Abstract
Theory predicts that if most mutations are deleterious to both overall fitness and condition-dependent traits affecting mating success, sexual selection will purge mutation load and increase nonsexual fitness. We explored this possibility with populations of mutagenized Drosophila melanogaster exhibiting elevated levels of deleterious variation and evolving in the presence or absence of male-male competition and female choice. After 60 generations of experimental evolution, monogamous populations exhibited higher total reproductive output than polygamous populations. Parental environment also affected fitness measures—flies that evolved in the presence of sexual conflict showed reduced nonsexual fitness when their parents experienced a polygamous environment, indicating trans-generational effects of male harassment and highlighting the importance of a common garden design. This cost of parental promiscuity was nearly absent in monogamous lines, providing evidence for the evolution of reduced sexual antagonism. There was no overall difference in egg-to-adult viability between selection regimes. If mutation load was reduced by the action of sexual selection in this experiment, the resultant gain in fitness was not sufficient to overcome the costs of sexual antagonism.
Keywords: Sexual selection, experimental evolution, mutation load, adaptation, good genes, indirect benefits, sexual conflict, mutagenesis, condition, sexually antagonistic coevolution
Introduction
The relationship between nonsexual and sexual fitness remains a fundamental question in evolutionary biology. One possibility, suggested first by Darwin (1859), is that sexual selection will “give its aid to ordinary selection”. This idea that sexual selection will favor well-adapted males developed into modern good genes theory, which explains how females can bear the cost of choosiness and suggests population-level benefits to the operation of sexual selection, including accelerated adaptation (Proulx, 1999; Lorch et al., 2003) and purification of the genome (reviewed in Whitlock & Agrawal, 2009). Alternatively, the conflict of interests between the sexes may lead to antagonistic coevolution and depress nonsexual fitness. There is a growing body of evidence that there are direct costs to female fitness traded off for direct benefits to male fitness in many systems (Arnqvist & Rowe, 2005).
A particularly powerful approach to measuring fitness effects has been manipulating the presence or absence of sexual selection in laboratory populations for one or several generations. This approach has given mixed results. Female choice leads to increased viability in some studies (Partridge, 1980; Promislow et al., 1998), and the presence of sexual selection can help populations adapt to novel environments (Fricke & Arnqvist, 2007) and purge deleterious variation (Hollis et al., 2009). However, the majority of recent work based on these multi-generation mating system manipulations has reported no benefit to populations experiencing sexual selection (Holland & Rice, 1999; Holland, 2002; Radwan et al., 2004; Rundle et al., 2006, Maklakov et al. 2009).
The fact that both increases and decreases in nonsexual fitness have been observed makes it clear that the real unknown is the balance of costs and benefits accrued through sexual selection. This is an empirical question and quantifying the relative magnitudes of the opposing forces will clarify whether there is a net advantage to populations experiencing sexual selection. An important caveat is that this balance is likely to differ across taxa with different mating systems; costs or benefits of sexual selection determined for a population of promiscuous insects may not apply to, for example, a monogamous bird with parental care. Still, uncovering the pattern of antagonistic adaptations and indicators of genetic quality in any one system will go a long ways towards strengthening our predictions across taxa.
The difficulty in establishing a clear picture is surprising, as we should expect the effects of sexual selection to be far-reaching. Sexual selection operates on more than just showy characters—indeed, whenever sexually-selected traits are costly to produce they should evolve to be an honest indicator of quality through the process of “genic capture” (Rowe & Houle, 1996). As genetic variation for sexually-selected traits is exhausted, theory predicts new variation will be recruited into building those traits. Through this process, much of the genome may become involved in the expression of sexually-selected traits.
If sexual selection targets loci throughout the genome, it should amplify the effects of nonsexual selection and help to reduce genetic load. Few studies have directly tested whether this happens in populations. Radwan et al. (2004) relaxed nonsexual selection in bulb mite populations and found that the resultant decline in fecundity as deleterious mutations accumulated was not influenced by the presence of sexual selection. However, Radwan (2004) later found in a single generation experiment that populations of irradiated bulb mites in which sexual selection operated showed elevated viability relative to populations where sexual selection was removed.
We addressed the question of whether or not sexual selection provides a benefit to populations with long term experimental evolution of Drosophila melanogaster exhibiting increased levels of deleterious genetic variation. We did this by exposing flies to a mutagen and then manipulating the presence or absence of male-male competition and female choice. We then measured fitness components of flies from both mating treatments after 60 generations of experimental evolution, allowing us to observe any differences between treatments that evolved.
Materials and Methods
Fly Stocks and Rearing
The experiments were carried out with a long-term laboratory population (the IV population) that was initiated from about 200 wild Drosophila melanogaster of each sex collected in Massachusetts in 1975 (Charlesworth & Charlesworth, 1985). The IV population is maintained at 25°C in 10 bottles on a 14-day schedule and a 12L:12D cycle with mixing between bottles every generation. The population is extremely crowded, increasing mortality at all phases of the life cycle. Selection on development time is particularly strong, as there is only time for one generation in each cycle. Previous work has characterized selection in these populations (Houle & Rowe, 2003) and shown only a narrow window of time (∼2 days) in which any laid eggs will reach adulthood before the next transfer. A particular advantage of the IV population is that it has adapted to this same environment for over 750 generations, and so what constitutes fitness is well-understood.
