Abstract
The adhesin known as Antigen I/II, P1 or PAc of the cariogenic dental pathogen Streptococcus mutans is a target of protective immunity and candidate vaccine antigen. Previously we demonstrated that immunization of mice with S. mutans complexed with anti-AgI/II monoclonal antibodies (MAbs) resulted in changes in the specificity, isotype and functionality of elicited anti-AgI/II antibodies in the serum of immunized mice compared to administration of bacteria alone. In the current study, an anti-AgI/II MAb reported in the literature to confer unexplained long term protection against S. mutans re-colonization following passive immunization in human clinical trials (MAb Guy’s 13), and expressed in tobacco plants (MAb Guy’s 13 plantibody), was evaluated for its potential immunomodulatory properties. Immunization of BALB/c mice with immune complexes of Guy’s 13 plantibody bound to S. mutans whole cells resulted in a similar change in specificity, isotype, and functionality of elicited anti-AgI/II antibodies as had been observed for other immunomodulatory MAbs. This new information, coupled with the recently solved crystal structure of the adhesin, now provides a rational explanation and plausible mechanism of action of passively administered Guy’s 13/Guy’s 13 plantibody in human clinical trials, and how long-term prevention of S. mutans carriage well past the application period of the therapeutic antibody could have been achieved.
Keywords: Immunomodulation, Passive immunization, Streptococcus, Monoclonal antibody
1. Introduction
As the primary agent of dental caries [1], a number of antigens expressed by Streptococcus mutans have been studied as potential vaccine candidates [2–6]. One of these is the cell-surface localized multifunctional adhesive molecule originally identified as Antigen I/II (AgI/II) [7], and also known as P1 [8], Antigen B [9], or PAc [10]. AgI/II-like polypeptides, which are produced by most species of oral streptococci, mediate interactions with host salivary constituents, cell matrix proteins such as fibronectin, fibrinogen, collagen, and other oral bacteria (reviewed in [11]). A schematic representation of the primary sequence of AgI/II is shown in Figure 1. The interaction of AgI/II with salivary components is complex and multivalent [12]. Depending upon whether its major physiologic receptor salivary agglutinin (SAG), an oligomeric protein complex consisting of the scavenger receptor glycoprotein gp340, sIgA and an 80 kDa protein [13, 14], is immobilized on a surface or in fluid phase, different regions of the receptor [15] and the AgI/II adhesin [16] are involved in the interaction. The interaction of AgI/II with immobilized SAG contained within the salivary pellicle that coats the tooth surface is believed to contribute to S. mutans adherence and colonization, and thus would be important to disrupt with protective antibodies. On the other hand aggregation of numerous pathogens, including S. mutans, by fluid-phase gp340 present in saliva has been reported to represent a mechanism of mucosal innate host defense [17]. Numerous studies in immunized animals and naturally-sensitized human subjects have demonstrated the relevance of the humoral immune response against S. mutans P1 (AgI/II, PAc) in protection against bacterial colonization and cariogenicity (reviewed in [3, 11, 18, 19]). Salivary as well as serum antibodies against S. mutans that gain access to the oral cavity via transudation through the gingival crevice have been reported to be protective [6, 20–25], or in some instances non-protective [26–28], depending on the study. This reiterates that subtle and potentially unapparent differences among measured immune responses can be key in determining the ultimate outcome. Numerous pathogens can persist in the face of an immune response and naturally dominant epitopes are often not optimal for protection [29]. Clearly such a balance exists for S. mutans and it is the fine specificity and functional activity of host antibodies, more so than the total amount, which likely determines whether colonization and/or cariogenicity is sufficiently perturbed to prevent disease.
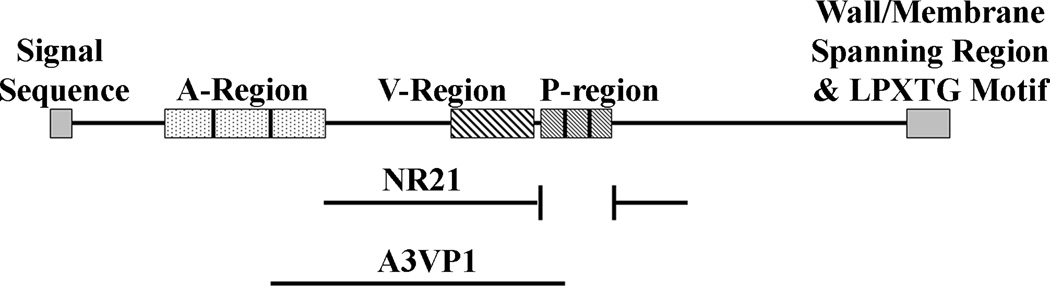
Figure 1.
Schematic representation of the primary structure of S.mutans Antigen I/II. The truncated NR21 and A3VP1 polypeptides used as antigens in the immunoassays described in this report are indicated.
The potential immunomodulatory properties of antibodies have long been recognized (reviewed in [18]) and researchers as early as Emil von Behring have sought to enhance protective immunity by administering exogenous antibody in combination with antigen [30]. In fact, antibody has been referred to as a natural adjuvant (reviewed in [31]). Long term effects of passively administered antibody are not uncommon and may stem from the deliberate or inadvertent formation of immune complexes in situ (see [18]). For example, an essential contribution of infected cell/antibody immune complexes in the enhancement of anti-viral immunity was recently demonstrated following passive immunization with a monoclonal antibody against murine leukemia virus [32]. Such studies highlight not only the potential power of harnessing the immunomodulatory properties of exogenously administered antibodies to engage desirable aspects of the adaptive response, but also elucidate potential mechanisms by which passive antibody can exert an effect.
The use of anti-AgI/II MAbs as a passive immunotherapy against S. mutans colonization of the oral cavity has been studied in non-human primates and in human clinical trials [33–38]. The initial human studies demonstrated that application of an anti-AgI/II MAb (Guy’s 1) to the tooth surface was able to decrease or eliminate colonization of implanted S. mutans following MAb treatment for over 100 days [38]. A follow-up study using four different anti-AgI/II MAbs revealed that protection against colonization by an exogenously applied S.mutans strain was conferred for over a year by two of these anti-AgI/II MAbs (Guy’s 1 and Guy’s 13). These results depended upon the epitope specificity, but not the isotype, of the passively administered MAbs [36]. The long duration of protection could not be accounted for by functional MAb remaining on the teeth and it was speculated that the ecological niche vacated by S. mutans was filled by other bacteria; however, no obvious alterations in the microbial flora were observed in these trials. A subsequent study reported a lack of re-colonization by indigenous S. mutans following oral chlorhexidine disinfection treatment of human subjects for up to 2 years in individuals who received MAb Guy’s 13 [37]. This MAb was subsequently re-engineered as a chimeric IgA/IgG Ab with rabbit secretory component for expression in tobacco plants, and the plant-derived antibody (MAb Guy’s 13 plantibody) was also reported to be effective in passive immunization trials in humans [39, 40]. However, an attempt by another group to reproduce the long-term protective effect of this agent did not replicate previous outcomes. The difference in findings was not understood, but speculated by the authors to result from a different patient demographic and/or insufficient tooth surface disinfection prior to application of the therapeutic antibody [41]. A satisfactory explanation of how MAb Guy’s 13 and the tobacco-expressed plantibody derivative exhibited long-term effects on S. mutans re-colonization in some, but not all, human trials, has remained elusive.
In previous studies, our laboratory evaluated six different anti-S. mutans P1 (AgI/II) MAbs for immunomodulatory properties using an active immunization approach in mice that incorporated the MAbs as part of immune complexes (IC) [42–47]. These studies showed that when administered as part of an IC with S. mutans whole cells certain anti-P1 MAbs redirected the resultant immune response toward one of increased efficacy when the ability of sera from the immunized mice to inhibit bacterial adherence to SAG was measured. Furthermore, we showed that the presence of the beneficial immunomodulatory MAbs, when included as part of an IC immunogen, altered the fine specificity and isotype composition of the elicited antibody response. These effects were concentration-dependent, Fc-independent, correlated with the epitope specificity of the immunomodulatory MAb, and appeared to stem from a structural effect of the MAb on the cell surface localized adhesin resulting in increased exposure of normally cryptic or subdominant epitopes [47].
