Abstract
The purpose of this study was to examine the pharmacologic plasticity of cholinergic, non-adrenergic non-cholinergic (NANC), and purinergic contractions in neurogenic bladder strips from spinal cord injured (SCI) rats. Bladder strips were harvested from female rats three to four weeks after T9–T10 spinal cord transection. The strips were electrically stimulated using two experimental protocols to compare the contribution of muscarinic and NANC/purinergic contractions in the presence and the absence of carbachol or muscarine. The endpoints of the study were: (1) percent NANC contraction that was unmasked by the muscarinic antagonist 4-DAMP, and (2) P2X purinergic contraction that was evoked by α,β–methylene ATP. NANC contraction accounted for 78.5% of the neurally evoked contraction in SCI bladders. When SCI bladder strips were treated with carbachol (10 µM) prior to 4-DAMP (500 nM), the percent NANC contraction decreased dramatically to only 13.1% of the neurally evoked contraction (p=0.041). This was accompanied by a substantial decrease in α,β–methylene ATP evoked P2X contraction, and desensitization of purinergic receptors (the ratio of subsequent over initial P2X contraction decreased from 97.2% to 42.1%, p=0.0017). Sequential activation of the cholinergic receptors with carbachol (or with muscarine in neurally intact bladders) and unmasking of the NANC response with 4-DAMP switched the neurally evoked bladder contraction from predominantly NANC to predominantly cholinergic. We conclude that activation of muscarinic receptors (with carbachol or muscarine) blocks NANC and purinergic contractions in neurally intact or in SCI rat bladders. The carbachol-induced inhibition of the NANC contraction is expressed more in SCI bladders compared to neurally intact bladders. Along with receptor plasticity, this change in bladder function may involve P2X-independent mechanisms.
Keywords: neurogenic bladder, bladder contractility, spinal cord injury, purinergic receptor, cholinergic receptor
1. Introduction
The muscarinic and purinergic pathways play important roles in urinary bladder contraction. During neurally evoked contractions of the bladder, acetylcholine (ACh) and ATP are co-released from parasympathetic nerve terminals and activate post-junctional muscarinic (M) and purinergic (P2X1) receptors, respectively, to elicit a bladder contraction in the rat [8]. Thus, muscarinic antagonists such as atropine or 4-DAMP are not fully effective in inhibiting neurally evoked bladder contraction due to a significant non-adrenergic, non-cholinergic (NANC) contractile component [1, 26, 30]. The P2X purinergic pathway contributes significantly to this NANC contraction since P2X antagonists abolish most of the remaining NANC bladder contraction after atropine treatment [15, 17, 28].
The relative contribution of cholinergic and NANC/purinergic transmission to neurally evoked bladder contraction is influenced by a number of factors, such as animal age [13, 22, 31], frequency of electrical stimulation, and pathology. For example, at higher frequency of nerve stimulation (10–40 Hz), more ACh is released and the bladder contraction becomes more cholinergic [23, 29]. After spinal cord injury (SCI), the rat bladder becomes more responsive to the cholinergic transmitter ACh and less responsive to the purinergic transmitter ATP [24, 26]. Thus, muscarinic antagonists, like atropine or 4-DAMP, exhibit greater inhibition on neurally evoked contractions subsequently following SCI [26].
We have recently shown that activation of muscarinic receptors by the cholinergic agonist carbachol, or by endogenous ACh induces a cascade of events that leads to reduced purinergic contractile response, and consequently inhibition of the bladder NANC response [16]. Correspondingly, in the presence of the cholinergic agonist carbachol, the muscarinic antagonist 4-DAMP becomes more effective in inhibiting neurally evoked bladder contractions, whereas the P2X receptor agonist α,β-methylene ATP (α,β-mATP) produced a diminished contractile response. This pharmacologic plasticity, i.e. an abrupt shift from NANC/purinergic contraction to cholinergic/muscarinic contraction, occurs rapidly after carbachol administration, and may be caused by the desensitization of P2X receptors [16].