Fitness assays involving a competitor used a second laboratory population derived from the IV population that carries a recessive ebony mutation (IVe). The IVe population was established in 1992 after a spontaneous ebony mutation was repeatedly backcrossed into the larger IV population. IVe has been maintained in the laboratory for more than 350 generations and, because of their darker coloration, provide a competitive standard easily distinguished from flies in our experimental populations.
Mutagenesis
Approximately 1000 male and 1000 female virgins were collected from the base IV population. These flies were starved for a 12 hour period and then placed for 12 hours in vials with filter paper soaked in a solution of 2.5 mM ethyl methanesulfonate (EMS) in 1% sucrose. EMS is a potent mutagen that produces primarily single base pair changes (Ashburner, 1989), and past work has shown lethal mutation rate elevated from 7-fold (Huang & Baker, 1976) to greater than 50-fold (Ohnishi, 1977) at this dose. Fitness effects at this dose are also well studied; Keightley (1998) showed significant decreases in viability, fecundity, hatchability, developmental time, longevity, and mating speed.
In order to directly estimate the amount of induced mutation in our experiment, a standard test for detecting induced lethal mutations was performed (Fig. 1). First, immediately after mutagenesis, 250 EMS-treated males were mated with 250 FM7 females. FM7 is a first chromosome (the sex chromosome) balancer that carries markers for yellow body coloration and white eyes and also prevents recombination, so the female progeny of these crosses received an FM7 chromosome from their mothers and an X chromosome from their mutagen-treated fathers. To determine whether that X chromosome is lethal in homozygous state, the female offspring of this cross, who are FM7/+, were crossed again to FM7 males. All male offspring from this cross were then collected and scored for phenotypically yellow body and white eyes. If the X chromosome treated with EMS carried a lethal mutation, all males had a yellow body and white eyes and no wild type males emerged from the cross. As a control, 80 untreated control males were crossed in the same manner.
Figure 1.
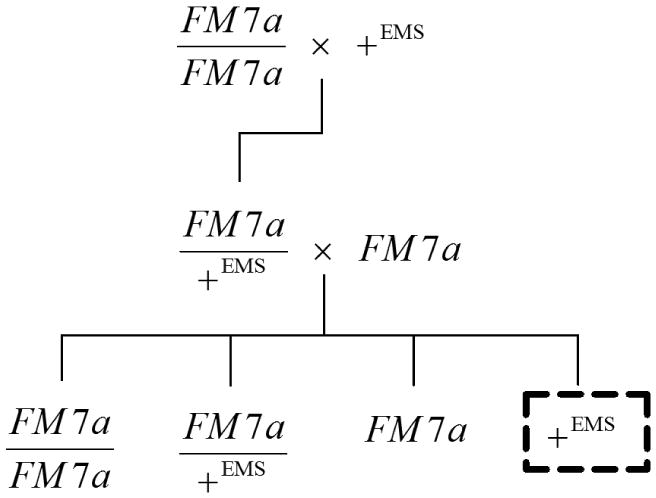
Crossing scheme to detect recessive lethal mutations on chromosome I (the sex chromosome). The absence of wild type males (indicated by the dashed box) implies a novel lethal mutation induced by mutagenesis.
Measuring fitness deficits of mutagenized flies
In order to assess the initial fitness deficits of the mutagenized flies from which our experimental populations would be drawn, three different assays were performed one generation after mutagenesis. Competitive egg-to-adult viability was measured by placing groups of 50 eggs from either the mutagenized population or our IV control population into bottles in which 50 IVe females had been laying eggs for 12 hours. IVe females were then returned to these bottles and continued laying eggs for the next 2 days, creating intense competition between IVe larvae and the larvae of interest. All eclosing flies were collected from day 9-14 and the number of the 50 transferred eggs reaching adulthood was scored.
Fecundity was measured by placing groups of 3-day old virgin males and females into vials (3 males, 3 females) and counting the number of eggs laid during a 12 hour light period. These groups were transferred to holding vials overnight and in the morning placed into fresh vials for another 12 hours of egg laying. This was repeated once more, so that each group of flies' eggs was counted across three consecutive broods, and the total number of eggs laid was used as a measure of fecundity.
Male mating success of control and mutagenized flies was measured by placing 5 virgin males of interest with 5 IVe virgin females and 5 IVe virgin male competitors. After 2 days all of the flies from these vials were discarded. Offspring emerging from days 9-14 were collected and the proportion of each brood that was phenotypically wild-type (as opposed to ebony-bodied) was used as a measure of male mating success.
Beginning the experiment
After one generation of mass breeding, the mutagenized IV population was subdivided into six replicate populations, each initiated from 100 virgin males and 100 virgin females. Three of these replicates experienced sexual selection (the polygamous mating system or S+) for the remainder of the experiment and three did not (the monogamous mating system or S-). Replicate numbers 1, 2, and 3 were arbitrarily assigned within each treatment.
