Abstract
While genes involved in the differentiation of the mechanosensory hair cells and the neurons innervating them have been identified, genes involved in balancing their relative numbers remain unknown. Six1a plays a dual role by promoting hair cell fate while inhibiting neuronal fate in these two lineages. Genes homologous to six1a act as either transcriptional activators or repressors, depending on the partners with which they interact. By assaying the in vivo and in vitro effects of mutations in presumptive protein-protein interacting and DNA-binding domains of Six1a, we show that, in the developing zebrafish inner ear, Six1a promotes hair cell fate by acting as a transcriptional activator and inhibits neuronal fate by acting as a transcriptional repressor. We also identify several potential partners for Six1a that differ between these two lineages. The dual role of Six1a in the developing otocyst provides a mechanism for balancing the relative number of hair cells and neurons during organogenesis of the inner ear.
Keywords: Inner Ear, Hair Cells, Neurons, Cell Fate, Six1a, Zebrafish
INTRODUCTION
The inner ear of vertebrates is a complex organ for hearing and maintaining balance, consisting of non-sensory epithelia and 6 to 9 precisely positioned sensory organs, which contain the mechanosensory hair cells (Fritzsch et al., 2002). These are innervated by neurons of the VIIIth cranial or stato-acoustic ganglion (SAG) (Rubel and Fritzsch, 2002). All inner ear cell types, including SAG neurons, are derived from an ectodermal thickening adjacent to the hindbrain, the otic placode (Fritzsch et al., 1998; Haddon and Lewis, 1996; Hudspeth, 1989). The lineage relationships between these different cell types are just beginning to be understood (Fekete and Wu, 2002). Single cell lineage studies have demonstrated that hair cells and neurons share a common progenitor in chickens (Satoh and Fekete, 2005). The auditory and vestibular functions of the vertebrate inner ear depend on the proper balance between number of hair cells and the neurons that innervate them (Chen and Segil, 1999; Chen et al., 2003).
The transcription factor Six1 has been shown in mouse to be necessary for the development of multiple organs and tissues, including the inner ear (Zheng et al., 2003). This role of Six1 in organogenesis involves it binding and interacting with co-factors such as Eyes absent (Eya) and Dachshund (Dach) (Li et al., 2003). Within this protein complex, Eya1, acting as a phosphatase, changes the Six1-Dach complex from a repressor to an activator of transcription (Li et al., 2003). Six genes have also been shown to interact directly with genes from the Eyes absent (Eya) family at the protein level through binding sites encoded within their Six domain (Ruf et al., 2004). The Six-Eya protein complexes can act as transcriptional activators as well (Brugmann et al., 2004). A related mechanism has been proposed to explain the role of the fly six1 homologue, sine oculis (so) in compound eye development (Silver et al., 2003), based on prospective binding to both Groucho (GRO), a general transcriptional repressor, and EYA. The model (Silver et al., 2003) predicts that in the absence of EYA, GRO is bound to SO leading to repression of so target genes, whereas in the presence of EYA, EYA displaces GRO and the newly formed SO-EYA complex can now activate transcription of so target genes. All these studies emphasize that the conversion of Six1 from a transcriptional repressor to an activator occurs within the same organ or cell type and is a required transition for organogenesis (Li et al., 2003; Silver et al., 2003).
We recently demonstrated that the transcription factor Six1a (formerly named Six1 (Bessarab et al., 2008)) plays a pivotal role in controlling the relative numbers of sensory hair cells and SAG neurons (Bricaud and Collazo, 2006). It is the only gene identified that differentially affects two distinct cell types in the developing inner ear. Six1a is expressed in the precursor population as well as the differentiated hair cells and neurons. It is notable as well that Six1a only acts on a sub-population of hair cells, those within the utricular macula sensory organ (Bricaud and Collazo, 2006). Utricular hair cells are the only hair cells of the inner ear to arise from the same region as SAG neurons (anterior-ventral otic epithelium) and at the same time in development (Haddon and Lewis, 1996). The function of Six1a in the developing zebrafish inner ear is to promote hair cell formation by increasing cell proliferation in the sensory lineage while concomitantly inhibiting SAG neuron formation by inducing cell death in the neuronal lineage (Fig. S1). That Six1a has opposite effects in these two lineages could be explained by the fact that Six proteins can either activate or repress downstream target genes, depending upon their interactions with certain co-factors (Brugmann et al., 2004; Kawakami et al., 2000; Kobayashi et al., 2001; Lagutin et al., 2003; Lopez-Rios et al., 2003; Ohto et al., 1999; Pignoni et al., 1997; Ruf et al., 2004; Zhu et al., 2002).
All Six proteins have at least two regions of relatively high homology, an N-terminal Six domain and a C-terminal Six-type homeodomain. The N-terminal Six domain is thought to be necessary for protein-protein interactions while the homeodomain is required for the binding of Six proteins to their DNA targets (Kawakami et al., 2000). Zebrafish Six1a also contains two engrailed homologue 1 related motifs (eh1) in its Six domain. These two domains are highly homologous to those found in human SIX1, mouse Six2, and fly so (Kobayashi et al., 2001; Laclef et al., 2003). These motifs have been demonstrated in vitro to be required for interactions with repressors of the Groucho (Gro) family (Kobayashi et al., 2001; Laclef et al., 2003). Furthermore, this Groucho-mediated transcriptional repression is thought to involve histone modifications resulting from the recruitment of histone deacetylases to the target gene (Chen and Courey, 2000).
How an organ balances the relative number of constituent cells is one of the least understood processes in development. To understand how Six1a can have opposite effects in two distinct inner ear cell lineages, we assayed the effects of perturbing the binding of Six1a to members of either the Gro or Eya family on the formation of hair cells and neurons within the developing inner ear. Our results shows that the integrity of the two eh1 binding sites is required for the proper function of Six1a in the neuronal lineage, whereas the Eya-binding site is necessary for Six1a to act in the sensory lineage. Mutations in the Six-homeodomain result in Six1a having no effect on either the sensory or neuronal lineages, suggesting that DNA binding is required in both lineages. These results combined with in vitro assays lead us to propose that Six1a acts as a transcriptional repressor in the neuronal lineage and as a transcriptional activator in the hair cell lineage, likely with an Eya-like factor. Six1a’s repressive role in the neuronal lineage not only seems to require an interaction with a Gro-like factor but also the presence of a histone deacetylase. We further propose that the dual role of Six1a in the developing otocyst could be a mechanism balancing the relative number of hair cells and neurons during organogenesis of the inner ear.
MATERIALS AND METHODS
Morpholino-modified oligonucleotides and mRNA injections
The method for injecting mRNA and morpholino-modified oligonucleotides (MO) into zebrafish embryos has been described elsewhere (Nasevicius and Ekker, 2000; Westerfield, 1995). The MO (Gene Tools, LLC, Philomath, OR) used were as follows (complementary bases to the predicted start codon are underlined):
gro1: 5′-GCCCTGCGGATACATCTTGAATGT-3′;
std: Gene Tools, LLC standard control oligo.
Whole mount immunofluorescence
Whole mount immunofluorescence was performed as described elsewhere (Bricaud et al., 2001). After staining, the larvae were mounted and documented with a Zeiss or Leica Laser Scanning Confocal Microscope by collecting and projecting confocal z-series. The antibody directed against HCS-1 (Gift from J.T. Corwin, University of Virginia, Charlottesville, VA; used at a concentration of 1:50) has been shown to specifically recognize otoferlin (Goodyear et al., 2010) and labels hair cells in fish, amphibians, birds and mammals (Finley et al., 1997; Gale et al., 2000; Goodyear et al., 2010). The antibody directed against HuC (Molecular Probes, Inc., Eugene, OR; used at a concentration of 1:1000) has been shown previously to label the neurons of cranial ganglia (Andermann et al., 2002; Marusich et al., 1994). As a cell proliferation marker, we used an antibody directed against human PhosphoHistoneH3 (EMD Biosciences, San Diego, CA; used at a concentration of 1:100). Programmed cell death was studied using an antibody directed against the human activated Caspase3 (R&D Systems, Inc., Minneapolis, MN; used at a concentration of 1:100).
Administration of histone deacetylase inhibitor, Trichostatin A
The method for administrating Trichostatin A (TSA) has already been described elsewhere (Miller et al., 2004). Briefly, the TSA (Sigma-Aldrich, St Louis, MO) was applied to live embryos at a concentration of 0.1 M in embryo water (Westerfield, 1995) with 0.003% DMSO. Control embryos were raised in embryo water containing just DMSO. To ensure both penetration and that the inhibition would be effective during the period hair cells and neurons are developing, embryos were incubated in the presence of the inhibitor from 15 hpf (prior to when hair cells and neurons form) to 3 dpf, a period encompassing the formation of both sensory hair cells and SAG neurons, and then assayed for the number of utricular hair cells and SAG neurons.