The pharmacologic plasticity of bladder cholinergic and NANC response was first demonstrated in normal rats [16]. In this paper we investigated the cholinergic-NANC plasticity in SCI rats where the micturition circuitry is reorganized due to permanent disruption of ascending and descending pathways to the brain. Specifically, we compared the effectiveness of 4-DAMP (a muscarinic receptor antagonist) to inhibit neurally evoked bladder contraction, and the effectiveness of α,β-mATP (a P2X purinergic receptor agonist) to evoke bladder contraction in the presence and absence of carbachol or muscarine. Our findings have significant importance because the plasticity of cholinergic, NANC, and purinergic transmission is poorly understood in the rat model of neurogenic bladder. Although treatment with antimuscarinic agents has been widely used in the management of neurogenic detrusor overactivity in humans, the efficacy is modest while the side effects may be substantial. Understanding the pharmacologic modulation of cholinergic and purinergic contractions in the rat model of SCI bladders, and the mechanisms underlying this modulation, may have potential impact on the management of neurogenic bladder conditions as long as the results are translatable to humans.
2. Materials and Methods
All the experimental procedures were approved by the Institutional Animal Care and Use Committee at Baylor College of Medicine and were conducted in accordance with NIH guidelines for the care and use of laboratory animals.
2.1 Spinal cord injury (SCI)
Experiments were performed on 31 neurally intact (NI) and 17 spinal cord transected (SCI) female Sprague-Dawley rats weighing 250–300 g as previously described [20]. Rats were anesthetized with isoflurane (1.5%). A midline dorsal incision and laminectomy were performed to expose the spinal segment T9–T10. The dura mater was opened and the spinal cord was completely transected. The overlying muscle and skin were closed and the rats were given 2 mL of saline solution subcutaneously. All rats received ampicillin (100 mg/kg) intramuscularly once a day for 3 days. Rat bladders were manually expressed twice a day until spinal reflex micturition developed.
2.2 Bladder strips preparation and experimental paradigms
Three to four weeks after spinal cord transection rats were euthanized, the urinary bladder was removed above the trigone, and 4 longitudinal bladder strips were prepared taking special care to preserve the integrity of the urothelial layer during preparation. The strips were mounted in 5 mL organ baths containing oxygenated Krebs (NaCl 113, KCl 4.7, CaCl2 1.25, MgSO4 1.2, NaHCO3 25, KH2PO4 1.2, D-glucose 11.5 mM) at 37°C. Pretension of 10 mN was applied to all strips, isometric contractions were measured with a force transducer (FT-2, World Precision Instruments, Sarasota, FL), and normalized based on strip cross-sectional area. Neurally evoked contractions were induced using electrical field stimulation via platinum wire electrodes. The data were collected in real-time using the WINDAQ data acquisition program (DataQ Instruments, Akron, OH) at a sampling rate of 20 Hz. Trains of square wave impulses (0.25 ms, 20 Hz, 200 shocks every 100s) were applied at a voltage (100 V) that produced maximal contractions. Drugs were added to the organ bath according to the experimental protocols A or B as outlined in figure 1. 10 µM α,β–mATP was applied at the beginning and at the conclusion of the experiment to evoke P2X purinergic bladder contractions. The two α,β–mATP applications (A1, A2) were approximately 35 minutes apart. After A1 and wash-out, neurally evoked contractions were applied until a stable baseline (expressed as B) was established. Bladder NANC contractions were unmasked by applying the muscarinic antagonist 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP). In paradigm A (figure 1A), 500 nM 4-DAMP was added between the α,β-mATP contractions while in paradigm B (figure 1B), 10 µM carbachol (or 10 µM muscarine) and 500 mM 4-DAMP were added sequentially. That portion of the neurally evoked contraction that was inhibited by 4-DAMP was considered cholinergic while the residual contraction (R) that remained after 4-DAMP administration was considered as the NANC response. There was no wash-out between carbachol (or muscarine), 4-DAMP, and the second application of α,β–mATP (A2). At the end of all experiments, the viability of bladder strips was confirmed by contracting the bladder strips with 100 mM K+. At 500 nM concentration, 4-DAMP loses its selectivity towards M3 and functions as a non-specific muscarinic antagonist [9, 10, 25]. We evaluated: (1) the percent NANC contraction that was unmasked by the muscarinic antagonist 4-DAMP (expressed as R/B X 100%), and (2) P2X purinergic contraction that was evoked by α,β–mATP. The A2/A1 ratio was used as a measure of the purinergic receptor desensitization.
Figure 1.
Experimental paradigms A (without pre-treatment) and B (with carbachol or muscarine pre-treatment) are illustrated. CCh, carbachol; A1, first α,β-mATP evoked contraction; A2, second α,β-mATP evoked contraction; B, baseline neurally evoked contraction; R, residual neurally evoked contraction after 4-DAMP treatment; K, potassium. Sample tracings from SCI bladder strips are shown following each stimulation protocol. Note that there was no washout between carbachol, 4-DAMP and A2. Note that in some experiments muscarine was used instead of carbachol.