Manipulation of sexual selection
In order to enforce monogamy in S- populations, each generation virgin females were randomly paired with one virgin male apiece and allowed to spend two days mating in these interaction vials. In contrast, in S+ populations groups of 5 virgin females were combined with groups of 5 virgin males in vials and also allowed to spend two days mating. In both S- and S+ treatments, several extra interaction vials were made each generation to make up for any mortality.
After two days of opportunity for sexual selection, males from both treatments were discarded as were the interaction vials. Females from each replicate were placed into two bottles, 50 females per bottle. The mated females spent the next three days laying eggs in these bottles before also being discarded. These bottles were the source of the next generation's flies. This transfer of females to bottles for egg-laying (in the absence of males) helps to confine our manipulation to pre- and post-copulatory sexual selection while equalizing nonsexual selection across treatments. Nine or ten days after the initial setup of these bottles, virgin collections began and continued until enough flies were collected to begin the next generation. These virgins were then passed back through the experimental treatment.
Measuring net reproductive rate
Net reproductive rate of S- and S+ populations was measured after 60 generations of experimental evolution. Prior to measurement, all experimental populations experienced two generations of a monogamous mating environment in order to remove any effects of antagonism between the sexes that persisted across generations.
Groups of 3 virgin females and 3 virgin males (all 3 days old) from each replicate population were placed in vials (n = 30 vials per replicate population). These flies were allowed to mate and deposit eggs continuously for three days before being discarded. The total number of flies produced by these vials represented net reproductive output.
Measuring fecundity and egg-to-adult viability
Fecundity and egg-to-adult viability of S- and S+ populations was also measured after 60 generations of experimental evolution. Preliminary measures during the course of experimental evolution had suggested a fecundity deficit in polygamous populations. The measured flies in these early estimates were collected directly from the population bottles, leaving open the possibility that trans-generational effects of polygamous and monogamous mating environments influenced our measures. In order to control for this, all experimental populations were simultaneously reared under both mating treatments for one generation before components of fitness were measured.
Groups of 3 virgin females and 3 virgin males (all 3 days old) from each replicate population were placed in vials for three consecutive 12-hour light cycles as in the original fecundity assay of the mutagenized population (n̄= 41 groups in the 12 replicate/parental mating environment combinations). Fecundity was measured as the number of eggs laid by each group of flies across these three broods. Egg-to-adult viability was determined by the proportion of these eggs that reached adulthood.
Statistical analysis
The fitness effects of mutagenesis were evaluated using a linear model with treatment (mutagenized or control) as a fixed effect. For the experimental evolution results, a generalized linear mixed model was fit using PROC GLIMMIX in SAS© version 9.1 (SAS Institute, 2003). Because parental environment was controlled (two generations of monogamy), the analysis of net reproductive rate included only the fixed effect of mating treatment and random replicate effects. Measures of fecundity and egg-to-adult viability were each modeled with mating treatment and parental mating system as fixed effects and replicates as a random effect. For the egg-to-adult viability data, where the measure obtained was a proportion, egg number was also included as a covariate because density is an important determinant of the proportion of flies reaching adulthood in a vial.
Results
Mutagenesis
Our mutagenized flies experienced a lethal mutation rate of .152 (29 out of 191 successful crosses resulted in no male offspring carrying the mutagenized first chromosome). There were no lethals detected in control crosses. Estimates of the spontaneous lethal mutation rate for the first chromosome vary between .001 and .003. Woodruff et al. (1983), summarizing the results of many studies, give a best estimate of .0016. At this spontaneous lethal rate, our mutagenesis treatment was roughly equivalent to 95 generations of spontaneous mutation accumulation.
Estimates of the fitness deficits for mutagenized flies are shown in Fig. 2. Egg-to-adult viability in the presence of a standardized competitor (IVe) was significantly higher in control populations (37.50 ± 1.06 flies) than in the mutagenized population (31.58 ± 1.33 flies) (Fig. 2A, F = 12.16, df = 1, 22, p < .01). Fecundity, however, was not different between populations, with control flies laying 122.08 ± 4.29 eggs and mutagenized flies laying 125.36 ± 3.32 eggs (Fig. 2B, F = 0.37, df = 1, 98, p =.55).
Figure 2.
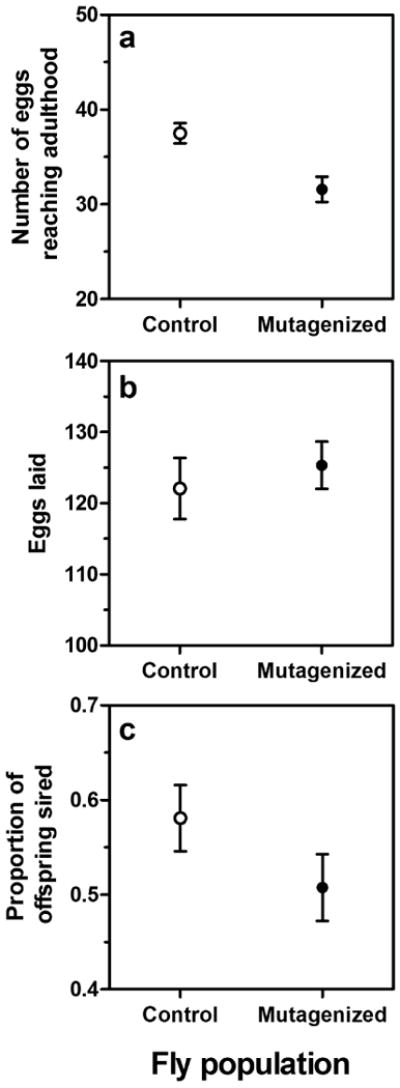
Fitness estimates for mutagenized and control populations. Mutagenized flies exhibited reduced competitive egg-to-adult viability (a) but were not significantly different than the control population in measures of (b) fecundity and (c) competitive mating success.
