Abstract
We report the discovery of two long-wavelength (365 nm) photoactivatable diaryltetrazoles through screening a small library of diaryltetrazoles that were designed using a ‘scaffold hopping’ strategy. A naphthalene-derived tetrazole showed excellent reactivity in the photoinduced cycloaddition reaction with methyl methacrylate under 365-nm photoirradiation in PBS buffer. Besides, the brightly fluorescent pyrazoline cycloadducts that were formed further increase the potential utility of these new diaryltetrazoles as “photoclick” reagents and as reporters in biological studies.
Keywords: bioorthogonal chemistry, photoclick chemistry, dipolar cycloaddition, tetrazoles, fluorophores
Light-induced chemical reactions regulate many important cellular and organismal functions in nature. For example, prokaryotes such as B. abortus employ a photoinduced flavin addition to cysteine of a histidine kinase to regulate bacterial chemotaxis, phototaxis and virulence.1 In mammals a photoinduced isomerization of 11-cis-retinyl Schiff base in rhodopsin forms the chemical basis for vision formation.2 To harness the power of light for cell biology studies, natural sensory photoreceptors such as channelrhodopsins have been successfully used as optogenetic modules in order to achieve photo-regulation of the function of neurons and other cells.3 However, because fusion of large photo-sensing proteins adds considerable mass to a target protein, an alternative approach has involved the introduction of small photoreactive chemical moieties, e.g., a photocaged4 or a photoisomerizable group,5 to a target protein sites-specifically followed by subsequent light-dependent functional manipulation.
Recently, we have developed a protein photo-modification protocol based on a bioorthogonal, photoinduced tetrazole-alkene cycloaddition reaction (“photoclick chemistry”).6 In this approach, a bioorthogonal alkene7 or tetrazole8 is introduced site-selectively into the proteins, which can be subsequently modified with the cognate reagents in live cells in a light-dependent manner. Since alkene structures are considerably smaller in size compared to tetrazoles, it is more straightforward to encode alkenes in proteins and use tetrazoles as the reagents to drive the protein photo-modification. To this end, robust tetrazole reagents need to be identified that show both high reactivity9 and long-wavelength photoactivatability.10 In addition, it is also desirable that the pyrazoline cycloadducts exhibit bright fluorescence in order to facilitate the monitoring of protein photo-modification in vivo. Here we report the synthesis of a small library of tetrazoles and subsequent identification of two new diaryltetrazoles that show 365-nm photoactivatability and cycloaddition products with good fluorescent properties.
Our previous study has identified several 365-nm photo-reactive tetrazoles containing either amino or styryl group at the para-position of the N-phenyl ring.10 The long-wavelength photoreactivity was attributed to increased absorption at the long-wavelength region in the UV-Vis spectra. However, the pyrazoline cycloadducts derived from the amino-substituted diphenyltetrazole showed rapid photo-bleaching, limiting its utility in biological studies. To overcome this problem, we borrowed a concept from medicinal chemistry dubbed ‘scaffold hopping’ where key pharmacophores are allowed to ‘jump’ from one scaffold to another in order to derive novel ligands with improved bioactivities.11 We hypothesized by ‘hopping’ the photoreactive tetrazole moiety from aniline to other known long-wavelength UV absorbing scaffolds, we may obtain new tetrazoles with increased photoreactivity and photostability.
