Summary
Sprouting angiogenesis expands the embryonic vasculature enabling survival and homeostasis. Yet how the angiogenic capacity to form sprouts is allocated among endothelial cells (ECs) to guarantee the reproducible anatomy of stereotypical vascular beds remains unclear. Here we show that Sema-PlxnD1 signaling, previously implicated in sprout guidance, represses angiogenic potential to ensure the proper abundance and stereotypical distribution of the trunk’s Segmental Arteries (SeAs). We find that Sema-PlxnD1 signaling exerts this effect by antagonizing the pro-angiogenic activity of Vascular Endothelial Growth Factor (VEGF). Specifically, Sema-PlxnD1 signaling ensures the proper endothelial abundance of soluble flt1 (sflt1), an alternatively spliced form of the VEGF receptor Flt1 encoding a potent secreted decoy. Hence Sema-PlxnD1 signaling regulates distinct but related aspects of angiogenesis: the spatial allocation of angiogenic capacity within a primary vessel and sprout guidance.
INTRODUCTION
Blood vessels form a pervasive tubular network that distributes oxygen, nutrients, hormones and immunity factors. The first blood vessels assemble de novo via EC coalescence or vasculogenesis. Later, they expand via angiogenesis, the growth of new blood vessels from preexisting ones. In some locales this process is stereotypic and vascular sprouts form with evolutionarily conserved and organ-specific distribution, abundance and shapes (Carmeliet, 2005; Isogai et al., 2001; Isogai et al., 2003). For example, zebrafish SeAs sprout bilaterally from the trunk’s aorta just anterior to each somite boundary (SB; Figure 1A). SeA sprouts contain migratory, proliferative and filopodia-rich arterial angiogenic ECs molecularly distinct from the sedentary “phalanx” ECs remaining in the aorta (De Bock et al., 2009; Siekmann and Lawson, 2007; Torres-Vazquez et al., 2004). Normally, only aortic ECs near SBs acquire angiogenic capacity (Ahn et al., 2000; Childs et al., 2002). It is thought that non-endothelial paracrine VEGF signals promote angiogenic capacity, while Notch-mediated lateral inhibition between ECs antagonizes it (Phng and Gerhardt, 2009; Siekmann et al., 2008). However, the mRNA expression of vegf-a and Notch pathway genes is inconsistent with the distribution of SeA sprouts. vegf-a is not transcribed along SBs, but rather expressed dorsal to the aorta at both the flanking somites’ centers and the hypochord, a midline endodermal cell row. Notch pathway genes are expressed continuously along the aorta or broadly through the body (Hogan et al., 2009b; Lawson et al., 2002; Leslie et al., 2007; Phng et al., 2009; Siekmann and Lawson, 2007) (C.M.G., J.B. and J. T-V., unpublished observations). Hence, other cascades likely modulate VEGF and/or Notch signaling at pre-sprouting stages to enable the stereotypical allocation of angiogenic capacity within the aorta. Perturbing these unidentified cascades might change the SeA sprouts’ reproducible number, distribution and/or the ratio of aortic ECs that acquire angiogenic capacity.
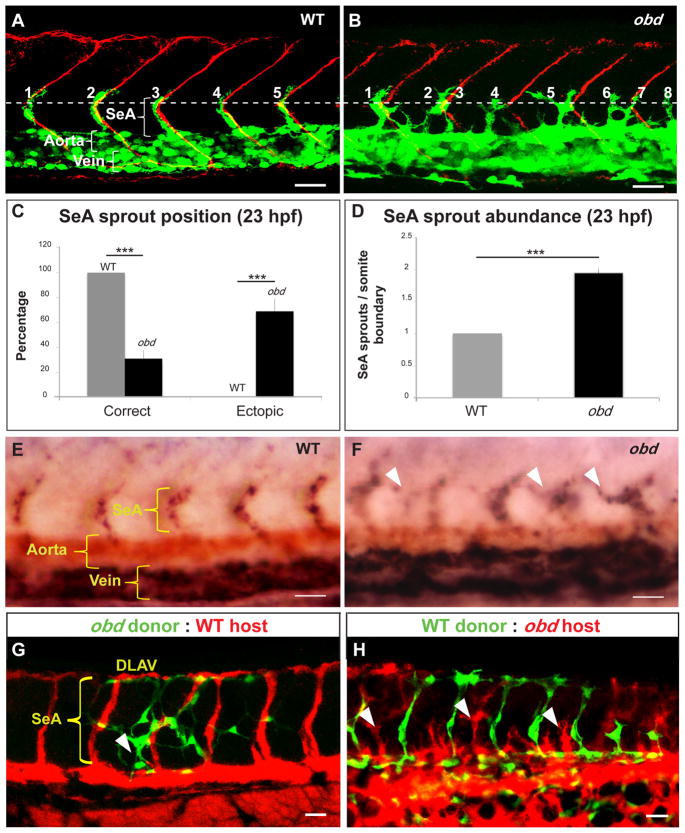
Figure 1. Sema-PlxnD1 signaling is cell autonomously required within the endothelium for proper SeA sprout abundance and distribution.
(A–B) 23 hpf vasculatures, green. SBs, red. Horizontal myoseptum (HM), white dotted line. SeA sprouts, numbered. (A) WT. (B) obd. SeA sprout position (C) and abundance (D) in 23 hpf WT and obd. (E–F) WISH, 28 hpf trunks. Ectopic SeA sprouts, white arrowheads. Riboprobes: flt4 (blue), cdh5 (red). WT (E). obd (F). (G–H) 32 hpf chimeric vasculatures with ECs of donor (green) and host (red) origin. Examples of ectopic SeA sprouts, white arrowheads. (C–D) n = 8 WT, 12 obd. Error bars, s.e.m. ***p < 0.001. (E–F) n = 10/10 WT, 10/10 obd. (A–B, E–H) Anterior, left; dorsal, up. Scale bars, 30 μm. See Figure S1.
Besides VEGF and Notch activity, proper SeA development requires paracrine Sema-Plxn signaling. Type 3 semas (sema3s) are repulsive guidance cues secreted by somites. Sema3s direct SeA sprout pathfinding by modulating cytoskeletal dynamics via the endothelial Sema3-receptor PlxnD1. Hence, sema3 or plxnD1 inactivation yields similar SeA sprout pathfinding defects in zebrafish and mice (Gay et al., 2011). Two observations made in zebrafish make Sema-PlxnD1 signaling a candidate modulator of angiogenic capacity. First, sema3 and plxnD1 expression begins hours before SeAs sprout from the aorta at ~21 hours post-fertilization (hpf). Second, loss of Sema-PlxnD1 signaling induces ectopic SeA sprout launching (Childs et al., 2002; Torres-Vazquez et al., 2004).
In wild type (WT) animals SeA sprouts grow dorsally with a chevron-like shape, bifurcate anteroposteriorly at the neural tube’s roof level and interconnect with their ipsilateral neighbors at ~32 hpf forming the paired Dorsal Longitudinal Anastomotic Vessels (DLAVs) (Isogai et al., 2003). In contrast, in plxnD1 (out of bounds - obd) mutants and plxnD1 morphants, SeA sprouts are misshaped and interconnect ectopically with their ipsilateral neighbors but form properly placed DLAVs (Childs et al., 2002; Torres-Vazquez et al., 2004). Thus, we further examined Sema-PlxnD1’s signaling role during zebrafish SeA development, finding that it plays a pre-sprouting role as a repressor of the aorta’s angiogenic potential - the probability that ECs acquire angiogenic capacity. This role stems from its ability to promote sflt1’s endothelial abundance and thus antagonize pro-angiogenic VEGF activity (Rahimi, 2006). We propose that Sema-PlxnD1 signaling allocates angiogenic capacity among aortic ECs in a reproducible spatial pattern, guaranteeing the proper abundance and distribution of SeA sprouts.
RESULTS
Lack of Sema-PlxnD1 signaling induces too many and ectopic SeA sprouts
To investigate if Sema-PlxnD1 signaling modulates angiogenic capacity we measured SeA sprout abundance and positioning in WT and obd at 23 hpf, when individual obd SeA sprouts are readily identifiable as they are yet to interconnect ectopically. We found obd has almost twice the WT’s number of SeA sprouts, with most of them launching ectopically (Figure 1A–D). Hence, Sema-PlxnD1 signaling limits the abundance and defines the position of SeA sprouts.
To molecularly verify the angiogenic character of ECs within obd SeA sprouts we used whole mount RNA in situ hybridization (WISH) (Moens, 2008) to visualize expression of the pan-endothelial marker cdh5 (Larson et al., 2004) and flt4/vegfr-3, which labels arterial angiogenic ECs within SeA sprouts and the vein (Covassin et al., 2006; Hogan et al., 2009b; Siekmann and Lawson, 2007). flt4 is expressed in all SeA sprouts and vein of WT and obd (Figure 1E–F), confirming the angiogenic character of ECs within obd’s SeA sprouts and the lack of artery/vein differentiation defects in obd (Torres-Vazquez et al., 2004).
Loss of Sema-PlxnD1 signaling yields more angiogenic cells
To determine if obd’s SeA sprout overabundance is associated with too many angiogenic ECs we compared the number of EC nuclei found within developing SeAs and DLAVs of WT and obd at 21, 23 and 32 hpf. We found that obd’s SeAs/DLAVs collectively harbor more angiogenic ECs than WT (Figure S1A–B). We next aimed to compare the WT and obd ratios of angiogenic to phalanx arterial ECs. However, SeA sprouts arise while the aorta and vein segregate from each other (Herbert et al., 2009), making the quantification of early aortic EC abundance unfeasible. We thus instead counted EC nuclei in the axial vasculature (aorta and vein taken together) and found that obd shows increased axial vasculature EC abundance (Figure S1A–B). Hence, loss of Sema-PlxnD1 signaling yields more angiogenic and axial vasculature ECs.