Male mating success appeared reduced in the mutagenized population relative to the control, with mutagenized flies securing approximately half of an IVe female's brood (.51 ± .04) while control flies sired a larger proportion in our trials (.58 ± .03), but this difference was not significant (Fig. 2C, F = 2.20, df = 1, 83, p = .12).
Experimental evolution
We measured fitness after 60 generations of mating system manipulation and two generations of monogamy (net reproductive rate) or one generation of rearing all populations in both mating environments (fecundity and egg-to-adult viability). Results of the statistical analysis of fitness measures are summarized in Table 1.
Table 1.
Results of mixed model analysis testing the effects of sexual selection on experimental populations' (a) net reproductive rate, (b) fecundity, and (c) egg-to-adult viability.
| Source | df | F | P |
|---|---|---|---|
| (a) Net reproductive rate | |||
| Mating system (S+ vs. S-) | 1, 4 | 8.62 | 0.043 |
| (b) Fecundity | |||
| Mating system (S- vs. S+) | 1, 4 | 0.12 | 0.751 |
| Parental environment (S- vs. S+) | 1, 4 | 8.38 | 0.044 |
| Mating system × Parental environment | 1, 4 | 6.74 | 0.060 |
| Brood | 2, 8 | 214.15 | < 0.001 |
| Mating system × Brood | 2, 8 | 14.06 | 0.002 |
| (c) Egg-to-adult viability | |||
| Mating system (S+ vs. S-) | 1, 4 | 0.50 | 0.520 |
| Parental environment (S- vs. S+) | 1, 4 | 0.75 | 0.435 |
| Mating system × Parental environment | 1, 4 | 3.13 | 0.151 |
| Brood | 2, 8 | 5.26 | 0.035 |
| Mating system × Brood | 2, 8 | 7.12 | 0.017 |
| Eggs | 1, 1424 | 14.20 | < 0.001 |
Net reproductive output after two generations of monogamous rearing was significantly higher in the S- treatment (126.59 ± 2.16 flies) than in the S+ treatment (117.60 ± 2.17 flies) (Fig. 3). This productivity assay is similar to the rearing protocol over the course of experimental evolution, with competition between adult flies extending several days and a high density of eggs and developing larvae.
Figure 3.
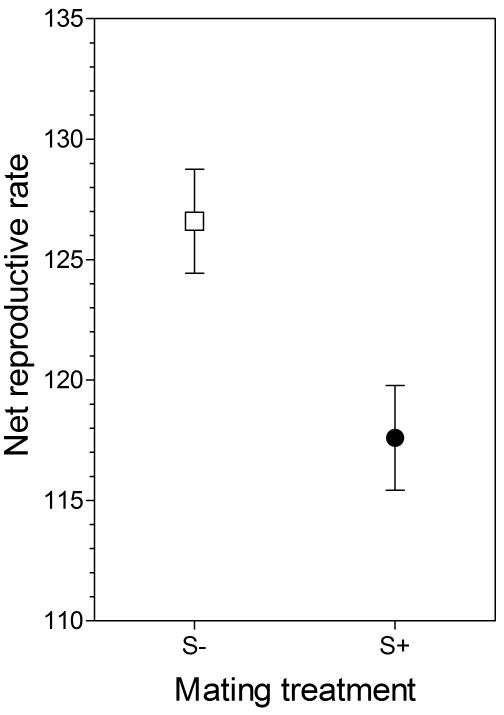
Net reproductive rate after 60 generations of experimental evolution and two consecutive generations of monogamous rearing.
Total fecundity—the sum of all eggs laid across three consecutive broods—was not significantly different between selection treatments. The mating environment of parents of measured flies did matter, however (Fig. 4A). This effect of parental rearing environment also differed between mating treatments (marginally significant, Table 1), with S+ flies showing a marked decline in the number of eggs laid if their parents experienced a polygamous mating environment (125.49 ± 6.86) versus a monogamous parental mating environment (140.23 ± 7.23). S- flies showed the opposite pattern (125.80 ± 7.04 with monogamous parents and 129.76 ± 6.64 with polygamous parents), indicating reduced costs of sexual interactions in monogamous populations. There was also a significant brood effect, with all lines laying more eggs in the first 12-hour window than in either of the later two laying periods (Fig. 4B). Lines from the S+ and S- treatments showed a different pattern of decline in the experiment (mating treatment × brood interaction in Table 1) with S+ fecundity dropping more quickly (48% decline from first to second brood if parents were monogamous, 46% decline if parents were polygamous) than S- fecundity (29% decline with monogamous parents, 34% decline if parents were polygamous).