In light of our prior work, we hypothesized that the anti-AgI/II Guy’s 13 MAb may also exhibit immunomodulatory properties. The Guy’s 13 epitope is similar to those of several immunomodulatory anti-P1 (AgI/II) MAbs used in our previous active immunization studies in that there is a requirement for an interaction of the discontinuous alanine- and proline-rich repeat regions for formation of its cognate epitope [43, 48]. In the current study we tested the Guy’s 13 plantibody for potential immunomodulatory properties as a logical explanation for the reported results of clinical trials and demonstrated that it also alters the specificity, isotype distribution, and functional activity of the resultant adaptive immune response in a concentration-dependent manner. This new information, now interpretable in the context of the recently elucided crystal structure of AgI/II (P1)[49, 50], reveals a pattern in which beneficial immunomodulatory MAbs map to different parts of the adhesin’s tertiary structure than those MAbs that inhibit adherence directly and serve to increase the immunogenicity of relevant target epitopes.
2. Materials and Methods
2.1 Bacterial strains, plasmids and growth conditions
Serotype c S. mutans strain NG8 was grown aerobically to stationary phase for 16 hr in Todd-Hewitt broth supplemented with 0.3% yeast extract (BBL, Cockeysville, MD). The Escherichia coli (E. coli) host strains used to express recombinant P1 polypeptides were DH5α (InVitrogen Corp., San Diego, CA) and M15 (pREP4) (Qiagen, Santa Clarita, CA). E. coli strains were grown aerobically at 37°C with vigorous shaking in Luria-Bertani broth (1% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract, 1% [wt/vol] NaCl) supplemented with ampicillin (50–100 µg/mL) or kanamycin (25–50 µg/mL) as appropriate. Plasmids pCR2.1 (InVitrogen Corp) and pMalp (New England Biolabs, Inc. [NEB], Beverly, MA) were used as cloning and expression vectors.
2.2 Expression and purification of AgI/II polypeptides
The two truncated polypeptides used in this study to evaluate changes in antibody specificity in immunized mice, NR21 and A3VP1, are illustrated in Figure 1. The spaP gene encodes S. mutans AgI/II (P1) [51]. Plasmid pNR21 was derived by PCR amplification [52] using as template an engineered variant of spaP that lacks DNA encoding the P-region [53]. E. coli containing pNR21 was grown as described [52]. Overnight cultures were diluted 1:100 into fresh media containing ampicillin (50–100 µg/mL), grown with shaking to OD600 of 0.45–0.6 and expression was induced with 0.1–0.5 mM IPTG (Fisher) for 3–5 hours at 37°C. The maltose binding protein-P1 fusion protein was then affinity purified from E. coli lysates by column chromatography using amylose resin (New England Biolabs, Inc. [NEB], Beverly, MA) according to the manufacturer’s protocol. The polypeptide A3VP1 was kindly provided by Dr. Champion Deivanayagam (Department of Physiology and Biophysics, University of Alabama, Birmingham, Alabama).
2.3 Mice
Six-eight week old female BALB/c mice were purchased from Charles River Laboratories, Wilmington, MA. Mice were housed in biosafety level 2 facilities under infectious disease conditions and fed a standard diet. Animal experiments were conducted under the auspices of the University of Florida Institutional Animal Care and Use Committee.
2.4 Source of antibodies
Anti-P1 MAbs 1-6F, 4-10A, and 5-5D were obtained from previously established hybridomas made in our laboratory [54]. These three MAbs were purified by column chromatography from ascites fluid using an ImmunoPure (A Plus) IgG Purification Kit (Pierce, Rockford, IL) according to the manufacturer’s protocol. Peroxidase-labeled and unlabeled secondary MAb reagents were obtained from Southern Biotech (Birmingham, AL). The Guy’s 13 plantibody was kindly provided by Planet Biotechnology Inc. (Hayward, CA).
2.5 Immunizations and sample collections
Groups of six mice were immunized intraperitoneally (IP) with ~1.5 × 109 CFUs of S. mutans in 150 µl of PBS, or with the same amount of S. mutans that had been incubated with Guy’s 13 plantibody (2.0 mg/ml) beginning with a 1:100 dilution then 3-fold dilutions up to 1:8100, washed in phosphate buffered saline, pH 7.2 (PBS), and resuspended to the original volume in PBS. In a separate experiment 1:100, 1:1000, and 1:10,000 dilutions of plantibody were used. To exclude the possibility of anti-idiotype effects and to control for other potential effects of the MAb that might occur independently of antigen, additional control groups received 150 µl of MAb alone at the highest concentration. Sera from these control mice were not reactive with purified P1 (AgI/II) or with S. mutans, nor were pre-bleed sera from experimental groups or sera from negative control mice that received PBS only (data not shown). Mice were pre-bled one week before the first inoculation, immunized on days 0 and 14 and exsanguinated on day 30–40.
2.6 BIAcore assay of S. mutans adherence to salivary agglutinin
Adherence of S. mutans whole cells to human salivary agglutinin immobilized on a CM3 sensor chip (GE Healthcare, Piscataway, NJ) was measured using the BIAcore 3000 machine (BIAcore AB, Uppsala, Sweden) as previously described [44]. Salivary agglutinin was prepared by a modification of the technique of Rundegren and Arnold [16, 55]. The sera from the individual mice within each immunization group were pooled and adherence of S. mutans cells reacted with a 1:50 dilution of sera pooled from mice immunized with bacteria alone versus those immunized with IC were compared. Inhibition of bacterial adherence by MAbs was tested using a 1:100 dilution of each MAb. All MAbs had undiluted starting concentrations of 1.8–2.0 mg/ml.
2.7 Biotin-labeling of MAb 1-6F and competition ELISA
Approximately 1 mg of purified anti-P1 (AgI/II) MAb 1-6F was labeled with biotin using EZ-Link™ Biotin-LC-Hydrazide (Pierce, Rockford, IL) according to the manufacturer’s protocol. ELISA plate wells were coated with S. mutans whole cells (~107 cfu/well) [54] in carbonate-bicarbonate buffer, pH 9.6. To determine the optimal dilution for each competition experiment, immune sera from S. mutans or IC-immunized mice were serially diluted three-fold beginning at 1:50 and added to the wells followed by biotin-labeled MAb 1-6F and incubated at 37°C for two hours. Plates were washed and avidin-HRP conjugate (Pierce, IL) was applied to the wells for 30 minutes at room temperature. Plates were washed again and developed with 0.1 M o-phenylenediamine dihydrochloride containing 0.012% hydrogen peroxide in 0.01 M phosphate citrate buffer. Percent inhibition of biotin-labeled MAb 1-6F binding was calculated as % Inhibition = [OD450 direct binding of biotin-labeled 1-6F - OD450 experimental well / OD450 direct binding of biotin-labeled 1-6F] X 100. Control wells contained non-labeled MAb 1-6F and avidin-HRP.
2.8 Quantitative subclass ELISA
Serum samples were assayed for anti-P1 polypeptide NR21 and anti-P1 polypeptide A3VP1 IgG1, IgG2a, and IgG2b isotype antibodies by quantitative subclass. ELISA plate wells were coated with NG8 whole cells or with 100 ng/well purified recombinant NR21 or A3VP1 in carbonate-bicarbonate buffer, pH 9.6. Murine sera were serially diluted three-fold beginning at 1:50 and added to the wells. Antibody reactivity was detected using affinity-purified peroxidase-labeled goat anti-mouse peroxidase conjugated subclass specific antibodies (Southern Biotech) at a 1:2000 dilution. Plates were developed with 0.1 M o-phenylenediamine dihydrochloride containing 0.012% hydrogen peroxide in 0.01 M phosphate citrate buffer. The concentrations of anti-A3VP1 or anti-NR21 IgG subclass antibodies were calculated by interpolation on standard curves generated using purified mouse subclass reagents (Southern Biotech, Birmingham, AL).
2.9 Statistics
Statistically significant differences between groups were determined by one- or two-way analysis of variance (ANOVA) using Graph Pad Prism 4.0. A p-value of less than 0.05 was considered significant. Differences between the different groups were determined by Tukey's Multiple Comparison Test.