Cell counts
To assess cell numbers accurately, multiple focal planes (confocal z-sections) encompassing all labeled cells around the inner ear were collected with a Zeiss or Leica Laser Scanning Confocal Microscope. Examples of collected images are shown in Figure S2. By careful comparison to the previous and following sections, we made sure to only count each labeled cell once. Sample sizes listed for the cell counts represent individual embryos since for each embryo, only the cells on one side have been scored. Statistical analyses were done using Student’s t-test and the Smith’s Statistical Package(Pomona College, Claremont, CA) with a P < 0.05 considered as statistically significant.
Six1a site-directed mutagenesis
Site-directed mutagenesis was performed using the GeneEditor™ in vitro Site-Directed Mutagenesis System (Promega, Inc., Madison, WI) according to the manufacturer’s instructions. The two Gro-binding sites (6-SFGFTQEQVACV-17 and 73-HQFSPHNHPKL-83), also called eh1 domains for engrailed homologue 1 domains, have been mutated in a similar fashion in zebrafish Six3 (Kobayashi et al., 2001). Briefly, the phenylalanines in position 7 and 9 and the one in position 75 were mutated into glutamic acid residues to generate the mutant forms subsequently called Six1aeh1a* (positions 7 and 9), Six1aeh1b* (position 75) and Six1aeh1a*b*, the latter having mutations in all 3 positions and both eh1 domains (Fig. S4). It is notable that Six1a contains two phenylalanines within its first eh1 domain, separated by one amino-acid, compared to six3 which only has one (Kobayashi et al., 2001). We individually mutated both phenylalanines in position 7 and 9 and the resulting mutated proteins behave identically to the proteins where both phenylalanines are mutated into glutamic acid (Data not shown). We therefore used the mutant form where both phenylalanine 7 and 9 are mutated for subsequent experiments. The mutations affecting the Eya-binding site (subsequently called Six1aR110W) and the DNA-binding sites (subsequently called Six1aY129C and Six1adelE133) have been described in humans as responsible for causing Branchio-Oto-Renal syndrome (Fig. S4) (Ruf et al., 2004). The R110W mutation where the arginine in position 110 is replaced by a tryptophan has been shown in vitro to prevent human EYA1 binding (Ruf et al., 2004). The two other mutations, Y129C and delE133, where the tyrosine in position 129 is replaced by a cysteine and where the glutamic acid in position 133 is deleted, respectively, have been shown in vitro to prevent human SIX1 binding to DNA (Ruf et al., 2004). The cellular behavior of the Six1a mutants has been verified by transient transfections (Fig. S3)
Transfections and luciferase assays
Transfections of the different constructs were performed in COS-7 cells (ATCC, Manassas, VA) using PolyFect Transfection Reagent (Qiagen, Inc., Valencia, CA) according to the manufacturer’s recommendations. Twenty-four hours after transfection, the cells were harvested and the luciferase assays were carried out according to the manufacturer’s protocol using Dual-Luciferase Reporter Assay Kit (Promega, Inc., Madison, WI). Luciferase activity were measured with a BD Moonlight 3010 Luminometer (BD Biosciences Pharmingen, San Diego, CA) using pRL-CMV (Promega, Inc., Madison, WI), expressing the Renilla luciferase under the control of the CytoMegaloVirus (CMV) promoter, as an internal control. As a reporter of six1a transcriptional activity, we used the ARE-luciferase construct (kindly provided by S. Silver, Whitehead Institute for Biomedical Research, Cambridge, MA) consisting of the firefly luciferase gene under the control of seven repeats of the AREC3 (SIX4) binding site (Silver et al., 2003). The expression in all the other constructs is driven by the CMV promoter. Absolute luciferase activity for 3 independent experiments have been averaged and normalized against the control experiments.
RESULTS
Six1a acts as a transcription factor by directly binding DNA in both the sensory and neuronal lineages of the developing zebrafish inner ear
To determine whether Six1a functions as a transcription factor in the developing zebrafish inner ear, we assayed the effects on hair cells and SAG neurons of the over-expression of mutant forms of Six1a, where the presumptive DNA-binding site is disrupted. Mutations where the tyrosine in position 129 is replaced by a cysteine or where the glutamic acid in position 133 is deleted have been shown in vitro to prevent human SIX1 binding to DNA (Ruf et al., 2004). We introduced these mutations into the zebrafish Six1a sequence, Six1aY129C and Six1adel133 respectively, and then assayed in vivo whether the over-expression of these mutant mRNAs had the same effect on inner ear development as the over-expression of wild-type Six1a (Fig. 1, S2). As the most striking effect of Six1a over-expression is a decrease in the number of SAG neurons and an increase in the number of utricular hair cells (Bricaud and Collazo, 2006), we first scored these two cell types at 3 days post fertilization (dpf) after over-expression of either Six1aY129C or Six1adel133, and compared the numbers to those observed in control and Six1a over-expressing embryos (Fig. 1A, S2). The numbers of SAG neurons and utricular hair cells, after over-expression of either Six1aY129C or Six1adel133, are indistinguishable from those observed in control, non-injected embryos (Fig. 1A; P values ranging fro 0.1 to 0.8). We also looked at programmed cell death and cell proliferation in embryos over-expressing the two mutant forms of Six1a (Fig. 1B). Instead of the increase in cell death and proliferation normally observed when Six1a is over-expressed (Bricaud and Collazo, 2006), we observed no change in number of cells undergoing death and proliferation in embryos over-expressing either Six1aY129C or Six1adel133 when compared to control embryos (Fig. 1B; P values ranging from 0.1 to 0.8). These results strongly suggest that the DNA-binding domain of Six1a is crucial for its function in both sensory and neuronal lineages.
Fig. 1.
Integrity of the Six1a DNA-binding domain is required for the proper function of Six1a in vivo. The decrease in neuronal numbers and increases in hair cell numbers, proliferation and cell death normally observed after six1a over-expression are not observed when using mutant constructs. Values represent mean cell counts (± standard deviation) with a sample size of 20 embryos for each experiment. (A) Average number of hair cells and neurons in 3 dpf utricular maculae and SAG. Hair cells and neurons were detected by HCS-1 and HuC immunolabeling, respectively, using confocal microscopy. (B) Quantification of cell proliferation and programmed cell death in otocysts at 28 hpf. Cells labeled with either PhosphoHistoneH3 or activated Caspase3. For cell proliferation and death, only cells within the otocyst were counted. Statistical analyses were performed for both panels with Student’s t test; all comparisons were made to embryos injected with the standard MO control or with six1a mRNA. Comparisons where P < 0.05 were considered statistically significant.
In order to confirm that Six1a functions as a transcription factor, we used an in vitro cellular system to study whether Six1a could trans-activate a reporter gene whose expression was controlled by a Sine oculis responsive element (Fig. 2A). When Six1a is expressed in our reporter system, the absolute luciferase activity (ALA) is increased by 35% compared to the baseline luciferase activity observed when the reporter construct is transfected alone (Fig. 2A), showing that Six1a can act as a transcriptional activator. Furthermore, this trans-activation is impaired when a Six1a DNA-binding site is mutated as the ALA, when either Six1aY129C or Six1adel133 is expressed (Fig. 2B, C), is indistinguishable from the negative control.
Fig, 2.
DNA-binding domain of Six1a is required for the function of Six1a in vitro. The in vitro trans-activation of Six1a targets is not observed when the DNA-binding domain of Six1a is mutated. The ARE firefly luciferase reporter ARE-luc was cotransfected with pc-Six1a (A), pc-Six1aY129C (B), pc-Six1adelE133 (C), pc-Eya1, pc-Gro1, or combinations of these into COS-7 cells. Firefly luciferase activity in the cell lysate was normalized to the Renilla luciferase activity of pRL-CMV as an internal control. The mean fold activation from three independent experiments is shown, expressed in arbitrary units. Statistical analyses were performed with Student’s t test. Comparisons where P < 0.05 were considered statistically significant. The sites mutated are shown in the insets in B and C.
In conclusion, we propose that Six1a’s principal function in both hair cell and neuronal lineages is to bind directly to its DNA-targets and either activate or repress transcription.
Zebrafish Six1a function requires a direct interaction with an Eya factor in the sensory but not neuronal lineage of the developing inner ear
Previous studies have shown that complexes formed between Sine oculis and Eyes absent proteins could act as transcriptional activators (Brugmann et al., 2004; Silver et al., 2003). To determine whether an interaction with an Eya factor was necessary for Six1a function in vivo, we over-expressed Six1aR110W, a mutant form of Six1a where Eya-binding is impaired and studied the effects of its over-expression on the development of the inner ear (Fig. 3, S2).
Fig. 3.