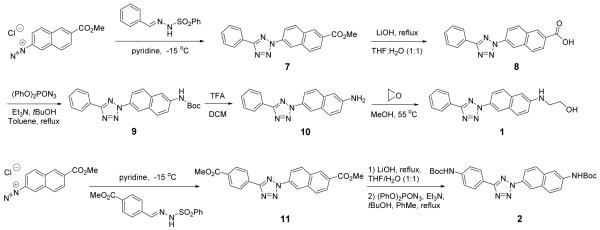
Based on the long-wavelength UV absorbance and synthetic accessibility, five chromophores were selected as alternative scaffolds for our new diaryltetrazoles 1-5 (Figure 1). Starting from 2-naphthalenediazonium salt, the 2-naphthoate tetrazoles 7 and 11 were efficiently prepared using the Kakehi procedure,12 with the yield of 26% and 62%, respectively (Scheme 1). Following LiOH-mediated hydrolysis, the tetrazole carboxylic acids were converted to the Boc-protected-6-aminonaphthyl-tetrazoles 9 and 2 via Curtius rearrangement in 67% and 46% yield, respectively. Subsequent deprotection of 9 with TFA followed by alkylation of naphthylamine with ethylene oxide produced a water-soluble tetrazole 1. Howevery, deprotection of 3 generated a chemically unstable compound so we decided to use the Boc-protected 2 directly in our reactivity studies.
Figure 1.
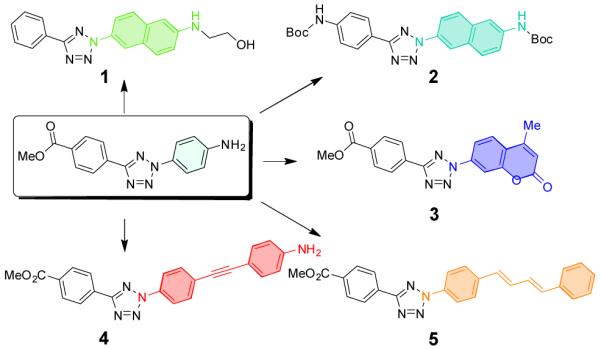
Structures of diaryltetrazoles with alternative scaffolds.
Scheme 1.
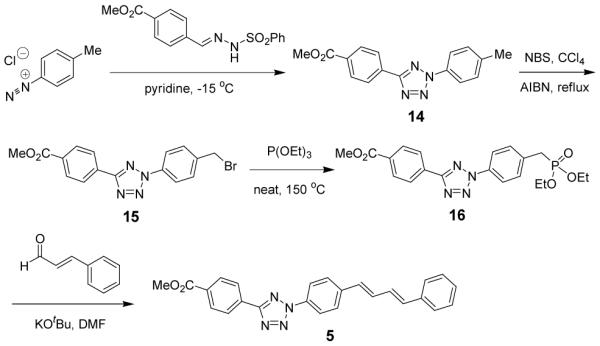
Since coumarin is a blue fluorophore and absorbs strongly in the UV region around 365 nm, we prepared a tetrazole-modified coumarin 3 from the commercially available 7-amino-4-methyl-coumarin with a yield of 81% (Scheme 2). Separately, since diarylacetylene and diaryl-1,3-butadiene have been used in the fluorophore design,13 we appended the photoreactive tetrazole to these scaffolds and generated tetrazoles 4 and 5. Tetrazole 4 was readily prepared from the Sonogashira coupling between 2-p-iodophenyltetrazole 13 and p-ethynylaniline (Scheme 2). On the other hand, the 1,4-dipenylbutadiene-conjugated tetrazole 5 was synthesized in four steps: (i) Kakehi tetrazole synthesis produced N-tolyltetrazole 14 in 86% yield; (ii) bromonation of 14 with NBS and catalytic amount of AIBN gave the intermediate 15 in 80% yield; (iii) heating of benzyl bromide 15 in triethylphosphite gave benzyl phosphonate 16 in 60% yield; and (iv) the Horner–Wadsworth–Emmons reaction between 16 and cinnamaldehyde afforded the final product tetrazole 5 in 39% yield (Scheme 3).
Scheme 2.
