Abstract
Best established as components of steroid hormone receptor complexes, it is now clear that the large molecular weight immunophilins, FKBP52 and FKBP51, play important regulatory roles elsewhere in the cell. This review outlines what is known about the organization of the genes, FKBP4 and FKBP5 respectively, encoding these proteins and describes their diverse actions in the nervous system, reproduction, and cancer. The organization of FKBP4 and FKBP5 is very similar among the chordates, and gene expression is influenced by both genetic and epigenetic mechanisms. Recent studies identifying roles of FKBP52 and FKBP51 in regulation of the microtubule-associated protein tau and microtubule assembly are discussed, as is their interaction with and influence on the transient receptor potential canonical subfamily of ion channel proteins.
Introduction
The FK506-binding proteins (FKBPs) are members of a large superfamily of peptidyl-prolyl isomerases (PPIase) that are widely distributed in nature and have diverse functions [1]. Some FKBPs, including FKBP52 and FKBP51, also possess C-terminal tetratricopeptide repeat (TPR) domains. Through the TPR domain, FKBP52 and FKBP51 compete for binding to Hsp90 complexes, especially those associated with steroid hormone receptors [2]. There is an extensive literature of how steroid hormones regulate these FKBPs, and how FKBP52 and FKBP51 in turn regulate steroid receptor activity. Recent work has revealed new roles for these and other FKBPs in cellular function, especially relating to cancer biology. Thus, a series of opinions highlighting our present understanding of relevant FKBPs at the genomic and protein level is warranted and timely. Here, we provide details of the organization of the human FKBP52 and FKBP51 genes (FKBP4 and FKBP5, respectively) and present recent studies on the diverse roles of these proteins in cell biology including regulation of microtubule function and the activity of transient receptor potential channels.
Organization of FKBP4 and FKBP5
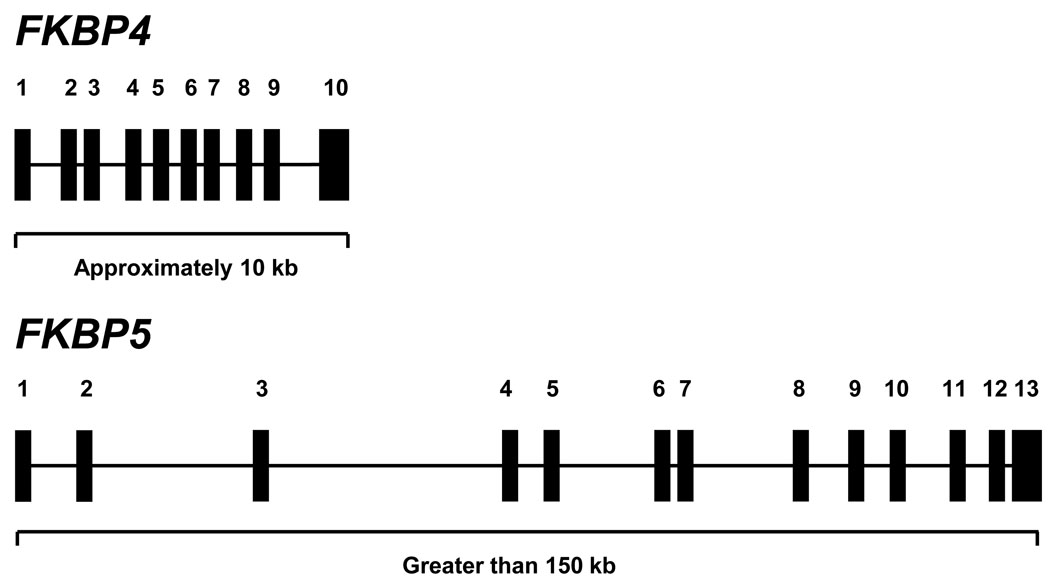
FKBP4 and FKBP5 map to chromosomes 12 (12p13.33) and 6 (6p21.31), respectively [data from the Gene, Genes and Mapped Phenotypes, portal of the National Center for Biotechnology Information website (URL: http://www.ncbi.nlm.nih.gov/gene)]. Eight pseudogenes for FKBP4 have been identified, seven on chromosome 9 and one on chromosome 4. Pseudogenes for FKBP5 have not been reported. The genes for human and rabbit FKBP52 were isolated from genomic DNA in 2003, and their promoters were partially characterized [3, 4]. The human and mouse FKBP51 genes were first isolated during the same time period [5, 6]. FKBP4 consists of 10 exons and 9 introns spanning approximately 10 kb of genomic DNA, whereas FKBP5 has 13 exons and 12 introns spanning more than 150 kb (Figure 1). The organization of FKBP5 is identical to that of FKBP4 with the exception that non-coding exons 1–3 in FKBP5 are absent in FKBP4. The exon-intron boundaries throughout the two genes are otherwise identical. The HomoloGene portal of the National Center of Biotechnology Information website (URL: http://www.ncbi.nlm.nih.gov/homologene) was utilized to detect genes homologous to FKBP4 and FKBP5. FKBP4 orthologues are found in chordates and in fruit fly Drosophila