MCR-07-0101. [DOI] [PubMed] [Google Scholar]
- 40.Sodhi A, Montaner S, Patel V, Zohar M, Bais C, Mesri EA, et al. The Kaposi's sarcoma-associated herpes virus G protein-coupled receptor up-regulates vascular endothelial growth factor expression and secretion through mitogen-activated protein kinase and p38 pathways acting on hypoxia-inducible factor 1alpha. Cancer Res. 2000;60:4873–4880. [PubMed] [Google Scholar]
- 41.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim JE, Han JM, Park CR, Shin KJ, Ahn C, Seong JY, et al. Splicing variants of the orphan G-protein-coupled receptor GPR56 regulate the activity of transcription factors associated with tumorigenesis. J Cancer Res Clin Oncol. 2009 doi: 10.1007/s00432-009-0635-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xu L, Begum S, Barry M, Crowley D, Yang L, Bronson RT, et al. GPR56 plays varying roles in endogenous cancer progression. Clin Exp Metastasis. 2010;27:241–249. doi: 10.1007/s10585-010-9322-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li S, Jin Z, Koirala S, Bu L, Xu L, Hynes RO, et al. GPR56 regulates pial basement membrane integrity and cortical lamination. J Neurosci. 2008;28:5817–5826. doi: 10.1523/JNEUROSCI.0853-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Little KD, Hemler ME, Stipp CS. Dynamic regulation of a GPCR-tetraspanin-G protein complex on intact cells: central role of CD81 in facilitating GPR56-Galpha q/11 association. Mol Biol Cell. 2004;15:2375–2387. doi: 10.1091/mbc.E03-12-0886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Silva JP, Lelianova V, Hopkins C, Volynski KE, Ushkaryov Y. Functional cross-interaction of the fragments produced by the cleavage of distinct adhesion G-protein-coupled receptors. J Biol Chem. 2009;284:6495–6506. doi: 10.1074/jbc.M806979200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.