Abstract
Wistar rats will self-administer cocaine directly into the nucleus accumbens shell (AcbSh), but not into the nucleus accumbens core. In human and animal literature, there is a genetic association between alcoholism and cocaine dependency. The current experiment examined whether selective breeding for high alcohol preference is also associated with greater sensitivity of the AcbSh to the reinforcing properties of cocaine. P and Wistar rats were given cocaine (0, 100, 200, 400, or 800 pmol/100 nl) to self-infuse into the AcbSh. Rats were given cocaine for the first 4 sessions (acquisition), artificial CSF for sessions 5 and 6 (extinction), and cocaine again in session 7 (reinstatement). During acquisition, P rats self-infused 200–800 pmol cocaine (59 infusions/session), whereas Wistar rats only reliably self-infused 800 pmol cocaine (38 infusions/session). Furthermore, P rats received a greater number of cocaine infusions in the 200, 400 and 800 pmol cocaine groups compared to respective Wistar groups during acquisition. Both P and Wistar rats reduced responding on the active lever when aCSF was substituted for cocaine, and reinstated responding in session 7 when cocaine was restored. However, P rats had significantly greater infusions during session 7 compared to session 4 at all concentrations of cocaine tested, whereas Wistar rats only displayed greater infusions during session 7 compared to session 4 at the 400 and 800 pmol cocaine concentrations. The present results suggest that, compared to Wistar rats, the AcbSh of P rats was more sensitive to the reinforcing effects of cocaine. The reinstatement data suggest that the AcbSh of P rats may have become sensitized to the reinforcing effects of cocaine. Overall, the findings from this study support a genetic association between high alcohol preference and greater sensitivity to the reinforcing effects of cocaine.
Keywords: intracranial self-administration, nucleus accumbens shell, reinforcement, alcohol-preferring rats, ethanol, cocaine
1. Introduction
Cocaine and alcohol are frequently co-abused. The majority of cocaine users (up to 90%) report co-administering EtOH during cocaine binges (Brookoff et al., 1996; Magura and Rosenblum, 2000). The high prevalence of co-abuse of alcohol with cocaine in humans has been postulated to be predicated upon both a common genetic factor that predispose an organism to abuse multiple substances, including alcohol, and the interaction of the drugs within the organism (Uhl 2004, 2006; Uhl et al., 2008). Individuals predisposed to abuse alcohol and other drugs of abuse are disproportionally reactive to alcohol and other drugs of abuse when given alone, and co-administration of alcohol and other drugs of abuse result in further divergence (Schuckit 1994a,b; Kareken et al., 2010; Uhl 2008; Piazza and LeMoal 1996).
In addition to previously mentioned literature, a number of studies have focused at directly assessing the genetic influence on alcohol dependency (AD) and cocaine dependency (CD) in humans. In a detailed COGA study, the rate of CD was approximately 2.5 fold higher in individuals with a genetic predisposition for alcoholism than the general population (Nurnberger et al., 2004). Similar findings were reported in a study that examined the effects of a family history of alcohol-related problems. For example, if a strict DSM-IV AD diagnosis was used for family history positive, the odds ratio was 1.6 for co-morbid CD and AD in family history positive individuals, or if the criterion for family positive was reduced to alcohol abuse and not AD, the odds ratio increased (Compton et al., 2002). In a twin study, there was strong linkage for familial factors between major depression, AD, and CD (Lin et al., 1996).
In humans, cocaine use increases the amount of alcohol consumed in polydrug users (Williamson et al., 1997). Conversely, alcohol consumption is associated with greater cocaine usage (Magura and Rosenblum, 2000). The rate of alcoholism in high-frequency cocaine users was approximately 60% compared to 37% percent in low-frequency cocaine users (Fox et al., 2005). Subjects with the diagnosis of alcohol dependence are more likely to become cocaine misusers and experience more adverse consequences of cocaine use (Heil et al., 2001; Staines et al., 2001). In addition, alcohol abuse is a common problem among cocaine dependent patients (Miller et al., 1989). Co-administration of alcohol during cocaine binges allows the user to prolong the euphoric effects and diminish the anxiogenic effects of cocaine (Williamson et al., 1997). Additionally, the likelihood to relapse to cocaine and/or alcohol use was greater in individuals who co-abused (Fox et al., 2005).
In animal studies, FAST and SLOW mice, selectively bred for their differential locomotor responses to ethanol, displayed parallel divergence for ethanol and cocaine (Bergstrom et al., 2003). Cocaine increases extracellular dopamine (DA) levels to a greater extent in the nucleus accumbens (Acb) and caudate-putamen in AA (Alko, Alcohol) rats compared to ANA (Alko nonalcohol) rats (Mikkola et al., 2001). In Wistar rats selected for high and low alcohol-preference, locomotor stimulation induced by cocaine was positively correlated with alcohol preference (Stromberg and Mackler, 2005). Additionally, high alcohol consuming Wistar rats were more sensitive to the reinforcing effects of cocaine than low alcohol consuming Wistar rats as measured by conditioned place preference (Stromberg and Mackler, 2005). Alcohol-preferring (P) rats are more resistant to extinguish cocaine self-administration and are more sensitive to a priming dose of cocaine to elicit cocaine-seeking behaviors than alcohol-nonpreferring (NP) rats (Le et al., 2006).
The intracranial self-administration (ICSA) technique has been used to identify specific brain regions involved in the initiation of response-contingent behaviors for the delivery of a reinforcer (Bozarth and Wise, 1980; Goeders and Smith, 1987; McBride et al., 1999). The ICSA procedure has successfully isolated discrete brain regions where opioids (Bozarth and Wise, 1981; Devine and Wise, 1994), amphetamine (Hoebel et al.,1983; Phillips et al., 1994), acetaldehyde (Rodd-Henricks et al., 2002), and ethanol (Gatto et al., 1994; Rodd-Henricks et al., 2000) produce their reinforcing effects. Previous ICSA research indicated that cocaine was self-administered into the medial prefrontal cortex (mPFC; Goeders and Smith, 1983) and posterior, but not anterior, ventral tegmental area (VTA; Rodd et al, 2005). Cocaine is self-administered by Wistar rats directly in the AcbSh, but not in the AcbC (Rodd-Henricks et al., 2002). In addition, ICSA studies have found that P rats have a greater sensitivity to the reinforcing actions of ethanol compared to Wistar rats in the AcbSh (Engleman et al., 2009).
The goal of the present study was to compare the dose-response effects for the self-infusion of cocaine into the AcbSh of selectively bred alcohol preferring (P) and Wistar rats. The Wistar rat is the founding stock of the P rat. The hypothesis to be tested is that selective breeding for high alcohol preference is also associated with increased sensitivity to the reinforcing effects of cocaine in the AcbSh.
2. Methods
2.1. Animals
Female P rats, from the 52nd and 53rd generations, and Wistar rats (Harlan, Indianapolis, IN) weighing 250–320 g at time of surgery were used. Female rats were used in the present study because (a) female rats were used in previous studies involving the ICSA of cocaine (Rodd et al., 2005; Rodd-Henricks et al., 2002, McKinzie et al., 1999), and (b) female rats appear to maintain their body weights and head size better than male rats for more accurate stereotaxic placements (Ikemoto et al., 1997a,b; Rodd-Henricks et al., 2000, 2002 and 2003). Rats were double-housed upon arrival and maintained on a 12-hr reverse light-dark cycle (lights off at 0900 hr). Although not systematically studied, the estrus cycle did not appear to have a significant effect on ICSA behavior in the present study, or in previous ICSA studies (Gatto et al., 1994; Ikemoto et al., 1997a,b; Rodd-Henricks et al., 2000; Rodd-Henricks et al., 2002; Rodd-Henricks et al., 2003), as indicated by no obvious fluctuations in self-adminstrations by female rats given the same dose over several consecutive sessions. Animals used in this study were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All research protocols were approved by the institutional animal care and use committee and are in accordance with the guidelines of the Institutional Care and Use Committee of the National Institute on Drug Abuse, NIH, and the Guide for the Care and Use of Laboratory Animals (National Research Council 1996).