melanogaster and soil nematode Caenorhabditis elegans genomes, although the exon-intron boundary organization was conserved only among the chordates. Using the same analysis, FKBP5 orthologues are found in chordates, but in neither D. melanogaster nor C. elegans genomes. Using clustering analysis, Galat [7] suggested that FKBP4 and FKBP5 likely evolved from an ancestral invertebrate gene, possibly fkb-6 [8], through a gene duplication event that occurred before the emergence of fishes. Regarding the expression of these genes, the molecular interactions that mediate constitutive or regulated activity of FKBP4 are largely unexplored. On the other hand, our understanding of FKBP5 expression is greater, where there is increasing evidence that both genetic and epigenetic variation in non-coding regions of FKBP5 may influence basal and hormone-stimulated expression of this gene. For example, single nucleotide polymorphisms in FKBP5 are associated with elevated expression of FKBP51 and impaired stress hormone regulation [9]. On the other hand, Lee, Tamashiro and colleagues [10] showed that long-term treatment with corticosteroid results in a site-specific decrease in DNA methylation in intron 5 of FKBP5 and an increase in FKBP51 mRNA in vivo and in vitro. And histone density is decreased at several regions of the gene by dexamethasone [11]. This fascinating area of research will be covered in more detail in other opinions.
Figure 1.
Gene organization of FKBP4 and FKBP5. Solid boxes represent exons and horizontal lines represent introns. FKBP4 spans approximately 10 kb, whereas FKBP5 spans approximately 150 kb. The organization of FKBP5 is identical to that of FKBP4 with the exception that non-coding exons 1–3 in FKBP5 are absent in FKBP4. The exon-intron boundaries throughout the two genes are otherwise identical.
Organization of FKBP52 and FKBP51
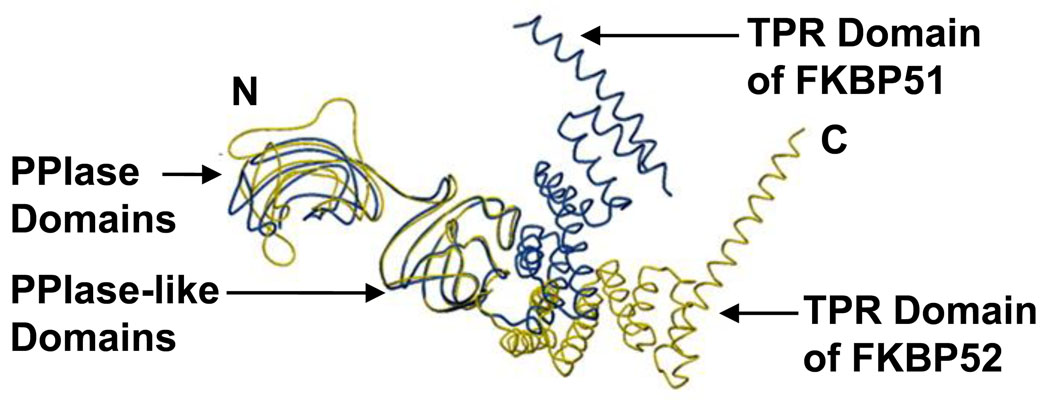
FKBP51 and FKBP52 are homologous proteins as demonstrated by their amino acid sequences, domain organization, and three-dimensional structures [12, 13]. They share 60% identity and 75% similarity in their amino acid sequences. Both proteins contain an N-terminal FK1 domain that is responsible for the PPIase activity and a PPIase-like FK2 domain, which shares 32% sequence homology with FK1 and exhibits no PPIase activity. These domains are highlighted in the crystallographic structures of FKBP52 and FKBP51 in Figure 2. Also shown is the C-terminal TPR domain, which is made up of three units of a consensus 34-amino acid motif. None of the PPIase or TPR domain boundaries in FKBP52 or FKBP51 coincide with exon-intron boundaries. Considering the similar domain organization and sequence of FKBP52 and FKBP51, redundancy in their actions might be expected. However, when the three-dimensional crystallographic structures of the two proteins were superimposed, differences in the domain-domain orientations were revealed (Figure 2). Thus, amino acid and structural differences are likely responsible for the antagonistic activities of the two proteins on steroid receptor activity [14, 15]. The different activities of FKBP52 and FKBP51 on microtubule dynamics and the transient receptor potential channel activity, described below, likely also reside in structural differences between two proteins.