EYA-binding site of Six1a is required in vivo for the function of Six1a in the sensory hair cell lineage of the developing zebrafish inner ear. Mutating the EYA-binding site within Six1a abolishes the increase in hair cell numbers and cell proliferation normally seen after six1a over-expression. Values represent mean cell counts (± standard deviation) with a sample size of 20 embryos for each experiment. (A) Average number of hair cells and neurons in 3 dpf utricular maculae and SAG. Hair cells and neurons were detected by HCS-1 and HuC immunolabeling, respectively, using confocal microscopy. (B) Quantification of cell proliferation and death in otocysts at 28 hpf. Cells labeled with either PhosphoHistoneH3 or activated Caspase3. For cell proliferation and death, only cells within the otocyst were counted. Statistical analyses were performed for both panels with Student’s t test; all comparisons were made to embryos injected with standard MO control or with six1a mRNA. Comparisons where P < 0.05 were considered statistically significant.
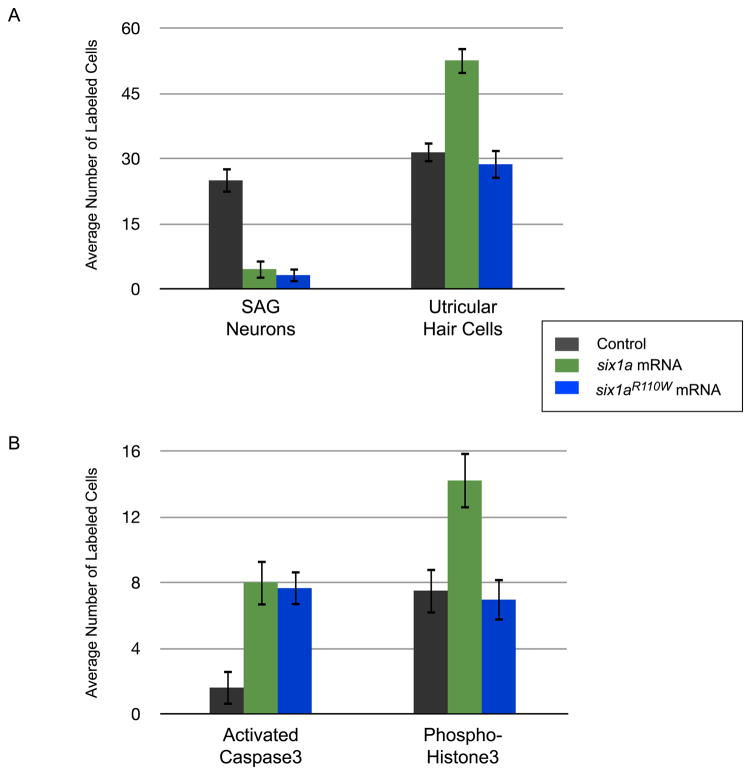
We first assayed the effects of Six1aR110W over-expression on the formation of SAG neurons and utricular hair cells (Fig. 3A, S2). The decreased number of SAG neurons seen is indistinguishable from that observed after wild-type Six1a over-expression (3.15 ± 1.63 (Six1aR110W) versus 4.55 ± 2.84 (Six1a); P = 0.03; n = 20), indicating that a direct interaction between Six1a and an Eya factor is not required for Six1a function in the neuronal lineage of the developing zebrafish inner ear. However, the increased number of utricular hair cells normally seen after wild-type Six1a over-expression is not observed when Six1aR110W is over-expressed (28.65 ± 3.38 (Six1aR110W) versus 52.55 ± 3.14 (Six1a); P < 0.005; n = 20). The number of hair cells observed after Six1aR110W over-expression is similar to that observed in control larvae (28.65 ± 3.38 (Six1aR110W) versus 29.9 ± 2.7 (STD); P = 0.2; n = 20). These results show that Six1a interaction with an Eya factor is necessary for Six1a function in the sensory but not neuronal lineage.
The increased number of utricular sensory hair cells seen after Six1a over-expression is mainly due to an increase in cell proliferation within the developing otocyst (Bricaud and Collazo, 2006). This observation led us to assay whether Six1a interaction with an Eya factor was required for Six1a-mediated control of cell proliferation in the sensory lineage (Fig. 3B). When Six1aR110W is over-expressed, we did not observe the increase in cell proliferation normally observed when wild-type Six1a is over-expressed (6.95 ± 1.28 (Six1aR110W) versus 14.2 ± 1.7 (Six1a); P < 0.005; n = 20). The number of dividing cells in the otocyst after Six1aR110W over-expression is indistinguishable from that observed in control larvae (6.95 ± 1.28 (Six1aR110W) versus 7.5 ± 1.4 (STD); P = 0.2; n = 20). Six1aR110W over-expression still results in the increased cell death normally observed after Six1a over-expression (7.65 ± 1.04 (Six1aR110W) versus 8 ± 1.41 (Six1a); P = 0.4; n = 20), an increase that we believe is solely occurring in the neuronal lineage (Bricaud and Collazo, 2006). These results strongly suggest that a direct interaction between Six1a and an Eya factor is required for Six1a to control cell proliferation in the sensory, but not neuronal lineage.
Zebrafish Six1a function requires a direct interaction with a Gro factor in the neuronal but not sensory lineage of the developing inner ear
As it has been shown that interactions between Sine oculis proteins and Groucho factors could turn Sine oculis into transcriptional repressors (Brugmann et al., 2004; Kobayashi et al., 2001; Lopez-Rios et al., 2003; Silver et al., 2003; Zhu et al., 2002), we assayed whether an interaction between Six1a and a Gro factor was relevant for Six1a function in the developing zebrafish inner ear. We over-expressed Six1aeh1a*, Six1aeh1b*, and Six1aeh1a*b*, mutant forms of Six1a where either one (Six1aeh1a* and Six1aeh1b*) or two (Six1aeh1a*b*) prospective Gro-binding site(s) (the engrailed homologue 1 related motifs) had been mutated and studied the effects of their over-expression on numbers of utricular hair cells and SAG neurons (Fig. 4, S2).
Fig. 4.
GRO-binding sites of Six1a are required in vivo for the function of Six1a in the neuronal lineage of the developing zebrafish inner ear. When both sites are mutated, the loss of neurons and increase in cell death normally observed after six1a over-expression is abolished. Mutations in one site result in an intermediate condition. Values represent mean cell counts (± standard deviation) with a sample size of 20 embryos for each experiment. (A) Average number of hair cells and neurons in 3 dpf utricular maculae and SAG. Hair cells and neurons were detected by HCS-1 and HuC immunolabeling, respectively, using confocal microscopy. (B) Quantification of cell proliferation and death in otocysts at 28 hpf. Cells labeled with either PhosphoHistoneH3 or activated Caspase3. For cell proliferation and death, only cells within the otocyst were counted. Statistical analyses were performed for both panels with Student’s t test; all comparisons were made to embryos injected with standard MO control or with six1a mRNA. Comparisons where P < 0.05 were considered statistically significant.
Over-expression of any of the 3 Six1a mutants perfectly phenocopies Six1a over-expression with respect to the number of utricular hair cells, i.e. an increase in their numbers (Fig. 4A, S2). In contrast, Six1aeh1a* or Six1aeh1b* over-expression results in only a moderate decrease in the number of SAG neurons (Fig. 4A, S2). The numbers are 14.5 ± 2.95 and 14 ± 2.53 for Six1aeh1a* or Six1aeh1b*, respectively, versus 24.95 ± 2.84 in control larvae (P < 0.005 for either mutant; n = 20) and 4.55 ± 2.24 in larvae over-expressing wild-type Six1a (P < 0.005; n = 20). When the construct with both sites mutated, Six1aeh1a*b*, is over-expressed, the number of SAG neurons is similar to that observed in control larvae (Fig. 4A, S2): 25.95 ± 1.96 compared to 24.95 ± 2.84 in controls (P = 0.2; n = 20). These results show that Six1a interaction with a Gro factor is necessary for Six1a function in the neuronal lineage but not sensory lineage.
The decrease in number of SAG neurons observed after Six1a over-expression is due to an increase in cell death (Bricaud and Collazo, 2006). Because of this observation, we assayed for the number of cells undergoing cell death after over-expression of Six1aeh1a*, Six1aeh1b*, and Six1aeh1a*b* (Fig. 4B). As observed with SAG neurons, the number of cells undergoing cell death in the otic vesicle is only moderately increased relative to control larvae when either Six1aeh1a* or Six1aeh1b* is over-expressed (Fig. 4B): 4.25 ± 1.65 and 4.2 ± 1.15, respectively, versus 1.6 ± 1.05 in control larvae (P < 0.005 for both mutants; n = 20) or 8 ± 1.41 after Six1a over-expression (P < 0.005 for both mutants; n = 20). In contrast, when Six1aeh1a*b* is over-expressed, the number of cells undergoing cell death in the developing otocyst is similar to that observed in control larvae (Fig. 4B), 1.95 ± 1.1 compared to 1.6 ± 1.05 in controls (P = 0.3; n = 20). Notably, the over-expression of these 3 mutants still results in the increased cell proliferation normally observed after Six1a over-expression (12.05 ± 1.61 (Six1aeh1a*), 14.3 ± 1.56 (Six1aeh1b*), and 13.4 ± 1.85 (Six1aeh1a*b*) versus 7.5 ± 1.40 (STD); P < 0.005 for the 3 mutants; n =20 or 14.2 ± 1.70 (Six1a); P = 0.002. 0.8, and 0.16 for the 3 mutants, respectively; n = 20). These results strongly suggest that a direct interaction between Six1a and a Gro factor is required for Six1a to control cell death in the neuronal, but not sensory cell lineage.