3. Results
3.1. Guy’s 13 plantibody IC immunization results in increased inhibition of S. mutans adherence
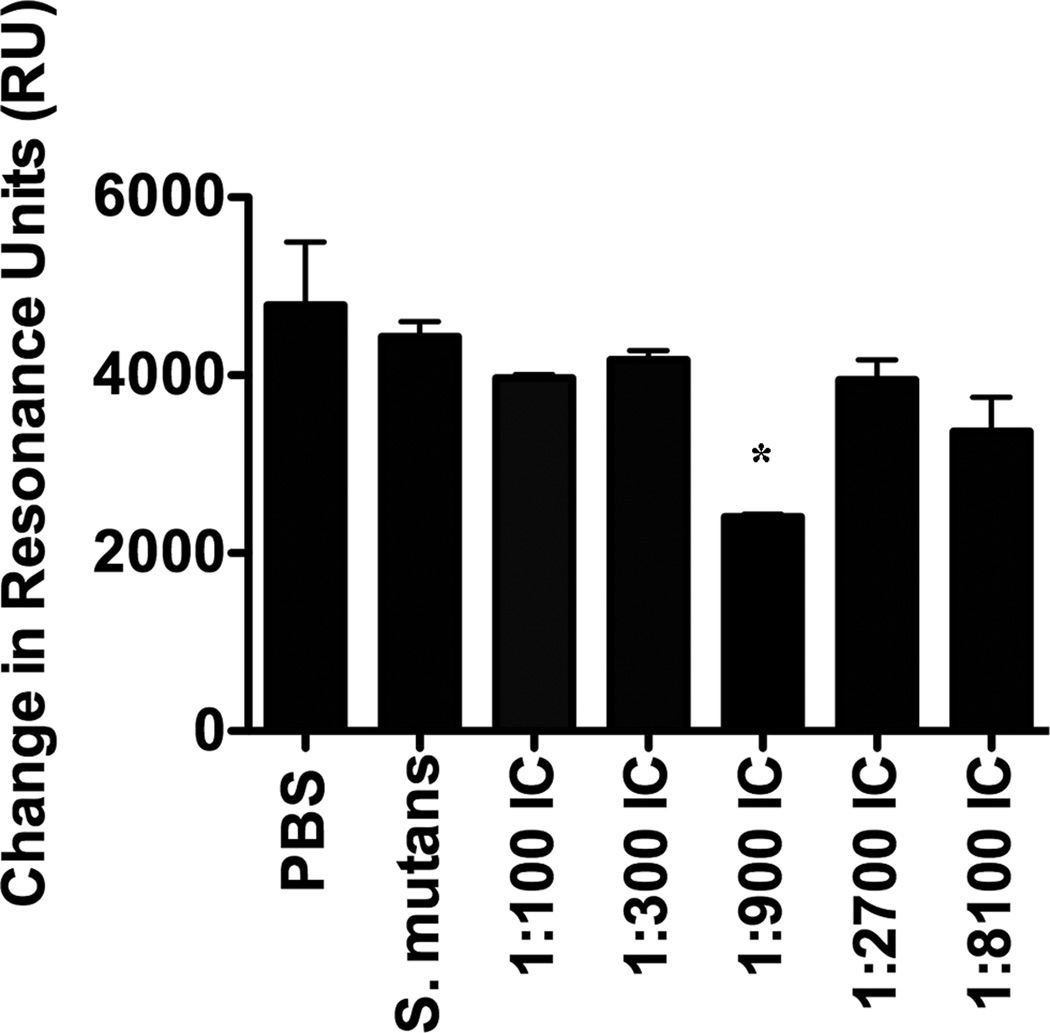
To assess potential immunomodulatory properties of Guy’s 13 plantibody and the role MAb concentration might play, mice were immunized with S. mutans or with S. mutans/plantibody IC containing MAb over a range of concentrations from 1:100 to 1:8100. Pooled sera from the different groups of immunized mice were evaluated by BIAcore SPR for their ability to inhibit S. mutans adherence to SAG. The Guy’s 13 plantibody was shown to enhance the formation of adherence inhibiting antibodies in the sera of mice immunized with IC containing an intermediate concentration of MAb (1:900) (Figure 2). This pronounced prozone-like concentration dependency was similar to that seen in our previous study using the anti-P1 (AgI/II) MAb 4-10A, which, like Guy’s 13 plantibody, also requires an A- and P-region interaction for formation of its epitope [47]. Prozone-like effects have been reported with other passively administered antibodies against infectious agents [56–58], and likely stem from the necessity for a particular antigen:antibody ratio to mediate the desired biological effect. In light of our current results, the concentration-dependency of the immunomodulatory effects of the Guy’s 13 plantibody is a variable that must be considered in future passive protection studies. While consistently achieving the optimal Ag-Ab balance for use as a direct therapy in patients may be challenging to accomplish, the information gained from studying how the MAb affects Ag structure or immunogenicity and thereby alters the downstream adaptive response will also facilitate the design of improved antigen-based therapies.
Figure 2.
Evaluation of S. mutans adherence to SAG in the presence of sera from immunized mice. Immunogens are indicated on the X-axis. BIAcore SPR analysis was used to evaluate bacterial adherence following incubation of S. mutans with sera collected from BALB/c mice immunized with PBS, S. mutans, or with immune complexes (IC) containing S. mutans and the Guy’s 13 plantibody at the indicated dilutions. For ease of presentation the changes in resonance units (ΔRU) detected over the entire 60 second injection cycle, rather than the sensograms, are shown. The assay was performed in triplicate and is representative of 4 separate SPR assays. Statistically significant differences between S. mutans and IC-immunized groups were evaluated by one-way analysis of variance (ANOVA) using Graph Pad Prism 4.0 *(p≤0.05)
3.2. Guy’s 13 plantibody increases immunogenicity against a key epitope
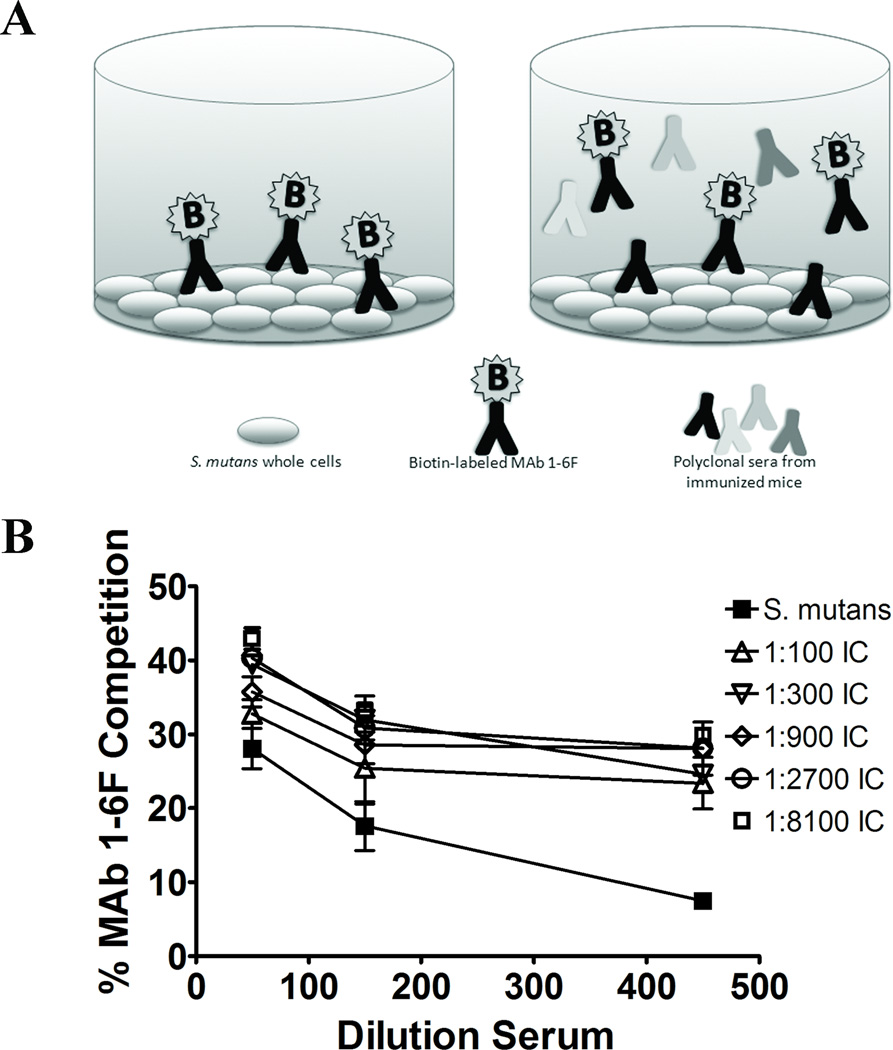
We demonstrated previously that binding of P1 (AgI/II) by beneficial immunomodulatory anti-P1 MAbs increases the exposure of the epitope recognized by MAb 1-6F [47]. The 1-6F MAb is a strong inhibitor of S. mutans adherence [16, 45]. Conversely, most of the immunodulatory MAbs themselves are not [45]. To determine if binding of the Guy’s 13 plantibody to S. mutans prior to immunization also increases the formation of 1-6F–like antibodies, sera from the S.mutans immunized mice and the IC immunized mice were compared for their ability to inhibit the binding of biotin-labeled MAb 1-6F to S. mutans whole cells in a competition ELISA (Figure 3A). As shown in Figure 3B, sera from mice immunized with IC containing Guy’s 13 plantibody at all MAb concentrations demonstrated significantly increased levels of 1-6F-like antibodies compared to the sera from mice immunized with S. mutans alone (p≤0.05-0.001).