Figure 2.
Crystallographic structures of FKBP52 (yellow) and FKBP51 (blue). Superimposing the three-dimensional structures of FKBP51 and FKBP52 shows their structural similarity and reveals differences in domain-domain orientations. The PPIase domain at the N-terminus (FK1) and the TPR domain at the C-terminus are oriented differently in the two proteins.
From: Proc Natl Acad Sci U S A. 2004 June 1; 101(22): 8348–8353. Published online 2004 May 24. doi: 10.1073/pnas.0305969101.
Functions of FKBP52 and FKBP51
Table 1 highlights recent studies, published in the last two years, which describe the diverse actions of FKBP52 and FKBP51. The table includes the well established roles of FKBP52 and FKBP51 in regulating steroid hormone receptor activity [15]. Other papers that directly involve the effect of FKBPs in steroid hormone receptor function are not included. The roles of FKBP52 in control of amyloid beta toxicity and copper homeostasis [16], modulation of α-synuclein aggregation [17], control of proto-oncogene RET [18], and regulation of peroxiredoxin-6 levels [19] are highlighted. Also included in the table are the actions of FKBP51 in control of apoptosis [20], regulation of the kinase Akt [21], and the association of FKBP5 polymorphisms with mood disorders [9]. In addition to the actions of FKBP52 and FKBP51 described in Table 1, these proteins have been shown to regulate microtubule dynamics and transient receptor potential channels. These actions are described in more detail below.
Table 1.
Diverse actions of FKBP52 and FKBP51.
| FKBP52 | Regulation of steroid hormone receptor function | [15] |
| Control of amyloid beta toxicity and copper homeostasis in Drosophila | [16] | |
| Modulation of α-synuclein aggregation | [17] | |
| Control of proto-oncogene RET in neurons | [18] | |
| Protection of pregnancy from oxidative stress through regulation of peroxiredoxin-6 levels | [19] | |
| FKBP51 | Regulation of steroid hormone receptor function | [15] |
| Inhibition apoptosis in irradiated melanoma cells | [20] | |
| Promotion of dephosphorylation of Akt and down-regulation of the Akt pathway | [21] | |
| Association of polymorphisms in FKBP5 in affective and anxiety disorders | [9] |
Regulation of microtubule dynamics
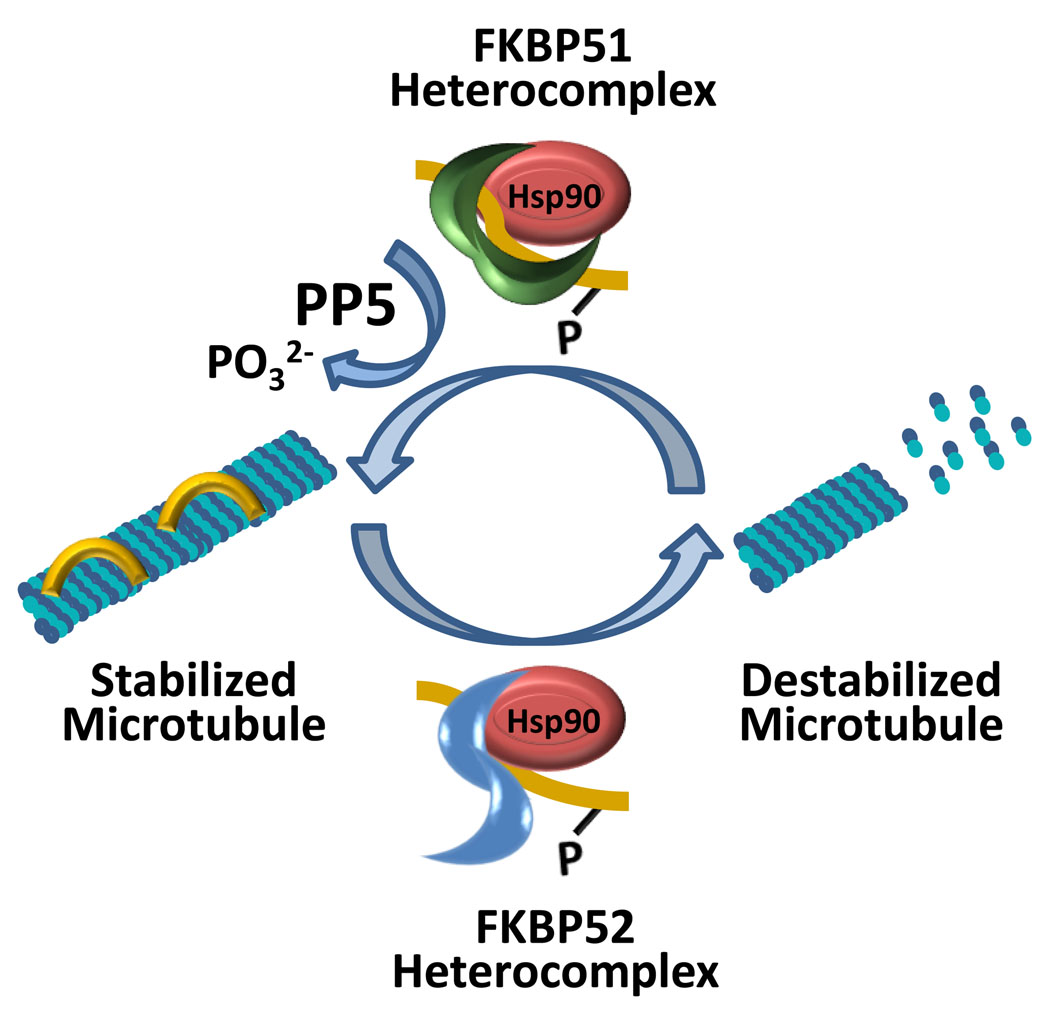