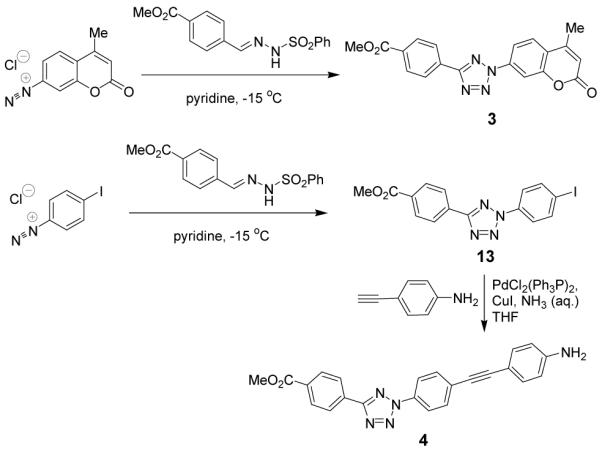
Scheme 3.
Since tetrazole photoreactivity of at any given wavelength is dependent on molar absorption coefficient at that particular wavelength,14 the UV-Vis spectra of tetrazoles 1-5 were taken (Figure S1 in supplemental materials) and the data were collected in Table 1. Compared to p-aminodiphenyltetrazole, all five tetrazoles showed significant bathochromic shift in λmax, ranging from 14 nm for tetrazole 2 to 50 nm for tetrazole 5. The strong absorption bands in 300 ~ 400 nm can be attributed to the π–π* electronic transition between HOMO and LUMO orbitals of the tetrazole-conjugated fluorophores. The extent of bathochromic shift appears to correlate with the calculated HOMO-LUMO gap as tetrazoles 4 and 5 with smaller gap showed larger λmax shifts.
Table 1.
Absorption maxima, molar absorption coefficients and molecular orbital energy calculation for diaryltetrazoles a
| tetrazole |
λmaxb (nm) |
ε302 (M−1 cm−1) |
ε365 (M−1 cm−1) |
ε395 (M−1 cm−1) |
HOMOd (eV) |
LUMOd (eV) |
Δ
LUMO-HOMOd (eV) |
|---|---|---|---|---|---|---|---|
| p-amino c | 310 | 20500 | 3500 | N.D. | −5.66 | −1.51 | 4.15 |
| 1 | 338 | 7103 | 11908 | 1935 | −5.64 | −1.40 | 4.24 |
| 2 | 324 | 16415 | 1813 | 1042 | −5.57 | −1.40 | 4.17 |
| 3 | 332 | 25838 | 907 | 682 | −6.57 | −2.22 | 4.35 |
| 4 | 346 | 28892 | 35177 | 6167 | −5.41 | −1.74 | 3.67 |
| 5 | 360 | 9035 | 30819 | 8184 | −5.60 | −1.99 | 3.61 |
UV-Vis was measured by dissolving tetrazoles in CHCl3 to derive the final concentrations of 25 μM.
λmax were derived from the wavelength region of 300 ~ 450 nm.
Data were taken from ref. 10.
DFT calculation were performed at the B3LYP/6-31G* level in vacuum using the SPARTAN’08 program.
To probe whether long-wavelength UV absorption leads to enhanced photoreactivity in biological systems, we initially attempted to test the reactivity of all five tetrazoles in a mixed acetonitrile/PBS buffer (1:1), pH 7.5, and found that tetrazoles 1 and 2 and their cycloaddition photoproducts were reasonably soluble but tetrazoles 3-5 were insoluble. Therefore, we performed a fluorescence-based screen of the photoreactivity of tetrazoles 1 and 2 under the 302-, 365-, or 395-nm UV irradiation in the aqueous buffer by taking advantage of the fact that pyrazoline cycloadducts are fluorescent. We found that tetrazole 2 showed rapid ‘turn-on’ fluorescence upon 302- and 365-nm photoirradiation (Figure 2) while tetrazole 1 showed no change in fluorescence after the photoirradiation even though tetrazole 1 itself was weakly fluorescent (Figure S2). To gain insight about the intrinsic photoreactivity of tetrazoles 3-5, we dissolved these compounds in chloroform and examined their reactivity towards methyl methacrylate under the 302-, 365- or 395-nm irradiation. Interestingly, the coumarin-fused tetrazole 3 showed bright ‘turn-on’ fluorescence after exposure to the 302- or 365-nm lamp (Figure 2); tetrazoles 4 and 5 were brightly fluorescent before and after the photoirradiation and the TLC monitoring showed no detectable new spots (Figure S3).