Figure 3.
Competitive inhibition of binding of anti-P1 (AgI/II) MAb 1–6F. (A) Schematic representation of the competition ELISA used to measure the levels of MAb 1-6F-like Abs in the sera of BALB/c mice. The adherence-inhibiting MAb 1-6F was labeled with biotin and the ability of sera from immunized mice to compete for binding to S. mutans cells immobilized in ELISA plate wells was measured. (B) Sera from mice immunized with ICs containing MAb Guy’s 13 plantibody were compared to those immunized with S. mutans alone. The percent inhibition of binding of biotin-labeled 1-6F to S. mutans by sera from the immunized mice at the indicated dilutions is shown. Results are expressed as mean ± SEM. Data are representative of at least three independent experiments. Statistically significant differences as determined by two-way ANOVA were detected between all groups receiving IC compared to S. mutans alone (1:100 p≤0.001; 1:300 p≤0.01; 1:900; p≤0.05; 1:2700 p≤0.01; 1:8100 p≤ 0.001).
3.3 Immunization with IC containing Guy’s 13 plantibody alters the Ab specificity and isotype against AgI/II (P1)
The ability to inhibit S. mutans adherence to SAG has been previously shown to correlate with the level of anti-P1 (AgI/II) antibodies of the IgG2a and IgG2b isotypes [45]. We also demonstrated previously that sera from IC-immunized mice contain increased levels of IgG2a and IgG2b, and in some cases IgG1 antibodies that recognize the P1-derived polypeptide, NR21 [47]. The NR21 polypeptide is a particularly useful tool in that it is recognized by the adherence inhibiting MAb 1-6F, but no others in our panel of eleven anti-P1 (AgI/II) MAbs [47]. Thus NR21 represents an informative antigen to use in immunassays to measure the amount and isotype of 1-6F-like antibodies.
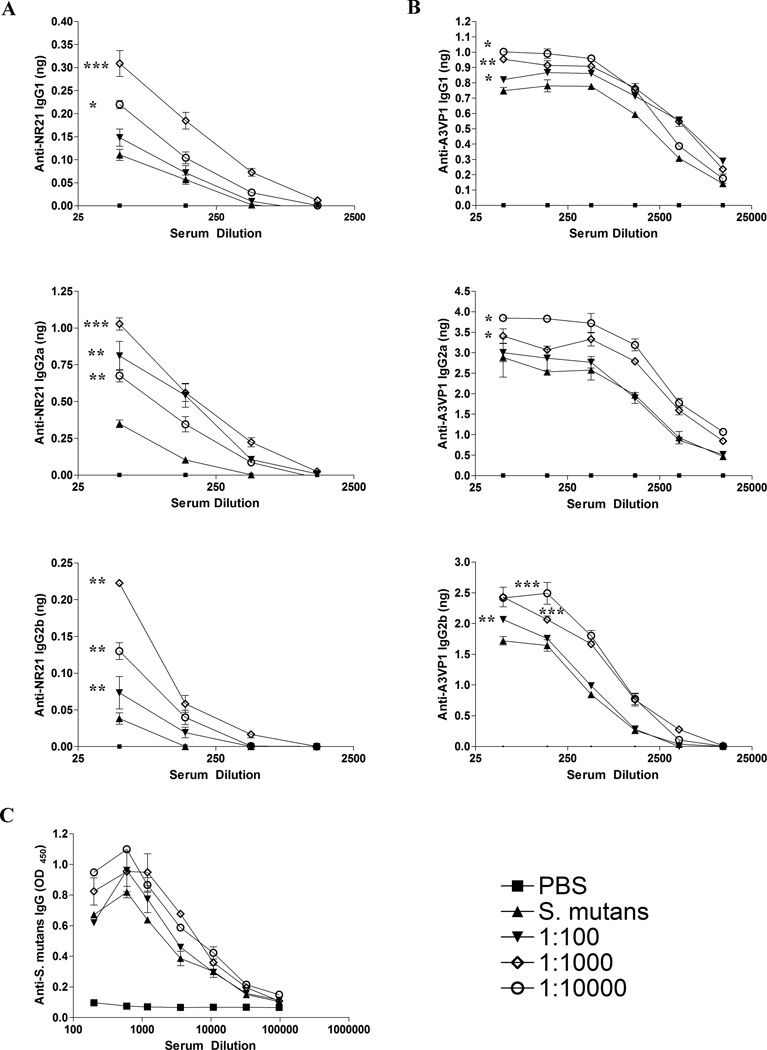
To confirm that immunization with IC containing Guy’s 13 plantibody reproducibly alters the specificity and isotype of the anti-P1 (AgI/II) antibody response, another set of mice were immunized to obtain additional serum samples. When the pooled sera from each of these groups was analyzed by quantitative ELISA for reactivity with the NR21 polypeptide, it was observed that immunization with IC containing Guy’s 13 plantibody, especially at the intermediate concentration (1:1000), resulted in increased levels of anti-NR21 IgG2a, IgG2b, and IgG1 compared to immunization with S. mutans alone (Figure 4A). With the exception of IgG1 for the 1:100 IC-immunized group, all the IC-immunized groups were significantly different from the group immunized with S. mutans only (p≤ 0.05 to 0.001). In addition to NR21, we also utilized the AgI/II derivative A3VP1 as an antigen for quantitative ELISA analysis (Figure 4B) because the crystal structure of this polypeptide has been solved and shown to represent a functionally active SAG binding fragment [50]. A3VP1 is recognized by several anti-P1 (AgI/II) MAbs that are potent inhibitors of S. mutans adherence, namely 1-6F, 4-9D and 4-10A [50]. Immunization with Guy’s 13 plantibody IC resulted in increased levels of anti-A3VP1 IgG2a, IgG2b, and IgG1 serum Abs compared to immunization with S. mutans alone (Figure 4B). In this case, the effects were most pronounced at the lower (1:10,000) followed by the intermediate (1:1000) concentration of MAb and more notable for the IgG2a and IgG2b isotypes. All IC-immunized groups were significantly different from the group immunized with S. mutans for all isotypes tested (p≤ 0.05-0.001), with the exception of the 1:100 IC group for IgG2a. Although the results for A3VP1 were similar to those observed for NR21, they were not identical. This suggests that incorporation of the Guy’s 13 plantibody in the IC immunogens likely influenced the immune response against epitopes other than just that recognized by MAb 1-6F, particularly at the higher dilution (1:10,000). When total anti-S. mutans IgG in the sera was evaluated by serial endpoint titration (Figure 4C), there were no obvious differences between groups. This indicates that the plantibody had more of an effect on altering the fine specificity of the immune response, rather than on influencing total serum IgG levels against S. mutans. As would be expected, relevant immunomodulatory effects and key features of changes in the immune response become obscured when simply measuring a total polyclonal response against an entire complex antigen.
Figure 4.