The roles of FKBP51 and FKBP52 in microtubule dynamics have focused on the microtubule-associated protein tau. In the last year, a model (Figure 3) has been proposed in which FKBP51 promotes microtubule stabilization through interaction with tau in a complex with Hsp90 [22, 23]. In this complex, phosphorylated tau is in a trans configuration. The PPIase activity of FKBP51 isomerizes tau to a cis configuration, which enhances dephosphorylation of tau by the phosphatase PP5. This is a critical event because dephosphorylated tau is recycled to microtubules and stabilizes them. On the other hand, highly phosphorylated tau exhibits reduced microtubule binding, leading to a loss of microtubule integrity [24]. In as much as FKBP51 plays a role in promoting microtubule stabilization, the work of Chambraud and colleagues [25, 26] suggests that FKBP52 plays an opposite role and promotes microtubule destabilization. In the initial study, it was observed that FKBP52 binds to tubulin and promotes microtubule depolymerization. While FKBP52 was able to directly interact with tubulin in vitro, the ability of FKBP52 to promote depolymerization of tubulin required the presence of a microtubule associated protein. In their subsequent study, it was demonstrated that FKBP52 binds to the microtubule associated protein tau, especially when in a phosphorylated or hyperphosphorylated state. If FKBP51 and FKBP52 possess PPIase activity and both are capable of binding phosphorylated tau, why is it that FKBP51 is selectively able to isomerize tau and promote microtubule polymerization? First, FKBP51 and FKBP52, and indeed cyclophilin 40 and PP5, compete for a common Hsp90 binding-site in steroid receptor complexes [2], and it seems likely that similar competition occurs in the Hsp90 complex containing phosphorylated tau and a TPR cochaperone (Figure 3). Like the steroid receptor heterocomplex, the actual composition of the Hsp90-tau complex is determined by a dynamic process in which the relative abundance and affinity of each TPR cochaperone play a role. Second, structural comparison of FKBP51 and FKBP52 revealed that the PPIase (FK) and TPR domains exhibit different spatial orientations (Figure 2). Riggs and colleagues have also emphasized the importance of specific residues within the FK1 domains of FKBP52 and FKBP51 in determining steroid receptor interactions and their impact on receptor activity [14]. Thus, it may be that FKBP51 achieves an optimal orientation in the complex that allows it to isomerize specific tau residues.
Figure 3.