Zebrafish Six1a can form a transcriptional activator complex with Eya1 in vitro
Our in vivo experiments suggest that a direct interaction between Six1a and an Eya factor is required for Six1a function in the sensory lineage within the developing zebrafish inner ear. In order to study whether Six1a’s function as an activator in the sensory lineage could be potentiated by its interaction with Eya1 (Sahly et al., 1999), we co-expressed Six1a and Eya1 in our in vitro reporter system and tested the effect of their co-expression on the reporter gene (Fig. 2A, 5).
Fig. 5.
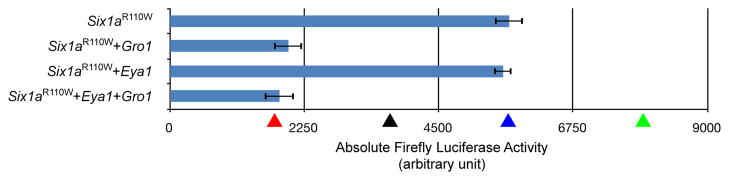
EYA-binding site of Six1a is necessary in vitro for Six1a’s interaction with zebrafish Eya1 and the subsequent trans-activation of Six1a targets. The increase in trans-activation of the Six1a targets normally observed in vitro when both six1a and eya1 are transfected is not observed when the EYA-binding domain of Six1a is mutated. The ARE firefly luciferase reporter ARE-luc was cotransfected with pc-Six1aR110W, pc-Eya1, pc-Gro1, or combinations of these into COS-7 cells. Firefly luciferase activity in the cell lysate was normalized to the Renilla luciferase activity of pRL-CMV as an internal control. The mean fold activation from three independent experiments is shown expressed in arbitrary units. Statistical analyses were performed with Student’s t test. Comparisons where P < 0.05 were considered statistically significant. Absolute firefly luciferase activities for control combinations are shown as arrowheads (red for pc-Six1a + pc-Gro1, black for the empty vector by itself, blue for pc-Six1a alone and green for pc-Six1a + pc-Eya1). The site mutated is shown in the schematic below the chart.
When Six1a is co-expressed with Eya1, the measured ALA increases by 34% compared to Six1a alone (Fig. 2A), showing that Eya1 and Six1a can act synergistically to activate the transcription of Six1a targets; Eya1 alone having no effect on the measured ALA (Fig. 2A). Moreover, this synergistic effect is most likely due to a direct interaction between Six1a and Eya1, since when the Six1aR110W mutant, in lieu of the wild-type Six1a, is co-expressed with Eya1, the measured ALA is similar to levels observed with Six1a alone (Fig. 5). Direct interactions between Six1a and Eya1 were confirmed by GST-pulldown experiments (Fig. S5).
Zebrafish Six1a can form a transcriptional repressor complex with Gro1 in vitro
Our in vivo experiments suggest that an interaction between Six1a and a Gro factor is required for Six1a function in the neuronal lineage of the developing zebrafish inner ear. To address this, we co-expressed Six1a and Gro1 (Wulbeck and Campos-Ortega, 1997), in our in vitro reporter system, and assayed for the expression of our reporter gene (Fig. 2A, 6).
Fig. 6.
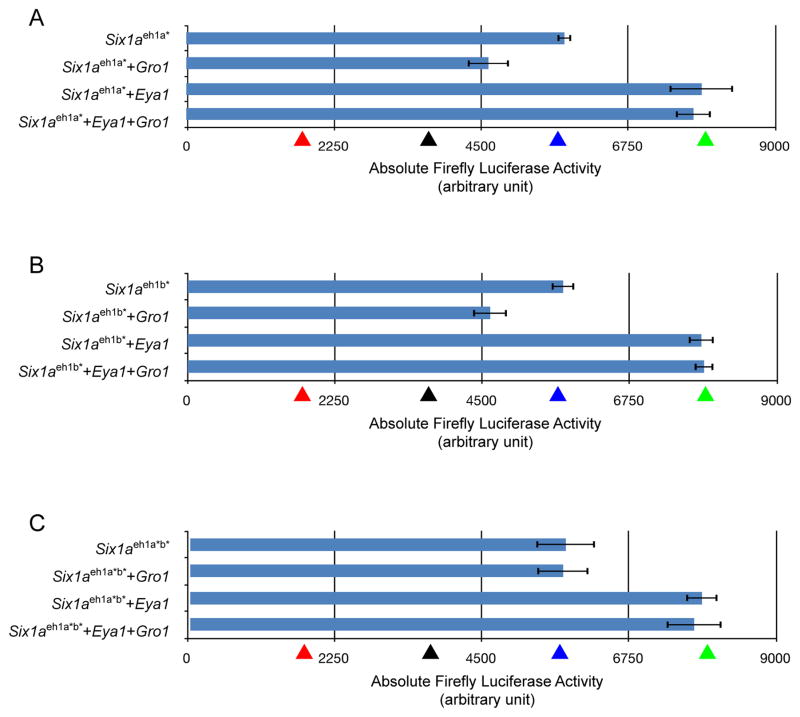
GRO-binding sites of Six1a are necessary in vitro for Six1a’s interaction with zebrafish Gro1 and the subsequent repression of Six1a targets. The repression of Six1a targets normally observed in vitro when both six1a and gro1 are transfected is not observed when the prospective GRO-binding sites of Six1a are mutated. The ARE firefly luciferase reporter ARE-luc was cotransfected with pc-Six1a, pc-Six1eh1a* (A), pc-Six1aeh1b* (B), pc-Six1eh1a*b* (C), pc-Eya1, pc-Gro1, or combinations of these into COS-7 cells. Firefly luciferase activity in the cell lysate was normalized to the Renilla luciferase activity of pRL-CMV as an internal control. The mean fold activation from three independent experiments is shown expressed in arbitrary units. Statistical analyses were performed with Student’s t test. Comparisons where P < 0.005 were considered statistically significant. Absolute firefly luciferase activities for control combinations are shown as arrowheads (red for pc-Six1a + pc-Gro1, black for the empty vector by itself, blue for pc-Six1a alone and green for pc-Six1a + pc-Eya1). The sites mutated are shown in the schematics below each chart.
When Six1a is co-expressed with Gro1, the ALA is reduced by 64% compared to Six1a alone (Fig. 2A), demonstrating that Six1a and Gro1 can act synergistically to repress the transcription of Six1a targets. Furthermore, this synergistic effect likely involved direct protein-protein interaction between both eh1 motifs of Six1a and Gro1 since when Six1aeh1a* or Six1aeh1b* is co-expressed with Gro1, in lieu of the wild-type Six1a, the measured ALA is only reduced by 25% and 22%, respectively (Fig. 6). When Six1aeh1a*b* is transfected, there is no decrease in the measured ALA which is not statistically different from that of wild-type Six1a alone (Fig. 6). The direct binding of Gro1 to Six1a was also confirmed by GST-pulldown experiments (Fig. S5).
The binding of Six1a to Eya1 is favored over that of Six1a to Gro1 in vitro
Using our in vitro reporter system, we next assayed whether the binding of Eya1 to Six1a was favored over that of Six1a to Gro1. The co-transfection of Six1a, Gro1, and Eya1 leads to an activation of the reporter similar to that observed when only Eya1 is co-transfected with Six1a (Fig. 2A), strongly suggesting that the presence of Gro1 has no effect on the Eya1-mediated enhancement of Six1a transcriptional activity. Furthermore, when Six1aR110W is co-transfected with both Gro1 and Eya1 (Fig. 5), the measured ALA is similar to the one measured when only wild type Six1a and Gro1 are co-transfected, demonstrating that it is the binding of Eya1 to Six1a that prevents Gro1 from modulating Six1a transcriptional activity. The preference of Six1a to bind Eya1 over Gro1 was also confirmed by GST-pulldown experiments (Fig. S5).