2.2. Drug and Vehicle
The artificial cerebrospinal fluid (aCSF) consisted of (in mM): 120.0 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.2 Mg SO4, 25.0 NaHCO3, 2.5 CaCl2, and 10.0 d-glucose. Cocaine (Sigma) was dissolved in the aCSF solution. When necessary, 0.1 N NaOH was added to adjust the pH to 7.4 ± 0.1.
2.3. Animal Preparation
While under isoflurane anesthesia, a unilateral 22-gauge guide cannula (Plastic One) was stereotaxically implanted in the right hemisphere of each subject, aimed 1.0 mm above the target region. Coordinates (Paxinos and Watson, 1998) for placements into the AcbSh were 1.2 mm anterior to bregma, 2.1 mm lateral to the midline, and 8.0 mm ventral from the surface of the skull at a 10-degree angle to the vertical. Between experimental sessions, a 28-gauge stylet was placed into the guide cannula and extended 0.5 mm beyond the tip of the guide. Following surgery, all rats were individually housed and allowed to recover 7–10 days. Animals were handled for at least 5 min daily following the fourth recovery day. Subjects were not acclimated to the test chamber prior to the commencement of data collection, nor did they receive any prior operant training.
2.4. General Test Condition
Testing was conducted in standard two-lever operant chambers as previously described (Ikemoto et al., 1997b; Rodd-Henricks et al., 2002; Rodd et al., 2005). The electrolytic microinfusion transducer (EMIT) system has also been described in detail (Bozarth and Wise, 1980). For testing, subjects were brought to the testing room, the stylet was removed, and the injection cannula screwed into place. To avoid trapping air at the tip of the injection cannula, the infusion current was delivered for 5 sec during insertion of the injector, which resulted in a single non-contingent administration of infusate at the beginning of the session. Injection cannulae extended 1.0 mm beyond the tip of the guide. The test chamber was equipped with two levers. Depression of the ‘active lever’ (FR1 schedule of reinforcement) caused the delivery of a 100-nl bolus of infusate over 5 sec followed by a 5-sec time-out period. During both the 5-sec infusion period and 5-sec time-out period, responses on the active lever did not produce further infusions. Responses on the ‘inactive lever’ were recorded, but did not result in infusions. The assignment of active and inactive lever with respect to the left or right position was counterbalanced among subjects. The active and inactive levers remained the same for each rat throughout the experiment. No shaping technique was used to facilitate the acquisition of lever responses. The number of infusions and responses on the active and inactive lever were recorded. The duration of each test session was 4 hr and sessions occurred every other day.
2.5. Dose Response
P (n = 7–8/dose) and Wistar (n = 8–12/dose) rats were randomly assigned to one of five groups. A vehicle group received infusions of aCSF for all seven sessions. The other groups received infusions of 100, 200, 400 or 800 pmol/100 nl cocaine for the first four sessions. During the fifth and sixth sessions, all animals received infusions of aCSF (extinction sessions). On the seventh session (reinstatement), rats were allowed to respond for their originally assigned infusate. In the learning field, reinstatement by definition is the return of the availability of a reinforcer following a period of unavailability, typically following extinction training (Flaherty, 1985). Reinstatement should not be confused with ‘reinstatement of responding’, the animal model of drug-induced seeking developed by Stewart and Shaham (Shaham et al., 2003). In the current experiment, reinstatement as defined by the learning field was examined during session 7. A previous study indicated that stable responding on the cocaine lever was attained by sessions 3 and 4, extinction was reached within 2 sessions, and responding on the active lever was reinstated within one session when cocaine was restored (Rodd-Henricks et al. 2002). A total of 37 P rats and 52 Wistar rats completed the ICSA dose-response experiment into the AcbSh. In addition, 8 P rats had cannulae implanted aimed at the nucleus accumbens core (AcbC). These rats were allowed to self-infuse either 400 or 800 pmol cocaine.
2.6. Histology
At the termination of the experiment, 1% bromophenol blue (0.5 µl) was injected into the infusion site. Subsequently, the animals were given a fatal dose of Nembutal and then decapitated. Brains were removed and immediately frozen at −70° C. Frozen brains were subsequently equilibrated at −15°C in a cryostat microtome and then sliced into 40 µm sections. Sections were then stained with cresyl violet and examined under a light microscope for verification of the injection site using the rat brain atlas of Paxinos and Watson (1998).
2.7. Statistical Analysis
Data analysis consisted of a line × concentration × session mixed ANOVA, with a repeated measure of ‘session’, performed on the number of infusions. Additionally, for each individual group, lever discrimination was determined by type (active or inactive) × day mixed ANOVA with a repeated measure of ‘session’.
3. Results
The AcbSh and AcbC were defined by Paxinos and Watson (1998), and the injection sites are illustrated in Figure 1. For the P rats with cannula aimed at the AcbSh, there were 3 misses (all in the AcbC), and two (1 – 400 pmol, 1 – 800 pmol) were added into the control condition. For the P rats with cannula aimed at the AcbC, there was one placement in the AcbSh (1–400 pmol); these data were included in the AcbSh group. Misses in Wistar rats tended to be ventral to the AcbSh (n = 4), and no self-administration was observed. Data for the P rats self-administering cocaine into the AcbC were not included in the statistical analyses (average responses/session were 13 ± 5 and 11 ± 7 on the active and inactive levers, respectively, which is in agreement with findings previously reported by Rodd-Henricks et al. 2002). Placements within the AcbSh of P rats overlapped with and were similarly located to the placements within the AcbSh of the Wistar rats.
Figure 1.
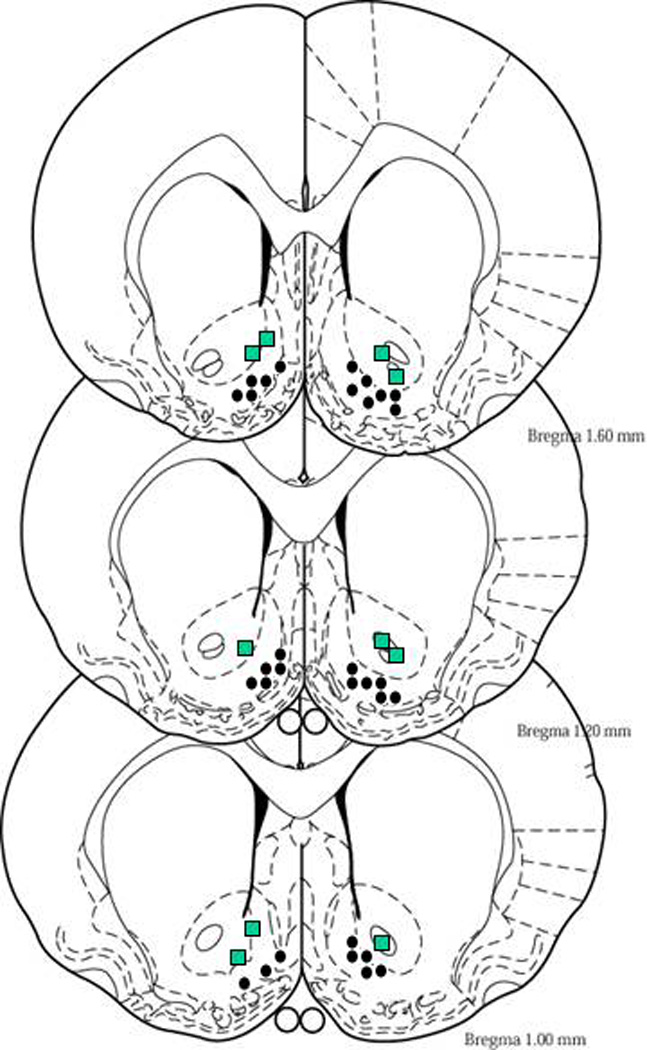
Representative injection sites in the AcbSh and AcbC of P and Wistar rats (overlapping sites are excluded). Circles represent sites of injection within the AcbSh and squares represent sites of injection within the AcbC. For illustrative purposes, injection sites for P and Wistar rats are depicted on the left and right sides of the figures, respectively.