Evaluation of the specificity and isotype of serum antibody responses by ELISA. (A) The amount of anti-NR21 specific IgG1, IgG2a, and IgG2b subclass antibody was measured by quantitative ELISA in sera collected from BALB/c mice immunized with S. mutans alone or immunized with IC containing the Guy’s 13 plantibody. Antibody concentrations were extrapolated using standard curves. (B) The amount of anti-A3VP1 specific IgG1, IgG2a, and IgG2b subclass antibody was measured by quantitative ELISA in sera collected from BALB/c mice immunized with S. mutans alone or immunized with IC containing the Guy’s 13 plantibody. Antibody concentrations were extrapolated using standard curves. (C) Levels of total anti-S. mutans IgG in the sera from S. mutans versus Guy’s 13 plantibody IC-immunized mice were evaluated by serial endpoint titration and standard ELISA. The key for the line graphs indicates whether the mice received the PBS buffer control, S. mutans alone, or the dilution of the Guy’s 13 plantibody within the S. mutans IC used for immunization. All results are expressed as mean ± SEM and data are representative of at least three independent experiments. Statistically significant differences between groups receiving IC compared to S. mutans alone were determined my two-way ANOVA and are indicated by *(p≤0.05); **(p≤0.01); ***(p≤0001).
It is not surprising that increased anti-NR21 and A3VP1 Ab isotypes were detected in the serum of mice receiving IC containing Guy’s 13 plantibody over a range of concentrations, while the improved adherence-inhibiting serum response was only detected in mice receiving a more finite (1:900/1:1000) dilution of the MAb within the immune complex. In the latter case, the functional activity of a highly defined yet minority population of epitope specific Abs in the serum of the immunized mice was measured. In contrast, when the ability of serum Abs to react with increasingly larger truncated P1 proteins was evaluated, the pool of measured polyclonal serum Abs was expanded as NR21, and A3VP1 even more so, would contain multiple potential epitopes for recognition.
3.4 The Guy’s 13 plantibody does not inhibit S. mutans adherence directly
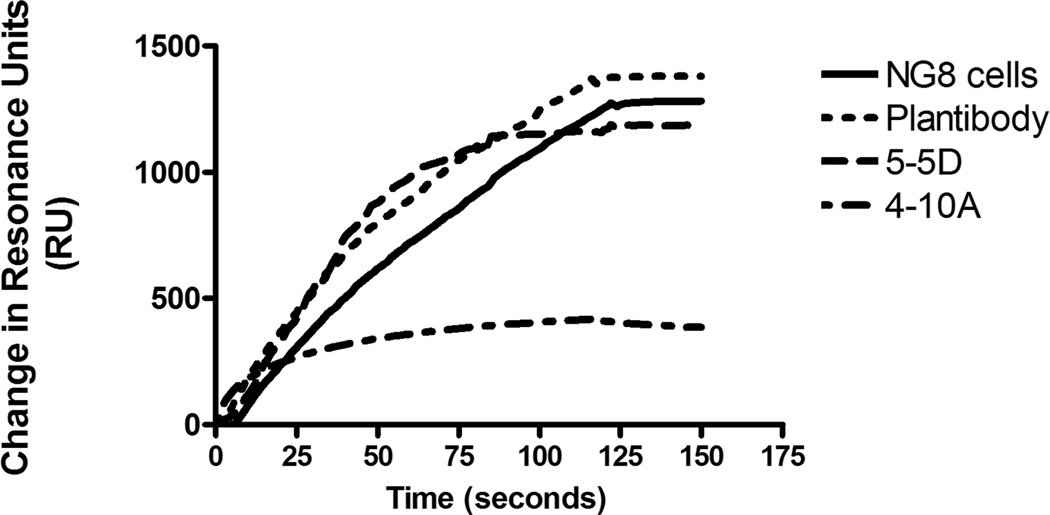
We previously observed an inverse correlation between the ability of anti-P1 (AgI/II) immunomodulatory MAbs to directly inhibit bacterial adherence compared to their ability to redirect the immune response toward one better able to inhibit bacterial adherence [43, 45]. To test whether this dichotomy is also seen with the Guy’s 13 MAb, BIAcore surface plasmon resonance was used to determine if the plantibody was capable of inhibiting S. mutans adherence to immobilized SAG (Figure 5). This was done in parallel with the previously characterized anti-P1 (AgI/II) MAbs 4-10A and 5-5D as positive and negative controls since these are known to represent adherence inhibiting and non-inhibiting MAbs, respectively. As can be seen by the overlaid sensograms, similar to MAb 5-5D, the Guy’s 13 plantibody did not inhibit the adherence of S. mutans to the immobilized SAG, whereas the MAb 4-10A did (Figure 5). Thus the behavior of Guy’s 13 is comparable to three other previously described beneficial immunomodulatory MAbs in that it does not directly inhibit bacterial adherence but promotes the formation of antibodies that do. As discussed below, this new information can now be interpreted in light of the recently available crystal structure and tertiary model of Ag I/II (P1) [49, 50].
Figure 5.
Evaluation of inhibition of S. mutans adherence to immobilized salivary agglutinin by anti-AgI/II (P1) MAbs. BIAcore surface plasmon resonance analysis was used to test the ability of S. mutans to bind to SAG immobilized on the gold chip surface in the absence of antibody (NG8 cells) or after pre-incubation of S. mutans with the Guy’s 13 plantibody, or with the previously characterized MAbs 4-10A or 5-5D.
4. Discussion
Consistent with our previous results with several anti-P1 (AgI/II) MAbs [43, 45, 47], immunization with Guy’s 13 plantibody/S. mutans immune complexes improved the ability of sera from immunized animals to inhibit bacterial adherence to SAG and resulted in a shift in the specificity and isotype of the host immune response toward less immunodominant epitopes when compared to sera from mice immunized with S. mutans alone. Also similar to our previous studies, the influence of the plantibody on the host antibody response demonstrated a notable prozone-like or concentration-dependent effect. Such a property is not unique to our system and other researchers have also reported that more is not necessarily better in that the beneficial effects of exogenous antibody can be improved by decreasing the dose [59]. The repeated observation of prozone-like effects associated with the immunomodulatory activity of anti-P1 (Ag I/II) Mabs is consistent with a structural mechanism of action requiring an optimal Ag:Ab ratio. The opportunity for the passively applied Guy’s 13 MAb to come into contact with the bacterium it recognizes in a clinical trial setting is suggested by the reported finding that S. mutans began to re-colonize sham-treated human subjects during that window of time during which the passive antibody treatment was still being applied to the tooth surface [35]. Hence, S. mutans may have been undetectable at early time points in the treated subjects because it was recognized and prevented from attachment by the antibody. Guy’s 13 and S. mutans, together, may have acted as an immune complex immunogen. A subsequent redirected and effective adaptive immune response could then have prevented re-colonization at the later time points. Because modifications of the anti-S. mutans adaptive response were not evaluated in the clinical trials described in the literature we undertook our current study to determine whether the Guy’s 13 reagent can in fact modulate the anti-AgI/II (P1) response. In previously reported work, protection against S. mutans re-colonization by Guy’s 13 was strictly dependent on the epitope specificity of the passively applied MAb, as a different anti-AgI/II MAb, Guy’s 11, was ineffective [36].
The crystal structure of major portions of AgI/II were recently solved enabling construction of a three dimensional model [49, 50]. This in turn has enabled the approximation of the binding sites, based on their primary sequence binding characteristics [11, 48, 60], of all of the MAbs we have evaluated to date for immunomodulatory activity (Figure 6). The adhesin molecule has a highly unusual tertiary structure in which the alanine-rich repeats form an α-helix that intertwines with the polyproline II helix of the proline-rich repeats to from a long and narrow hybrid helical stalk [50]. There are two globular domains within the molecule that lie on either end of the extended helical stalk. The extreme C-terminus of AgI/II contains the LPXTG motif recognized by sortase, which covalently links its substrates to the cell wall peptidoglycan [61].