Gro-Six1a transcriptional inhibition could be mediated by histone modifications in the neuronal lineage
At least one Groucho gene, gro1, is expressed in the developing inner ear at the same developmental stages as six1a (Wulbeck and Campos-Ortega, 1997). To test whether Groucho plays a role in the developing inner ear, we studied the effects of knocking down gro1 by injecting a morpholino-modified oligonucleotide (MO) directed against gro1 into zebrafish embryos and assaying for the number of utricular hair cells and SAG neurons at 3 dpf (Fig. 7, S6). After gro1-MO injection, the observed number of utricular hair cells is similar to that observed in control larvae (Fig. 7; 31.05 ± 2.95 compared to 31.4 ± 2.3 in control larvae; P = 0.7; n = 20), demonstrating that Gro1 function is not required in the sensory lineage of the developing inner ear. However, in the neuronal lineage, knocking down gro1 results in a dramatic reduction in the number of SAG neurons at 3 dpf (Fig. 7; 5.1 ± 2.00 compared to 24.95 ± 2.84 in control larvae; P < 0.005; n = 20). This is similar to what is observed when six1a is over-expressed (4.55 ± 2.24; P = 0.4; n = 20). These results, combined with our in vitro data, suggest that Gro1 and Six1a function together to repress transcription in the neuronal lineage of the developing zebrafish inner ear.
Fig. 7.
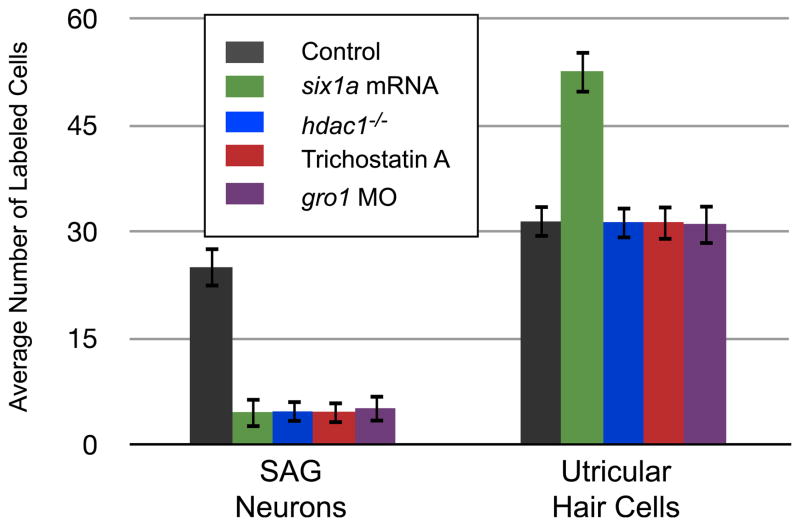
The function of Six1a as a transcriptional repressor in the neuronal lineage of the developing zebrafish inner ear likely involves Hdac1 and Gro1. Loss of function of either hdac1 or gro1 leads to a decrease in number of neurons similar to that observed when six1a is over-expressed. Effects of perturbing these molecules are restricted to the neuronal lineage. Average number of hair cells and neurons in 3 dpf utricular maculae and SAG. Hair cells and neurons were detected by HCS-1 and HuC immunolabeling, respectively, using confocal microscopy. Values represent mean cell counts (± standard deviation) with a sample size of 20 for each experiment. Statistical analyses were performed for both panels with Student’s t test; all comparisons were made to embryos injected with standard MO control (STD) or with six1a mRNA. Comparisons where P < 0.05 were considered statistically significant.
One of the currently favored models for Gro-mediated repression (Chen and Courey, 2000) involves the recruitment of histone deacetylases, which remove acetyl groups from the histones, resulting in the compaction of the chromatin (de Ruijter et al., 2003; Marks et al., 2003). These chromatin modifications make the gene being regulated refractory to the binding of transcription factors, repressing its transcription. In order to test whether such a mechanism could be at play in the neuronal lineage, we assayed for the role of zebrafish Hdac1, a histone deacetylase expressed in the developing inner ear (Cunliffe, 2004). We first used Trichostatin A (TSA), a general pharmacological inhibitor of histone deacetylases (Miller et al., 2004). While the number of utricular hair cells observed after TSA treatment is indistinguishable from that observed in control larvae (Fig. 7; 31.3 ± 2.61 compared to 31.4 ± 2.30 (control); P = 0.9; n = 20), the number of SAG neurons observed after TSA treatment is dramatically reduced (Fig. 7; 4.6 ± 1.73 compared to 24.95 ± 2.84 in control larvae; P < 0.005; n = 20) and is comparable to the number observed when either gro1 is knocked down (Fig. 7; 5.1 ± 2.00; P = 0.4; n = 20) or six1a is over-expressed (Fig. 7; 4.55 ± 2.24; P = 0.9; n = 20). We also assayed for the numbers of utricular hair cells and SAG neurons in 3 dpf larvae in which the hdac1 locus has been mutated (larvae that contain a null hdac1 insertional mutation, hi1618 (Golling et al., 2002), generously provided by V.T. Cunliffe). The observed results are similar to those obtained with TSA treatment (Fig. 7). The number of utricular hair cells is not affected in larvae homozygous for the hdac1 mutation (Fig. 7; 31.3 ± 2.61 compared to 31.4 ± 2.30 in wild-type larvae; P = 0.9; n = 20) whereas the number of SAG neurons is dramatically reduced to levels similar to those observed when larvae are either treated with TSA, gro1 is knocked down, or six1a is over-expressed (Fig. 7; 4.65 ± 1.6 compared to 24.95 ± 2.84 (wild-type; P < 0.005; n = 20), 4.60 ± 1.73 (TSA; P = 0.9; n =20), 5.10 ± 2.00 (gro1 MO; P = 0.4; n = 20 and 4.55 ± 2.24 (six1a over-expression; P = 0.9; n = 20)).
These results strongly suggest that Six1a-mediated transcriptional repression in the SAG neuronal lineage acts through the recruitment of Gro1. The newly formed Six1a-Gro1 complex in turn may interact with Hdac1, a histone deacetylase, which decreases the level of acetylation of the targeted region of chromatin, further inhibiting transcription.
DISCUSSION
Six1a acts as a transcriptional activator in the sensory hair cell lineage and as a transcriptional repressor in the SAG lineage
The results presented here suggest dual and opposing functions for Six1a during the formation of sensory hair cells and the neurons innervating them, in the developing zebrafish inner ear. In the sensory hair cell lineage, Six1a likely acts as a transcriptional activator whereas in the SAG neuronal lineage its function is to repress transcription. This lineage-dependent function of Six1a is most likely due to the presence of modifying co-factors that are able to physically interact with Six1a at the protein level and, subsequently, confer Six1a activating or repressing activities.
Combining the results presented in this paper with those previously reported (Bricaud and Collazo, 2006) lead us to propose a model (Fig. 8) for how Six1a acts in the developing zebrafish inner ear that may provide insights into inner ear development in other vertebrates. In the precursors to both the sensory hair cells and SAG neurons, the homeodomain of Six1a physically interacts with specific DNA sequences within the promoter of Six1a target genes. In the sensory lineage, a co-factor, likely a member of the EYA gene family, is present and being bound by the Six domain of Six1a. The newly formed heterocomplex is then able to promote the transcription of Six1a targets that trigger the proliferation of hair cells while protecting them from programmed cell death, thereby increasing the number of sensory hair cells. Such a role for a Six1a-Eya1 complex is not unlike the role described for Six1-Eya1 complexes in Xenopus cranial placodes where a Six1-Eya1 complex is able to maintain a proliferative state which blocks the expression of neuronal genes in placode derived ganglia (Schlosser et al., 2008). Conversely, in the SAG neuronal lineage, the absence or unavailability of this particular Eya-like co-factor enables another co-factor, most likely a member of the GRO gene family, to bind to the eh1 motifs of Six1a. This newly formed complex, in turn, recruits a histone deacetylase, which modifies histones, resulting in the compaction of chromatin in these regions, silencing genes and inhibiting the transcription of Six1a targets. This inhibition blocks the proliferation of cells in this lineage and triggers programmed cell death, leading to fewer SAG neurons. The different roles in these two lineages result in Six1a balancing the relative numbers of inner ear sensory cells and neurons.
Fig. 8.
Model of Six1a’s dual and opposing roles in the sensory and neuronal lineages of the developing zebrafish inner ear. In the sensory lineage, Six1a is able to interact with an Eya-like factor. They subsequently recruit an activator complex, leading to the transcriptional activation of Six1a targets, which, in turn, increases cell proliferation while decreasing programmed cell death. The result is the promotion of hair cell formation in the developing otocyst. Conversely, in the neuronal lineage, Six1a is able to interact with a Gro-like factor. They then recruit a repressor complex, leading to the transcriptional repression of Six1a targets, inhibiting cell proliferation and activating programmed cell death, resulting in an inhibition of neuron formation in the developing inner ear. Both roles require Six1a binding to DNA.