The number of animals indicated for each experiment represents 96 % of the total number that underwent surgery; about 4 % of the animals were not included for analyses mainly due to the loss of the guide cannula before completion of all experimental sessions. The data for these animals were not used because their injection sites could not be verified.
Cocaine concentrations of 100, 200, 400 and 800 pmol/100 nl were tested in the present study to determine the response-contingent behaviors of P and Wistar rats (Fig. 2). The overall analysis examined the number of infusions received across all 7 sessions and indicated that there was a significant effect of line (F1,79 = 110.6; p < 0.0001), concentration (F4,79 = 64.6; p < 0.0001), session (F6,74 = 77.6; p < 0.0001), and a line × concentration × session interaction (F24,308 = 3.8; p < 0.0001). Reducing the analysis to the average number of infusions received during the initial 4 sessions (acquisition; Fig. 2) revealed that there was a significant effect of line (F1,79 = 84.5; p < 0.0001), concentration (F4,79 = 58.0; p < 0.0001), and a line × concentration interaction (F4,79 = 12.9; p < 0.0001). In the P rats, there was a significant effect of cocaine concentration (F4,32 = 24.5; p < 0.0001), with post-hoc comparisons (Tukey; p < 0.05) indicating that P rats self-administering 200, 400 or 800 pmol cocaine received significantly more infusions than P rats self-administering 0 or 100 pmol cocaine, and that P rats self-administering 400–800 pmol cocaine self-infused more than P rats self-administering 200 pmol cocaine.
Figure 2.
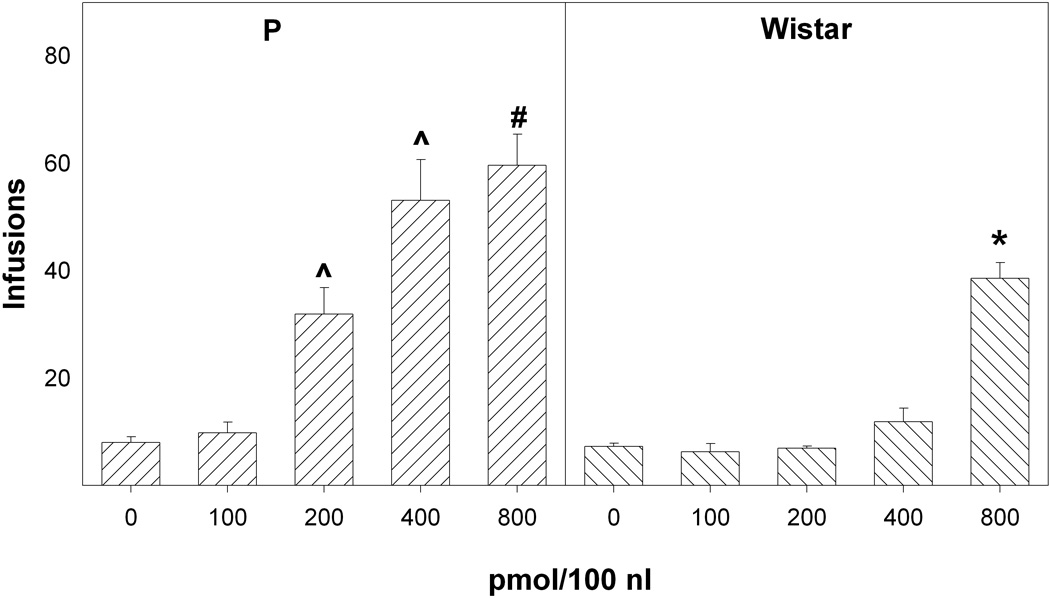
The effects of the concentration of cocaine infused into the nucleus accumbens shell on the mean (± SEM) number of infusions received over the initial 4 sessions (acquisition) by P (left panel) and Wistar (right panel) rats. ^ indicates a significantly higher number of infusions than the 0 or 100 pmol/100 nl groups by P rats. # indicates significantly more infusions compared to the 200 pmol/100 nl group by P rats. * indicates significantly higher infusions compared to the 0, 100, 200 and 400 pmol/100 nl groups by Wistar rats. ^ also indicates significantly greater infusions by P rats compared to Wistar rats for the 200, 400 and 800 pmol/100 nl concentrations of cocaine.
For Wistar rats, there was a significant effect of cocaine concentration (F4,47 = 48.9; p < 0.001), with post-hoc comparisons (Tukey; p < 0.05) indicating that Wistar rats self-administering 800 pmol cocaine received significantly more infusions than Wistar rats self-administering all other concentrations. Additionally, the interaction term was further examined by comparing the number of infusions received for each cocaine concentration between the P and Wistar rats. There were no significant differences between P and Wistar rats self-administering 0, or 100 pmol cocaine (F values1, 16 < 2.0; p values > 0.18). However, for rats self-administering 200, 400, or 800 pmol cocaine, there were significant differences between P and Wistar rats (F values1, 16 > 11.5; p values < 0.005), with P rats having higher self-administrations (Fig. 2).
Throughout the sessions, the number of lever presses by the aCSF group for both P and Wistar rats did not differ (p = 0.46) between the active and inactive lever (Fig. 3). Overall, statistical analysis of the active lever responses indicated there was a significant effect of line (F1,79 = 83.1; p < 0.0001), concentration (F4,79 = 54.5; p < 0.0001), session (F6,74 = 66.8; p < 0.0001), and a line × concentration × session interaction (F24,308 = 4.1; p < 0.0001). Active lever responses (Figs. 3 and 4; left panels) were higher in P rats self-administering 200, 400 and 800 pmol/100 nl cocaine than in P rats self-administering aCSF or 100 pmol/100 nl cocaine during sessions 1–4 (F values4,32 > 12.1; p values < 0.001; Tukey’s b p < 0.05). In sessions 5 and 6, when aCSF was substituted for cocaine, P rats in the 200–800 pmol/100 nl cocaine groups displayed reduced responding on the active lever. In P rats self-administering 200–800 pmol/100 nl cocaine (Figs. 3 and 4; left panels), there was a significant effect of session when contrasting the active lever responses among sessions 4, 5, and 6 (F values2,6 > 12.6; p values < 0.007). Post-hoc comparisons indicated that active lever responses were lower during session 5 and 6 than during session 4. In session 7, when cocaine was restored, P rats self-administering 100–800 pmol/100 nl cocaine increased the number of active lever responses compared to session 6 (F values1,6 > 22.9; p < 0.001). In addition, responses on the active lever for P rats given 100–400 pmol/100 nl cocaine were significantly (F values1,7 > 17.2; p < 0.004) higher in session 7 than session 4 (Figs. 3 and 4; left panels).
Figure 3.