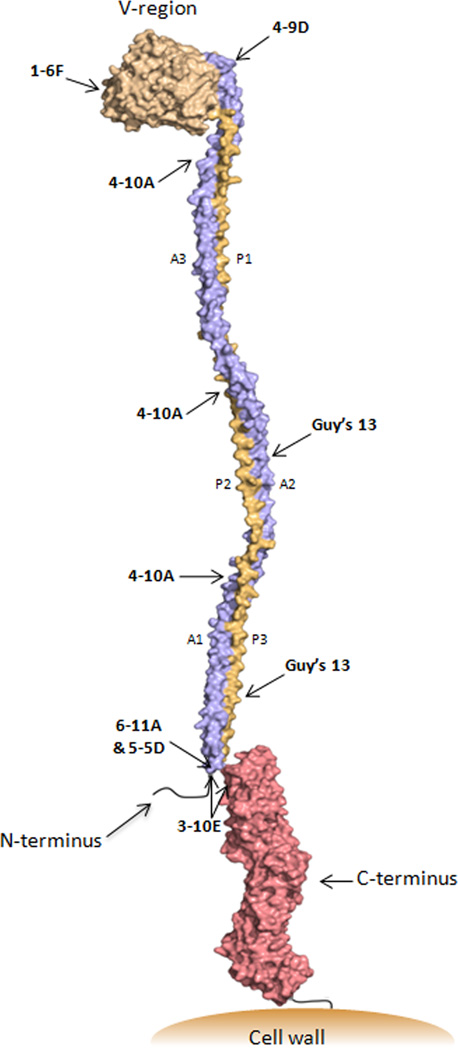
Figure 6.
Schematic representation of the tertiary structure of Antigen I/II. Approximate binding sites of anti-AgI/II (P1) MAbs are indicated. The modeled structure was deduced based on the solved crystal structure of the A3VP1 fragment coupled with the estimated dimensions of the full-length molecule determined by velocity centrifugation [50] and the solved crystal structure of the complete C-terminus [49]. The extended stalk formed by the interaction of the three alanine-rich repeats (blue) with the three proline-rich repeats (orange) brings the pre-A-region into close proximity with the globular C-terminal region. AgI/II family molecules are anchored to the cell wall via sortase following cleavage at the C-terminal LPXTG consensus motif [61].
The characteristics of anti-P1 (AgI/II) Mabs are summarized in Table 1. Interestingly, they fall into discrete groups with regard to their immunomodulatory properties and adherence-inhibiting behavior [43–45]. The novel tertiary structure now lends insight into this partitioning. Antibodies such as 1-6F and 4-9D that are strong inhibitors of adherence map to the globular V-region at the apex of the stalk (Figure 6). When complexed with S. mutans prior to immunization, these two MAbs impede the formation of an effective adherence inhibiting response by immunized animals [45]. Conversely, MAbs such as 6-11A, 5-5D, and 3-10E which map to the other end of the helical stalk do not inhibit adherence themselves, but promote the formation of adherence-inhibiting antibodies when they are included as part of an immune complex immunogen [42, 43, 45–47]. Their epitopes depend on an interaction between the A- and P-regions and are also contributed to by a segment immediately amino-terminal to the A-region that when eliminated results in notably increased recognition of P1 (AgI/II) by MAb 1-6F [60]. Thus, destabilization of structure appears to impact both the antigenicity and immunogenicity of AgI/II, particularly the 1-6F epitope.
Table 1.
Summary of Characteristics of Anti-P1 MAbs
| 3-10E | 6-11A | 5-5D | 4-10A | Plantibody | 1-6F | 4-9D | |
|---|---|---|---|---|---|---|---|
| Directly inhibits adherence of S. mutans to salivary agglutinin | − | − | − | + | − | + | + |
| IC promotes formation of adherence-inhibiting Abs | + | + | + | + | + | − | − |
| IC promotes formation of MAb 1-6F-like Abs | + | + | + | + | + | − | − |
| Maps to the V-region | − | − | − | − | − | + | + |
| A and P-region interaction contributes to epitope | + | + | + | + | + | − | − |
| Pre-A region sequence contributes to epitope | + | + | + | − | − | − | − |
The immunomodulatory MAb 4-10A maps exclusively to the stalk itself. It both inhibits adherence directly and can also promote the formation of adherence inhibiting antibodies that are 1-6F-like in nature [47]. Its immunomodulatory effects are markedly prozone-like and best observed at intermediate concentrations. The Guy’s 13 MAb, demonstrated in this report to possess concentration-dependent immunomodulatory activity, is similar to 4-10A in that it requires only an A-P interaction for formation of its epitope (reviewed in [11]), but the location of binding of these two MAbs along the stalk differs. The P-region amino acids required for the Guy’s 13 epitope have been defined and are present in P2 and P3, but not the P1 segment [48]. In contrast, the 4-10A epitope is likely repeated three times along the stalk and has been experimentally reconstituted by an interaction between the P1 and A3 segments [50, 60, 62]. Therefore, Guy’s 13 shares some properties with 4-10A, but is also similar to the imunomodulatory MAbs 6-11A, 5-5D, and 3-10E in that it maps away from the apical V-region and does not inhibit adherence directly when assayed by BiaCore surface plasmon resonance. The immunomodulatory activities of MAbs 4-10A, 6-11A, 5-5D, and 3-10E are Fc- independent and appear to stem from a perturbation of antigen structure [47]. Whether this happens via an influence on the tertiary structure, or on a quaternary structure on the bacterial cell surface, or both, is not yet known. The in vivo protective effect of Guy’s 13 against S. mutans re-colonization is Fc-independent, and was conferred with bivalent F(ab)2, but not Fab fragments [35]. This suggests that the passively applied antibody did not act merely by blocking a simple monovalent adhesive interaction, but may have acted by a higher order structural mechanism.
The results of the current study indicate clearly that the Guy’s 13 plantibody modulates the specificity and isotype of the humoral response against S. mutans AgI/II (P1) toward one associated with increased inhibition of bacterial adherence to SAG. This offers an alternative mechanistic explanation to the proposed shift in microbial ecology and niche displacement for the long-term effect reported following passive immunotherapy with the Guy’s 13 MAb [35]. Because of the concentration dependence of MAbs that modulate the anti-P1 immune response toward one of enhanced inhibition of S. mutans adherence in a murine model, use of therapeutic passive immunization against S. mutans colonization in humans should address antibody dosage as a function of efficacy. In addition, information gained by characterizing the antigenic changes mediated by beneficial immunomodulatory MAbs, and the downstream effect on the adherence-inhibiting adaptive anti-Ag I/II (P1) immune response, will help enable the design of more effective protein variant or peptide immunogens for potential vaccines in future studies.