Figure 2.
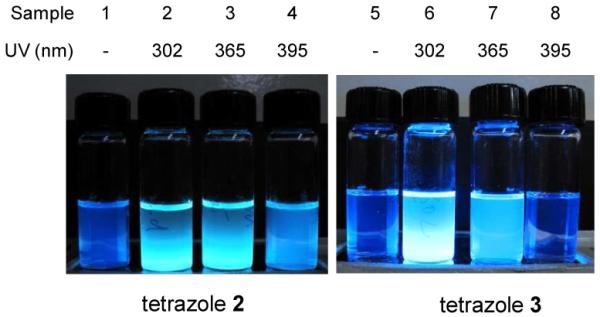
Fluorescence images of tetrazoles 2 and 3 after the photo-irradiation with methyl methacrylate in either acetonitrile/PBS buffer (1:1) or chloroform; λex = 365 nm. The concentrations of tetrazole and methyl methacrylate were 100 μM and 10 mM, respectively, and the durations of photoirradiation were 10 min.
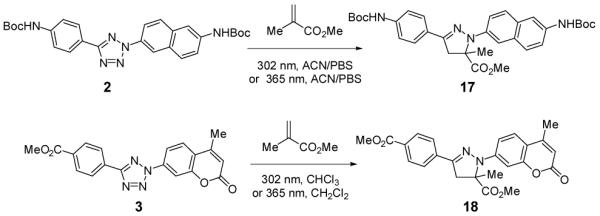
To confirm the fluorescence screening results, we carried out the photoinduced cycloaddition reactions with tetrazoles 2 and 3 on a preparative scale (Scheme 4). For tetrazole 2 under the 302-nm UV irradiation for 2 hours, the expected pyrazoline product 17 was obtained in 89% isolated yield. Using the purified 17 as a positive control in the HPLC-based analysis (Figure S4), the same reaction on an analytical scale showed a conversion of 90% under the 365-nm photoirradiation for only 5 min. Similarly, the pyrazoline product 18 was isolated in 68% yield after tetrazole 3 was irradiated at 302 nm in chloroform for 1.5 hours, and 13% yield when 365-nm UV lamp was used. The reduced yield seen at 365 nm was presumably due to the significantly lower molar absorption coefficient at 365 nm compared to 302 nm (Table 1).
Scheme 4.
In summary, we have identified two long-wavelength photoactivatable diaryltetrazoles containing either naphthalene or coumarin chromophore. The naphthalene-derived tetrazole showed excellent long-wavelength photoreactivity in an acetonitrile-PBS mixed solvent. Additionally, both tetrazoles produced bright pyrazoline fluorophores upon the cycloaddition reactions, which may prove critical for their use as “photoclick” reagents in visualizing and perturbing the alkene-tagged proteins in living cells.7 Detailed characterization of these two compounds and additional structural modifications are currently underway and will be reported in due course.