Sema-PlxnD1 signaling is cell-autonomously required within the endothelium
To ask if Sema-PlxnD1 signaling acts cell autonomously to limit the number and define the position of SeA sprouts we performed cell transplants (Carmany-Rampey and Moens, 2006) to make heterogenotypic WT:obd (WT-to-obd and obd-to-WT) chimeras. We analyzed these at ~32 hpf to determine SeA sprout abundance and distribution and examine the SeA contribution of ECs from donors and hosts (Figure 1G–H). We found too many SeA sprouts in WT:obd chimeras. As in obd, some SeA sprouts launched ectopically and others were positioned correctly. WT ECs were found only within properly positioned SeA sprouts, while obd ECs contributed to misshapen SeAs sprouts at both ectopic and correct positions (Figure 1G–H and S1C). Control homogenotypic (WT-to-WT and obd-to-obd) chimeras also showed mosaic SeAs with both host and donor ECs (Figure S1E). Hence, SeAs are not necessarily of clonal origin, in agreement with results from prior transplantation and mosaic transgenic labeling experiments (Childs et al., 2002; Siekmann and Lawson, 2007).
obd ECs found within WT hosts contribute to SeAs/DLAVs much more often than WT ECs contribute to these angiogenic vessels in obd hosts (Figures S1C-D and S2C). Since obd ECs show exacerbated angiogenic capacity in a WT environment this property is not caused by axial vasculature EC over-abundance. Finally, non-endothelial obd cells, like ventral somitic muscle fibers (Childs et al., 2002), did not influence the abundance, distribution or anatomical patterning of SeA sprouts (Figure S1C), consistent with plxnD1’s endothelial-specific expression (Torres-Vazquez et al., 2004) and the identical vascular phenotypes of mice with global or EC-specific plxnD1 inactivation (Gay et al., 2011). Thus, Sema-PlxnD1 signaling acts cell autonomously within the endothelium to limit angiogenic potential and ensure the proper abundance and positioning of SeA sprouts.
Aortic ECs with less Sema-PlxnD1 signaling (obd/+) become angiogenic tip cells more often and are enriched in the aorta’s dorsal side
Each SeA sprout has a spearheading tip cell that becomes “T” shaped during DLAV formation and which is trailed by a few stalk cells (Siekmann and Lawson, 2007). Tip cells embody an enhanced angiogenic state promoted by increased pro-angiogenic signaling and characterized by exacerbated filopodia dynamics whose acquisition and/or maintenance involves cell competition (Jakobsson et al., 2010; Leslie et al., 2007; Roca and Adams, 2007).
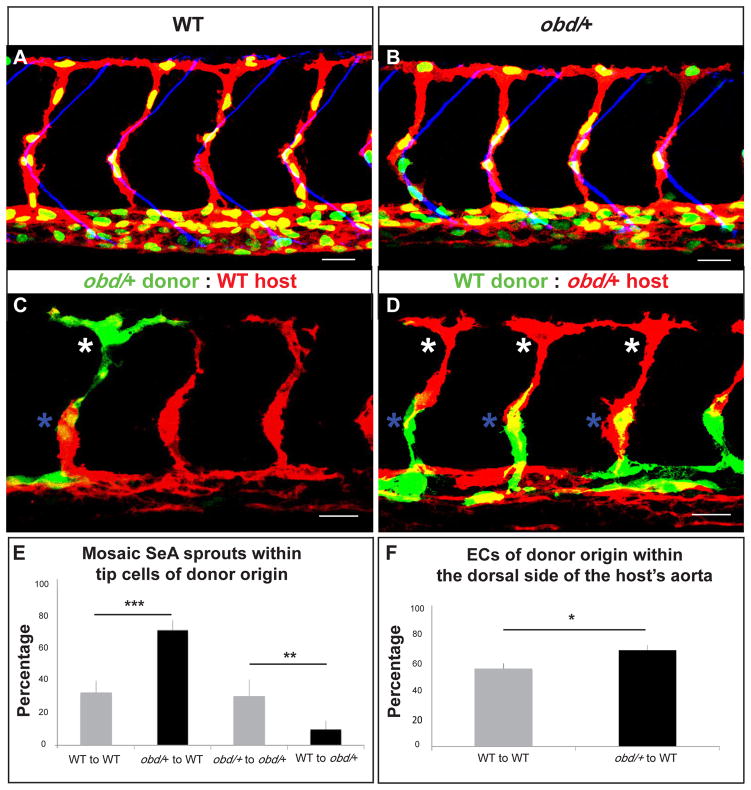
Thus, if Sema-PlxnD1 signaling antagonizes angiogenic potential then ECs with reduced Sema-PlxnD1 signaling levels should acquire an enhanced angiogenic state more often. To test this hypothesis we used cell transplantation experiments to compare the properties of ECs from WT and obd/+ heterozygotes. These embryos have the same number of ECs within both the SeAs/DLAVs and the axial vasculature (Figure S2A) and identical SeA sprout abundance, positioning and patterning (Figure 2A–B). We determined the frequency at which donor ECs become tip cells in homogenotypic (WT-to-WT and obd/+-to-obd/+) and heterogenotypic (WT-to-obd/+ and obd/+-to-WT) chimeras. To ensure competition between donor and host ECs had occurred we only scored mosaic SeAs harboring both donor and host ECs. All chimeras showed correctly patterned and positioned SeA sprouts (Figure 2C–D and data not shown) and both kinds of homogenotypic chimeras showed identical donor tip cell percentages (Figure 2E). In contrast, the donor tip cell percentage was significantly larger in obd/+-to-WT chimeras and smaller in WT-to-obd/+ chimeras (Figure 2E).
Figure 2. ECs with less Sema-PlxnD1 signaling tend to become tip cells and occupy the aorta’s dorsal side.
(A–B) 32 hpf vasculatures. EC nuclei (green), membranes (red). SBs, blue. (A) WT. (B) obd/+. (C–D) 28 hpf vasculatures with ECs of donor (green) and host (red) origin. Asterisks: Tip cells (white), stalk cells (blue). (E) Percentage of mosaic SeAs with tip cells of donor origin in homogenotypic (grey bars) and heterogenotypic (black bars) chimeras. (F) Percentage of ECs of donor origin found within the dorsal side of the host’s arterial tree in homogenotypic (grey bar) and heterogenotypic (black bar) chimeras. (E–F) *p < 0.05, **p < 0.01, ***p < 0.001. Error bars, s.e.m. (E) n = 27 WT to obd/+, n = 18 obd/+ to obd/+, n = 38 obd/+ to WT, n = 34 WT to WT. Error bars, s.e.m. (F) n = 24 WT to WT, n = 32 obd/+ to WT. (A–D) Anterior, left; dorsal, up. Scale bars, 30 μm. See Figure S2 and Movie S1.
Hence, the angiogenic capacity and angiogenic positional fate of aortic ECs is not pre-specified but is acquired and/or maintained competitively, agreeing with prior related observations (Jakobsson et al., 2010; Siekmann and Lawson, 2007). Indeed, within developing SeA sprouts angiogenic cell nuclei swap positions (Movie S1), suggesting that angiogenic cells within SeA sprouts can exchange places. Thus, the SeA tip cell population scored in Figure 2C–E likely includes both the angiogenic cells that launched first from the aorta and kept their leading position and those that trailed the original tip cell but later overtook it. Prior studies suggest that migration speed is similar between cells with differential abilities to acquire/maintain a tip cell positional status (Jakobsson et al., 2010). Of note, both WT and obd/+ embryos form DLAVs at similar times, suggesting that their SeA sprouts grow with matching speeds. Thus, independently of its roles in guiding SeA sprouts (Gay et al., 2011) and limiting EC abundance (Figure S1A–B), Sema-PlxnD1 signaling antagonizes angiogenic responses.
Both the angiogenic potential of obd ECs and the angiogenic response of obd/+ ECs within WT hosts is enhanced, suggesting that Sema-PlxnD1 signaling acts prior to SeA sprouting. To investigate this possibility and determine its potential cellular basis we made obd/+-to-WT and WT-to-WT chimeras and plotted the distribution of donor ECs within the host’s trunk vasculature shortly after SeA sprouts launch (Figure S2B). Consistent with Sema-PlxnD1 signaling’s dispensability for artery-vein differentiation (Torres-Vazquez et al., 2004) ECs from both donors contributed to the WT host’s aorta equally. However, ECs from obd/+ donors were enriched along the aorta’s dorsal side (Figure 2F) and obd ECs also preferentially occupy this locale in WT hosts (Figures S1C and S2C). In contrast, ECs with a cell autonomous impairment in downstream VEGF signaling that abrogates SeA angiogenesis localize to the aorta’s ventral side within WT hosts (Covassin et al., 2009).
The aorta’s dorsal side lies near the trunk’s paracrine sources of pro-angiogenic VEGF (Lawson et al., 2002) and is the aortic angiogenic region (Ahn et al., 2000; Wilkinson et al., 2009). Importantly, obd/+ lacks aortic dorso-ventral polarization defects: both WT and obd display similar expression of the aortic dorsal side marker tbx20 (data not shown) and make red blood cells, which derive from the aorta’s ventral side (data not shown) (Wilkinson et al., 2009).
Hence, Sema-PlxnD1 signaling plays a pre-sprouting role in SeA angiogenesis and the cellular basis for the enhanced angiogenic response of obd/+ arterial ECs is, at least, related to their ability to localize early within the WT host’s aortic roof, a property likely due to increased VEGF responsiveness. Notably, in heterogenotypic chimeras plxnD1 genetic dosage affects aortic cell distribution (Figure 2F) and tip cell positional status (Figure 2E) similarly but to different extents. Hence, Sema-PlxnD1 signaling likely exerts other pre-and/or post-sprouting effects, like modulating the angiogenic cell’s launching schedule and/or positional persistence (Childs et al., 2002; Jakobsson et al., 2010; Kearney et al., 2004).