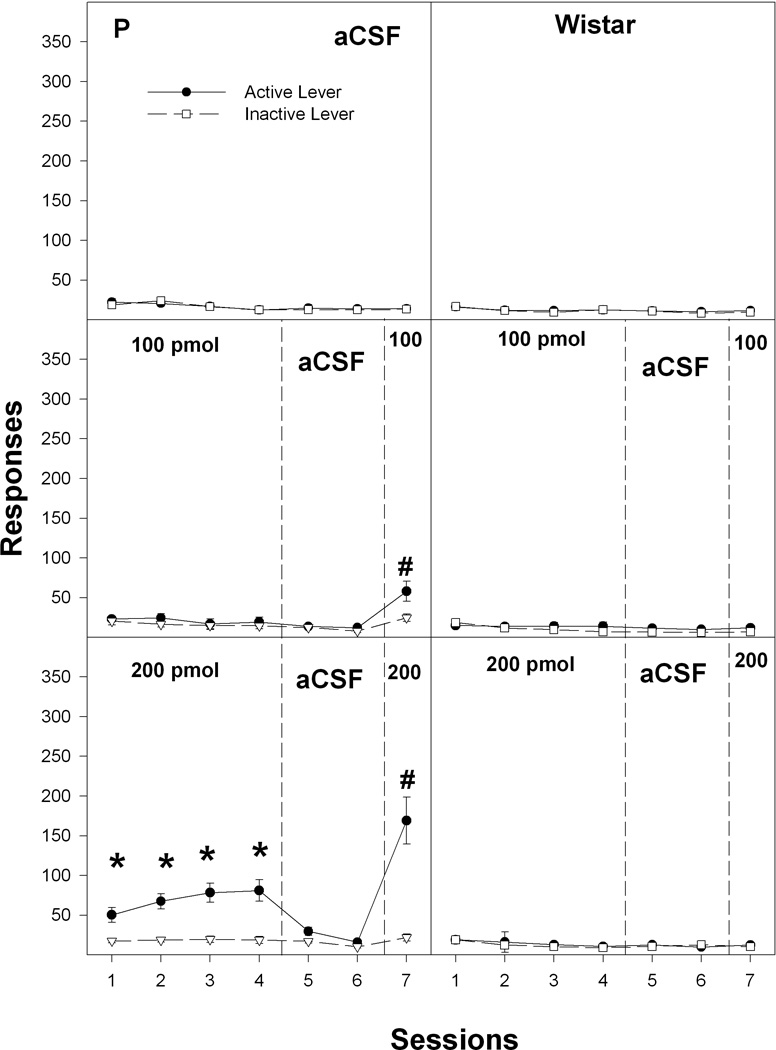
The effects of the concentration of cocaine infused into the nucleus accumbens shell on the mean (± SEM) number of responses on the active and inactive levers by P (left panel) and Wistar (right panel) rats for the 0, 100 and 200 pmol/100 nl groups. * indicates significantly greater active lever responses by P rats for the 200 pmol/100 nl group compared to respective inactive lever responses and compared to active lever responses for the 0 or 100 pmol/100 nl groups during sessions 1–4. # indicates greater responses during session 7 compared to session 4 by P rats for both the 100 and 200 pmol/100 nl groups and greater responses compared to the aCSF (0 pmol/100 nl) group.
Figure 4.
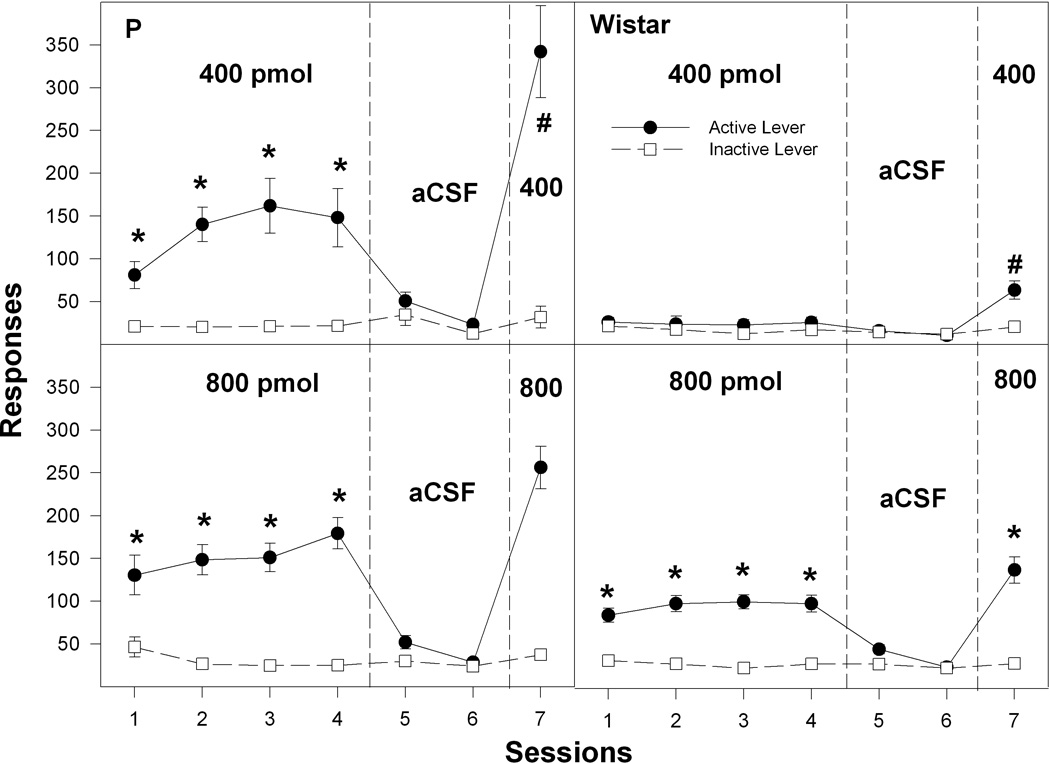
The effects of the cocaine infused into the nucleus accumbens shell on the mean (± SEM) number of responses on the active and inactive levers by P (left panel) and Wistar (right panel) rats for the 400 and 800 pmol/100 nl groups. * indicates significantly greater active lever responses by P rats compared to respective inactive lever responses and greater active lever responses for the 400 and 800 pmol/100 nl group compared to active lever responses for 0 or 100 pmol/100 nl groups during sessions 1–4. * also indicates significantly greater active lever responses by Wistar rats (right panels) for the 800 pmol/100 nl group compared to the 0, 100, 200 and 400 pmol/100 nl groups during sessions 1–4 and session 7. # indicates significantly greater active lever responses during session 7 compared to session 4 by P rats for the 400 pmol/100 nl group. # also indicates significantly greater active lever responses during session 7 compared to session 4 by Wistar rats for the 400 pmol/100 nl group.
Active lever responses (Figs. 3 and 4; right panels) were higher in Wistar rats self-administering only 800 pmol/100 nl cocaine than in Wistar rats self-administering aCSF or any other concentration of cocaine during session 1–4 (F values4,51 > 28.7; p values < 0.0001; post-hocs Tukey’s b p < 0.05). Additionally, Wistar rats self-administering 800 pmol/100 nl cocaine (Fig. 4; right panels) reduced responding on the active lever when aCSF was substituted for Cocaine (session 4 vs sessions 5 and 6, F values2,6 > 50.8; p values < 0.0001). Wistar rats, self-administering 400 or 800 pmol/100 nl cocaine, also reinstated active lever responding when Cocaine was returned (session 6 vs 7, F values1,7 > 87.8; p < 0.0001). In addition, responses on the active lever for Wistar rats given 400 pmol/100 nl cocaine were significantly (F1,11 = 24.2; p < 0.001) higher in session 7 than session 4 (Fig. 4).
A line × concentration × day mixed ANOVA, with a repeated measure of ‘session’, performed on the number of infusions self-administered during session 4 and 7 revealed a significant effect of line (F1,79 = 123.7; p < 0.0001), concentration (F4,79 = 56.5; p < 0.0001), session (F1,79 = 209.9; p < 0.0001), and a line × concentration × session interaction (F4,79 = 10.8; p < 0.0001). For Wistar rats (Fig. 5, right panel), the number of infusions received during session 7 was greater than infusions during session 4 in the gr 800 pmol/100 nl cocaine (session: Fvalues 1, 11 > 21.4; p values < 0.001). For P rats (Fig 5, left panel), the number of infusions was higher in sessions 7 compared to session 4 for all cocaine concentrations (F values 1,6 > 42.3; p values < 0.001).