2.3 Drugs
All pharmacologic agents and the constituents of Krebs solution were obtained from Sigma-Aldrich (St Louis, MO, USA).
2.4 Statistics
The data are expressed as mean ± S.E.M. For statistical analysis, one way ANOVA followed by a Bonferoni’s post-test was used for analyzing the results obtained with carbachol and 4-DAMP in NI and SCI bladders. Paired two-tailed t-tests were performed for data obtained with muscarine and 4-DAMP in neurally intact bladders. Statistical analysis and figure preparation were performed with PRISM 4 (GraphPad Prism Software, San Diego, CA, USA). Statistical significance was considered at a level of P ≤ 0.05.
3. Results
3.1 Effect of activation of muscarinic receptors on the magnitude of NANC contraction in NI and SCI rat bladders
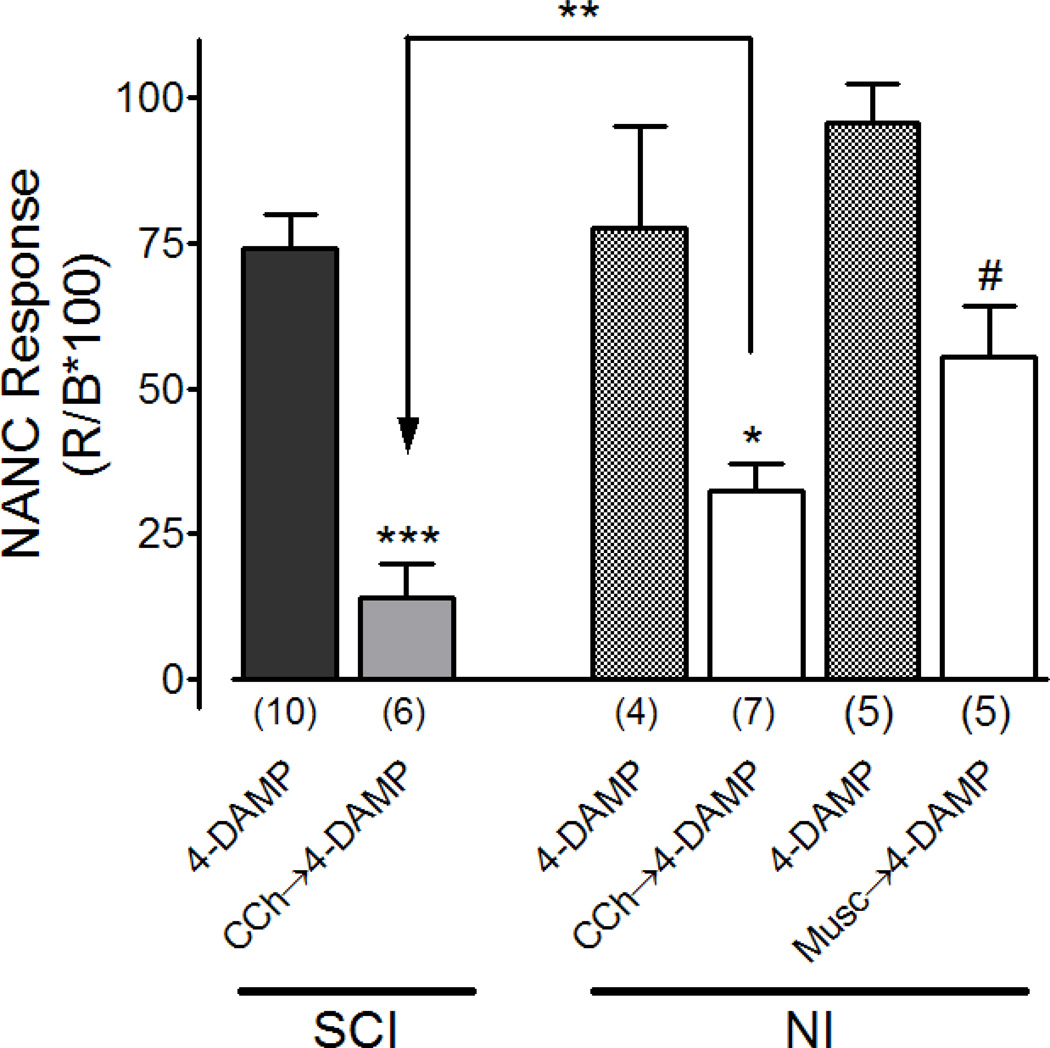
In SCI rats, the muscarinic antagonist 4-DAMP (500 nM) decreased the amplitude of neurally evoked bladder contractions by approximately 21% (figure 2, n=10). Thus, the NANC contraction accounted for 78.5 ± 21.9% of the neurally evoked contraction in SCI rat bladder strips. The experimental paradigm is shown in figure 1A. When SCI bladder strips were treated with the mixed cholinergic agonist carbachol (10 µM) prior to 4-DAMP application (500 nM) (paradigm B in figure 1), 4-DAMP decreased the amplitude of neurally evoked bladder contraction by 87% (figure 2, n=6). Therefore, the NANC contraction only accounted for 13.1 ± 5.6% of the neurally evoked bladder strip contraction. This percent NANC contraction was significantly smaller than that without carbachol pre-treatment (13.1 ± 5.6% versus 78.5 ± 21.9%, p=0.041). In other words, in the presence of cholinergic receptor activation (with 10 µM carbachol), the percent NANC contraction became smaller while the percent cholinergic contraction became more prominent.
Figure 2.