Model of FKBP51- and FKBP52-mediated regulation of microtubule dynamics. Microtubules are stabilized by the binding of cis tau, but are destabilized when tau becomes phosphorylated and can no longer bind. Phosphorylated tau in a trans configuration can be part of an FKBP51 heterocomplex or an FKBP52 heterocomplex. These heterocomplexes consist of at least FKBP51/FKBP52, phosphorylated trans tau, and Hsp90. In the FKBP51 heterocomplex, the TPR domain of FKBP51 (green) mediates interaction with Hsp90, and its FK1 domain is spatially oriented towards phosphorylated trans tau (yellow). In this orientation, FKBP51 catalyzes isomerization to the cis configuration that allows the phosphatase PP5 access to tau. PP5 dephosphorylates cis tau, which recycles to bind to and stabilize microtubules. In the FKBP52 heterocomplex, while the TPR domain of FKBP52 (blue) mediates binding to Hsp90, its FK1 domain is oriented away from phosphorylated trans tau thereby preventing PPIase activity on tau. Tau remains in a phosphorylated trans configuration unable to bind to microtubules, which leads to microtubule destabilization.
What are some of the functional consequences of changes in microtubule stability? Chambraud et al. [26] demonstrated that FKBP52-mediated microtubule destabilization plays a role in determining neurite length. Overexpression of FKBP52 reduced neurite outgrowth in response to nerve growth factor in PC12 cells. This finding is consistent with the previous study of Ruan et al. [27], who showed that RNAi knockdown of FKBP52 enhanced neurite outgrowth in cortical neurons. As neurite outgrowth is dependent on microtubule assembly [28], it is proposed that the mechanism underlying decreased neurite length involves FKBP52 binding to phosphorylated tau and preventing the stabilizing interaction of tau with microtubules. Because FKBP51 may isomerize tau and stabilize microtubules, it is of interest to ask whether FKBP51 promotes increased neurite length. A recent report by Galigniana’s group [29] supports the idea that FKBP52 and FKBP51 are indeed antagonistic in neuronal cells. However, in their study a modest knockdown of FKBP51 in N2a murine neuroblastoma cells enhanced FK506-stimulated neurite outgrowth. These results indicate that FKBP51 inhibits neurite outgrowth and suggest that the effects of FKBPs may be cell type- or condition-specific.
That FKBP52 and FKBP51 are important effectors of neurite length may explain, at least in part, the observations that immunophilin ligands such as FK506 and rapamycin exhibit neurotrophic effects. FK506 has been shown to promote neuroprotective and neuroregenerative effects in a number of injury models [30]. And rapamycin analogs WYE-592 and ILS-920 increase neurite outgrowth in cultured cells and improve neurological recovery in an in vivo stroke model, effects that were ascribed to FKBP52 inhibition [27]. Thus, therapeutic agents that target the large molecular weight immunophilins are proving to be valuable in neuronal regeneration and protection in the adult central nervous system.
Regulation of transient receptor potential channels
Recent work has uncovered a role for FKBP52 in regulation of the TRPC1 calcium channel. TRPC1 is a member of the transient receptor potential canonical (TRPC) subfamily of ion channel proteins that belong to the TRP superfamily [31]. Proteins in the TRP superfamily are six transmembrane spanning domain cation channels that mediate diverse cellular responses [32]. Shim and colleagues [33] showed in Xenopus spinal neurons that the downstream effect of FKBP52-regulated TRPC1 channel opening is chemotropic turning of neuronal growth cones. They showed that the PPIase activity of FKBP52 is capable of catalyzing the cis/trans isomerization of L-P bonds corresponding to regions within both the N- and C-termini regions of TRPC1. This suggests that peptidyl-prolyl isomerization is a key element of FKBP52 regulation of channel function.
From these observations, other questions arise. What is the effect of FKBP51 on growth cone movement? Do FKBP52 and FKBP51 interact with and regulate other TRPC channels? What are the physiological effects of these interactions? Do FKBP52 and FKBP51 have opposing actions, and is Hsp90 a part of a scaffolding heterocomplex? Schilling and colleagues [34, 35] demonstrated specific interaction of FKBP52 with TRPC1, TRPC4, and TRPC5 as well as TRPL. TRPV5 was also shown by Gkika et al. to be a target of FKBP52 [36]. With regards to FKBP51, reports showing potential interactions with TRP channels have yet to be published. However, preliminary work from our laboratories (unpublished data) has shown that FKBP51 inhibits thapsigargin-mediated store-operated calcium entry through the ISOC channel, of which at least TRPC1 and TRPC4 are subunits. These results suggest a functional interaction between FKBP51 and these channels, but direct evidence of interaction awaits further investigation.