Six1a acts independently of Eya1 in the neuronal lineage of the developing zebrafish inner ear
To account for the dual function of Six1a, we currently favor a model (Fig. 8) in which Six1a preferentially binds to Eya1 over Gro1 when both are present. However, eya1, gro1, and six1a mRNAs are all expressed in both sensory and neuronal lineages, which suggests their protein products are as well (Bricaud and Collazo, 2006; Sahly et al., 1999; Wulbeck and Campos-Ortega, 1997). While having Eya1 expressed in the sensory lineage is consistent with our model, its expression in the neuronal lineage is more difficult to reconcile because it would seem to prevent Six1a from binding to Gro1 in this lineage. Interestingly, eya1 loss-of-function results in fewer sensory hair cells and SAG neurons, which indicates that it is necessary in both lineages (Kozlowski et al., 2005). We would argue that Eya1 and Six1a most likely function independently of one another in the neuronal lineage.
The reasons for the presumed lack of interaction between Eya1 and Six1a in the neuronal lineage are unknown. However, three possible reasons are: (1) a co-factor for which Eya1 has more affinity than Six1a is present in the neuronal lineage, preventing Eya1 binding to Six1a and allowing Gro1 to bind Six1a in this lineage; (2) either Six1a, Eya1, or both, are modified at the post-translational level (for example, by phosphorylation or methylation) in the neuronal lineage and such modifications prevent their mutual interactions, leaving Six1a available to interact with Gro1; or (3) the Eya1-Six1a complex is more labile in vivo than it is in vitro and such a complex needs to be stabilized by a third partner only expressed in the sensory lineage but absent in the neuronal lineage, such Eya1 co-factors have been recently characterized in mice and zebrafish (Landgraf et al. 2010).
While we emphasize Six1a interaction with Eya1 in the sensory hair cell lineage there are other eya genes expressed in the developing inner ear and Six1a could be interacting with another Eya protein. For example, eya4 is also expressed in the developing zebrafish inner ear and eya4 loss-of-function results in fewer hair cells (Schonberger et al., 2005; Wang et al., 2008). The effect of eya4 loss-of-function on the formation of SAG neurons has not been studied (Wang et al., 2008). Although Eya4 can interact physically with Six1 in both mouse and zebrafish, Eya4-Six1 complexes have only weak transcriptional activity, making Eya4 an unlikely partner for Six1a in the developing zebrafish inner ear (Schonberger et al., 2005; Zhang et al., 2004).
Six genes act as switches between developmental programs within a morphogenetic field by modulating their transcriptional abilities
Six1a seems to function in opposite ways within two lineages of cells that arise at about the same time and from the same region, or morphogenetic field, of the developing inner ear. Single cell lineage analysis in the chicken inner ear showed that hair cells and SAG neurons share a common progenitor but more interesting was that this was only true for hair cells from the utricular macula which forms closest to the region from where neurons arise (Satoh and Fekete, 2005). While the interactions between Six and Eya or Six and Gro proteins have been reported in Drosophila and vertebrates (Giot et al., 2003; Ikeda et al., 2002; Ohto et al., 1999; Pignoni et al., 1997; Silver et al., 2003), new to this study is the revelation that six1a can both activate and repress transcription within a population of lineally related cells. Also of note is that Six1a and not some other cofactor provides the switch between these two functions, as it is the only gene known to differentially affect these two inner ear cell types. Such a dual function for Six1 has been suggested, based on indirect evidence, for ectoderm specification in Xenopus (Brugmann et al., 2004). During development, the region of ectoderm directly surrounding the neural plate gives rise to the intervening neural crest and the pre-placodal ectoderm (PPE) (Baker and Bronner-Fraser, 2001). This particular region of the ectoderm has been dubbed the lateral neurogenic ectoderm (LNE). In Xenopus, Six1, Eya1, and at least three members of the Gro family are expressed in a pattern consistent with their involvement in PPE formation (Choudhury et al., 1997; David et al., 2001; Molenaar et al., 2000; Pandur and Moody, 2000). Six1, in Xenopus, is necessary for promoting PPE fate and, concomitantly, inhibiting neural crest fates medially and epidermal fates laterally (Brugmann et al., 2004). Furthermore, the authors showed that PPE genes are transcriptionally activated by Six1, most likely through its interaction with Eya1, whereas neural crest and epidermal genes are transcriptionally repressed by Six1, likely through interactions with a Gro factor (Brugmann et al., 2004).
The Xenopus study and our present findings lead us to propose that Six proteins, and Six1 in particular, might have been co-opted during evolution to fulfill the role of molecular switches in morphogenetic fields where cells are faced with a binary choice. For instance, cells in the Xenopus LNE can adopt either a PPE or a neural crest fate, depending upon whether Six1 acts as a transcriptional activator or repressor. While we cannot rule out a role in cell differentiation, our data are more consistent with Six1a serving as a molecular switch driving a given cell population to undergo either proliferation or programmed cell death. In the developing inner ear, this means turning cell proliferation on and cell death off in the sensory lineage while turning cell death on and cell proliferation off in the neuronal lineage. The ultimate result of the dual and opposing roles for Six1a as a transcriptional activator and inhibitor is to balance the relative number of sensory hair cells and SAG neurons developing from the anterior-ventral otic epithelium.
Zebrafish can be used as a Branchio-Oto-Renal syndrome model
Branchio-Oto-Renal (BOR) syndrome is an autosomal dominant developmental disorder of kidney and urinary tract malformations with hearing loss (Melnick et al., 1976). Branchio-Oto (BO) syndrome is a related disorder without renal anomalies. BOR/BO syndromes show a prevalence of 1:40,000 in the general population and are responsible for 2% of profoundly deaf children (Fraser et al., 1980). The major feature of BOR syndrome is hearing loss (93% of patients), which can be conductive, sensorineural, or both and varies in age and onset (Hone and Smith, 2001). It has been demonstrated that BOR and BO syndromes can be caused by mutations in EYA1 and that there are multiple allelic variants (Abdelhak et al., 1997a; Abdelhak et al., 1997b; Vervoort et al., 2002; Vincent et al., 1997). However, approximately 60% of BOR patients do not have mutations in EYA1 (Kochhar et al., 2007). A genome-wide search for linkage to BOR syndrome uncovered a secondary locus responsible for BOR syndrome (Ruf et al., 2003). This particular locus encompasses SIX1, SIX4 and SIX6 (Ruf et al., 2003). By sequencing genes from this locus in BOR patients with no mutation in the EYA1 gene, the authors were able to uncover 3 mutations in the SIX1 gene, thus identifying SIX1 as another gene whose mutation can cause BOR/BO syndromes (Ruf et al., 2004). Two of the mutations were found to be located in the DNA-binding domain and the third mutation in the prospective EYA-binding domain, demonstrating that perturbations of the EYA1-SIX1 transcriptional complex can result in BOR/BO syndromes (Ruf et al., 2004).
The three mutations found in SIX1 in BOR patients are the same ones we used in the present studies to assay the transcriptional function of Six1a in zebrafish otic development, thus, validating the use of zebrafish as a BOR/BO animal model. Recently, five more mutations in SIX1 have been sequenced in BOR patients (Kochhar et al., 2008). However, the molecular effects of these mutations remain largely unknown (Patrick et al., 2009). Studying the behavior of these mutations in the context of the development of the zebrafish inner ear as assayed here, should yield interesting insights on not only the role of Six1a in the development of the zebrafish inner ear but also on the molecular mechanisms responsible for causing BOR/BO syndromes.