Effect of carbachol and muscarine on the NANC contractions in SCI and NI rat bladder strips. 4-DAMP represents paradigm A; CCh → 4-DAMP or muscarine → 4-DAMP represent paradigm B. In the presence of CCh or muscarine, the percent of NANC contraction became smaller in both SCI and NI rats (more evident in SCI than NI rats). Note that experiments with muscarine were performed only in NI rat bladder strips. *P<0.05, **P<0.01, ***P<0.005, one-way ANOVA after Bonferroni’s post-test. #P<0.05, paired t-test for the experiments using muscarine.
When the experiments were performed in neurally intact (NI) normal rat bladders (figure 2), a similar reduction of the NANC response and an enhancement of the cholinergic contractile component were also observed when carbachol (10 µM) was applied prior to 4-DAMP (500 nM) [16]. In the presence of carbachol, the NANC response in SCI rat bladders (13.1 ± 5.6%) was significantly smaller than the NANC response in NI rat bladder (32.0 ± 3.2%) (p=0.0059). In other words, the negative effect of carbachol on bladder NANC contractions is more prominent in SCI rats than in NI rats.
In another series of experiments the selective muscarinic agonist, muscarine was applied instead of carbachol in NI rat bladder strips. As shown in figure 2, application of muscarine (10µM) before 4-DAMP produced a significant inhibition of NANC contraction by 42% (P<0.05, n=5) as compared to 4-DAMP only treated strips. The significantly reduced contractions to electrical stimulation obtained after the application of muscarine and 4 DAMP confirms that muscarinic receptors mediate the enhanced inhibition of the NANC response.
3.2. Effect of activation of muscarinic receptors and 4-DAMP on α,β-methylene ATP evoked purinergic receptor desensitization
In paradigms A and B (figure 1), the P2X-type receptor selective agonist α,β–mATP (10 µM) was applied at the beginning and at the conclusion of the experiments to evoke two purinergic bladder contractions (A1 and A2) approximately 35 minutes apart. The change in the purinergic contractile response was expressed as the ratio between the amplitudes of the two α,β-mATP evoked bladder contractions (A2/A1 ratio).