Figure 4.
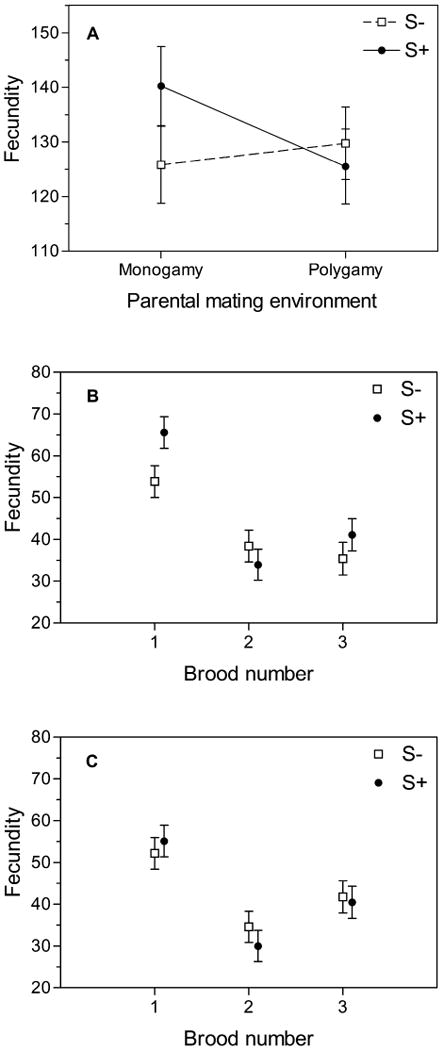
Fecundity of S+ and S- lines after 60 generations of experimental evolution when the parents of assay flies were reared in either monogamy or polygamy. (A) Total fecundity over three sequential broods. (B) Fecundity of evolved lines when parents were monogamous, by brood. (C) Fecundity of evolved lines when parents were polygamous, by brood.
Egg-to-adult viability followed a generally similar pattern to the fecundity results. There was no significant difference between S- and S+ treatments in overall egg-to-adult viability and there was no significant effect of the parental mating environment (Fig. 5A). As with the fecundity measures, egg-to-adult viability decreased in the S+ treatment when parents of assay flies were reared in a polygamous environment (76.4 ± 2.6% of eggs reaching adulthood) versus parents reared in a monogamous environment (79.7 ± 2.4% of eggs reaching adulthood) but stayed relatively constant in the S- treatment (80.4 ± 2.3% versus 80.1 ± 2.4%). Unlike for the fecundity measures, however, this mating treatment × parental environment interaction was not significant. In addition to an effect of brood number and egg density on egg-to-adult viability, there was a mating treatment × brood interaction (Table 1) where the S- treatment showed increased egg-to-adult viability in later broods while S+ egg-to-adult viability remained relatively constant (Fig. 5B).
Figure 5.
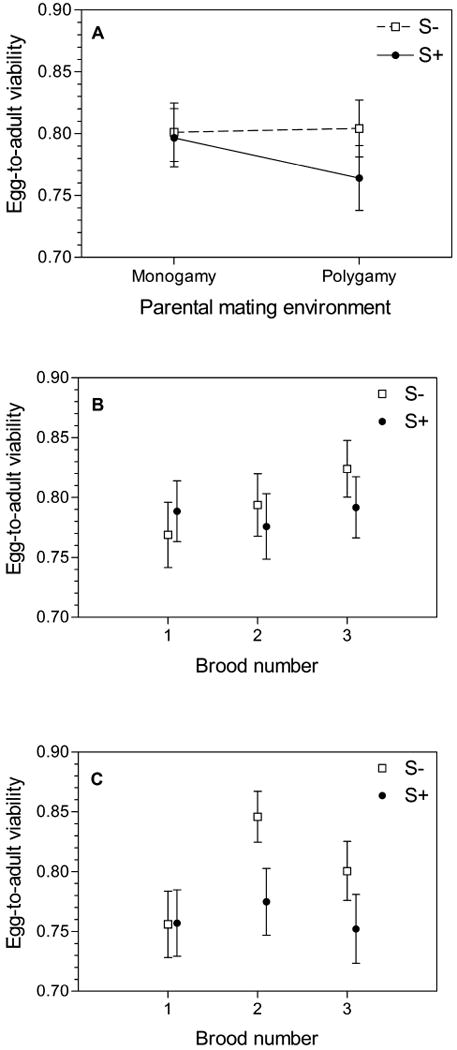
Egg-to-adult viability of S+ and S- lines after 60 generations of experimental evolution when the parents of assay flies were reared in either monogamy or polygamy. (A) Total egg-to-adult viability over three sequential broods. (B) Egg-to-adult viability of evolved lines when parents were monogamous, by brood. (C) Egg-to-adult viability of evolved lines when parents were polygamous, by brood.