Supplementary Material
Acknowledgments
This work was supported by RO1 Grant DC004061 to A. Collazo from the National Institutes of Health (NIH) and National Institute for Deafness and Other Communication Disorders (NIDCD). This work was also supported by the Oberkotter Foundation. We thank the members of Andy K. Groves, Neil Segil and Andres Collazo laboratories for their stimulating discussions of the data presented here. We also thank Drs. Vincent T. Cunliffe and S. Silver for generously providing us with the hdac1hi1618 embryos and pARE-luciferase plasmid, respectively. Core resources were provided by the Ahmanson Foundation and NIH NIDCD P-30 grant DC006276 to D. J. Lim.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Levi-Acobas F, Cruaud C, Le Merrer M, Mathieu M, Konig R, Vigneron J, Weissenbach J, Petit C, Weil D. Clustering of mutations responsible for branchio-oto-renal (BOR) syndrome in the eyes absent homologous region (eyaHR) of EYA1. Hum Mol Genet. 1997a;6:2247–55. doi: 10.1093/hmg/6.13.2247. [DOI] [PubMed] [Google Scholar]
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, Bitner-Glindzicz M, Francis M, Lacombe D, Vigneron J, Charachon R, Boven K, Bedbeder P, Van Regemorter N, Weissenbach J, Petit C. A human homologue of the Drosophila eyes absent gene underlies branchio-oto-renal (BOR) syndrome and identifies a novel gene family. Nat Genet. 1997b;15:157–64. doi: 10.1038/ng0297-157. [DOI] [PubMed] [Google Scholar]
- Andermann P, Ungos J, Raible DW. Neurogenin1 Defines Zebrafish Cranial Sensory Ganglia Precursors. Dev Biol. 2002;251:45–58. doi: 10.1006/dbio.2002.0820. [DOI] [PubMed] [Google Scholar]
- Baker CV, Bronner-Fraser M. Vertebrate cranial placodes I. Embryonic induction. Dev Biol. 2001;232:1–61. doi: 10.1006/dbio.2001.0156. [DOI] [PubMed] [Google Scholar]
- Bessarab DA, Chong SW, Srinivas BP, Korzh V. Six1a is required for the onset of fast muscle differentiation in zebrafish. Dev Biol. 2008;323:216–28. doi: 10.1016/j.ydbio.2008.08.015. [DOI] [PubMed] [Google Scholar]
- Bricaud O, Chaar V, Dambly-Chaudiere C, Ghysen A. Early efferent innervation of the zebrafish lateral line. J Comp Neurol. 2001;434:253–61. doi: 10.1002/cne.1175. [DOI] [PubMed] [Google Scholar]
- Bricaud O, Collazo A. The transcription factor six1 inhibits neuronal and promotes hair cell fate in the developing zebrafish (Danio rerio) inner ear. J Neurosci. 2006;26:10438–51. doi: 10.1523/JNEUROSCI.1025-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugmann SA, Pandur PD, Kenyon KL, Pignoni F, Moody SA. Six1 promotes a placodal fate within the lateral neurogenic ectoderm by functioning as both a transcriptional activator and repressor. Development. 2004;131:5871–81. doi: 10.1242/dev.01516. [DOI] [PubMed] [Google Scholar]
- Chen G, Courey AJ. Groucho/TLE family proteins and transcriptional repression. Gene. 2000;249:1–16. doi: 10.1016/s0378-1119(00)00161-x. [DOI] [PubMed] [Google Scholar]
- Chen P, Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–90. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen P, Zindy F, Abdala C, Liu F, Li X, Roussel MF, Segil N. Progressive hearing loss in mice lacking the cyclin-dependent kinase inhibitor Ink4d. Nat Cell Biol. 2003;5:422–6. doi: 10.1038/ncb976. [DOI] [PubMed] [Google Scholar]
- Choudhury BK, Kim J, Kung HF, Li SS. Cloning and developmental expression of Xenopus cDNAs encoding the Enhancer of split groucho and related proteins. Gene. 1997;195:41–8. doi: 10.1016/s0378-1119(97)00150-9. [DOI] [PubMed] [Google Scholar]
- Cunliffe VT. Histone deacetylase 1 is required to repress Notch target gene expression during zebrafish neurogenesis and to maintain the production of motoneurones in response to hedgehog signalling. Development. 2004;131:2983–2995. doi: 10.1242/dev.01166. [DOI] [PubMed] [Google Scholar]
- David R, Ahrens K, Wedlich D, Schlosser G. Xenopus Eya1 demarcates all neurogenic placodes as well as migrating hypaxial muscle precursors. Mech Dev. 2001;103:189–92. doi: 10.1016/s0925-4773(01)00355-0. [DOI] [PubMed] [Google Scholar]
- de Ruijter AJ, van Gennip AH, Caron HN, Kemp S, van Kuilenburg AB. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–49. doi: 10.1042/BJ20021321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fekete DM, Wu DK. Revisiting cell fate specification in the inner ear. Curr Opin Neurobiol. 2002;12:35–42. doi: 10.1016/s0959-4388(02)00287-8. [DOI] [PubMed] [Google Scholar]
- Finley JR, Xia B, Corwin JT. Monoclonal antibodies raised as markers for supporting cells and hair cells. Assoc Res Otolaryngol Abstr. 1997;20:134. [Google Scholar]
- Fraser FC, Sproule JR, Halal F. Frequency of the branchio-oto-renal (BOR) syndrome in children with profound hearing loss. Am J Med Genet. 1980;7:341–9. doi: 10.1002/ajmg.1320070316. [DOI] [PubMed] [Google Scholar]
- Fritzsch B, Barald KF, Lomax MI. Early embryology of the vertebrate ear. In: Rubel EW, Popper AN, Fay RR, editors. Development of the Auditory System. Vol. 9. Springer; New York: 1998. pp. 80–145. [Google Scholar]
- Fritzsch B, Beisel KW, Jones K, Farinas I, Maklad A, Lee J, Reichardt LF. Development and evolution of inner ear sensory epithelia and their innervation. J Neurobiol. 2002;53:143–56. doi: 10.1002/neu.10098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale JE, Meyers JR, Corwin JT. Solitary hair cells are distributed throughout the extramacular epithelium in the bullfrog’s saccule. J Assoc Res Otolaryngol. 2000;1:172–82. doi: 10.1007/s101620010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giot L, Bader JS, Brouwer C, Chaudhuri A, Kuang B, Li Y, Hao YL, Ooi CE, Godwin B, Vitols E, Vijayadamodar G, Pochart P, Machineni H, Welsh M, Kong Y, Zerhusen B, Malcolm R, Varrone Z, Collis A, Minto M, Burgess S, McDaniel L, Stimpson E, Spriggs F, Williams J, Neurath K, Ioime N, Agee M, Voss E, Furtak K, Renzulli R, Aanensen N, Carrolla S, Bickelhaupt E, Lazovatsky Y, DaSilva A, Zhong J, Stanyon CA, Finley RL, Jr, White KP, Braverman M, Jarvie T, Gold S, Leach M, Knight J, Shimkets RA, McKenna MP, Chant J, Rothberg JM. A protein interaction map of Drosophila melanogaster. Science. 2003;302:1727–36. doi: 10.1126/science.1090289. [DOI] [PubMed] [Google Scholar]
- Golling G, Amsterdam A, Sun Z, Antonelli M, Maldonado E, Chen W, Burgess S, Haldi M, Artzt K, Farrington S, Lin SY, Nissen RM, Hopkins N. Insertional mutagenesis in zebrafish rapidly identifies genes essential for early vertebrate development. Nat Genet. 2002 doi: 10.1038/ng896. [DOI] [PubMed] [Google Scholar]
- Goodyear RJ, Legan PK, Christiansen JR, Xia B, Korchagina J, Gale JE, Warchol ME, Corwin JT, Richardson GP. Identification of the hair cell soma-1 Antigen, HCS-1, as Otoferlin. J Assoc Res Otolaryngol. 2010;11:573–86. doi: 10.1007/s10162-010-0231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddon C, Lewis J. Early ear development in the embryo of the zebrafish, Danio rerio. J Comp Neurol. 1996;365:113–28. doi: 10.1002/(SICI)1096-9861(19960129)365:1<113::AID-CNE9>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Hone SW, Smith RJ. Genetics of hearing impairment. Semin Neonatol. 2001;6:531–41. doi: 10.1053/siny.2001.0094. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ. How the ear’s works work. Nature. 1989;341:397–404. doi: 10.1038/341397a0. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Watanabe Y, Ohto H, Kawakami K. Molecular interaction and synergistic activation of a promoter by Six, Eya, and Dach proteins mediated through CREB binding protein. Mol Cell Biol. 2002;22:6759–66. doi: 10.1128/MCB.22.19.6759-6766.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami K, Sato S, Ozaki H, Ikeda K. Six family genes--structure and function as transcription factors and their roles in development. Bioessays. 2000;22:616–26. doi: 10.1002/1521-1878(200007)22:7<616::AID-BIES4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Nishikawa K, Suzuki T, Yamamoto M. The homeobox protein Six3 interacts with the Groucho corepressor and acts as a transcriptional repressor in eye and forebrain formation. Dev Biol. 2001;232:315–26. doi: 10.1006/dbio.2001.0185. [DOI] [PubMed] [Google Scholar]
- Kochhar A, Fischer SM, Kimberling WJ, Smith RJ. Branchio-oto-renal syndrome. Am J Med Genet A. 2007;143A:1671–8. doi: 10.1002/ajmg.a.31561. [DOI] [PubMed] [Google Scholar]