Conclusions
FKBP4 and FKBP5 likely emerged by a gene duplication event from a common, ancestral invertebrate gene. Although expression of FKBP4 and FKBP5 is quite different, the gene structures are homologous and the protein sequences are 75% similar. Both FKBP52 and FKBP51 bind Hsp90 through C-terminal TPR domains and share an FK1 domain that exhibits PPIase activity. Despite these similarities, FKBP52 and FKBP51 have diverse and often opposite actions. Antagonistic roles of these proteins were first described in their effects on steroid hormone receptor activity. Now, in light of recent observations, it is interesting to consider that competitive association with Hsp90 through the TPR domain is integral to immunophilin action, and this antagonistic behavior is a fundamental characteristic of the two proteins. To date, our understanding of the functions of FKBP52 and FKBP51 is incomplete, as is the role of their PPIase activity and/or FK1 domain in regulating downstream targets.
Acknowledgments
Dr. Cioffi is supported by a National Institutes of Health (NIH) Pathway to Independence Award 5R00HL089361. Some of the work described in this review was supported by the American Heart Association, Southeast Affiliate, and grant 13200 from the National Center for Research Resources (NCRR), a component of the NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NCRR or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Donna L. Cioffi, Email: dlcioffi@usouthal.edu.
Tina R. Hubler, Email: trhubler@una.edu.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Galat A. Functional drift of sequence attributes in the FK506-binding proteins (FKBPs) J Chem Inf Model. 2008;48:1118–1130. doi: 10.1021/ci700429n. [DOI] [PubMed] [Google Scholar]
- 2.Smith DF, Toft DO. Minireview: the intersection of steroid receptors with molecular chaperones: observations and questions. Mol Endocrinol. 2008;22:2229–2240. doi: 10.1210/me.2008-0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Massol N, Lebeau MC, Schumacher M, Baulieu EE. Promoter activity and gene structure of rabbit FKBP52. DNA Cell Biol. 2003;22:505–511. doi: 10.1089/10445490360708919. [DOI] [PubMed] [Google Scholar]
- 4.Scammell JG, Hubler TR, Denny WB, Valentine DL. Organization of the human FK506-binding immunophilin FKBP52 protein gene (FKBP4) Genomics. 2003;81:640–643. doi: 10.1016/s0888-7543(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 5.Scammell JG, Denny WB, Valentine DL, Smith DF. Overexpression of the FK506-binding immunophilin FKBP51 is the common cause of glucocorticoid resistance in three New World primates. Gen Comp Endocrinol. 2001;124:152–165. doi: 10.1006/gcen.2001.7696. [DOI] [PubMed] [Google Scholar]
- 6.Hubler TR, Denny WB, Valentine DL, Cheung-Flynn J, Smith DF, Scammell JG. The FK506-binding immunophilin FKBP51 is transcriptionally regulated by progestin and attenuates progestin responsiveness. Endocrinology. 2003;144:2380–2387. doi: 10.1210/en.2003-0092. [DOI] [PubMed] [Google Scholar]
- 7.Galat A. A note on clustering the functionally-related paralogues and orthologues of proteins: a case of the FK506-binding proteins (FKBPs) Comput Biol Chem. 2004;28:129–140. doi: 10.1016/j.compbiolchem.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 8.Richardson JM, Dornan J, Opamawutthikul M, Bruce S, Page AP, Walkinshaw MD. Cloning, expression and characterisation of FKB-6, the sole large TPR-containing immunophilin from C. elegans. Biochem Biophys Res Commun. 2007;360:566–572. doi: 10.1016/j.bbrc.2007.06.080. [DOI] [PubMed] [Google Scholar]
- 9. Binder EB. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34 Suppl 1:S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. • An influential review outlining how polymorphisms in FKBP5 are associated with higher levels of FKBP51 and mood and anxiety disorders.
- 10. Lee RS, Tamashiro KL, Yang X, Purcell RH, Harvey A, Willour VL, Huo Y, Rongione M, Wand GS, Potash JB. Chronic corticosterone exposure increases expression and decreases deoxyribonucleic acid methylation of Fkbp5 in mice. Endocrinology. 2010;151:4332–4343. doi: 10.1210/en.2010-0225. • One of the first studies showing that DNA methylation in non-coding sequences of FKBP5 mediates the effect of hormones on FKBP51 expression.