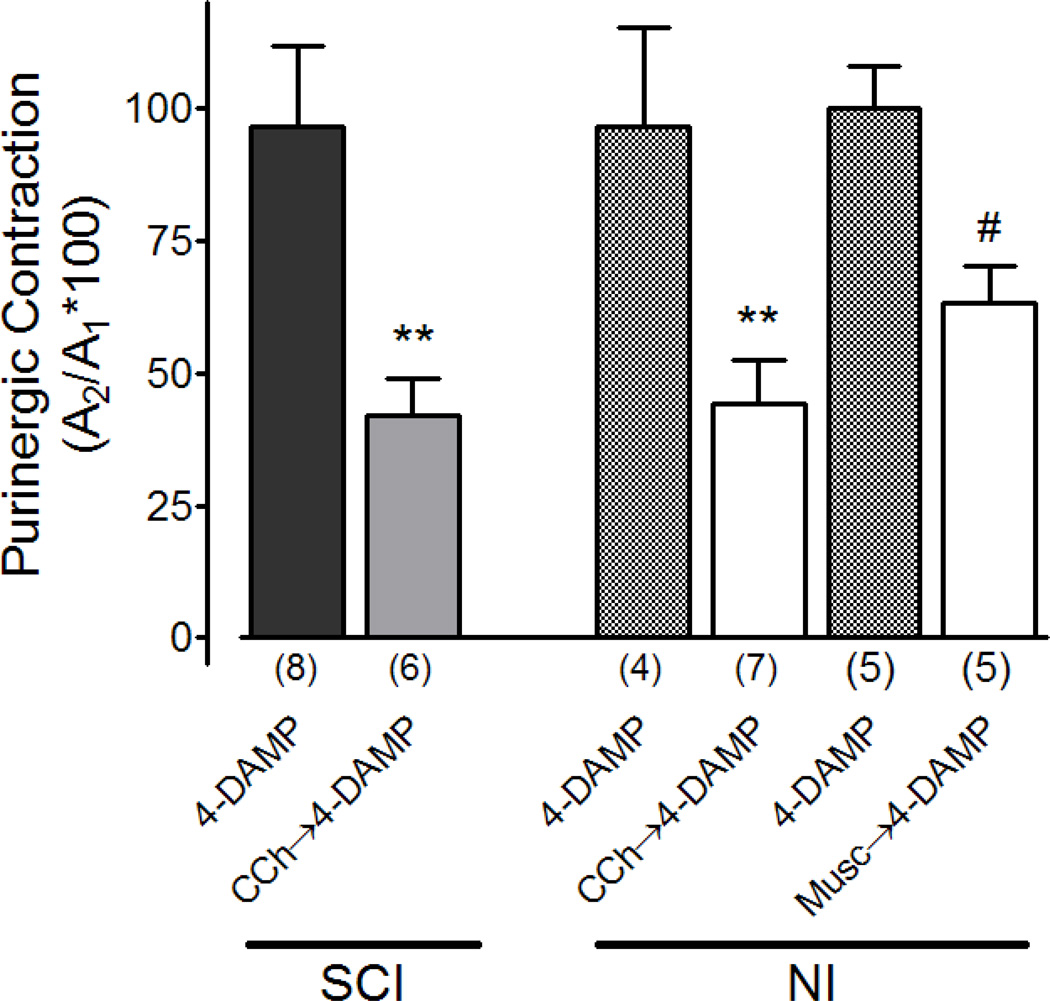
In SCI rats, when 4-DAMP (500 nM) was applied between the two α,β–mATP bladder contractions (paradigm A), the amplitude of A2 was identical to A1 (A2/A1 ratio = 97.2 ± 14.8%, figure 3, n=8). This indicates that there was no desensitization of purinergic receptors when the two applications of α,β–mATP were separated by 35 minutes while 4-DAMP was present in the solution, i.e. the P2X receptors remained equally sensitive to α,β–mATP when it was applied the second time. When the SCI bladder strips were treated with carbachol (10 µM) prior to 4-DAMP (500 nM) (paradigm B), the amplitude of A2 was significantly smaller than A1 (A2/A1 ratio = 42.1 ± 11.6%, figure 3, n=6). This A2/A1 ratio was also significantly smaller than that without carbachol pre-treatment (i.e. 42.1 ± 11.6% versus 97.2 ± 14.8%, p=0.017). In other words, in the presence of carbachol and 4-DAMP, the purinergic contractile response to α,β–mATP in SCI rat bladders was reduced and indicating a significant desensitization of P2X receptors after activation of cholinergic receptors.
Figure 3.
Effect of carbachol and 4-DAMP on α,β–methylene ATP evoked P2X purinergic bladder contractions in SCI and NI rat bladders or muscarine and 4-DAMP in NI rat bladders. In the presence of CCh and 4-DAMP, purinergic receptor desensitization was noted in both SCI and NI rats (A2/A1 < 0.5). The extent of purinergic desensitization was similar between SCI and NI rats (P>0.1). *P<0.05, one-way ANOVA following Bonferroni’s post-test. Note that muscarine also induced enhanced desensitization in NI bladders #P<0.05, paired t test
When the experiments were performed in neurally intact (NI) normal rat bladders (figure 3), a similar reduction of A2/A1 ratio was also observed when carbachol (10 µM) was applied prior to 4-DAMP (500 nM) [16]. In the presence of carbachol and 4-DAMP, the A2/A1 ratio in NI rats (43.4 ± 7.1%) was smaller than 1, but it was not significantly different from the A2/A1 ratio found in SCI rats (figure 3, p=0.92). The same results were obtained with muscarine (10µM) applied instead of carbachol. We found that the enhanced desensitization of purinergic receptors still took place (A2/A1: 63±7% p<0.05; n=5) indicating that activation of muscarinic receptors play an important role in the desensitization of P2X-type purinergic receptors.