Sema-PlxnD1 signaling regulates the abundance of the VEGF antagonist encoded by soluble flt1 (sflt1)
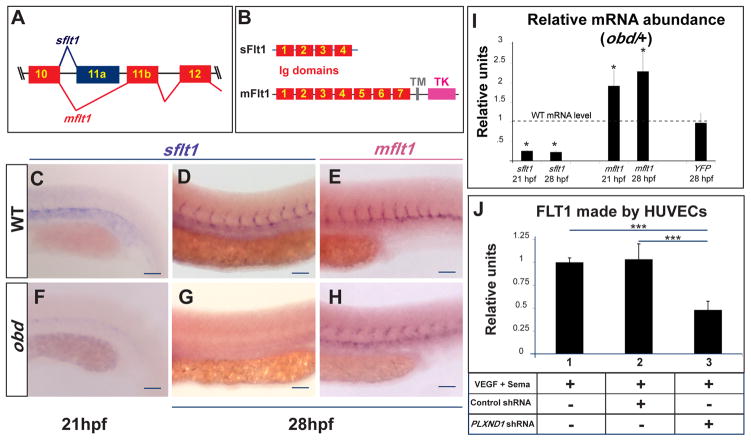
To determine the molecular mechanism by which Sema-PlxnD1 signaling represses angiogenic potential we used WISH (Moens, 2008) to visualize the expression of twelve components and targets of the VEGF and Notch signaling cascades, including artery-vein differentiation markers (see Supplemental Information). Only flt1 (fms-related tyrosine kinase/vegf receptor 1) (Bussmann et al., 2007; Krueger et al., 2011) expression was visibly affected in obd. We found that zebrafish flt1 pre-mRNA is alternatively spliced into transcripts encoding products similar to the soluble (sFlt1) and membrane (mFlt1) mammalian proteins that function as high-affinity VEGF decoys or receptor/co-receptor tyrosine kinases, respectively (Figure 3A–B) (Krueger et al., 2011; Rahimi, 2006). Using isoform-specific riboprobes we detected sflt1 and mflt1 transcripts in the WT trunk arterial tree at 21–28 hpf (Figure 3C–E) (Krueger et al., 2011). In contrast, sflt1 was barely detectable in obd despite robust mflt1 staining (Figure 3F–H), suggesting that Sema-PlxnD1 signaling modulates the relative abundance of flt1 isoforms and/or flt1 transcription. We used qPCR to compare the mRNA levels of WT and obd/+, which have identical EC abundances. We measured the transcript levels of both flt1 isoforms and, separately, quantified the YFP mRNA output of the flt1 transcriptional reporter Tg(flt1:YFP)hu4624 (Hogan et al., 2009a). obd/+ shows reduced sflt1 (four-fold) and increased mflt1 (two-fold) levels, but unaltered flt1 transcriptional levels (Figures 3I), and, confocal imaging reveals no clear differences in Tg(flt1:YFP)hu4624 expression between WT and obd (Figure S3C). Finally, ELISA-based measurements of FLT1 from extracts of HUVECs (human umbilical vein ECs) exposed to both VEGF and the canonical PlxnD1 ligand Sema3E reveal that shRNA-mediated PLXND1 knockdown reduces FLT1 without decreasing FLT1 transcription (Figure 3J and S3A, see also S3B).
Figure 3. Sema-PlxnD1 signaling ensures the proper endothelial abundance of sflt1.
(A) Alternative flt1 splicing yields sflt1 and mflt1 isoforms with unique eleventh exons. Exons, colored boxes. Introns, black lines. (B) sflt1 encodes a soluble 474 aa protein. mflt1 encodes a 1,273 aa transmembrane protein. Protein domains: Immunoglobulin (Ig, red numbered boxes), transmembrane (TM, grey box), tyrosine kinase (TK, pink box). (C–H) WISH, embryo trunks (genotypes and ages indicated) hybridized with sflt1 (C–D, F–G) and mflt1 (E, H) riboprobes (blue). (I) qPCR measurements. Relative mRNA abundance of sflt1, mflt1 and YFP (from Tg(flt1:YFP)hu4624/+) in 28 hpf obd/+ (WT level = 1, dashed line). Error bars, coefficient of variance *p < 0.05. (J) ELISA-based quantification of FLT1 prepared from cell extracts of HUVECs treated with both VEGF and Sema3E and the control or PLXND1-targeting shRNAs. Error bars, s.e.m. ***p < 0.001. (C–H) n = 10 embryos per riboprobe, stage and genotype. Pictures of representative examples of stainings observed (10/10 embryos in each category). Anterior, left; dorsal, up. Scale bars, 50 μm. See Figure S3.
We conclude that Sema-PlxnD1 signaling acts via a post-transcriptional mechanism to ensure sflt1’s proper abundance within the trunk’s arterial tree and propose this model: sflt1 acts as a PlxnD1 effector that antagonizes pro-angiogenic VEGF signaling to limit angiogenic potential.
Partial reduction of both plxnD1 and sflt1 enhances SeA angiogenesis
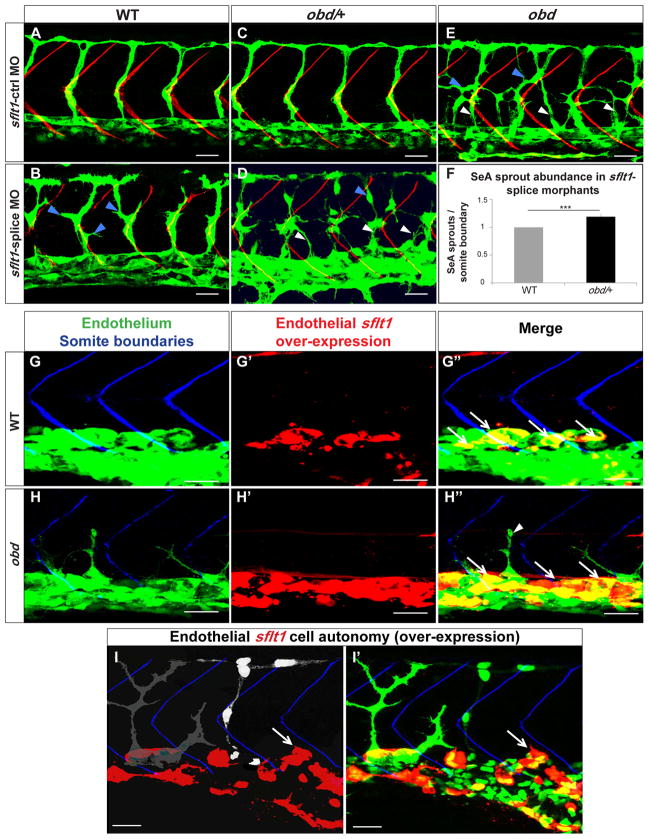
If the proposed model is true, plxnD1 and sflt1 should interact genetically to limit SeA angiogenesis. We tested this prediction with a morpholino (MO) (Morcos, 2007) that inhibits the alternative splicing event that yields sflt1 (Figure S4A–B). The sflt1-splice MO induces aberrantly branched SeA sprouts in WT and obd-like SeA sprout defects such as ectopic launching and aberrant branching in obd/+ heterozygotes (Figures 4B, D, E–F and S4C). Similarly, a pan-flt1 splice-blocking MO (Rottbauer et al., 2005) targeting both sflt1 and mflt1 also induces obd-like SeA sprout defects in obd/+ (Figure S4E–F). Of note, a different pan-flt1 MO also induces SeA misbranching in WT (Krueger et al., 2011). Both the expressivity and penetrance of these abnormalities is greater in sflt1-splice than in pan-flt1 morphants, likely due to differences in knockdown efficiencies and the combined effects of inactivating flt1 isoforms with opposite roles (Figure 4F and S4F) (Rahimi, 2006). In contrast, WT and obd/+ treated with mismatched control sflt1 splice-blocking MO or an mflt1-specific splice-blocking MO (Rottbauer et al., 2005) display normal SeA sprouts (Figure 4A, C, S4D, F).
Figure 4. plxnD1 and sflt1 interact genetically, sflt1 limits SeA angiogenesis cell autonomously.
(A–I) 32 hpf trunk vasculatures, green. (A–E) SBs, red. White arrowheads, ectopic SeA sprouts. Blue arrowheads, ectopic SeA branching. (A, C, E) Embryos treated with 20 ng of sflt1-ctrl MO: WT (A), obd/+ (C), obd (E). Embryos treated with 20 ng of sflt1-splice MO: WT (B), obd/+ (D). (F) 23 hpf SeA sprout abundance in WT (left, grey bar) and obd/+ (right, black bar) sflt1-splice morphants. n = 20 WT and n = 19 obd/+. Error bars, s.e.m. ***p < 0.001. (G–I′) SBs, blue. GAL4FF/UAS-mediated endothelial-specific sflt1 over-expression, red. White arrows, missing SeA sprouts. (G′–H″) Endothelial sflt1 over-expression inhibits SeA sprouting. WT (G-G″). obd (H-H″), note lack of sflt1 over-expression (red) in remaining SeA sprout (white arrowhead). (I-I′) Mosaic vasculature with ECs from both obd donor and WT host. Endothelial-specific and mosaic sflt1 and DsRed co-expression restricted to the WT endothelium (red, I-I′). obd ECs express cytosolically-targeted EGFP (grey in I; green in I′). WT ECs express nuclear-targeted EGFP (white in I; green in I′). obd and WT ECs without sflt1 over-expression (DsRed-) from SeA sprouts even next to sflt1 over-expressing WT ECs (DsRed+). WT ECs over-expressing sflt1 (DsRed+) fail to form SeA sprouts (white arrows, I-I′). (G-H″) n = 30 embryos with over-expression per genotype, all showing suppression of SeA sprouting. Anterior, left; dorsal, up. Scale bars, 30 μm. See Figure S4.
These observations agree with the vascular organization roles of plxnD1 (Gay et al., 2011) and flt1 (Krueger et al., 2011; Rahimi, 2006), the differential activities of flt1 isoforms (Chappell et al., 2009; Kappas et al., 2008; Rahimi, 2006) and sflt1’s low level in obd/+ (Figure 3I). In short, plxnD1 and sflt1 (but not mflt1) interact genetically to modulate SeA sprout positioning, abundance and patterning.