- 11.Paakinaho V, Makkonen H, Jaaskelainen T, Palvimo JJ. Glucocorticoid receptor activates poised FKBP51 locus through long-distance interactions. Mol Endocrinol. 2010;24:511–525. doi: 10.1210/me.2009-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sinars CR, Cheung-Flynn J, Rimerman RA, Scammell JG, Smith DF, Clardy J. Structure of the large FK506-binding protein FKBP51, an Hsp90-binding protein and a component of steroid receptor complexes. Proc Natl Acad Sci U S A. 2003;100:868–873. doi: 10.1073/pnas.0231020100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu B, Li P, Liu Y, Lou Z, Ding Y, Shu C, Ye S, Bartlam M, Shen B, Rao Z. 3D structure of human FK506-binding protein 52: implications for the assembly of the glucocorticoid receptor/Hsp90/immunophilin heterocomplex. Proc Natl Acad Sci U S A. 2004;101:8348–8353. doi: 10.1073/pnas.0305969101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Riggs DL, Cox MB, Tardif HL, Hessling M, Buchner J, Smith DF. Noncatalytic role of the FKBP52 peptidyl-prolyl isomerase domain in the regulation of steroid hormone signaling. Mol Cell Biol. 2007;27:8658–8669. doi: 10.1128/MCB.00985-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schulke JP, Wochnik GM, Lang-Rollin I, Gassen NC, Knapp RT, Berning B, Yassouridis A, Rein T. Differential impact of tetratricopeptide repeat proteins on the steroid hormone receptors. PLoS One. 2010;5:e11717. doi: 10.1371/journal.pone.0011717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanokawa-Akakura R, Cao W, Allan K, Patel K, Ganesh A, Heiman G, Burke R, Kemp FW, Bogden JD, Camakaris J, et al. Control of Alzheimer's amyloid beta toxicity by the high molecular weight immunophilin FKBP52 and copper homeostasis in Drosophila. PLoS One. 2010;5:e8626. doi: 10.1371/journal.pone.0008626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerard M, Deleersnijder A, Daniels V, Schreurs S, Munck S, Reumers V, Pottel H, Engelborghs Y, Van den Haute C, Taymans JM, et al. Inhibition of FK506 binding proteins reduces alpha-synuclein aggregation and Parkinson's disease-like pathology. J Neurosci. 2010;30:2454–2463. doi: 10.1523/JNEUROSCI.5983-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fusco D, Vargiolu M, Vidone M, Mariani E, Pennisi LF, Bonora E, Capellari S, Dirnberger D, Baumeister R, Martinelli P, et al. The RET51/FKBP52 complex and its involvement in Parkinson disease. Hum Mol Genet. 2010;19:2804–2816. doi: 10.1093/hmg/ddq181. [DOI] [PubMed] [Google Scholar]
- 19.Hirota Y, Acar N, Tranguch S, Burnum KE, Xie H, Kodama A, Osuga Y, Ustunel I, Friedman DB, Caprioli RM, et al. Uterine FK506-binding protein 52 (FKBP52)-peroxiredoxin-6 (PRDX6) signaling protects pregnancy from overt oxidative stress. Proc Natl Acad Sci U S A. 2010;107:15577–15582. doi: 10.1073/pnas.1009324107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romano S, D'Angelillo A, Pacelli R, Staibano S, De Luna E, Bisogni R, Eskelinen EL, Mascolo M, Cali G, Arra C, et al. Role of FK506-binding protein 51 in the control of apoptosis of irradiated melanoma cells. Cell Death Differ. 2010;17:145–157. doi: 10.1038/cdd.2009.115. [DOI] [PubMed] [Google Scholar]
- 21.Pei H, Li L, Fridley BL, Jenkins GD, Kalari KR, Lingle W, Petersen G, Lou Z, Wang L. FKBP51 affects cancer cell response to chemotherapy by negatively regulating Akt. Cancer Cell. 2009;16:259–266. doi: 10.1016/j.ccr.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jinwal UK, Koren J, 3rd, Borysov SI, Schmid AB, Abisambra JF, Blair LJ, Johnson AG, Jones JR, Shults CL, O'Leary JC, 3rd, et al. The Hsp90 cochaperone, FKBP51, increases Tau stability and polymerizes microtubules. J Neurosci. 2010;30:591–599. doi: 10.1523/JNEUROSCI.4815-09.2010. •• The paper presents a model of how FKBP51 works with the Hsp90 complex to recycle tau for microtubule stabilization.