Figure 5.
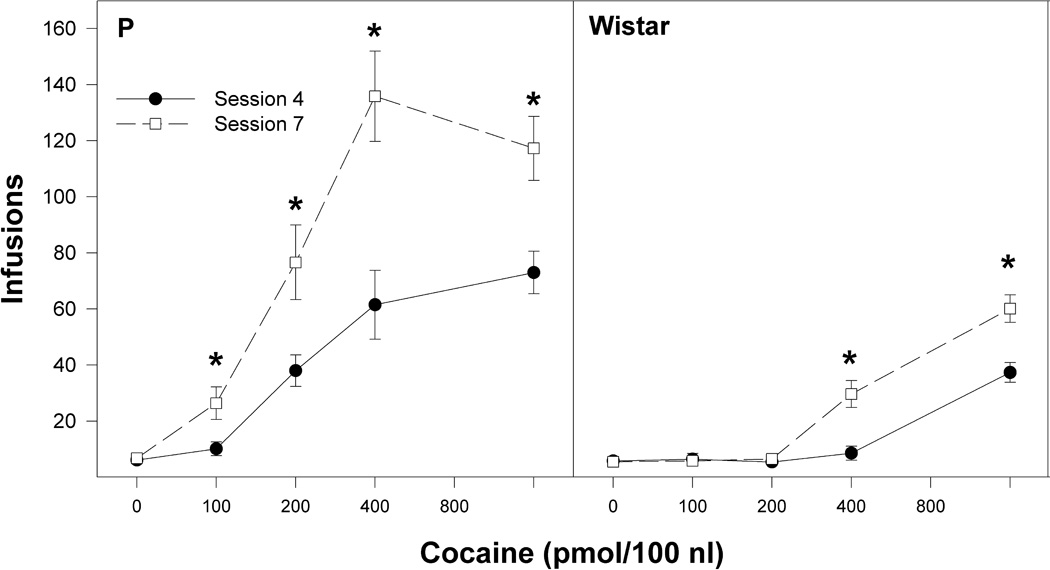
The effects of the concentration of cocaine (0, 100, 200, 400 and 800 pmol/100 nl) infused into the nucleus accumbens shell on the average (± SEM) number of infusions received during sessions 4 (acquisition) and 7 (reinstatement) by P (left panel) and Wistar (right panel) rats. * indicates significantly greater infusions during session 7 by P rats (left panel) for the 100, 200, 400 and 800 pmol/100 nl groups compared to session 4, and greater infusions during session 7 by Wistar rats (right panel) for the 400 and 800 pmol/100 nl groups compared to session 4.
4. Discussion
The present findings indicate that both P and Wistar rats will acquire and maintain the self-administration of cocaine directly into the AcbSh (Figs 2, 3 and 4), which suggests that the AcbSh is critically involved in the reinforcing effects of cocaine. Furthermore, the current results support previous findings in our laboratory that Wistar rats will initiate and maintain the self-infusion of cocaine into the AcbSh, but not the AcbC (Rodd-Henricks et al 2002). The self-infusion of cocaine into the AcbSh does not appear to be a result of a general increase in behavioral activity, because rats in this study learned to discriminate the active from the inactive lever for the self-infusion of 200, 400 and 800 pmol cocaine for P rats and 800 pmol cocaine for Wistar rats (Figs. 3 and 4). Regardless of line, animals that demonstrated significant lever discrimination also displayed decreased responding on the active lever when aCSF was substituted for cocaine and reinstated responding when the cocaine was restored (Figs 3, 4 and 5). A genetic effect was also apparent because P rats demonstrated greater sensitivity to cocaine, as indicated by self-infusion of 200, 400 and 800 pmol into the AcbSh, whereas Wistar rats did not demonstrate self-administration until 800 pmol was given (Fig. 2). In our previous study (Rodd-Henricks et al., 2002), Wistar rats infused 400 pmol cocaine at a slightly higher rate than aCSF (15 ± 4.2 vs 4.4 ± 3.1 infusions), but was significantly lower than Wistar rats self-administrating 800 pmol cocaine. The lack of significance for the self-administration of 400 pmol cocaine into the AcbSh in Wistar rats in the current experiment was due to a reduction in the average number of infusions (11.9 ± 2.5) and more infusions in the aCSF group (5.3 ± 1.1). A small meta-analysis (collapsing all groups across the Rodd-Henricks et al., 2002 report and the current data set) indicated that 400 pmol is not significantly self-administered into the AcbSh in Wistar rats (post-hoc Tukey’s b). Nevertheless, P rats displayed significantly more responses for cocaine than Wistar rats at the 200 and 400 pmol concentrations (Figs. 3 and 4). Overall, the data suggest that cocaine is reinforcing in the AcbSh and selective breeding for high alcohol intake is associated with increased sensitivity of this brain region to the reinforcing properties of cocaine.
P rats received a greater number cocaine infusions into the AcbSh for the 200, 400 and 800 pmol compared to the 0 or 100 pmol cocaine groups and received a greater number of infusions at the 400 and 800 pmol compared to the 200 pmol cocaine groups (Fig. 2; left panel). In contrast, Wistar rats only received a greater number of infusions of cocaine into the AcbSh in the 800 pmol cocaine group (Fig. 2; right panel). These findings indicate that P, but not Wistar rats, show a concentration-dependent increase in the number of infusions of cocaine self-administered into the AcbSh. Furthermore, P rats received a greater number of cocaine infusions in the 200, 400 and 800 pmol cocaine groups compared to respective Wistar groups (Fig. 2), which suggests that P rats are more sensitive to the reinforcing effects of cocaine self-administered into the AcbSh than Wistar rats during acquisition. These findings are in agreement with previous findings from our laboratory in which Wistar rats self-administered 400 to 1600 pmol cocaine concentrations into the AcbSh, but not AcbC (Rodd-Henricks et al 2002).
Compared to session 4, the number of self-infusions by P rats during reinstatement sessions was significantly higher at all concentrations of cocaine tested, whereas Wistar rats only received significantly more infusions at the 400 and 800 pmol concentrations. These findings suggest that the AcbSh of P rats, and to a lesser degree the AcbSh of Wistar rats, was associated with neuronal adaptations resulting from prior cocaine self-administration and extinction that modified the reinforcing properties of cocaine within this nucleus. Repeated, intermittent exposure to cocaine has been shown to result in numerous molecular, neurochemical, and behavioral alterations that have been hypothesized to be the basis for cocaine sensitization (for reviews, see Koob and Nestler, 1997; Pierce and Kalivas, 1997). Repeated administration of cocaine results in a supersensitivity in the efficacy of reuptake blockade by cocaine (Henry and White, 1995). Similarly, repeated injections of amphetamine produced sensitization to the locomotor stimulation effect of a challenge dose of amphetamine administered directly into the Acb (Paulson and Robinson, 1991). Evidence suggests that cocaine-induced psychomotor sensitization involves neuroadaptations in mesocorticolimbic dopamine (DA) and glutamatergic terminal fields, such as the Acb (Nestler 2002; Wolf et al 2004; Everitt and Robbins 2005; Kalivas and O’Brien 2008). It has been generally accepted that psychomotor stimulants produce sensitization by increasing extracellular concentrations of DA in the Acb (Robinson and Becker 1986; Kalivas and Stewart 1991; White and Wolf 1991). Other dopaminergic mechanisms contributing to the expression of cocaine sensitization may be supersensitivity of DA D1 receptor-mediated responses in the Acb (Henry and White 1992; White et al., 1992, 1995). For example, repeated cocaine administration enhances the inhibitory efficacy of DA and DA D1 receptor agonists on Acb neurons (Henry et al., 1989; Henry and White 1991). Neuroadaptations in accumbal glutamatergic neurotransmission have also been implicated in psychomotor cocaine sensitization, as withdrawal from cocaine potentiates glutamatergic neurotransmission and increases α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor (AMPA) glutamate receptor surface expression (Boudreau and Wolf 2005; Boudreau et al 2007; Kourrich et al 2007). In general, the data indicate that subthreshold concentrations of cocaine produced a “kindling-like” effect after an abstinence period (a total of 6 days from session 4 to 7).