Endothelial over-expression of sflt1, but not mflt1, inhibits SeA angiogenesis
Based on our model sflt1, like Sema-PlxnD1 signaling, should inhibit SeA angiogenesis. We tested this idea by over-expressing sflt1 in an endothelial-specific fashion in both WT and obd via the GAL4/UAS system (Figure S4G). We found that sflt1 over-expression suppresses SeA sprouting in WT and obd (Figure 4G–H″). To determine if mflt1 plays similar vascular roles during SeA angiogenesis we analyzed the effects of mflt1 over-expression. This treatment does not abrogate SeA sprouting but instead induces ectopic SeA sprouting at low frequency, consistent with the weak mflt1 pro-angiogenic activity reported (Rahimi, 2006) (Figure S4H). Hence, within the trunk vasculature sflt1 and mflt1 play distinct roles, with sflt1 acting as an inhibitor of SeA angiogenesis.
sflt1 inhibits SeA angiogenesis cell autonomously
Based on the model proposed, sflt1, like plxnD1, should act cell autonomously within the trunk’s endothelium to suppress SeA angiogenesis. Given the lack of flt1 mutants we tested this prediction by combining sflt1 over-expression with cell transplantation experiments using donors and hosts carrying different endothelial reporters to distinguish ECs according to their genotype. We made chimeras to determine if over-expressed sflt1 inhibits SeA sprouting non cell-autonomously. We transplanted obd cells into WT hosts with GAL4/UAS system-dependent mosaic endothelial co-expression of sflt1 and fluorescent DsRed protein. We found that WT aortic ECs over-expressing sflt1 (DsRed+) fail to form SeA sprouts. However, neighboring obd donor and WT host aortic ECs without sflt1 over-expresion (DsRed−) form SeA sprouts (Figure 4I-I′). In another experiment we transplanted cells from obd donors with endothelial sflt1 over-expression (DsRed+) into WT hosts. While the obd aortic ECs with sflt1 over-expression (DsRed+) failed to form SeA sprouts, neighboring WT and donor obd ECs not over-expressing sflt1 (DsRed−) formed SeA sprouts (Figure S4I). Thus, sflt1 acts cell autonomously despite the potential diffusible nature of its encoded product.
The exacerbated SeA angiogenesis of obd requires VEGF signaling
sflt1 encodes a VEGF signaling antagonist whose levels are greatly reduced in obd (Figure 3). To test if VEGF signaling is required for obd’s SeA angiogenesis we chemically inhibited VEGF receptor activation with SU5416 (Herbert et al., 2009). SU5416, but not its vehicle (DMSO), abrogates SeA sprouting in WT and obd (Figure 5A–B, E–F; see also Figure S5B). Similarly, MO-induced vegfa activity reduction also abrogates obd’s SeA angiogenesis (Childs et al., 2002). These findings indicate obd’s excessive SeA angiogenesis is VEGF-dependent.
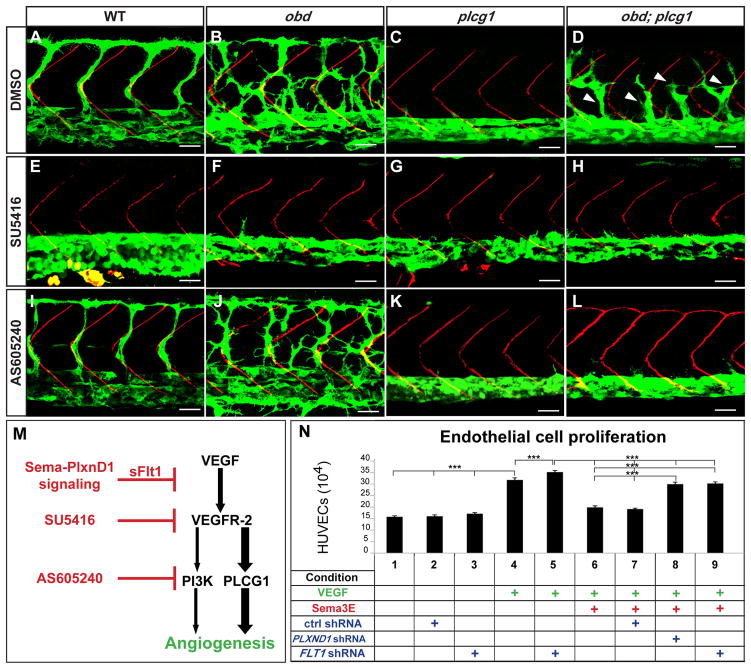
Figure 5. Enhanced VEGF signaling causes obd’s exacerbated SeA angiogenesis.
A–L, 32 hpf trunk vasculatures. WT, obd, plcg1 and obd; plcg1 treated with DMSO, SU5416 (VEGFR inhibitor) or AS605240 (PI3K inhibitor). Genotypes, top; treatments, left. Endothelium, green. SBs, red. White arrowheads, recovered SeA sprouts in obd; plcg1. Anterior, left; dorsal, up. Scale bars, 30 μm. n = 18 embryos per genotype and treatment. Pictures show representative phenotypes (18/18 embryos per category). (M) Diagram of the VEGF cascade and steps inhibited by sflt1 and drugs used in (E–L). (N) HUVEC proliferation in response to combinations of VEGF, Sema3E and shRNAs (control, PLXND1 and FLT1). ***p < 0.001. Error bars, s.e.m. See Figure S5.
VEGF signaling is enhanced in obd
The VEGF cascade splits downstream of the VEGF receptors into PLCG1 (phospholipase C gamma1; plcg1) and PI3Kp110a (phosphoinositide 3-kinase p110a isoform)-dependent pro-angiogenic branches (Figure 5M) (Covassin et al., 2009; Graupera et al., 2008). Our model predicts enhanced VEGF signaling in obd. Hence, angiogenic deficits due to impaired VEGF signaling, such as those of plcg1 mutants, should be ameliorated in an obd background. plcg1 lacks SeA sprouts (Figure 5C) (Covassin et al., 2009). However, obd; plcg1 double mutants show too many and ectopic SeA sprouts (Figure 5D and S5A) that express flt4 and a trunk arterial tree with reduced sflt1 abundance (data not shown). obd; plcg1’s SeA sprouting recovery requires VEGF signaling, since SU5416 suppresses it (Figure 5H). These observations support the notion that Sema-PlxnD1 cascade inactivation enhances VEGF signaling, suggesting that obd; plcg1’s angiogenic recovery is VEGF/PI3Kp110-dependent.
We tested this possibility via chemical inhibition of PI3Kp110 activity with AS605240 (Herbert et al., 2009). PLCG function removal has a greater impact on angiogenesis than PI3Kp110a inactivation (Covassin et al., 2009; Graupera et al., 2008). Accordingly, AS605240 neither abrogates SeA angiogenesis in WT or obd nor ameliorates plcg1’s angiogenic deficit (Figure 5I–K). However, AS605240 blocks SeA sprouting in obd; plcg1 (Figure 5L), indicating that pro-angiogenic VEGF/PI3Kp110 activity is limiting under plcg1-deficient conditions. Hence, compared with obd (Figure 5B), obd; plcg1 show fewer and stunted SeA sprouts that fail to form DLAVs (Figures 5D, 1D and 6L).
Figure 6. Notch signaling loss does not phenocopy obd.
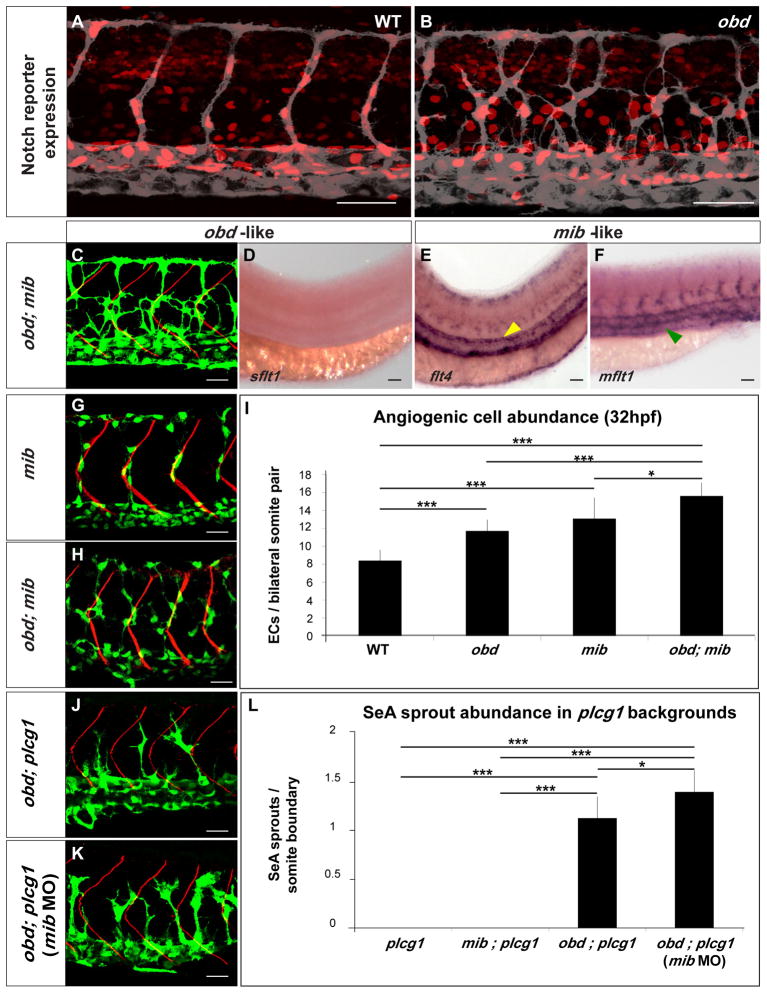
(A–B) Expression of Notch’s activity nuclear reporter Tg(Tp1bglob:hmgb1-mCherry)jh11 (red) in the endothelium (grey) of WT (A) and obd (B). (C–F) obd; mib. (C) Endothelium, green. SBs, red. (D–F) WISH with sflt1, flt4 and mflt1 riboprobes, as indicated. Double mutant phenotypes classed as obd-like (C–D) or mib-like (E–F) based on the mutant they resemble most. Note lack of sflt (as in Fig. 3G) and ectopic aortic flt4 (yellow arrowhead; as in Fig. S6A) and venous mflt1 stainings (green arrowhead, as in Fig. S6B). (G–I) Angiogenic cell abundance within the trunk’s arterial tree of WT, obd, mib (G) and obd; mib (H) in Tg(fli1:nEGFP)y7 embryos. (G–H) EC nuclei, green. SBs, red. (I) Quantification; n= 10 per genotype. (J–L) SeA sprout abundance in plcg, mib; plcg, obd; plcg (J) and obd; plcg embryos injected with 10 ng of mib MO (mib MO) (K). (J–K) Endothelium, green. SBs, red. (L) n = 8, 7, 11 and 9 for plcg, mib; plcg, obd; plcg and obd; plcg (mib MO), respectively. Scale bars: 50 μm (A–B, D–F), 30 μm (C, G–H, J–K). (I, L) *p <0.05, ***p < 0.001. Error bars: s.e.m. (A–F, G–H, J–K) Anterior, left; dorsal, up. Trunk images and quantifications: 32 hpf (A–C, G–L), 28 hpf (D–F). See Figure S6.