Acknowledgements
This work was supported by the National Institute for Dental and Craniofacial Research R01-DE13882 to L.J.B. and training grant T32-DE07200 to R.A.R. We thank Keith Wycoff of Planet Biotechnology Inc. for providing the Guy’s 13 plantibody, and Champion Deivanayagam of the University of Alabama for providing the A3VP1 polypeptide. We also thank the late Arnold S. Bleiweis for his insight and many helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hamada S, Slade HD. Biology, immunology, and cariogenicity of Streptococcus mutans. Microbiol Rev. 1980 Jun;44(2):331–384. doi: 10.1128/mr.44.2.331-384.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taubman MA, Nash DA. The scientific and public-health imperative for a vaccine against dental caries. Nat Rev Immunol. 2006 Jul;6(7):555–563. doi: 10.1038/nri1857. [DOI] [PubMed] [Google Scholar]
- 3.Michalek SM, Katz J, Childers NK. A vaccine against dental caries: an overview. BioDrugs. 2001;15(8):501–508. doi: 10.2165/00063030-200115080-00002. [DOI] [PubMed] [Google Scholar]
- 4.Smith DJ, Shoushtari B, Heschel RL, King WF, Taubman MA. Immunogenicity and protective immunity induced by synthetic peptides associated with a catalytic subdomain of mutans group streptococcal glucosyltransferase. Infect Immun. 1997 Nov;65(11):4424–4430. doi: 10.1128/iai.65.11.4424-4430.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith DJ, Taubman MA. Experimental immunization of rats with a Streptococcus mutans 59-kilodalton glucan-binding protein protects against dental caries. Infect Immun. 1996 Aug;64(8):3069–3073. doi: 10.1128/iai.64.8.3069-3073.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang P, Jespersgaard C, Lamberty-Mallory L, Katz J, Huang Y, Hajishengallis G, et al. Enhanced immunogenicity of a genetic chimeric protein consisting of two virulence antigens of Streptococcus mutans and protection against infection. Infect Immun. 2002 Dec;70(12):6779–6787. doi: 10.1128/IAI.70.12.6779-6787.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russell MW, Lehner T. Characterisation of antigens extracted from cells and culture fluids of Streptococcus mutans serotype c. Arch Oral Biol. 1978;23(1):7–15. doi: 10.1016/0003-9969(78)90047-x. [DOI] [PubMed] [Google Scholar]
- 8.Forester H, Hunter N, Knox KW. Characteristics of a high molecular weight extracellular protein of Streptococcus mutans. J Gen Microbiol. 1983 Sep;129(Pt 9):2779–2788. doi: 10.1099/00221287-129-9-2779. [DOI] [PubMed] [Google Scholar]
- 9.Russell RR. Wall-associated protein antigens of Streptococcus mutans. J Gen Microbiol. 1979 Sep;114(1):109–115. doi: 10.1099/00221287-114-1-109. [DOI] [PubMed] [Google Scholar]
- 10.Okahashi N, Sasakawa C, Yoshikawa M, Hamada S, Koga T. Molecular characterization of a surface protein antigen gene from serotype c Streptococcus mutans, implicated in dental caries. Mol Microbiol. 1989 May;3(5):673–678. doi: 10.1111/j.1365-2958.1989.tb00215.x. [DOI] [PubMed] [Google Scholar]
- 11.Brady LJ, Maddocks SE, Larson MR, Forsgren N, Persson K, Deivanayagam CC, et al. The changing faces of Streptococcus antigen I/II polypeptide family adhesins. Mol Microbiol. 2010 Jul;77(2):276–286. doi: 10.1111/j.1365-2958.2010.07212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hajishengallis G, Koga T, Russell MW. Affinity and specificity of the interactions between Streptococcus mutans antigen I/II and salivary components. J Dent Res. 1994 Sep;73(9):1493–1502. doi: 10.1177/00220345940730090301. [DOI] [PubMed] [Google Scholar]
- 13.Oho T, Yu H, Yamashita Y, Koga T. Binding of salivary glycoprotein-secretory immunoglobulin A complex to the surface protein antigen of Streptococcus mutans. Infect Immun. 1998 Jan;66(1):115–121. doi: 10.1128/iai.66.1.115-121.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prakobphol A, Xu F, Hoang VM, Larsson T, Bergstrom J, Johansson I, et al. Salivary agglutinin, which binds Streptococcus mutans and Helicobacter pylori, is the lung scavenger receptor cysteine-rich protein gp-340. J Biol Chem. 2000 Dec 22;275(51):39860–39866. doi: 10.1074/jbc.M006928200. [DOI] [PubMed] [Google Scholar]
- 15.Loimaranta V, Jakubovics NS, Hytonen J, Finne J, Jenkinson HF, Stromberg N. Fluid- or surface-phase human salivary scavenger protein gp340 exposes different bacterial recognition properties. Infect Immun. 2005 Apr;73(4):2245–2252. doi: 10.1128/IAI.73.4.2245-2252.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brady LJ, Piacentini DA, Crowley PJ, Oyston PC, Bleiweis AS. Differentiation of salivary agglutinin-mediated adherence and aggregation of mutans streptococci by use of monoclonal antibodies against the major surface adhesin P1. Infect Immun. 1992 Mar;60(3):1008–1017. doi: 10.1128/iai.60.3.1008-1017.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Madsen J, Mollenhauer J, Holmskov U. Review: Gp-340/DMBT1 in mucosal innate immunity. Innate Immun. 2010;16(3):160–167. doi: 10.1177/1753425910368447. [DOI] [PubMed] [Google Scholar]
- 18.Brady LJ. Antibody-mediated immunomodulation: a strategy to improve host responses against microbial antigens. Infect Immun. 2005 Feb;73(2):671–678. doi: 10.1128/IAI.73.2.671-678.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Michalek SM, Childers NK. Development and outlook for a caries vaccine. Crit Rev Oral Biol Med. 1990;1(1):37–54. doi: 10.1177/10454411900010010401. [DOI] [PubMed] [Google Scholar]
- 20.Challacombe SJ, Bergmeier LA, Rees AS. Natural antibodies in man to a protein antigen from the bacterium Streptococcus mutans related to dental caries experience. Arch Oral Biol. 1984;29(3):179–184. doi: 10.1016/0003-9969(84)90051-7. [DOI] [PubMed] [Google Scholar]
- 21.Lehtonen OP, Grahn EM, Stahlberg TH, Laitinen LA. Amount and avidity of salivary and serum antibodies against Streptococcus mutans in two groups of human subjects with different dental caries susceptibility. Infect Immun. 1984 Jan;43(1):308–313. doi: 10.1128/iai.43.1.308-313.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tenovuo J, Lehtonen OP, Aaltonen AS. Caries development in children in relation to the presence of mutans streptococci in dental plaque and of serum antibodies against whole cells and protein antigen I/II of Streptococcus mutans. Caries Res. 1990;24(1):59–64. doi: 10.1159/000261240. [DOI] [PubMed] [Google Scholar]
- 23.Ebersole JL. Humoral immune responses in gingival crevice fluid: local and systemic implications. Periodontol 2000. 2003;31:135–166. doi: 10.1034/j.1600-0757.2003.03109.x. [DOI] [PubMed] [Google Scholar]
- 24.Huang Y, Hajishengallis G, Michalek SM. Induction of protective immunity against Streptococcus mutans colonization after mucosal immunization with attenuated Salmonella enterica serovar typhimurium expressing an S. mutans adhesin under the control of in vivo-inducible nirB promoter. Infect Immun. 2001 Apr;69(4):2154–2161. doi: 10.1128/IAI.69.4.2154-2161.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lehner T, Russell MW, Caldwell J, Smith R. Immunization with purified protein antigens from Streptococcus mutans against dental caries in rhesus monkeys. Infect Immun. 1981 Nov;34(2):407–415. doi: 10.1128/iai.34.2.407-415.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregory RL, Hobbs LC, Kindle JC, VanTo T, Malmstrom HS. Immunodominant antigens of Streptococcus mutans in dental caries-resistant subjects. Hum Antibodies Hybridomas. 1990;1(3):132–136. [PubMed] [Google Scholar]
- 27.Kelly CG, Todryk S, Kendal HL, Munro GH, Lehner T. T-cell, adhesion, and B-cell epitopes of the cell surface Streptococcus mutans protein antigen I/II. Infect Immun. 1995 Sep;63(9):3649–3658. doi: 10.1128/iai.63.9.3649-3658.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hocini H, Iscaki S, Bouvet JP, Pillot J. Unexpectedly high levels of some presumably protective secretory immunoglobulin A antibodies to dental plaque bacteria in salivas of both caries-resistant and caries-susceptible subjects. Infect Immun. 1993 Sep;61(9):3597–3604. doi: 10.1128/iai.61.9.3597-3604.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Casadevall A, Pirofski LA. Exploiting the redundancy in the immune system: vaccines can mediate protection by eliciting 'unnatural' immunity. J Exp Med. 2003 Jun 2;197(11):1401–1404. doi: 10.1084/jem.20030637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas LF. Neurological stamp: Paul Ehrlich (1854–1915) and Emil Adolf von Behring (1854–1917) J Neurol Neurosurg Psychiatry. 2001 May;70(5):678. doi: 10.1136/jnnp.70.5.678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Getahun A, Heyman B. How antibodies act as natural adjuvants. Immunol Lett. 2006 Apr 15;104(1–2):38–45. doi: 10.1016/j.imlet.2005.11.005. [DOI] [PubMed] [Google Scholar]