The 800 pmol concentration of cocaine appeared to be the most reinforcing in P rats compared to other doses tested, since it maintained high active lever responding during acquisition (179 ± 18) and resulted in a high number of reinstatement responses (342 ± 54; Fig. 4). In Wistar rats, the most reinforcing concentration of cocaine was also 800 pmol. However, levels of responding in Wistar rats were lower than P rats at this same concentration (104 ± 9; Fig. 4). The infusions of cocaine at significantly higher levels by P than Wistar rats suggest that cocaine could have a greater reward saliency in the P rats. Alternatively, it is possible that P rats are less sensitive than Wistar rats to the aversive effects of cocaine at high doses, since P rats self-infused more cocaine than Wistar rats at the highest concentration tested in the current study.
P rats will self-administer EtOH directly into the AcbSh at a lower concentration (75 mg%; 16.5 mM) than Wistar rats (100 mg%; 22 mM; Engleman et al., 2009). Thus, selective breeding for high alcohol preference resulted in a significant (1.3 fold) alteration in the sensitivity of the AcbSh to the reinforcing actions of EtOH when compared to Wistar rats. In contrast, P rats displayed a four-fold (200 pmol/100 nl vs. 800 pmol/100 nl; 2 mM vs. 8 mM) greater sensitivity to the reinforcing actions of cocaine in the AcbSh compared to Wistar rats (Figs. 2–4). Therefore, selective breeding for high alcohol preference may have produced a greater divergence in sensitivity to the reinforcing actions of cocaine in the AcbSh than EtOH. Burst-firing activity within the VTA has been demonstrated to be greater in P than Wistar rats (Morzorati, 1998), which suggests that alcohol-preferring rats may release more DA in the NAC compared with alcohol-nonpreferring rats under basal conditions. Also, animals with a high propensity to self-administer drugs of abuse (HR rats) exhibit a greater locomotor response to systemically administered cocaine and amphetamine (Hooks et al., 1991; Piazza et al., 1989) and a greater dopaminergic response within the NAC to cocaine (15 mg/kg; ip) than animals with a low propensity to self-administer substances of abuse (LR rats) (Hooks et al., 1992). Furthermore, HR rats display a higher locomotor response than do LR rats to the intra-accumbens infusion of dopamine and cocaine (Hooks et al., 1993, 1994). These data may explain why alcohol-preferring animals are more sensitive to the dopamine-dependent aspects of the reinforcing properties of ethanol and cocaine. That is, alcohol and cocaine may be more reinforcing in animals exhibiting an enhanced dopaminergic response to these drugs, which may be associated with high-alcohol preference.
P rats displayed a greater sensitivity to the intracranially self-infused cocaine compared to ethanol, which may reflect cocaine having a greater impact on DA neurotransmission than ethanol, which would magnify any potential differences between P and Wistar rats’ sensitivity to the reinforcing effects of cocaine. Indeed, cocaine administration has been shown to enhance Acb DA efflux from 200 to 600% of baseline levels (Hemby et al 1997; Smith et al 2006; Zocchi et al 2003), whereas ethanol administration has been shown to enhance Acb DA by only 150 to 200% of baseline levels in rats and mice (Zocchi et al 2003; Weiss et al 1993; Yoshimoto et al 1992; Katner and Weiss 2001).
The results of the present study provide important information regarding how genetic factors influence a predisposition to high alcohol drinking and abuse of other drugs, and how genetic factors that influence alcohol drinking can also influence the effects of other drugs of abuse. In humans, concurrent use of alcohol and other drugs of abuse have been reported in numerous clinical studies and the high prevalence of alcohol and cocaine co-abuse in humans has been postulated to be predicated upon both a common genetic factor that predisposes an organism to abuse multiple substances and the interaction of drugs within the organism (Uhl 2004, 2006; Uhl et al., 2008). Furthermore, research indicates that individuals genetically predisposed to abuse alcohol and other drugs of abuse are disproportionally reactive to alcohol and other drugs of abuse when given alone (Schuckit 1994a,b; Kareken et al., 2010; Uhl 2008; Piazza and LeMoal 1996). These findings in humans are supported by the present findings in which rats selectively bred for high alcohol preference are also more sensitive to the reinforcing actions of cocaine and, therefore, support the hypothesis that there is a common genetic basis for the abuse potential of alcohol and cocaine.
In summary, the results of the present study support the AcbSh as being a neuroanatomical substrate involved in cocaine self-administration. In addition, the AcbSh of P rats is more sensitive than Wistar rats to the reinforcing effects of cocaine, and a brief history of cocaine self-administration and extinction is accompanied by neuronal adaptations that enhanced the reinforcing properties of cocaine in the AcbSh.
Highlights.
P rats self-infused 200–800 pmol and Wistar rats only self-infused 800 pmol cocaine.
P rats received more cocaine infusions compared to Wistar rats during acquisition.
P rats infused more cocaine than Wistar rats during reinstatement.
Compared to Wistar rats, the AcbSh of P rats was more sensitive to cocaine.
The AcbSh of P rats may have become sensitized to the reinforcing effects of cocaine.