We further confirmed the link between Sema-PlxnD1 and VEGF signaling by observing that hypomorphic mutants of kdrl, which encodes the duplicate canonical VEGF pathway component VEGF receptor 2/VEGFR-2/KDR, show SeA angiogenic deficits (Covassin et al., 2009; Habeck et al., 2002) that are ameliorated in an obd background (Figure S5C).
To selectively determine Sema-PlxnD1 signaling’s effect on VEGF-induced cellular responses we used a HUVEC proliferation assay (Figure 5N). We found that VEGF-induced HUVEC proliferation is reduced by Sema3E exposure and that the latter effect is abrogated via PLXND1 (Bellon et al., 2010; Fukushima et al., 2011; Sakurai et al., 2010; Uesugi et al., 2009) or FLT1 knockdown (Figure 5N and S5D). Accordingly, VEGF/Sema3E-treated HUVECs make less FLT1 protein upon PLXND1 knockdown (Figure 3J). Of note, PLXND1 knockdown in HUVECs does not affect FLT1 transcription (Figure S5D), paralleling our in vivo data indicating that Sema-PlxnD1 signaling modulates sflt1 abundance post-transcriptionally (Figures 3C–I and S3C).
WISHs suggest that sflt1’s level in the trunk’s arterial tree is independent of VEGF signaling: SU5416 treatment does not reduce sflt1 abundance in WT nor increases it in obd (Figure S5B). Hence, obd’s decreased sflt1 abundance is not secondary to enhanced VEGF signaling but rather at least one of its causes.
Sema-PlxnD1 and Notch signaling play distinct and additive roles in SeA angiogenesis
Notch signaling also negatively regulates SeA sprouting (Leslie et al., 2007; Siekmann and Lawson, 2007). We thus compared the arterial tree phenotypes induced by loss of Sema-PlxnD1 and Notch signaling. We found that unlike obd, SeA sprout abundance and distribution are normal in mind bomb (mib) mutants, in which a ubiquitin ligase required for Notch signaling is inactive (Figure S6A) (Itoh et al., 2003; Lawson et al., 2002; Lawson and Weinstein, 2002). Likewise, Notch pathway inactivation via mutations in either mib or delta-like ligand 4 (dll4), which encodes a Notch ligand expressed in the trunk’s arterial tree (Leslie et al., 2007), fails to ameliorate the angiogenic deficit of plcg1 (Figure S6C).
Studies in other systems and/or vascular beds suggest Notch signaling promotes flt1 expression (Bussmann et al., 2011; del Toro et al., 2010; Funahashi et al., 2010; Harrington et al., 2008; Jakobsson et al., 2010; Suchting et al., 2007), prompting us to ask if Notch signaling is reduced in obd or modulates the trunk’s arterial tree expression of flt1 and its isoforms.
WISH expression analysis of Notch pathway components (deltac, dll4 notch5 and gridlock) and targets (gridlock, ephrin-B2a, flt4 and ephB4a) fails to uncover evidence for reduced Notch signaling in obd (data not shown) and, endothelial expression of the transgenic Notch signaling reporters Tg(Tp1bglob:hmgb1-mCherry)jh11 and Tg(Tp1bglob:eGFP)um14 (Nicoli et al., 2010; Parsons et al., 2009) is similar in WT and obd (Figure 6A–B and data not shown), consistent with the notion that in obd Notch activity is preserved.
Visual comparison of the flt1 transcriptional reporter expression (Hogan et al., 2009a; Hogan et al., 2009b) in WT, obd mutants and mib morphants (Figure S3C) reveals no significant differences. Tg(flt1:YFP)hu4624 expression is also unaffected in dll4 morphants (Geudens et al., 2010). Moreover, WISH of mib mutants reveals no visible reduction in sflt1 or mflt1 abundance but rather a mild enhancement in sflt1 and mflt1 venous expression (Figure S6B). Consistent with the role of Notch signaling in artery/vein differentiation and angiogenesis, mib displays ectopic aortic flt4 expression (Figure S6A) (Lawson et al., 2001; Siekmann and Lawson, 2007).
To elucidate the relationship between Sema-PlxnD1 and Notch signaling we analyzed the anatomical, cellular and molecular vascular phenotypes of obd; mib and the combined impact of inactivating both pathways on plcg1’s SeA angiogenesis deficit. We found that within the arterial tree obd; mib show obd-like SeA anatomical organization and sflt1 abundance (Figure 6C–D) but mib-like flt4 and mflt1 expression patterns (Figure 6E–F). This mix of obd- and mib-like phenotypes reveals that Sema-PlxnD1 and Notch signaling play distinct vascular roles.
Yet we also find additive genetic interactions between both pathways: obd; mib have greater angiogenic cell abundance than obd or mib (Figure 6G–I) (Leslie et al., 2007; Siekmann and Lawson, 2007). Likewise, silencing mib (Itoh et al., 2003) in obd; plcg further increases their SeA sprout abundance (Figure 6J–L). Hence, in this sensitized background Notch signaling seems to play a minor role as a negative regulator of SeA sprout abundance, consistent with the loss of SeA sprouting induced by over-expression of constitutive-active Notch forms, the complex interplay between VEGF and Notch signaling and the lateral inhibition role of the latter (Jakobsson et al., 2010; Roca and Adams, 2007; Siekmann and Lawson, 2007). While these additive interactions suggest that Sema-PlxnD1 and Notch signaling modulate common aspects of angiogenic development, these pathways clearly make qualitatively and quantitatively different contributions via molecularly distinct mechanisms. For example, while both pathways antagonize VEGF signaling, they modulate different pathway components, namely sflt1 and flt4. Together, these observations indicate that Notch signaling remains active in obd and that Sema-PlxnD1 signaling functions without Notch activity (Figure 7A), underscoring the distinct roles of Sema-PlxnD1 and Notch signaling in SeA angiogenesis.
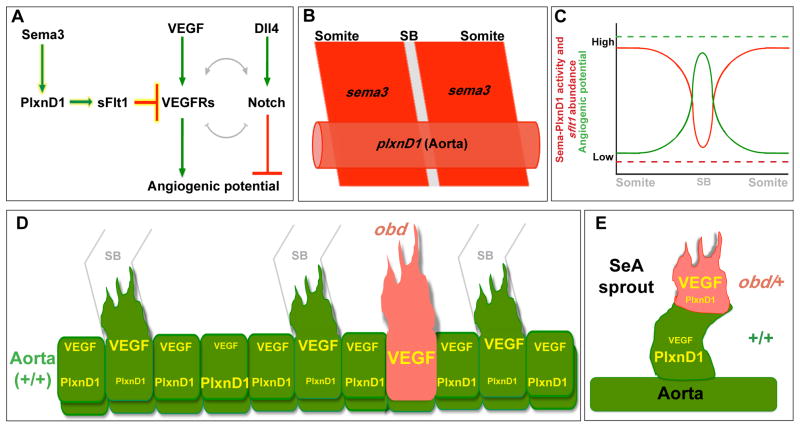
Figure 7. Model for how Sema3-PlxnD1 signaling restricts angiogenic potential along the aorta and limits angiogenic responses within SeA sprouts.
(A) Sema3-PlxnD1 signaling inhibits VEGF’s pro-angiogenic effects via sFlt1, limiting angiogenic potential. The complex cross-regulation (grey lines) between the VEGF and Notch cascades implies Sema-PlxnD1 signaling impacts Notch activity indirectly. (B) Somitic sema3s (dark red) and endothelial plxnD1 (light red) expression precedes SeA sprouting (SB, grey) (Roos et al., 1999; Torres-Vazquez et al., 2004; Yee et al., 1999). (C) WT aortic Sema-PlxnD1 signaling levels (red solid line) are highest in ECs next to the somites and lowest in ECs next to SBs, where angiogenic potential (green solid line) is highest. obd lacks Sema-PlxnD1 activity and thus sflt1 abundance is greatly reduced (red dotted line), leading to uniformly enhanced angiogenic potential levels (green dotted line) that yield too many and ectopic SeA sprouts. (D–E) VEGF signaling and angiogenic responses are cell autonomously enhanced by loss (obd) or decreased (obd/+) endothelial plxnD1 activity, as exemplified by obd to WT (D) and obd/+ to WT (E) chimeras. VEGF signaling and PlxnD1 activity levels are indicated by font size.
DISCUSSION
Our findings reveal that Sema-PlxnD1 signaling acts as a pre-sprouting repressor of angiogenic potential in the trunk’s arterial tree. We posit that Sema-PlxnD1 signaling fulfills this role, at least in part, by maintaining sflt1’s proper endothelial abundance to antagonize pro-angiogenic VEGF signaling (Figure 7A). We propose that the somitic sema3a and endothelial plxnD1 expression preceding SeA sprouting (Torres-Vazquez et al., 2004) (Figure 7B) reproducibly yield differences in Sema-PlxnD1 signaling level, and thus in sflt1 abundance, along the aorta (Figure 7C). Although the proposed variation in WT sflt1 aortic levels appears beyond the resolution of WISH, we find that ECs from obd/+ donors (which have less sflt1) are more likely to become angiogenic in WT hosts. Indeed, ECs with the lowest Flt1 abundance make the angiogenic sprouts of WT and Flt1lacZ/+ mouse retinas and ES cell-derived vessels (Chappell et al., 2009).
Our WISH and qPCR data indicates that loss or reduction of Sema-PlxnD1 signaling leads to low sflt1 abundance within both the aorta and SeA sprouts. Accordingly, our cell transplants show that Sema-PlxnD1 signaling acts cell autonomously to spatially restrict the aorta’s angiogenic capacity (Figure 7D) and limit the angiogenic responses of ECs within SeA sprouts (Figure 7E).
While sFlt1 can act non-cell autonomously (Ambati et al., 2006); (Chappell et al., 2009; Kearney et al., 2004), its effective range is context-dependent (Goldman et al., 1998; James et al., 2009; Kendall and Thomas, 1993). In the trunk’s arterial tree the anti-angiogenic effects of endothelial-specific sflt1 over-expression appear cell autonomous. sFlt1 forms VEGF-bridged inhibitory complexes with the pro-angiogenic receptors Flk1/Kdr (Bussmann et al., 2008; Kendall et al., 1996) and mFlt1 (Kendall and Thomas, 1993) and binds to the endothelial extracellular matrix, which abundantly surrounds the aorta (Jin et al., 2005; Orecchia et al., 2003), both observations suggest how sFlt1’s effective range might be limited within the aorta. Alternatively, sFlt1 might act in an intracrine manner, as proposed for mFlt1 (Lee et al., 2007b).