- 32.Michaud HA, Gomard T, Gros L, Thiolon K, Nasser R, Jacquet C, et al. A crucial role for infected-cell/antibody immune complexes in the enhancement of endogenous antiviral immunity by short passive immunotherapy. PLoS Pathog. 2010;6(6):e1000948. doi: 10.1371/journal.ppat.1000948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lehner T, Caldwell J, Smith R. Local passive immunization by monoclonal antibodies against streptococcal antigen I/II in the prevention of dental caries. Infect Immun. 1985 Dec;50(3):796–799. doi: 10.1128/iai.50.3.796-799.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehner T, Ma JK, Kelly CG. A mechanism of passive immunization with monoclonal antibodies to a 185,000 M(r) streptococcal antigen. Adv Exp Med Biol. 1992;327:151–163. doi: 10.1007/978-1-4615-3410-5_17. [DOI] [PubMed] [Google Scholar]
- 35.Ma JK, Hunjan M, Smith R, Kelly C, Lehner T. An investigation into the mechanism of protection by local passive immunization with monoclonal antibodies against Streptococcus mutans. Infect Immun. 1990 Oct;58(10):3407–3414. doi: 10.1128/iai.58.10.3407-3414.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma JK, Hunjan M, Smith R, Lehner T. Specificity of monoclonal antibodies in local passive immunization against Streptococcus mutans. Clin Exp Immunol. 1989 Sep;77(3):331–337. [PMC free article] [PubMed] [Google Scholar]
- 37.Ma JK, Lehner T. Prevention of colonization of Streptococcus mutans by topical application of monoclonal antibodies in human subjects. Arch Oral Biol. 1990;35 Suppl:115S–225S. doi: 10.1016/0003-9969(90)90140-6. [DOI] [PubMed] [Google Scholar]
- 38.Ma JK, Smith R, Lehner T. Use of monoclonal antibodies in local passive immunization to prevent colonization of human teeth by Streptococcus mutans. Infect Immun. 1987 May;55(5):1274–1278. doi: 10.1128/iai.55.5.1274-1278.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ma JK, Hiatt A, Hein M, Vine ND, Wang F, Stabila P, et al. Generation and assembly of secretory antibodies in plants. Science. 1995 May 5;268(5211):716–719. doi: 10.1126/science.7732380. [DOI] [PubMed] [Google Scholar]
- 40.Ma JK, Hikmat BY, Wycoff K, Vine ND, Chargelegue D, Yu L, et al. Characterization of a recombinant plant monoclonal secretory antibody and preventive immunotherapy in humans. Nat Med. 1998 May;4(5):601–606. doi: 10.1038/nm0598-601. [DOI] [PubMed] [Google Scholar]
- 41.Weintraub JA, Hilton JF, White JM, Hoover CI, Wycoff KL, Yu L, et al. Clinical trial of a plant-derived antibody on recolonization of mutans streptococci. Caries Res. 2005 May–Jun;39(3):241–250. doi: 10.1159/000084805. [DOI] [PubMed] [Google Scholar]
- 42.Brady LJ, van Tilburg ML, Alford CE, McArthur WP. Monoclonal antibody-mediated modulation of the humoral immune response against mucosally applied Streptococcus mutans. Infect Immun. 2000 Apr;68(4):1796–1805. doi: 10.1128/iai.68.4.1796-1805.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Isoda R, Robinette RA, Pinder TL, McArthur WP, Brady LJ. Basis of beneficial immunomodulation by monoclonal antibodies against Streptococcus mutans adhesin P1. FEMS Immunol Med Microbiol. 2007 Oct;51(1):102–111. doi: 10.1111/j.1574-695X.2007.00279.x. [DOI] [PubMed] [Google Scholar]
- 44.Oli MW, McArthur WP, Brady LJ. A whole cell BIAcore assay to evaluate P1-mediated adherence of Streptococcus mutans to human salivary agglutinin and inhibition by specific antibodies. J Microbiol Methods. 2005 Oct 17; doi: 10.1016/j.mimet.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 45.Oli MW, Rhodin N, McArthur WP, Brady LJ. Redirecting the humoral immune response against Streptococcus mutans antigen P1 with monoclonal antibodies. Infect Immun. 2004 Dec;72(12):6951–6960. doi: 10.1128/IAI.72.12.6951-6960.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rhodin NR. Immunomodulation by an anti-streptococcus mutans monoclonal antibody [Ph.D.] Gainesville, Florida: University of Florida; 2003. [Google Scholar]
- 47.Robinette RA, Oli MW, McArthur WP, Brady LJ. Beneficial immunomodulation by Streptococcus mutans anti-P1 monoclonal antibodies is Fc independent and correlates with increased exposure of a relevant target epitope. J Immunol. 2009 Oct 1;183(7):4628–4638. doi: 10.4049/jimmunol.0803300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Dolleweerd CJ, Kelly CG, Chargelegue D, Ma JK. Peptide mapping of a novel discontinuous epitope of the major surface adhesin from Streptococcus mutans. J Biol Chem. 2004 May 21;279(21):22198–22203. doi: 10.1074/jbc.M400820200. [DOI] [PubMed] [Google Scholar]
- 49.Larson MR, Rajashankar KR, Crowley PJ, Kelly C, Mitchell TJ, Brady LJ, et al. Crystal structure of the C-terminus of Streptococcus mutans antigen I/II and characterization of salivary agglutinin adherence domains. J Biol Chem. 2011 Apr 19; doi: 10.1074/jbc.M111.231100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larson MR, Rajashankar KR, Patel MH, Robinette RA, Crowley PJ, Michalek S, et al. Elongated fibrillar structure of a streptococcal adhesin assembled by the high-affinity association of alpha- and PPII-helices. Proc Natl Acad Sci U S A. 2010 Mar 30;107(13):5983–5988. doi: 10.1073/pnas.0912293107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kelly C, Evans P, Bergmeier L, Lee SF, Progulske-Fox A, Harris AC, et al. Sequence analysis of the cloned streptococcal cell surface antigen I/II. FEBS Lett. 1989 Nov 20;258(1):127–132. doi: 10.1016/0014-5793(89)81632-1. [DOI] [PubMed] [Google Scholar]
- 52.Rhodin NR, Cutalo JM, Tomer KB, McArthur WP, Brady LJ. Characterization of the Streptococcus mutans P1 epitope recognized by immunomodulatory monoclonal antibody 6-11A. Infect Immun. 2004 Aug;72(8):4680–4688. doi: 10.1128/IAI.72.8.4680-4688.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Brady LJ, Cvitkovitch DG, Geric CM, Addison MN, Joyce JC, Crowley PJ, et al. Deletion of the central proline-rich repeat domain results in altered antigenicity and lack of surface expression of the Streptococcus mutans P1 adhesin molecule. Infect Immun. 1998 Sep;66(9):4274–4282. doi: 10.1128/iai.66.9.4274-4282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ayakawa GY, Boushell LW, Crowley PJ, Erdos GW, McArthur WP, Bleiweis AS. Isolation and characterization of monoclonal antibodies specific for antigen P1, a major surface protein of mutans streptococci. Infect Immun. 1987 Nov;55(11):2759–2767. doi: 10.1128/iai.55.11.2759-2767.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rundegren J, Arnold RR. Differentiation and interaction of secretory immunoglobulin A and a calcium-dependent parotid agglutinin for several bacterial strains. Infect Immun. 1987 Feb;55(2):288–292. doi: 10.1128/iai.55.2.288-292.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Casadevall A. Antibody-mediated immunity against intracellular pathogens: two-dimensional thinking comes full circle. Infect Immun. 2003 Aug;71(8):4225–4228. doi: 10.1128/IAI.71.8.4225-4228.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Casadevall A, Cleare W, Feldmesser M, Glatman-Freedman A, Goldman DL, Kozel TR, et al. Characterization of a murine monoclonal antibody to Cryptococcus neoformans polysaccharide that is a candidate for human therapeutic studies. Antimicrob Agents Chemother. 1998 Jun;42(6):1437–1446. doi: 10.1128/aac.42.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Casadevall A, Pirofski LA. New concepts in antibody-mediated immunity. Infect Immun. 2004 Nov;72(11):6191–6196. doi: 10.1128/IAI.72.11.6191-6196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taborda CP, Rivera J, Zaragoza O, Casadevall A. More is not necessarily better: prozone-like effects in passive immunization with IgG. J Immunol. 2003 Apr 1;170(7):3621–3630. doi: 10.4049/jimmunol.170.7.3621. [DOI] [PubMed] [Google Scholar]
- 60.McArthur WP, Rhodin NR, Seifert TB, Oli MW, Robinette RA, Demuth DR, et al. Characterization of epitopes recognized by anti-Streptococcus mutans P1 monoclonal antibodies. FEMS Immunol Med Microbiol. 2007 Aug;50(3):342–353. doi: 10.1111/j.1574-695X.2007.00260.x. [DOI] [PubMed] [Google Scholar]
- 61.Ton-That H, Marraffini LA, Schneewind O. Protein sorting to the cell wall envelope of Gram-positive bacteria. Biochim Biophys Acta. 2004 Nov 11;1694(1–3):269–278. doi: 10.1016/j.bbamcr.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 62.Seifert TB, Bleiweis AS, Brady LJ. Contribution of the alanine-rich region of Streptococcus mutans P1 to antigenicity, surface expression, and interaction with the proline-rich repeat domain. Infect Immun. 2004 Aug;72(8):4699–4706. doi: 10.1128/IAI.72.8.4699-4706.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]