Discussion
The multitude of predictions about sexual selection's potential benefits to populations—increased average nonsexual fitness, accelerated adaptation, purified genomes, and an alleviated cost of sexual reproduction—are intuitively appealing because they offer an explanation for why females might bear the costs of being choosy. Also, because of the strong theoretical foundation behind “good genes” thinking and evidence for the benefits of sexual selection in manipulated populations (Fricke and Arnqvist 2007, Hollis et al. 2009), these predictions are not easily dismissed. The main result of the experiment reported here is clear: even when substantial deleterious variation is present in a laboratory population of D. melanogaster, any benefits conferred by the operation of sexual selection are outweighed by its costs.
In our measures of components of fitness, there was no overall effect of mating treatment on either fecundity or egg-to-adult viability. However, there was a significant decline in fecundity for S+ lines if parents were polygamous and this decline was absent in S- lines. Egg-to-adult viability showed a similar but non-significant trend. This indicates trans-generational effects of the mating environment of assayed flies' parents on measures of fitness, a phenomenon that has been reported in other invertebrates (Bernasconi and Keller 2001, Tregenza et al. 2003, Kozielska et al. 2004). Making any comparison between treatments without controlling for these parental effects is a major problem that renders the findings of several studies (e.g. Fricke et al. 2007 and Rundle et al. 2006) difficult to interpret.
In addition to the trans-generational costs of sexual antagonism to components of fitness, there is also evidence for within-generation costs. Fecundity of S+ flies declines more sharply in later broods than does the fecundity of S- flies. There is also a significant difference between the treatments in the change in egg-to-adult viability across broods. Viability increases in later broods of S- flies while staying relatively constant in S+ flies. These results are consistent with the marked difference between treatments in our net reproductive rate assay, as this harsher environment compounds effects of antagonism between the sexes. The detectable difference between treatments in net reproductive rate after 60 generations of experimental evolution but not individual components of fitness highlights the context-sensitivity of fitness measures and also the inherent difficulties of estimating absolute fitness from individual components.
The likely explanation for increased reproductive output in monogamous populations is the evolution of decreased manipulation of females by males. This is because the relaxation of competition for mating opportunities in the monogamy treatment and the ample two-day window for mating relaxes selection on male competitive ability, while any costs of male persistence to female reproductive success must necessarily reduce male reproductive success.
The differing effects of parental polygamy (costly for evolved S+ lines but not for evolved S- lines), along with the greater reduction in measures of fitness in S+ lines across broods as they are housed with S+ males, all provide evidence for the evolution of reduced male harm in monogamous populations. This change in males could be either precopulatory, for example through less vigorous courtship and harassment of females, or it could be occurring in a postcopulatory fashion, for example by reduced transfer of harmful male accessory gland proteins (ACPs). There is evidence that both pre- and post-copulatory events can increase male representation in future generations while simultaneously harming female reproductive success (Linder and Rice, 2005; Stewart et al., 2005; Chapman et al., 1995). Our results are consistent with postcopulatory male manipulation, as the role of many ACPs aligns with our data (e.g. increased egg laying for only one day after mating, Herndon and Wolfner 1995; a cost to mating for females, Chapman et al. 1995, Wigby and Chapman 2005) and past work has shown the evolution of enlarged accessory glands in populations with elevated promiscuity (Crudgington et al. 2009).
Future research into the proximal mechanisms of changes in components of fitness after relaxation of sexual selection is likely to be informative, particularly in light of a similar pattern of reduced female fecundity detected in Drosophila pseudoobscura (Crudgington et al. 2010). One way to approach this question is with functional studies of ACPs (e.g. Mueller et al., 2008) or other sexually antagonistic traits coupled with genome-wide sequence and expression analysis of experimentally-evolved monogamous and polygamous populations.
Although our work makes it clear that in laboratory populations of D. melanogaster sexual selection depresses nonsexual fitness by imposing a sexual conflict load, a role for sexual selection in facilitating the purging of deleterious mutations remains plausible. It is possible that in polygamous populations sexual selection is cleansing the genome while males and females concurrently experience an ongoing sexual arms race. This may be difficult to detect in experimental evolution studies lasting only tens of generations. The experiment reported here has “stacked the deck” in favor of picking up this adaptive signal in several ways. First, females have the opportunity to choose between males and remate, but their period of confinement with males is limited to two days. Also, unlike in similar studies with heavily male-skewed sex ratios, females in the polygamous treatments in this experiment experience an equal sex ratio during the mating phase. Finally, substantial deleterious variation was present in these populations at the outset. These factors taken together should amplify the potential for sexual selection to accelerate adaptation while reducing the effects of conflict, and yet still no advantage to sexual selection is seen. It is worth noting, however, that in the one situation in our experiment in which the negative effects of the sexual arms race are reduced (the first brood of flies, before there has been extended opportunity for harm to females, and when parents were reared monogamously and trans-generational effects removed) S+ flies demonstrate dramatically higher fecundity than S- flies.