Our model implies that PlxnD1 signaling in response to paracrine Sema3 cues is key for the proper spatial modulation of angiogenic capacity within the aorta (Gay et al., 2011). Yet our findings do not rule out the potential involvement of autocrine Sema3 cues in PlxnD1 signaling prior to and/or during SeA sprouting (Banu et al., 2006; Kutschera et al., 2011; Lamont et al., 2009; Serini et al., 2003; Toyofuku et al., 2007). Similarly, endothelial Sema-PlxnD1 signaling could impact the pro-angiogenic activity of both paracrine and autocrine VEGFs (Childs et al., 2002; Covassin et al., 2006; da Silva et al., 2010; Hogan et al., 2009b; Lee et al., 2007a; Siekmann and Lawson, 2007; Tammela et al., 2008).
Our study reveals a key mechanistic link between Sema-PlxnD1 and VEGF signaling (Bellon et al., 2010; Fukushima et al., 2011; Sakurai et al., 2010; Uesugi et al., 2009). Consistent with defects in exon selection during flt1’s alternative splicing and/or alterations in the mRNA stability of flt1 isoforms, impaired Sema-PlxnD1 signaling leads to contrasting post-transcriptional changes in sflt1 and mflt1 abundance. Sema-PlxnD1 signaling inactivates Ras-related proteins, antagonizes integrin and PI3K signaling and modulates cytoskeletal dynamics (Gay et al., 2011). How these PlxnD1-mediated events are connected to flt1’s post-transcriptional regulation and angiogenesis will be addressed by future studies.
Here we show that Sema-PlxnD1 and Notch signaling can function independently of each other and play largely distinct cellular and molecular roles. However, Sema-PlxnD1 activity antagonizes VEGF responsiveness and Notch and VEGF signaling are linked by complex feedback loops (Jakobsson et al., 2009; Lobov et al., 2007; Williams et al., 2006). Hence, we anticipate functional interactions between both pathways via the VEGF cascade. For example, it is likely that the enhanced VEGF signaling of ECs with less Sema-PlxnD1 activity allows them to exert a stronger Dll4/Notch-mediated lateral inhibition upon their neighbors, enabling the former to more often become angiogenic and/or, acquire and/or keep a tip cell positional status (Jakobsson et al., 2010; Leslie et al., 2007; Siekmann and Lawson, 2007). Remarkably, the combined loss of both Sema-PlxnD1 (plxnD1) and Notch signaling (mib) signaling does not enable every aortic EC to sprout, suggesting that other pathways and/or mechanisms limit the trunk’s arterial tree angiogenic capacity.
Together with prior studies (Gay et al., 2011), our findings indicate that Sema-PlxnD1 signaling regulates distinct yet interconnected aspects of angiogenic development: the spatial allocation of angiogenic capabilities and the guidance of growing sprouts. It is likely that these roles, and their bases, are evolutionarily conserved (see (Gay et al., 2011)). Changes in sflt1 abundance induce congenital vascular malformations (Acevedo and Cheresh, 2008), gestational hypertension (Rahimi, 2006) and are associated with cancer (Aref et al., 2005). Hence, mutations and polymorphisms that affect Sema-PlxnD1 signaling are likely modifiers of these diseases. Conversely, alterations in sflt1 abundance and/or activity might impact Sema-PlxnD1 signaling dependent processes like cardiovascular and nervous system development and both tumor angiogenesis and metastasis (Gay et al., 2011; Raab and Plate, 2007; Takahashi and Shibuya, 2005). Overall, the regulation of sflt1 abundance via Sema-PlxnD1 signaling has broad biomedical implications beyond angiogenesis and provides a new way of understanding how Sema and VEGF signals might be integrated in many contexts.
EXPERIMENTAL PROCEDURES
Zebrafish
Embryos and adults kept and handled using standard laboratory conditions under New York University IACUC guidelines. Zebrafish stocks and genotyping methods/reagents described in the Supplemental Information.
Imaging
Live and fluorescently immunostained embryos imaged via confocal microscopy, whole mount RNA in situ hybridized embryos and drug treated animals imaged via transmitted light microscopy. All embryos mounted sideways. Details in the Supplemental Information.
SeA sprout abundance and position quantification
Quantifications done using confocal images of immunofluorescently stained 23 hpf Tg(fli:EGFP)y1 embryos. SeA sprouts: individual EGFP-positive aortic dorsal projections that reach or surpass the Horizontal Myoseptum (HM; see Figure 1). SeA sprout positions: Correct (SeA base abbuts directly the anterior side of neighboring somite boundary), ectopic (all other base locations). SeA sprouts were counted in four adjacent anterior trunk segments and averaged to yield a SeA sprouts/somite boundary ratio. Student’s t-test (homocedastic, two-tail distribution) was used to analyze the differences between the means of cell number data sets.
Endothelial cell abundance quantification
21, 23 and 32 hpf Tg(fli1:nEGFP)y7; Tg(flk1:ras-mCherry)s896 and Tg(flk1:EGFP-NLS); Tg(flk1:ras-mCherry)s896 immunofluorescently stained embryos were used to visualize EC nuclei and vascular anatomy. Confocal sections across the width of the anterior trunk were collected and 3D-projected with Imaris 6.2.1 software (Bitplane AG). EGFP-positive nuclei were marked (measurement point application) and counted. Since WT SeAs launch next to somite boundaries (SBs) but obd SeAs arise from these and other sites we divided the trunk vasculature into segments delimited by the posterior and anterior halves of consecutive bilateral somite pairs and counted EC nuclei within each segment. Based on their location, EC nuclei were assigned to the axial vessels (AxV; aorta and vein), the SeAs and/or DLAVs. AxV (rather than aortic- and venous-specific) EC abundance was scored since the aorta and vein are not fully distinct at 21 and 23 hpf (Herbert et al., 2009). We counted ECs in three consecutive trunk segments (located dorsal to the yolk extension) and averaged them to obtain ECs/bilateral somite pair ratios for each location. Student’s t-test (homocedastic, two-tail distribution) was used to analyze the differences between the means of EC number data sets. Note: Not every EC whose nucleus is labeled by Tg(fli1:nEGFP)y7 (green) is marked by Tg(flk1:ras-mCherry)s896 (red) due to the latter’s expression mosaicism (Figure S1A).
Cell transplants
Cell transplants done with 3–4 hpf donor and host blastula-stage embryos as in (Carmany-Rampey and Moens, 2006). 30–50 cells were aspirated from the donor’s animal pole and placed into the host’s lateral margin zone. Donors and hosts carried distinct endothelial-specific reporters to easily identify the source of ECs within chimeras. plxnD1’s cell autonomy: We used both WT and obd as Tg(fli:EGFP)y1 donors and as Tg(flk1:ras-mcherry)s896 hosts. 1 nl of a 5% solution of lineage tracer (dextran Alexa Fluor 647; Invitrogen) was injected into 1-cell stage donors. Chimeras fixed at 32 hpf. Quantification of mosaic SeA sprouts with tip cells of donor origin: We used both WT and obd/+ as Tg(fli:EGFP)y1 donors and as Tg(flk1:ras-mcherry)s896 hosts. Chimeras fixed at 28 hpf. Quantification of the distribution of ECs of donor origin within the trunk vasculature of chimeras: We used both WT and obd/+ as Tg(flk1:EGFP-NLS) donors. Tg(flk1:ras-mcherry)s896 used as hosts. Chimeras fixed at 21–23 hpf. Embryos with ECs of donor origin within the trunk’s vascular tree were selected. Confocal images of their whole trunk vasculature were taken and analyzed as described in Figure S2B. sflt1’s cell autonomy: We used Tg(fli:EGFP)y1 donors and Tg(flk1:EGFP-NLS) hosts. Endothelial-specific, sflt1 mosaic over-expression in donors or hosts done using the Tg(fliep:gal4ff)ubs4 GAL4 driver line and the bidirectional UAS vector pTol[DsRed::UAS::sFlt1].
Whole mount RNA in situ hybridization (WISH)
WISH performed as in (Moens, 2008). The list of analyzed genes and riboprobe synthesis protocols are in the Supplemental Experimental Procedures.
Morpholino oligo (MO) injection
MOs (Gene Tools, LLC) were injected into 1-cell stage Tg(fli1:EGFP)y1 embryos as in (Morcos, 2007). MO sequences and validation methods are in the Supplemental Experimental Procedures.
Drug treatments
Embryos were dechorionated before treatment. Treatments began at 16 (Figure 5A–L) or 20 hpf (Figure S5B; to prevent the dramatic aortic size reduction induced by earlier treatments). Control embryos were treated with 0.025% dimethyl sulfoxide (DMSO; Sigma) in water. Inhibitor-treated embryos were incubated in 0.25 μM AS605240 or 0.5μM SU5416 (Sigma) aqueous solutions of 0.025% DMSO.
Quantitative real time polymerase chain reaction (qPCR)
Total mRNA (zebrafish embryos) and RNA (HUVECs) extraction and cDNA synthesis done as per Supplementary Experimental Procedures. qPCR DNA products amplified with Power SYBR Green 2X Master Mix (Applied Biosystems) as per manufacturer’s instructions. Whole embryo qPCR products were quantified with a 7900HT Real-Time PCR System (Applied Biosystems). Relative sflt1, mflt1 and YFP mRNA levels normalized to bactin2 transcript abundance. For shRNA control experiments, products were quantified with a PRISM 7900 (Applied Biosystems). Relative PLXND1 and FLT1 levels normalized to glyceraldehyde-3-phosphate (GAPDH) abundance. Primer sequences are in the Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Sema-PlxnD1 signaling promotes expression of the secreted VEGF decoy sflt1
Sema-PlxnD1 signaling allocates angiogenic capacity in the trunk’s arterial tree
Sema-PlxnD1 signaling limits angiogenic responses within sprouts
Sema-PlxnD1 and Notch signaling play distinct roles in SeA angiogenesis
Acknowledgments
We thank N.C. Chi, C-B. Chien, S. Childs, A. Chitnis, S.L. Johnson, K. Kawakami, N.D. Lawson, M. Parsons, S. Schulte-Merker, D. Stainier, B.M. Weinstein, and the Zebrafish International Resource Center for reagents; G. Fishell, E.J.A. Hubbard, H. Knaut, J.F. Nance, D.B. Rifkin, K.L. Targoff, J.E. Treisman, S.R. Schwab, F. Ulrich, K.A. Yaniv and D. Yelon for discussions; J. Zavadil (NYU Cancer Institute Genomics Facility), D. Dalfo and J-Y. Roignant for qPCR advice; D. Chan for administrative help. Support: A.K. (Werner Siemens-Foundation; Switzerland), C.M.G. (NICHD Training Program Grant 5T32HD007520-05), J.T-V (The David Himelberg Foundation and NHLBI). We apologize to authors not cited due to limited space.