4. Discussion
In the present study, we demonstrate a reduction of the non-adrenergic, non-cholinergic (NANC) bladder contractions after activating the cholinergic receptors with carbachol in SCI rats. The muscarinic antagonist 4-DAMP exhibits a more significant inhibition of the neutrally evoked contraction after carbachol application as compared to the inhibition produced by 4-DAMP alone. This carbachol-induced NANC inhibition is observed in normal rat bladders too [16], and as we demonstrated in the present study muscarine, a specific muscarinic agonist also evokes the enhanced inhibition. However, the muscarinic receptor activation induced inhibition is more pronounced in SCI bladders. Furthermore, our results show that sequential activation of the muscarinic receptors with carbachol or muscarine and unmasking of the NANC response with a muscarinic antagonist reduces the purinergic contraction evoked by α,β–mATP, and switches the neurally evoked bladder contraction from predominantly NANC to predominantly cholinergic in SCI rats. Such modulation was not observed if carbachol was not applied prior to 4-DAMP.
Previous studies have shown that the contribution of cholinergic and NANC transmission to neurally evoked bladder contraction is influenced by a number of factors, such as animal age [22, 31], frequency of nerve stimulation [23, 26], and pathology such as SCI [24, 26]. In the present study, we demonstrate that the neurally evoked bladder contractions of SCI and NI bladders are also remarkably plastic to the pharmacologic milieu of the bladder. The relative contribution of NANC/purinergic versus cholinergic/muscarinic contractions may be altered very rapidly (within minutes) and substantially by pharmacologic modulation of the bladder. Our results show that muscarinic antagonists exert a higher inhibitory effect on the neurally evoked NANC contraction when given in the presence of a cholinergic agonist. This observation may have important clinical significance. Although in the normal human bladder the NANC contractions are not significant, in pathologic conditions the NANC/purinergic contractions become more prominent [3]. Understanding how the cholinergic-induced NANC inhibition is mediated, and how NANC and cholinergic signaling interact in the bladder may yield important insights for the treatment of neurogenic urinary incontinence.
In our experiments, the NANC response was unmasked by the muscarinic antagonist 4-DAMP. 4-DAMP is a selective inhibitor of the M3 muscarinic receptors in the 5–20 nM concentration range, but at the 500 nM concentration used in our experiments, 4-DAMP loses its selectivity towards M3, i.e. it functions as a non-specific muscarinic antagonist [9, 10, 25]. The bladder NANC contraction is mediated primarily by purinergic neurotransmission on smooth muscle P2X1 receptors [17, 28], although non-purinergic components have also been described [12, 27]. The purinergic contractions (A1, A2 in figure 1) were evoked by α,β–mATP, a stable ATP analog that is resistant to ecto-ATP-ase activity and selectively activates P2X-type purinergic receptors. Serial applications of α,β–mATP have been shown to cause short-term desensitization of P2X receptors and reduce the contraction amplitude at subsequent application [4]. In rat bladder strips, it typically takes ~35 minutes for P2X desensitization to reverse [4, 16]. In our experimental paradigm, α,β–mATP was applied at the beginning and the end of the experiments. Since the two applications were about 35 minutes apart, the purinergic desensitization caused by the first α,β–mATP application (A1) is reversed by the time α,β–mATP (A2) was re-applied resulting in an A2/A1 ratio close to 1. This was shown in figure 3 paradigm A. However, when carbachol was applied before the second α,β–mATP (paradigm B), the α,β–mATP-evoked contraction became significantly smaller, suggesting that carbachol exerted an inhibitory effect on smooth muscle P2X1 receptors. Since it has been shown that ATP release is enhanced by carbachol in the bladder [18] as well as other smooth muscle preparations [19], this carbachol-induced release of ATP may desensitize the P2X1 receptors in SCI rat bladders, rendering them less responsive to α,β–mATP. We have previously described this effect in NI rat bladders [16] and presented evidence in the current study that the use of muscarine instead of carbachol generated the same effect. A similar cascade of events may also occur in neurogenic bladder from SCI rats (see figure 4).