- 23.Salminen A, Ojala J, Kaarniranta K, Hiltunen M, Soininen H. Hsp90 regulates tau pathology through co-chaperone complexes in Alzheimer's disease. Prog Neurobiol. 2011;93:99–110. doi: 10.1016/j.pneurobio.2010.10.006. [DOI] [PubMed] [Google Scholar]
- 24.Cowan CM, Bossing T, Page A, Shepherd D, Mudher A. Soluble hyper-phosphorylated tau causes microtubule breakdown and functionally compromises normal tau in vivo. Acta Neuropathol. 2010;120:593–604. doi: 10.1007/s00401-010-0716-8. [DOI] [PubMed] [Google Scholar]
- 25.Chambraud B, Belabes H, Fontaine-Lenoir V, Fellous A, Baulieu EE. The immunophilin FKBP52 specifically binds to tubulin and prevents microtubule formation. Faseb J. 2007;21:2787–2797. doi: 10.1096/fj.06-7667com. [DOI] [PubMed] [Google Scholar]
- 26. Chambraud B, Sardin E, Giustiniani J, Dounane O, Schumacher M, Goedert M, Baulieu EE. A role for FKBP52 in Tau protein function. Proc Natl Acad Sci U S A. 2010;107:2658–2663. doi: 10.1073/pnas.0914957107. •• The paper shows that FKBP52 interacts with tau and inhibits its ability to promote microtubule assembly.
- 27.Ruan B, Pong K, Jow F, Bowlby M, Crozier RA, Liu D, Liang S, Chen Y, Mercado ML, Feng X, et al. Binding of rapamycin analogs to calcium channels and FKBP52 contributes to their neuroprotective activities. Proc Natl Acad Sci U S A. 2008;105:33–38. doi: 10.1073/pnas.0710424105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Drubin DG, Feinstein SC, Shooter EM, Kirschner MW. Nerve growth factor-induced neurite outgrowth in PC12 cells involves the coordinate induction of microtubule assembly and assembly-promoting factors. J Cell Biol. 1985;101:1799–1807. doi: 10.1083/jcb.101.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quinta HR, Maschi D, Gomez-Sanchez C, Piwien-Pilipuk G, Galigniana MD. Subcellular rearrangement of hsp90-binding immunophilins accompanies neuronal differentiation and neurite outgrowth. J Neurochem. 2010;115:716–734. doi: 10.1111/j.1471-4159.2010.06970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Utuk A, Sarikcioglu L, Demirel BM, Demir N. The immunosuppressive agent FK506 prevents subperineurial degeneration and demyelination on ultrastructural and functional analysis. Curr Neurovasc Res. 2009;6:252–258. doi: 10.2174/156720209789630320. [DOI] [PubMed] [Google Scholar]
- 31.Gees M, Colsoul B, Nilius B. The role of transient receptor potential cation channels in Ca2+ signaling. Cold Spring Harb Perspect Biol. 2010;2 doi: 10.1101/cshperspect.a003962. a003962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Song MY, Yuan JX. Introduction to TRP channels: structure, function, and regulation. Adv Exp Med Biol. 2010;661:99–108. doi: 10.1007/978-1-60761-500-2_6. [DOI] [PubMed] [Google Scholar]
- 33. Shim S, Yuan JP, Kim JY, Zeng W, Huang G, Milshteyn A, Kern D, Muallem S, Ming GL, Worley PF. Peptidyl-prolyl isomerase FKBP52 controls chemotropic guidance of neuronal growth cones via regulation of TRPC1 channel opening. Neuron. 2009;64:471–483. doi: 10.1016/j.neuron.2009.09.025. •• This paper is the first to demonstrate the role of FKBP52 in chemotropic nerve guidance through gating of TRPC1 channel activity.
- 34.Goel M, Garcia R, Estacion M, Schilling WP. Regulation of Drosophila TRPL channels by immunophilin FKBP59. J Biol Chem. 2001;276:38762–38773. doi: 10.1074/jbc.M104125200. [DOI] [PubMed] [Google Scholar]
- 35.Sinkins WG, Goel M, Estacion M, Schilling WP. Association of immunophilins with mammalian TRPC channels. J Biol Chem. 2004;279:34521–34529. doi: 10.1074/jbc.M401156200. [DOI] [PubMed] [Google Scholar]
- 36.Gkika D, Topala CN, Hoenderop JG, Bindels RJ. The immunophilin FKBP52 inhibits the activity of the epithelial Ca2+ channel TRPV5. Am J Physiol Renal Physiol. 2006;290:F1253–F1259. doi: 10.1152/ajprenal.00298.2005. [DOI] [PubMed] [Google Scholar]