Footnotes
The authors declare no competing financial interests.
Author Contributions
T.Z., C.M.G., J.T-V. (ideas, experiments, data analysis, fish lines, plasmids, writing); J.B., C.M. (experiments, data analysis); K.M.F. (experiments, fish lines, husbandry support, writing); M.S. (ideas, cell culture experiments, data analysis, writing); L.H., A.K., H-G. B., M.A. (Tg(fliep:gal4ff)ubs4 line); J.A.E. (ideas, data analysis). All authors commented on the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acevedo LM, Cheresh DA. Suppressing NFAT increases VEGF signaling in hemangiomas. Cancer Cell. 2008;14:429–430. doi: 10.1016/j.ccr.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Ahn DG, Ruvinsky I, Oates AC, Silver LM, Ho RK. tbx20, a new vertebrate T-box gene expressed in the cranial motor neurons and developing cardiovascular structures in zebrafish. Mech Dev. 2000;95:253–258. doi: 10.1016/s0925-4773(00)00346-4. [DOI] [PubMed] [Google Scholar]
- Ambati BK, Nozaki M, Singh N, Takeda A, Jani PD, Suthar T, Albuquerque RJ, Richter E, Sakurai E, Newcomb MT, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature. 2006;443:993–997. doi: 10.1038/nature05249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aref S, El Sherbiny M, Goda T, Fouda M, Al Askalany H, Abdalla D. Soluble VEGF/sFLt1 ratio is an independent predictor of AML patient out come. Hematology. 2005;10:131–134. doi: 10.1080/10245330500065797. [DOI] [PubMed] [Google Scholar]
- Banu N, Teichman J, Dunlap-Brown M, Villegas G, Tufro A. Semaphorin 3C regulates endothelial cell function by increasing integrin activity. FASEB J. 2006;20:2150–2152. doi: 10.1096/fj.05-5698fje. [DOI] [PubMed] [Google Scholar]
- Bellon A, Luchino J, Haigh K, Rougon G, Haigh J, Chauvet S, Mann F. VEGFR2 (KDR/Flk1) signaling mediates axon growth in response to semaphorin 3E in the developing brain. Neuron. 2010;66:205–219. doi: 10.1016/j.neuron.2010.04.006. [DOI] [PubMed] [Google Scholar]
- Bussmann J, Bakkers J, Schulte-Merker S. Early endocardial morphogenesis requires Scl/Tal1. PLoS Genet. 2007;3:e140. doi: 10.1371/journal.pgen.0030140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann J, Lawson N, Zon L, Schulte-Merker S. Zebrafish VEGF receptors: a guideline to nomenclature. PLoS Genet. 2008;4:e1000064. doi: 10.1371/journal.pgen.1000064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussmann J, Wolfe SA, Siekmann AF. Arterial-venous network formation during brain vascularization involves hemodynamic regulation of chemokine signaling. Development. 2011;138:1717–1726. doi: 10.1242/dev.059881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmany-Rampey A, Moens CB. Modern mosaic analysis in the zebrafish. Methods. 2006;39:228–238. doi: 10.1016/j.ymeth.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Chappell JC, Taylor SM, Ferrara N, Bautch VL. Local guidance of emerging vessel sprouts requires soluble Flt-1. Dev Cell. 2009;17:377–386. doi: 10.1016/j.devcel.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Childs S, Chen JN, Garrity DM, Fishman MC. Patterning of angiogenesis in the zebrafish embryo. Development. 2002;129:973–982. doi: 10.1242/dev.129.4.973. [DOI] [PubMed] [Google Scholar]
- Covassin LD, Siekmann AF, Kacergis MC, Laver E, Moore JC, Villefranc JA, Weinstein BM, Lawson ND. A genetic screen for vascular mutants in zebrafish reveals dynamic roles for Vegf/Plcg1 signaling during artery development. Dev Biol. 2009;329:212–226. doi: 10.1016/j.ydbio.2009.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covassin LD, Villefranc JA, Kacergis MC, Weinstein BM, Lawson ND. Distinct genetic interactions between multiple Vegf receptors are required for development of different blood vessel types in zebrafish. Proc Natl Acad Sci U S A. 2006;103:6554–6559. doi: 10.1073/pnas.0506886103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva RG, Tavora B, Robinson SD, Reynolds LE, Szekeres C, Lamar J, Batista S, Kostourou V, Germain MA, Reynolds AR, et al. Endothelial alpha3beta1-integrin represses pathological angiogenesis and sustains endothelial-VEGF. Am J Pathol. 2010;177:1534–1548. doi: 10.2353/ajpath.2010.100043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Bock K, De Smet F, Leite De Oliveira R, Anthonis K, Carmeliet P. Endothelial oxygen sensors regulate tumor vessel abnormalization by instructing phalanx endothelial cells. J Mol Med. 2009;87:561–569. doi: 10.1007/s00109-009-0482-z. [DOI] [PubMed] [Google Scholar]
- del Toro R, Prahst C, Mathivet T, Siegfried G, Kaminker JS, Larrivee B, Breant C, Duarte A, Takakura N, Fukamizu A, et al. Identification and functional analysis of endothelial tip cell-enriched genes. Blood. 2010;116:4025–4033. doi: 10.1182/blood-2010-02-270819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Okada M, Kataoka H, Hirashima M, Yoshida Y, Mann F, Gomi F, Nishida K, Nishikawa S, Uemura A. Sema3E-PlexinD1 signaling selectively suppresses disoriented angiogenesis in ischemic retinopathy in mice. J Clin Invest. 2011;121:1974–1985. doi: 10.1172/JCI44900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funahashi Y, Shawber CJ, Vorontchikhina M, Sharma A, Outtz HH, Kitajewski J. Notch regulates the angiogenic response via induction of VEGFR-1. J Angiogenes Res. 2010;2:3. doi: 10.1186/2040-2384-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gay CM, Zygmunt T, Torres-Vazquez J. Diverse functions for the semaphorin receptor PlexinD1 in development and disease. Dev Biol. 2011;349:1–19. doi: 10.1016/j.ydbio.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geudens I, Herpers R, Hermans K, Segura I, Ruiz de Almodovar C, Bussmann J, De Smet F, Vandevelde W, Hogan BM, Siekmann A, et al. Role of delta-like-4/Notch in the formation and wiring of the lymphatic network in zebrafish. Arterioscler Thromb Vasc Biol. 2010;30:1695–1702. doi: 10.1161/ATVBAHA.110.203034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman CK, Kendall RL, Cabrera G, Soroceanu L, Heike Y, Gillespie GY, Siegal GP, Mao X, Bett AJ, Huckle WR, et al. Paracrine expression of a native soluble vascular endothelial growth factor receptor inhibits tumor growth, metastasis, and mortality rate. Proc Natl Acad Sci U S A. 1998;95:8795–8800. doi: 10.1073/pnas.95.15.8795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graupera M, Guillermet-Guibert J, Foukas LC, Phng LK, Cain RJ, Salpekar A, Pearce W, Meek S, Millan J, Cutillas PR, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453:662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- Habeck H, Odenthal J, Walderich B, Maischein H, Schulte-Merker S. Analysis of a zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr Biol. 2002;12:1405–1412. doi: 10.1016/s0960-9822(02)01044-8. [DOI] [PubMed] [Google Scholar]
- Harrington LS, Sainson RC, Williams CK, Taylor JM, Shi W, Li JL, Harris AL. Regulation of multiple angiogenic pathways by Dll4 and Notch in human umbilical vein endothelial cells. Microvasc Res. 2008;75:144–154. doi: 10.1016/j.mvr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Herbert SP, Huisken J, Kim TN, Feldman ME, Houseman BT, Wang RA, Shokat KM, Stainier DY. Arterial-venous segregation by selective cell sprouting: an alternative mode of blood vessel formation. Science. 2009;326:294–298. doi: 10.1126/science.1178577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BM, Bos FL, Bussmann J, Witte M, Chi NC, Duckers HJ, Schulte-Merker S. Ccbe1 is required for embryonic lymphangiogenesis and venous sprouting. Nat Genet. 2009a;41:396–398. doi: 10.1038/ng.321. [DOI] [PubMed] [Google Scholar]
- Hogan BM, Herpers R, Witte M, Helotera H, Alitalo K, Duckers HJ, Schulte-Merker S. Vegfc/Flt4 signalling is suppressed by Dll4 in developing zebrafish intersegmental arteries. Development. 2009b;136:4001–4009. doi: 10.1242/dev.039990. [DOI] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Isogai S, Lawson ND, Torrealday S, Horiguchi M, Weinstein BM. Angiogenic network formation in the developing vertebrate trunk. Development. 2003;130:5281–5290. doi: 10.1242/dev.00733. [DOI] [PubMed] [Google Scholar]
- Itoh M, Kim CH, Palardy G, Oda T, Jiang YJ, Maust D, Yeo SY, Lorick K, Wright GJ, Ariza-McNaughton L, et al. Mind bomb is a ubiquitin ligase that is essential for efficient activation of Notch signaling by Delta. Dev Cell. 2003;4:67–82. doi: 10.1016/s1534-5807(02)00409-4. [DOI] [PubMed] [Google Scholar]
- Jakobsson L, Bentley K, Gerhardt H. VEGFRs and Notch: a dynamic collaboration in vascular patterning. Biochem Soc Trans. 2009;37:1233–1236. doi: 10.1042/BST0371233. [DOI] [PubMed] [Google Scholar]
- Jakobsson L, Franco CA, Bentley K, Collins RT, Ponsioen B, Aspalter IM, Rosewell I, Busse M, Thurston G, Medvinsky A, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nat Cell Biol. 2010;12:943–953. doi: 10.1038/ncb2103. [DOI] [PubMed] [Google Scholar]
- James JM, Gewolb C, Bautch VL. Neurovascular development uses VEGF-A signaling to regulate blood vessel ingression into the neural tube. Development. 2009;136:833–841. doi: 10.1242/dev.028845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SW, Beis D, Mitchell T, Chen JN, Stainier DY. Cellular and molecular analyses of vascular tube and lumen formation in zebrafish. Development. 2005;132:5199–5209. doi: 10.1242/dev.02087. [DOI] [PubMed] [Google Scholar]