Acknowledgements
This study was supported in part by AA07611, AA07462, and AA012262.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bergstrom HC, Palmer AA, Wood RD, Burkhart-Kasch S, McKinnon CS, Phillips TJ. Reverse selection for differential response to the locomotor stimulant effects of ethanol provides evidence for pleiotropic genetic influence on locomotor response to other drugs of abuse. Alcohol Clin Exp Res. 2003;27:1535–1547. doi: 10.1097/01.ALC.0000091226.18969.B9. [DOI] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Cell surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activation of mitogen-activated protein kinases. J Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Electrolytic microinfusion transducer system: an alternative method of intracranial drug application. J Neurosci Methods. 1980;2(3):273–275. doi: 10.1016/0165-0270(80)90016-3. [DOI] [PubMed] [Google Scholar]
- Bozarth MA, Wise RA. Intracranial self-administration of morphine into the ventral tegmental area in rats. Life Sci. 1981;28(5):551–555. doi: 10.1016/0024-3205(81)90148-x. [DOI] [PubMed] [Google Scholar]
- Brookoff D, Rotondo MF, Shaw LM, Campbell EA, Fields L. Cocaethylene levels in patients who test positive for cocaine. Ann Emerg Med. 1996;27:316–320. doi: 10.1016/s0196-0644(96)70266-4. [DOI] [PubMed] [Google Scholar]
- Churchill L, Swanson CJ, Urbina M, Kalivas PW. Repeated cocaine alters glutamate receptor subunit levels in the nucleus accumbens and ventral tegmental area of rats that develop behavioral sensitization. J Neurochem. 1999;72:2397–2403. doi: 10.1046/j.1471-4159.1999.0722397.x. [DOI] [PubMed] [Google Scholar]
- Compton WM, Cottler LB, Ridenour T, Ben-Abdallah A, Spitznagel EL. The specificity of family history of alcohol and drug abuse in cocaine abusers. Am J Addict. 2002;11:85–94. doi: 10.1080/10550490290087866. [DOI] [PubMed] [Google Scholar]
- Devine DP, Wise RA. Self-administration of morphine, DAMGO, and DPDPE into the ventral tegmental area of rats. J Neurosci. 1994;14(4):1978–1984. doi: 10.1523/JNEUROSCI.14-04-01978.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engleman EA, Ding ZM, Oster SM, Toalston JE, Bell RL, Murphy JM, McBride WJ, Rodd ZA. Ethanol is self-administered into the nucleus accumbens shell, but not the core: evidence of genetic sensitivity. Alcohol Clin Exp Res. 2009;33(12):2162–2171. doi: 10.1111/j.1530-0277.2009.01055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci. 2005;8:1481–1489. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- Flaherty CF. Animal learning and cognition. McGraw-Hill; 1985. [Google Scholar]
- Fox HC, Talih M, Malison R, Anderson GM, Kreek MJ, Sinha R. Frequency of recent cocaine and alcohol use affects craving and associated responses to stress and drug-related cues. Psychoneuroendo. 2005;30:880–891. doi: 10.1016/j.psyneuen.2005.05.002. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11(6):557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221(4612):773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Intracranial self-administration methodologies. Neurosci Biobehav Rev. 1987;11(3):319–329. doi: 10.1016/s0149-7634(87)80017-9. [DOI] [PubMed] [Google Scholar]
- Heil SH, Badger GJ, Higgins ST. Alcohol dependence among cocaine-dependent outpatients: demographics, drug use, treatment outcome and other characteristics. J Studies Alcohol. 2001;62:14–22. doi: 10.15288/jsa.2001.62.14. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133(1):7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Henry DJ, Greene MA, White FJ. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system: repeated administration. J Pharmacol Exp Ther. 1989;251(3):833–839. [PubMed] [Google Scholar]
- Henry DJ, White FJ. Repeated cocaine administration causes persistent enhancement of D1 dopamine receptor sensitivity within the rat nucleus accumbens. J Pharmacol Exp Ther. 1991;258(3):882–890. [PubMed] [Google Scholar]
- Henry DJ, White FJ. Electrophysiological correlates of psychomotor stimulant-induced sensitization. Ann N Y Acad Sci. 1992;654:88–100. doi: 10.1111/j.1749-6632.1992.tb25958.x. [DOI] [PubMed] [Google Scholar]
- Henry DJ, White FJ. The persistence of behavioral sensitization to cocaine parallels enhanced inhibition of nucleus accumbens neurons. J Neurosci. 1995;15(9):6287–6299. doi: 10.1523/JNEUROSCI.15-09-06287.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoebel BG, Monaco AP, Hernandez L, Aulisi EF, Stanley BG, Lenard L. Self-injection of amphetamine directly into the brain. Psychopharmacology (Berl) 1983;81(2):158–163. doi: 10.1007/BF00429012. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Smith AD, Neill DB, Justice JB., Jr Individual differences in locomotor activity and sensitization. Pharmacol Biochem Behav. 1991;38:467–470. doi: 10.1016/0091-3057(91)90308-o. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Colvin AC, Juncos JL, Justice JB., Jr Individual differences in basal and cocaine-stimulated extracellular dopamine in the nucleus accumbens using quantitative microdialysis. Brain Res. 1992;587:306–312. doi: 10.1016/0006-8993(92)91012-4. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Jones GH, Hemby SE, Justice JB., Jr Environmental and pharmacological sensitization: Effects of repeated administration of systemic or intra-nucleus accumbens cocaine. Psychopharmacology (Berl) 1993;111:109–116. doi: 10.1007/BF02257416. [DOI] [PubMed] [Google Scholar]
- Hooks MS, Juncos JL, Justice JB, Jr, Meiergerd SM, Povlock SL, Schenk JO, Kalivas PW. Individual locomotor response to novelty predicts selective alterations in D1 and D2 receptors and mRNAs. J Neurosci. 1994;14:6144–6152. doi: 10.1523/JNEUROSCI.14-10-06144.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikemoto S, Kohl RR, McBride WJ. GABAA receptor blockade in the anterior ventral tegmental area increase extracellular levels of dopamine in the nucleus accumbens of rats. J Neuorchem. 1997a;69:137–143. doi: 10.1046/j.1471-4159.1997.69010137.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto S, Murphy JM, McBride WJ. Self-infusion of GABAA receptor antagonists directly into the ventral tegmental area and adjacent regions. Behav Neurosci. 1997b;110:331–345. doi: 10.1037//0735-7044.111.2.369. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, O’Brien C. Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology. 2008;33:166–180. doi: 10.1038/sj.npp.1301564. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Stewart J. Dopamine transmission in the initiation and expression of drug-and stress-induced sensitization of motor activity. Brain Res Brain Res Rev. 1991;16(3):223–244. doi: 10.1016/0165-0173(91)90007-u. [DOI] [PubMed] [Google Scholar]
- Katner SN, McBride WJ, Lumeng L, Li T-K, Murphy JM. Effects of cholinergic agents on locomotor activity of P and NP rats. Alcohol Clin Exp res. 1996;20:1004–1010. doi: 10.1111/j.1530-0277.1996.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Katner SN, Weiss F. Neurochemical characteristics associated with ethanol preference in selected alcohol-preferring and -nonpreferring rats: a quantitative microdialysis study. Alcohol Clin Exp Res. 2001;25(2):198–205. [PubMed] [Google Scholar]
- Kareken DA, Bragulat V, Dzemidzic M, Cox C, Talavage T, Davidson D, O'Connor SJ. Family history of alcoholism mediates the frontal response to alcoholic drink odors and alcohol in at-risk drinkers. Neuroimage. 2010;50(1):267–276. doi: 10.1016/j.neuroimage.2009.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9(3):482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- Kourrich S, Rothwell PE, Klug JR, Thomas MJ. Cocaine experience controls bidirectional synaptic plasticity in the nucleus accumbens. J Neurosci. 2007;27:7921–7928. doi: 10.1523/JNEUROSCI.1859-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê AD, Li Z, Funk D, Shram M, Li TK, Shaham Y. Increased vulnerability to nicotine self-administration and relapse in alcohol-naive offspring of rats selectively bred for high alcohol intake. J Neurosci. 2006;26(6):1872–1879. doi: 10.1523/JNEUROSCI.4895-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N, Eisen SA, Scherrer JF, Goldberg J, True WR, Lyones MJ, Tsuang MT. The influence of familial and non-familial factors on the association between major depression and substance abuse/dependence in 1874 monozygotic male twin pairs. Drug Alcohol Depend. 1996;43:49–55. doi: 10.1016/s0376-8716(96)01287-2. [DOI] [PubMed] [Google Scholar]