- Kochhar A, Orten DJ, Sorensen JL, Fischer SM, Cremers CW, Kimberling WJ, Smith RJ. SIX1 mutation screening in 247 branchio-oto-renal syndrome families: a recurrent missense mutation associated with BOR. Hum Mutat. 2008;29:565. doi: 10.1002/humu.20714. [DOI] [PubMed] [Google Scholar]
- Kozlowski DJ, Whitfield TT, Hukriede NA, Lam WK, Weinberg ES. The zebrafish dog-eared mutation disrupts eya1, a gene required for cell survival and differentiation in the inner ear and lateral line. Dev Biol. 2005;277:27–41. doi: 10.1016/j.ydbio.2004.08.033. [DOI] [PubMed] [Google Scholar]
- Laclef C, Souil E, Demignon J, Maire P. Thymus, kidney and craniofacial abnormalities in Six1 deficient mice. Mech Dev. 2003;120:669–79. doi: 10.1016/s0925-4773(03)00065-0. [DOI] [PubMed] [Google Scholar]
- Lagutin OV, Zhu CC, Kobayashi D, Topczewski J, Shimamura K, Puelles L, Russell HR, McKinnon PJ, Solnica-Krezel L, Oliver G. Six3 repression of Wnt signaling in the anterior neuroectoderm is essential for vertebrate forebrain development. Genes Dev. 2003;17:368–79. doi: 10.1101/gad.1059403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf K, Bollig F, Trowe MO, Besenbeck B, Ebert C, Kruspe D, Kispert A, Hanel F, Englert C. Sipl1 and Rbck1 are novel Eya1-binding proteins with a role in craniofacial development. Mol Cell Biol. 2010;30:5764–75. doi: 10.1128/MCB.01645-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, Nigam SK, Aggarwal AK, Maas R, Rose DW, Rosenfeld MG. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–54. doi: 10.1038/nature02083. [DOI] [PubMed] [Google Scholar]
- Lopez-Rios J, Tessmar K, Loosli F, Wittbrodt J, Bovolenta P. Six3 and Six6 activity is modulated by members of the groucho family. Development. 2003;130:185–95. doi: 10.1242/dev.00185. [DOI] [PubMed] [Google Scholar]
- Marks PA, Miller T, Richon VM. Histone deacetylases. Curr Opin Pharmacol. 2003;3:344–51. doi: 10.1016/s1471-4892(03)00084-5. [DOI] [PubMed] [Google Scholar]
- Marusich MF, Furneaux HM, Henion PD, Weston JA. Hu neuronal proteins are expressed in proliferating neurogenic cells. J Neurobiol. 1994;25:143–55. doi: 10.1002/neu.480250206. [DOI] [PubMed] [Google Scholar]
- Melnick M, Bixler D, Nance WE, Silk K, Yune H. Familial branchio-oto-renal dysplasia: a new addition to the branchial arch syndromes. Clin Genet. 1976;9:25–34. doi: 10.1111/j.1399-0004.1976.tb01546.x. [DOI] [PubMed] [Google Scholar]
- Miller CT, Maves L, Kimmel CB. moz regulates Hox expression and pharyngeal segmental identity in zebrafish. Development. 2004;131:2443–2461. doi: 10.1242/dev.01134. [DOI] [PubMed] [Google Scholar]
- Molenaar M, Brian E, Roose J, Clevers H, Destree O. Differential expression of the Groucho-related genes 4 and 5 during early development of Xenopus laevis. Mech Dev. 2000;91:311–5. doi: 10.1016/s0925-4773(99)00259-2. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–20. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Ohto H, Kamada S, Tago K, Tominaga SI, Ozaki H, Sato S, Kawakami K. Cooperation of six and eya in activation of their target genes through nuclear translocation of Eya. Mol Cell Biol. 1999;19:6815–24. doi: 10.1128/mcb.19.10.6815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandur PD, Moody SA. Xenopus Six1 gene is expressed in neurogenic cranial placodes and maintained in the differentiating lateral lines. Mech Dev. 2000;96:253–7. doi: 10.1016/s0925-4773(00)00396-8. [DOI] [PubMed] [Google Scholar]
- Patrick AN, Schiemann BJ, Yang K, Zhao R, Ford HL. Biochemical and Functional Characterization of Six SIX1 Branchio-oto-renal Syndrome Mutations. J Biol Chem. 2009;284:20781–90. doi: 10.1074/jbc.M109.016832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignoni F, Hu B, Zavitz KH, Xiao J, Garrity PA, Zipursky SL. The eye-specification proteins So and Eya form a complex and regulate multiple steps in Drosophila eye development. Cell. 1997;91:881–91. doi: 10.1016/s0092-8674(00)80480-8. [DOI] [PubMed] [Google Scholar]
- Rubel EW, Fritzsch B. Auditory system development: primary auditory neurons and their targets. Annu Rev Neurosci. 2002;25:51–101. doi: 10.1146/annurev.neuro.25.112701.142849. [DOI] [PubMed] [Google Scholar]
- Ruf RG, Berkman J, Wolf MT, Nurnberg P, Gattas M, Ruf EM, Hyland V, Kromberg J, Glass I, Macmillan J, Otto E, Nurnberg G, Lucke B, Hennies HC, Hildebrandt F. A gene locus for branchio-otic syndrome maps to chromosome 14q21.3-q24.3. J Med Genet. 2003;40:515–9. doi: 10.1136/jmg.40.7.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruf RG, Xu PX, Silvius D, Otto EA, Beekmann F, Muerb UT, Kumar S, Neuhaus TJ, Kemper MJ, Raymond RM, Jr, Brophy PD, Berkman J, Gattas M, Hyland V, Ruf EM, Schwartz C, Chang EH, Smith RJ, Stratakis CA, Weil D, Petit C, Hildebrandt F. SIX1 mutations cause branchio-oto-renal syndrome by disruption of EYA1-SIX1-DNA complexes. Proc Natl Acad Sci U S A. 2004;101:8090–5. doi: 10.1073/pnas.0308475101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahly I, Andermann P, Petit C. The zebrafish eya1 gene and its expression pattern during embryogenesis. Dev Genes Evol. 1999;209:399–410. doi: 10.1007/s004270050270. [DOI] [PubMed] [Google Scholar]
- Satoh T, Fekete DM. Clonal analysis of the relationships between mechanosensory cells and the neurons that innervate them in the chicken ear. Development. 2005;132:1687–97. doi: 10.1242/dev.01730. [DOI] [PubMed] [Google Scholar]
- Schlosser G, Awtry T, Brugmann SA, Jensen ED, Neilson K, Ruan G, Stammler A, Voelker D, Yan B, Zhang C, Klymkowsky MW, Moody SA. Eya1 and Six1 promote neurogenesis in the cranial placodes in a SoxB1-dependent fashion. Dev Biol. 2008;320:199–214. doi: 10.1016/j.ydbio.2008.05.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonberger J, Wang L, Shin JT, Kim SD, Depreux FF, Zhu H, Zon L, Pizard A, Kim JB, Macrae CA, Mungall AJ, Seidman JG, Seidman CE. Mutation in the transcriptional coactivator EYA4 causes dilated cardiomyopathy and sensorineural hearing loss. Nat Genet. 2005;37:418–422. doi: 10.1038/ng1527. [DOI] [PubMed] [Google Scholar]
- Silver SJ, Davies EL, Doyon L, Rebay I. Functional dissection of eyes absent reveals new modes of regulation within the retinal determination gene network. Mol Cell Biol. 2003;23:5989–99. doi: 10.1128/MCB.23.17.5989-5999.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vervoort VS, Smith RJ, O’Brien J, Schroer R, Abbott A, Stevenson RE, Schwartz CE. Genomic rearrangements of EYA1 account for a large fraction of families with BOR syndrome. Eur J Hum Genet. 2002;10:757–66. doi: 10.1038/sj.ejhg.5200877. [DOI] [PubMed] [Google Scholar]
- Vincent C, Kalatzis V, Abdelhak S, Chaib H, Compain S, Helias J, Vaneecloo FM, Petit C. BOR and BO syndromes are allelic defects of EYA1. Eur J Hum Genet. 1997;5:242–6. [PubMed] [Google Scholar]
- Wang L, Sewell WF, Kim SD, Shin JT, MacRae CA, Zon LI, Seidman JG, Seidman CE. Eya4 regulation of Na+/K+-ATPase is required for sensory system development in zebrafish. Development. 2008;135:3425–34. doi: 10.1242/dev.012237. [DOI] [PubMed] [Google Scholar]
- Westerfield M. A Guide for the Laboratory Use of Zebrafish (Danio rerio) University of Oregon Press; Eugene, OR: 1995. The Zebrafish Book. [Google Scholar]
- Wulbeck C, Campos-Ortega JA. Two zebrafish homologues of the Drosophila neurogenic gene groucho and their pattern of transcription during early embryogenesis. Dev Genes Evol. 1997;207:156–166. doi: 10.1007/s004270050103. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Knosp BM, Maconochie M, Friedman RA, Smith RJ. A comparative study of Eya1 and Eya4 protein function and its implication in branchio-oto-renal syndrome and DFNA10. J Assoc Res Otolaryngol. 2004;5:295–304. doi: 10.1007/s10162-004-4044-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Huang L, Wei ZB, Silvius D, Tang B, Xu PX. The role of Six1 in mammalian auditory system development. Development. 2003;130:3989–4000. doi: 10.1242/dev.00628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CC, Dyer MA, Uchikawa M, Kondoh H, Lagutin OV, Oliver G. Six3-mediated auto repression and eye development requires its interaction with members of the Groucho-related family of co-repressors. Development. 2002;129:2835–49. doi: 10.1242/dev.129.12.2835. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.