- Kappas NC, Zeng G, Chappell JC, Kearney JB, Hazarika S, Kallianos KG, Patterson C, Annex BH, Bautch VL. The VEGF receptor Flt-1 spatially modulates Flk-1 signaling and blood vessel branching. J Cell Biol. 2008;181:847–858. doi: 10.1083/jcb.200709114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearney JB, Kappas NC, Ellerstrom C, DiPaola FW, Bautch VL. The VEGF receptor flt-1 (VEGFR-1) is a positive modulator of vascular sprout formation and branching morphogenesis. Blood. 2004;103:4527–4535. doi: 10.1182/blood-2003-07-2315. [DOI] [PubMed] [Google Scholar]
- Kendall RL, Thomas KA. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993;90:10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall RL, Wang G, Thomas KA. Identification of a natural soluble form of the vascular endothelial growth factor receptor, FLT-1, and its heterodimerization with KDR. Biochem Biophys Res Commun. 1996;226:324–328. doi: 10.1006/bbrc.1996.1355. [DOI] [PubMed] [Google Scholar]
- Krueger J, Liu D, Scholz K, Zimmer A, Shi Y, Klein C, Siekmann A, Schulte-Merker S, Cudmore M, Ahmed A, et al. Flt1 acts as a negative regulator of tip cell formation and branching morphogenesis in the zebrafish embryo. Development. 2011;138:2111–2120. doi: 10.1242/dev.063933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kutschera S, Weber H, Weick A, De Smet F, Genove G, Takemoto M, Prahst C, Riedel M, Mikelis C, Baulande S, et al. Differential endothelial transcriptomics identifies semaphorin 3G as a vascular class 3 semaphorin. Arterioscler Thromb Vasc Biol. 2011;31:151–159. doi: 10.1161/ATVBAHA.110.215871. [DOI] [PubMed] [Google Scholar]
- Lamont RE, Lamont EJ, Childs SJ. Antagonistic interactions among Plexins regulate the timing of intersegmental vessel formation. Dev Biol. 2009;331:199–209. doi: 10.1016/j.ydbio.2009.04.037. [DOI] [PubMed] [Google Scholar]
- Larson JD, Wadman SA, Chen E, Kerley L, Clark KJ, Eide M, Lippert S, Nasevicius A, Ekker SC, Hackett PB, et al. Expression of VE-cadherin in zebrafish embryos: a new tool to evaluate vascular development. Dev Dyn. 2004;231:204–213. doi: 10.1002/dvdy.20102. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. In vivo imaging of embryonic vascular development using transgenic zebrafish. Dev Biol. 2002;248:307–318. doi: 10.1006/dbio.2002.0711. [DOI] [PubMed] [Google Scholar]
- Lee S, Chen TT, Barber CL, Jordan MC, Murdock J, Desai S, Ferrara N, Nagy A, Roos KP, Iruela-Arispe ML. Autocrine VEGF signaling is required for vascular homeostasis. Cell. 2007a;130:691–703. doi: 10.1016/j.cell.2007.06.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TH, Seng S, Sekine M, Hinton C, Fu Y, Avraham HK, Avraham S. Vascular endothelial growth factor mediates intracrine survival in human breast carcinoma cells through internally expressed VEGFR1/FLT1. PLoS Med. 2007b;4:e186. doi: 10.1371/journal.pmed.0040186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. doi: 10.1242/dev.003244. [DOI] [PubMed] [Google Scholar]
- Lobov IB, Renard RA, Papadopoulos N, Gale NW, Thurston G, Yancopoulos GD, Wiegand SJ. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci U S A. 2007;104:3219–3224. doi: 10.1073/pnas.0611206104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens C. Cold Spring Harb Protoc. 2008. Whole Mount RNA In Situ Hybridization on Zebrafish Embryos. [DOI] [PubMed] [Google Scholar]
- Morcos PA. Achieving targeted and quantifiable alteration of mRNA splicing with Morpholino oligos. Biochem Biophys Res Commun. 2007;358:521–527. doi: 10.1016/j.bbrc.2007.04.172. [DOI] [PubMed] [Google Scholar]
- Nicoli S, Standley C, Walker P, Hurlstone A, Fogarty KE, Lawson ND. MicroRNA-mediated integration of haemodynamics and Vegf signalling during angiogenesis. Nature. 2010;464:1196–1200. doi: 10.1038/nature08889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orecchia A, Lacal PM, Schietroma C, Morea V, Zambruno G, Failla CM. Vascular endothelial growth factor receptor-1 is deposited in the extracellular matrix by endothelial cells and is a ligand for the alpha 5 beta 1 integrin. J Cell Sci. 2003;116:3479–3489. doi: 10.1242/jcs.00673. [DOI] [PubMed] [Google Scholar]
- Parsons MJ, Pisharath H, Yusuff S, Moore JC, Siekmann AF, Lawson N, Leach SD. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phng LK, Gerhardt H. Angiogenesis: a team effort coordinated by notch. Dev Cell. 2009;16:196–208. doi: 10.1016/j.devcel.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Phng LK, Potente M, Leslie JD, Babbage J, Nyqvist D, Lobov I, Ondr JK, Rao S, Lang RA, Thurston G, et al. Nrarp coordinates endothelial Notch and Wnt signaling to control vessel density in angiogenesis. Dev Cell. 2009;16:70–82. doi: 10.1016/j.devcel.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raab S, Plate KH. Different networks, common growth factors: shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol. 2007;113:607–626. doi: 10.1007/s00401-007-0228-3. [DOI] [PubMed] [Google Scholar]
- Rahimi N. VEGFR-1 and VEGFR-2: two non-identical twins with a unique physiognomy. Front Biosci. 2006;11:818–829. doi: 10.2741/1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca C, Adams RH. Regulation of vascular morphogenesis by Notch signaling. Genes Dev. 2007;21:2511–2524. doi: 10.1101/gad.1589207. [DOI] [PubMed] [Google Scholar]
- Roos M, Schachner M, Bernhardt RR. Zebrafish semaphorin Z1b inhibits growing motor axons in vivo. Mech Dev. 1999;87:103–117. doi: 10.1016/s0925-4773(99)00153-7. [DOI] [PubMed] [Google Scholar]
- Rottbauer W, Just S, Wessels G, Trano N, Most P, Katus HA, Fishman MC. VEGF-PLCgamma1 pathway controls cardiac contractility in the embryonic heart. Genes Dev. 2005;19:1624–1634. doi: 10.1101/gad.1319405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai A, Gavard J, Annas-Linhares Y, Basile JR, Amornphimoltham P, Palmby TR, Yagi H, Zhang F, Randazzo PA, Li X, et al. Semaphorin 3E initiates antiangiogenic signaling through plexin D1 by regulating Arf6 and R-Ras. Mol Cell Biol. 2010;30:3086–3098. doi: 10.1128/MCB.01652-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serini G, Valdembri D, Zanivan S, Morterra G, Burkhardt C, Caccavari F, Zammataro L, Primo L, Tamagnone L, Logan M, et al. Class 3 semaphorins control vascular morphogenesis by inhibiting integrin function. Nature. 2003;424:391–397. doi: 10.1038/nature01784. [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Covassin L, Lawson ND. Modulation of VEGF signalling output by the Notch pathway. Bioessays. 2008;30:303–313. doi: 10.1002/bies.20736. [DOI] [PubMed] [Google Scholar]
- Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. doi: 10.1038/nature05577. [DOI] [PubMed] [Google Scholar]
- Suchting S, Freitas C, le Noble F, Benedito R, Breant C, Duarte A, Eichmann A. The Notch ligand Delta-like 4 negatively regulates endothelial tip cell formation and vessel branching. Proc Natl Acad Sci U S A. 2007;104:3225–3230. doi: 10.1073/pnas.0611177104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- Tammela T, Zarkada G, Wallgard E, Murtomaki A, Suchting S, Wirzenius M, Waltari M, Hellstrom M, Schomber T, Peltonen R, et al. Blocking VEGFR-3 suppresses angiogenic sprouting and vascular network formation. Nature. 2008;454:656–660. doi: 10.1038/nature07083. [DOI] [PubMed] [Google Scholar]
- Torres-Vazquez J, Gitler AD, Fraser SD, Berk JD, Van NP, Fishman MC, Childs S, Epstein JA, Weinstein BM. Semaphorin-plexin signaling guides patterning of the developing vasculature. Dev Cell. 2004;7:117–123. doi: 10.1016/j.devcel.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Toyofuku T, Yabuki M, Kamei J, Kamei M, Makino N, Kumanogoh A, Hori M. Semaphorin-4A, an activator for T-cell-mediated immunity, suppresses angiogenesis via Plexin-D1. EMBO J. 2007;26:1373–1384. doi: 10.1038/sj.emboj.7601589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesugi K, Oinuma I, Katoh H, Negishi M. Different requirement for Rnd GTPases of R-Ras GAP activity of Plexin-C1 and Plexin-D1. J Biol Chem. 2009;284:6743–6751. doi: 10.1074/jbc.M805213200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson RN, Pouget C, Gering M, Russell AJ, Davies SG, Kimelman D, Patient R. Hedgehog and Bmp polarize hematopoietic stem cell emergence in the zebrafish dorsal aorta. Dev Cell. 2009;16:909–916. doi: 10.1016/j.devcel.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CK, Li JL, Murga M, Harris AL, Tosato G. Up-regulation of the Notch ligand Delta-like 4 inhibits VEGF-induced endothelial cell function. Blood. 2006;107:931–939. doi: 10.1182/blood-2005-03-1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee CS, Chandrasekhar A, Halloran MC, Shoji W, Warren JT, Kuwada JY. Molecular cloning, expression, and activity of zebrafish semaphorin Z1a. Brain Res Bull. 1999;48:581–593. doi: 10.1016/s0361-9230(99)00038-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.