- Magura S, Rosenblum A. Modulating effect of alcohol use on cocaine use. Addict Behav. 2000;25:117–122. doi: 10.1016/s0306-4603(98)00128-2. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Murphy JM, Ikemoto S. Localization of brain reinforcement mechanisms: intracranial self-administration and intracranial place-conditioning studies. Behav Brain Res. 1999;101(2):129–152. doi: 10.1016/s0166-4328(99)00022-4. [DOI] [PubMed] [Google Scholar]
- McKinzie DL, Rodd-Henricks ZA, Dagon CT, Murphy JM, McBride WJ. Cocaine is self-administered into the shell region of the nucleus accumbens in Wistar rats. Ann N Y Acad Sci. 1999;877:788–791. doi: 10.1111/j.1749-6632.1999.tb09323.x. [DOI] [PubMed] [Google Scholar]
- Mikkola JA, Honakanen A, Piepponen TP, Kiianmaa K, Ahtee L. Effects of repeated cocaine treatment on striatal dopamine release in alcohol-preferring AA and alcoholavoiding ANA rats. N Sch Arch Pharm. 2001;363:209–214. doi: 10.1007/s002100000367. [DOI] [PubMed] [Google Scholar]
- Miller NS, Millman RB, Keskinen S. The diagnosis of alcohol, cocaine, and other drug dependence in an inpatient treatment population. J Subst Abuse Treat. 1989;6(1):37–40. doi: 10.1016/0740-5472(89)90018-4. [DOI] [PubMed] [Google Scholar]
- Morzorati SL. VTA dopamine neuron activity distinguishes alcohol-preferring (P) rats from Wistar rats. Alcohol Clin Exp Res. 1998;22(4):854–857. [PubMed] [Google Scholar]
- Nestler EJ. Common molecular and cellular substrates of addiction and memory. Neurobiol Learn Mem. 2002;78:637–647. doi: 10.1006/nlme.2002.4084. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Wiegand R, Bucholz K, O’Connor S, Meyer ET, Reich T, Rice J, Schuckit M, King L, et al. COGA. A family study of alcohol dependence: coaggrgations of multiple disorders in relatives of alcohol-dependent probands. Arch Gen Psychiatry. 2004;61:1246–1256. doi: 10.1001/archpsyc.61.12.1246. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Robinson TE. Sensitization to systemic amphetamine produces an enhanced locomotor response to a subsequent intra-accumbens amphetamine challenge in rats. Psychopharmacology (Berl) 1991;104(1):140–141. doi: 10.1007/BF02244569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips GD, Robbins TW, Everitt BJ. Bilateral intra-accumbens self-administration of d-amphetamine: antagonism with intra-accumbens SCH-23390 and sulpiride. Psychopharmacology (Berl) 1994;114(3):477–485. doi: 10.1007/BF02249339. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Le Moal ML. Pathophysiological basis of vulnerability to drug abuse: role of an interaction between stress, glucocorticoids, and dopaminergic neurons. Annu Rev Pharmacol Toxicol. 1996;36:359–378. doi: 10.1146/annurev.pa.36.040196.002043. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H. Factors that predict individual vulnerability to amphetamine self-administration. Science. 1989;245:1511–1513. doi: 10.1126/science.2781295. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25(2):192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Becker JB. Enduring changes in brain and behavior produced by chronic amphetamine administration: a review and evaluation of animal models of amphetamine psychosis. Brain Res. 1986;396(2):157–198. doi: 10.1016/s0006-8993(86)80193-7. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Kuc KA, Zhang Y, Murphy JM, McBride WJ. Intracranial self-administration of cocaine within the posterior VTA of Wistar rats: Evidence for the involvement of serotonin-3 receptors and dopamine neurons. J Pharmcol Exp Ther. 2005;313:134–145. doi: 10.1124/jpet.104.075952. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Crile RS, Murphy JM, McBride WJ. Regional heterogeneity for the intracranial self-administration of ethanol within the ventral tegmental area of female Wistar rats. Psychopharmacology (Berl) 2000;149(3):217–224. doi: 10.1007/s002139900347. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Li T-K, Murphy JM, McBride WJ. Cocaine is self-administered into the shell, but not the core, of the nucleus accumbens of Wistar rats. J Pharmacol Exp Ther. 2002;303:1216–1226. doi: 10.1124/jpet.102.038950. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Melendez RI, Berry N, Murphy JM, McBride WJ. Effects of serotonin-3 receptor antagonists on the intracranial self-administration of ethanol within the ventral tegmental area of Wistar rats. Psychopharmacology (Berl) 2003;165(3):252–259. doi: 10.1007/s00213-002-1300-2. [DOI] [PubMed] [Google Scholar]
- Rodd ZA, Bell RL, Melendez RI, Kuc KA, Lumeng L, Li T-K, Murphy JM, McBride WJ. Comparison of intracranial self-administration of ethanol within the posterior ventral tegmental area between alcohol-preferring (P) and Wistar rats. Alcohol Clin Exp Res. 2004b;28:1212–1219. doi: 10.1097/01.alc.0000134401.30394.7f. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. A clinical model of genetic influences in alcohol dependence. J Stud Alcohol. 1994a;55(1):5–17. doi: 10.15288/jsa.1994.55.5. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Low level of response to alcohol as a predictor of future alcoholism. Am J Psychiatry. 1994b;151(2):184–189. doi: 10.1176/ajp.151.2.184. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology, and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Staines GL, Magura S, Foote J, Deluca A, Kosanke N. Polysubstance use among alcoholics. Journal of Addictive Diseases. 2001;20:53–69. doi: 10.1300/j069v20n04_06. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Coller MD, Hemby SE, Martin TJ. Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology. 2006;31(1):139–150. doi: 10.1038/sj.npp.1300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stromberg MF, Mackler SA. The effects of cocaine on the expression of motor activity and conditioned place preference in high and low alcohol-preferring Wistar rats. Pharmcol Biochem Behav. 2005;82:314–319. doi: 10.1016/j.pbb.2005.08.021. [DOI] [PubMed] [Google Scholar]
- Uhl GR, Liu Q-R, Walther D, Hess J, Naiman D. Polysubstance abuse-vulnerability genes: genome scans for association, using 1,004 subjects and 1,494 single-nucleotide polymorphisms. Am J Hum Genet. 2001;69:1290–1300. doi: 10.1086/324467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR. Molecular genetics of substance abuse vulnerability: remarkable recent convergence of genome scan results. Ann N Y Acad Sci. 2004;1025:1–13. doi: 10.1196/annals.1316.001. [DOI] [PubMed] [Google Scholar]
- Uhl GR. Molecular genetics of addiction vulnerability. NeuroRx. 2006;3(3):295–301. doi: 10.1016/j.nurx.2006.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl GR, Drgon T, Johnson C, Li CY, Contoreggi C, Hess J, Naiman D, Liu QR. Molecular genetics of addiction and related heritable phenotypes: genome-wide association approaches identify "connectivity constellation" and drug target genes with pleiotropic effects. Ann N Y Acad Sci. 2008;1141:318–381. doi: 10.1196/annals.1441.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FJ, Wolf ME. Psychomotor stimulants. In: Pratt JA, editor. The biological basis of drug tolerance and dependence. London: Academic; 1991. pp. 153–197. [Google Scholar]
- White FJ, Henry DJ, Hu X-T, Jeziorski M, Ackerman JM. Electrophysiological effects of cocaine in the mesoaccumbens dopamine system. In: Lakoski JM, Galloway MP, White FJ, editors. Cocaine: pharmacology, physiology and clinical strategies. Boca Raton, FL: CRC; 1992. pp. 261–293. [Google Scholar]
- White FJ, Hu X-T, Henrv DJ, Zhang X-F. Neurouhvsiological alterations in the mesocorticolimbic dopamine system during repeated cocaine administration. In: Hammer RP Jr, editor. The neurobiology of cocaine: cellular and molecular mechanisms. Boca Raton, FL: CRC; 1995. pp. 95–115. [Google Scholar]
- Williamson S, Gossop M, Powis B, Griffiths P, Fountain J, Strang J. Adverse effects of stimulant drugs in a community sample of drug users. Drug Alcohol Depend. 1997;44:87–94. doi: 10.1016/s0376-8716(96)01324-5. [DOI] [PubMed] [Google Scholar]
- Wolf ME, Sun X, Mangiavacchi S, Chao SZ. Psychomotor stimulants and neuronal plasticity. Neuropharmacology. 2004;47 Suppl 1:61–79. doi: 10.1016/j.neuropharm.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K, McBride WJ, Lumeng L, Li TK. Alcohol stimulates the release of dopamine and serotonin in the nucleus accumbens. Alcohol. 1992;9(1):17–22. doi: 10.1016/0741-8329(92)90004-t. [DOI] [PubMed] [Google Scholar]
- Zocchi A, Girlanda E, Varnier G, Sartori I, Zanetti L, Wildish GA, Lennon M, Mugnaini M, Heidbreder CA. Dopamine responsiveness to drugs of abuse: A shell-core investigation in the nucleus accumbens of the mouse. Synapse. 2003;50(4):293–302. doi: 10.1002/syn.10271. [DOI] [PubMed] [Google Scholar]