The potential benefits of sexual selection are expected to be persistent due to an unremitting supply of slightly deleterious mutations. It is possible that the mutations induced by mutagenesis in this study were highly deleterious and therefore purged completely by populations from both selection regimes before measurements of fitness were conducted at generation 60. This is unlikely, however, as a large body of work on EMS mutagenesis indicates changes in trait values are caused by many mutations of small effect (Keightley and Ohnishi 1998, Yang et al. 2001). For example, at the dose used in this study, the average homozygous effect of a single mutation on traits related to fitness is on the order of a few percent (Keightley and Ohnishi 1998). This suggests that the elevated mutation load in our EMS-treated fly populations was caused by many new mutations of small average effect, each present primarily in heterozygous state, and persisted beyond the completion of the experiment.
The opposite scenario, in which fitness was measured before the benefits of sexual selection could be detected, is more likely. As suggested by Whitlock and Agrawal (2009), populations experiencing sexual selection may benefit on a time scale ignored in all experimental evolution studies, including this one. In order to pick up any signal of purged mutation load, future work will need to either include ambitious long-term experiments in a system amenable to this (e.g. the yeast mating-type system, Rogers and Greig 2009) or specifically target known deleterious variation.
Acknowledgments
We thank J.D. Aponte and F. Hollis for helping to maintain fly lines. J.D. Aponte, J. Dornell, S. Schwinn, and J. Nye all provided tremendous assistance in the measurement of fitness. This research was supported by NSF DEB 0910281, NSF DEB 0129219, and NIH U54 RR021813.
Literature Cited
- Arnqvist G, Rowe L. Sexual conflict. Princeton University Press; Princeton, NJ: 2005. [Google Scholar]
- Ashburner M. Drosophila: A laboratory manual. Cold Spring Harbor Laboratory; New York: 1989. [Google Scholar]
- Bernasconi G, Keller L. Female polyandry affects their sons' reproductive success in the red flour beetle Tribolium castaneum. J Evol Biol. 2001;14:186–193. doi: 10.1046/j.1420-9101.2001.00247.x. [DOI] [PubMed] [Google Scholar]
- Chapman T, Liddle LF, Kalb JM, Wolfner MF, Partridge L. Cost of mating in Drosophila melanogaster females is mediated by male accessory gland products. Nature. 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- Charlesworth B, Charlesworth D. Genetic variation in recombination in Drosophila. I. Responses to selection and preliminary genetic analysis. Heredity. 1985;54:71–83. [Google Scholar]
- Crudgington HS, Fellows S, Badcock NS, Snook RR. Experimental manipulation of sexual selection promotes greater male mating capacity but does not alter sperm investment. Evolution. 2009;63:926–938. doi: 10.1111/j.1558-5646.2008.00601.x. [DOI] [PubMed] [Google Scholar]
- Crudgington HS, Fellows S, Snook RR. Increased opportunity for sexual conflict promotes harmful males with elevated courtship frequencies. J Evol Biol. 2010;23:440–446. doi: 10.1111/j.1420-9101.2009.01907.x. [DOI] [PubMed] [Google Scholar]
- Darwin C. On the origin of species. Murray; London: 1859. [Google Scholar]
- Fricke C, Arnqvist G. Rapid adaptation to a novel host in a seed beetle (Callosobruchus maculates): the role of sexual selection. Evolution. 2007;61:440–454. doi: 10.1111/j.1558-5646.2007.00038.x. [DOI] [PubMed] [Google Scholar]
- Herndon LA, Wolfner MF. A Drosophila seminal fluid protein, Acp26Aa, stimulates egg laying in females for 1 day after mating. Proc Natl Acad Sci. 1995;92:10114–10118. doi: 10.1073/pnas.92.22.10114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland B, Rice WR. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes a reproductive load. Proc Natl Acad Sci USA. 1999;96:5083–5088. doi: 10.1073/pnas.96.9.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland B. Sexual selection fails to promote adaptation to a new environment. Evolution. 2002;56:721–730. doi: 10.1111/j.0014-3820.2002.tb01383.x. [DOI] [PubMed] [Google Scholar]
- Hollis B, Fierst JL, Houle D. Sexual selection accelerates the elimination of a deleterious mutant in Drosophila melanogaster. Evolution. 2009;63:324–333. doi: 10.1111/j.1558-5646.2008.00551.x. [DOI] [PubMed] [Google Scholar]
- Houle D, Rowe L. Natural selection in a bottle. Am Nat. 2003;161:50–67. doi: 10.1086/345480. [DOI] [PubMed] [Google Scholar]
- Huang SL, Baker BS. The mutability of the Minute loci of Drosophila melanogaster with ethyl methanesulfonate. Mutat Res. 1976;34:407–414. doi: 10.1016/0027-5107(76)90218-9. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Møller AP, Petrie M. Sexually selected traits and adult survival: a meta-analysis. Q Rev Biol. 2001;76:3–36. doi: 10.1086/393743. [DOI] [PubMed] [Google Scholar]