Supplementary Material
Acknowledgments
We gratefully acknowledge the National Institutes of Health R01 GM 085092 for financial support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and notes
- 1.Swartz TE, Tseng T-S, Frederickson MA, Paris G, Comerci DJ, Rajashekara G, Kim J-G, Mudgett MB, Splitter GA, Ugalde RA, Goldbaum FA, Briggs WR, Bogomolni RA. Science. 2007;317:1090. doi: 10.1126/science.1144306. [DOI] [PubMed] [Google Scholar]
- 2.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Science. 2000;289:739. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 3.Hegemann P, Möglich A. Nature Methods. 2011;8:39. doi: 10.1038/nmeth.f.327. [DOI] [PubMed] [Google Scholar]
- 4.(a) Curley K, Lawrence DS. J. Am. Chem. Soc. 1998;120:8573. [Google Scholar]; (b) Ghosh M, Song X, Mouneimne G, Sidani M, Lawrence DS, Condeelis JS. Science. 2004;304:743. doi: 10.1126/science.1094561. [DOI] [PubMed] [Google Scholar]; (c) Wu N, Deiters A, Cropp A, King D, Schultz PG. J. Am. Chem. Soc. 2004;126:14306. doi: 10.1021/ja040175z. T. [DOI] [PubMed] [Google Scholar]; (d) Chou C, Young DD, Deiters A. Angew. Chem. Int. Ed. 2009;48:5950. doi: 10.1002/anie.200901115. [DOI] [PubMed] [Google Scholar]; (e) Gautier A, Deiters A, Chin JW. J. Am. Chem. Soc. 2011;133:2124. doi: 10.1021/ja1109979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Lien L, Jaikaran DCJ, Zhang Z, Woolley GA. J. Am. Chem. Soc. 1996;118:12222. [Google Scholar]; (b) Banghart MR, Volgraf M, Trauner D. Biochemistry. 2006;45:15290. doi: 10.1021/bi0618058. [DOI] [PubMed] [Google Scholar]; (c) Volgraf M, Goostiza P, Numano R, Kramer RH, Isacoff EY, Trauner D. Nat. Chm. Biol. 2006;2:47. doi: 10.1038/nchembio756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Wang Y, Rivera Vera CI, Lin Q. Org. Lett. 2007;9:4155. doi: 10.1021/ol7017328. [DOI] [PubMed] [Google Scholar]; (b) Song W, Wang Y, Qu J, Madden MM, Lin Q. Angew. Chem. Int. Ed. 2008;47:2832. doi: 10.1002/anie.200705805. [DOI] [PubMed] [Google Scholar]
- 7.(a) Song W, Wang Y, Qu J, Lin Q. J. Am. Chem. Soc. 2008;130:9654. doi: 10.1021/ja803598e. [DOI] [PubMed] [Google Scholar]; (b) Wang Y, Song W, Hu WJ, Lin Q. Angew. Chem., Int. Ed. 2009;48:5330. doi: 10.1002/anie.200901220. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Song W, Wang Y, Yu Z, Vera CI, Qu J, Lin Q. ACS Chem. Biol. 2010;5:875. doi: 10.1021/cb100193h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Wang Y, Lin Q. Org. Lett. 2009;10:3570. doi: 10.1021/ol901300h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wang J, Zhang W, Song W, Wang Y, Yu Z, Li J, Wu M, Wang L, Zang J, Lin Q. J. Am. Chem. Soc. 2010;132:14812. doi: 10.1021/ja104350y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu Z, Lim RK, Lin Q. Chem. Eur. J. 2010;16:13325. doi: 10.1002/chem.201002360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Y, Hu WJ, Song W, Lim RKV, Lin Q. Org. Lett. 2008;10:3725. doi: 10.1021/ol801350r. [DOI] [PubMed] [Google Scholar]
- 11.Böhm H-J, Flohr A, Stahl M. Drug Disc. Today. 2004;1:217. doi: 10.1016/j.ddtec.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 12.Ito S, Tanaka Y, Kakehi A, Kondo K. Bull. Chem. Soc. Jpn. 1976;49:1920. [Google Scholar]
- 13.For recent examples, see: Saito Y, Suzuki A, Imai K, Nemoto N, Saito I. Tetrahedron Lett. 2010;51:2606. Saito Y, Koda M, Shinohara Y, Saito I. Tetrahedron Lett. 2011;52:491.
- 14.Turro NJ. Modern Molecular Photochemistry. Benjamin / Cummins Pub. Co.; California: 1978. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.