- Keightley PD, Ohnishi O. EMS-Induced Polygenic Mutation Rates for Nine Quantitative Characters in Drosophila melanogaster. Genetics. 1998;148:753–766. doi: 10.1093/genetics/148.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozielska M, Krzeminska A, Radwan J. Good genes and the maternal effects of polyandry on offspring reproductive success in the bulb mite. Proc R Soc B. 2004;271:165–170. doi: 10.1098/rspb.2003.2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder JE, Rice WR. Natural selection and genetic variation for female resistance to harm from males. J Evol Biol. 2005;18:568–575. doi: 10.1111/j.1420-9101.2004.00872.x. [DOI] [PubMed] [Google Scholar]
- Lorch PD, Proulx S, Rowe L, Day T. Condition-dependent sexual selection can accelerate adaptation. Evol Ecol Res. 2003;5:867–881. [Google Scholar]
- Maklakov AA, Bonduriansky R, Brooks RC. Sex differences, sexual selection, and ageing: an experimental evolution approach. Evolution. 2009;63:2491–2503. doi: 10.1111/j.1558-5646.2009.00750.x. [DOI] [PubMed] [Google Scholar]
- Mueller JL, Linklater JR, Ravi Ram K, Chapman T, Wolfner MF. Targeted gene deletion and phenotypic analysis of the Drosophila melanogaster seminal fluid protease inhibitor Acp62F. Genetics. 2008;178:1605–1614. doi: 10.1534/genetics.107.083766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller JL, Linklater JR, Ravi Ram K, Chapman T, Wolfner MF. Targeted Gene Deletion and Phenotypic Analysis of the Drosophila melanogaster Seminal Fluid Protease Inhibitor Acp62F. Genetics. 2008;178:1605–1614. doi: 10.1534/genetics.107.083766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi O. Spontaneous and ethyl methanesulfonate induced mutations controlling viability in Drosophila melanogaster. I. Recessive lethal mutations. Genetics. 1977;87:519–527. doi: 10.1093/genetics/87.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge L. Mate choice increases a component of offspring fitness in fruit flies. Nature. 1980;283:290–291. [Google Scholar]
- Promislow DL, Smith EA, Pearse L. Adult fitness consequences of sexual selection in Drosophila melanogaster. Proc Natl Acad Sci USA. 1998;95:10687–10692. doi: 10.1073/pnas.95.18.10687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proulx SR. Mating systems and the evolution of niche breadth. Am Nat. 1999;154:89–98. doi: 10.1086/303218. [DOI] [PubMed] [Google Scholar]
- Radwan J. Effectiveness of sexual selection in removing mutations induced with ionizing radiation. Ecol Lett. 2004;7:1149–1154. [Google Scholar]
- Radwan J, Unrug J, Śnigórska K, Gawrońska K. Effectiveness of sexual selection in preventing fitness deterioration in bulb mite populations under relaxed natural selection. J Evol Biol. 2004;17:94–99. doi: 10.1046/j.1420-9101.2003.00646.x. [DOI] [PubMed] [Google Scholar]
- Rogers DW, Greig D. Experimental evolution of a sexually selected display in yeast. Proc R Soc B. 2009;276:543–549. doi: 10.1098/rspb.2008.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe L, Houle D. The lek paradox and the capture of genetic variance by condition-dependent traits. Proc Roy Soc B. 1996;263:1415–1421. [Google Scholar]
- Rundle HD, Chenoweth SF, Blows MW. The roles of natural and sexual selection during adaptation to a novel environment. Evolution. 2006;60:2218–2225. [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS System for Windows, Release 9.1. SAS Institute; Cary, NC: 2003. [Google Scholar]
- Sharp NP, Agrawal AF. Mating density and the strength of sexual selection against deleterious alleles in Drosophila melanogaster. Evolution. 2008;62:857–867. doi: 10.1111/j.1558-5646.2008.00333.x. [DOI] [PubMed] [Google Scholar]
- Stewart AD, Morrow EH, Rice WR. Assessing putative interlocus sexual conflict in Drosophila melanogaster using experimental evolution. Proc R Soc Lond B. 2005;272:2029–2035. doi: 10.1098/rspb.2005.3182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregenza T, Wedell N, Hosken DJ, Ward PI. Maternal effects on offspring depend on female mating pattern and offspring environment in yellow dung flies. Evolution. 2003;57:297–304. doi: 10.1111/j.0014-3820.2003.tb00264.x. [DOI] [PubMed] [Google Scholar]
- Whitlock MC, Agrawal AF. Purging the genome with sexual selection: Reducing mutation load through selection on males. Evolution. 2009;63:569–582. doi: 10.1111/j.1558-5646.2008.00558.x. [DOI] [PubMed] [Google Scholar]
- Wigby S, Chapman T. Sex peptide causes mating costs in female Drosophila melanogaster. Curr Biol. 2005;15:316–321. doi: 10.1016/j.cub.2005.01.051. [DOI] [PubMed] [Google Scholar]
- Woodruff RC, Slatko BE, Thompson JN., Jr . Factors affecting mutation rates in natural populations. In: Ashburner M, et al., editors. The genetics and biology of Drosophila. 3c. Academic Press; London: 1983. pp. 37–124.pp. 427–428. [Google Scholar]
- Yang HP, Tanikawa AY, Van Voorhies WA, Silva JC, Kondrashov AS. Whole-genome effects of ethyl methanesulfonate-induced mutation on nine quantitative traits in outbred Drosophila melanogaster. Genetics. 157:1257–1265. doi: 10.1093/genetics/157.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]