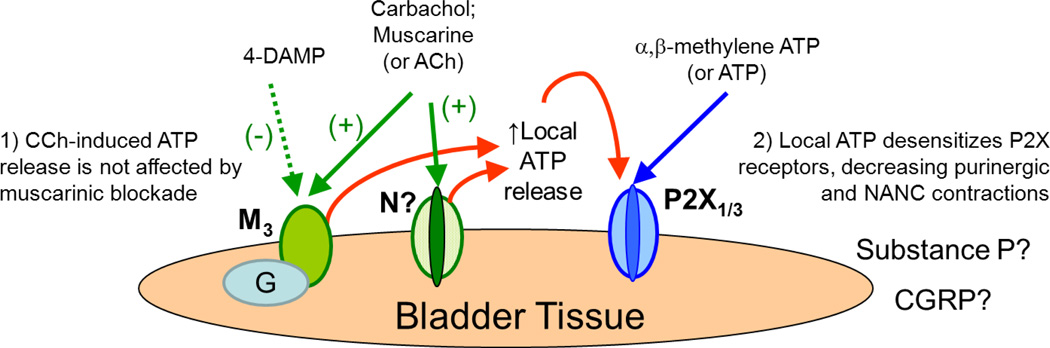
Figure 4.
A model of pharmacologic plasticity in SCI bladders. Carbachol evokes ATP release from the bladder smooth muscle [18, 20], however this release is not affected by subsequent muscarinic blockade by 4-DAMP [20]. The released ATP acts locally and desensitizes P2X-type purinergic receptors, rendering the bladder less responsive to the effects of the α,β-mATP (A2) that was applied shortly after carbachol and 4-DAMP. Other mediators such as CGRP or substance P may also be involved. M, muscarinic receptors; N, nicotinic receptors; P2X, purinergic receptors.
If the purinergic inhibition we observed here is the consequence of receptor desensitization by the carbachol or muscarine-induced ATP release, we can speculate that muscarinic receptor activation may enhance the purinergic receptor desensitization in SCI rat bladders. This kind of desensitization observed with muscarinic receptor activation has been described in nicotinic receptors in the enteric ganglia [5]. However whether there is any putative interaction between muscarinic receptors and purinergic receptors in the bladder needs further investigation. Since after inhibiting the muscarinic receptors with 4-DAMP the remaining site of action of carbachol would be with nicotinic receptors, it is plausible to hypothesize that nicotinic receptors might modulate purinergic receptor desensitization. Recently, several nicotinic receptor subtypes have been described in vascular smooth muscle [6]. Although we have not provided any direct evidence of putative interaction between nicotinic and purinergic receptors in the bladder, other studies have shown that nicotinic and purinergic receptors can negatively interact with each other in the neural cells of sympathetic or enteric ganglia [2, 21, 33]. On the other hand, the application of the selective muscarinic receptor agonist muscarine also negatively affects the purinergic desensitization and supports a role for muscarinic rather than nicotinic modulation.
Interestingly the extent of P2X receptor desensitization using paradigm B was identical in SCI and NI bladders and did not change as a consequence of spinal cord transection (A2/A1 ratio of CCh→4-DAMP in figure 3), despite a dramatic reduction of the % for NANC contraction in SCI bladders compared to NI bladders under the identical experimental paradigm (see % of NANC contraction for CCh→4-DAMP in figure 2). This difference suggests the NANC contractions in SCI neurogenic bladders may involve additional, P2X receptor-independent mechanisms. By definition, a NANC contraction may involve other excitatory transmitters besides ATP, such as substance P and CGRP [13]. In the primary afferents of SCI neurogenic bladders, there is a significant sprouting of substance P containing nerve terminals [32] as well as overexpression of substance P and CGRP in the dorsal root ganglia at S1 and L6 levels [29] that can cause elevated peptide levels in the spinal cord and in the bladder [7]. It has been demonstrated that cholinergic receptor activation induces the release of CGRP from the sensory system via the activation of nicotinic and muscarinic receptors [14]. In our experiments carbachol or muscarine may evoke CGRP release which may then modulate or reduce NANC contractions after 4-DAMP application [11]. Finally there is a possibility that ATP release by itself might be affected by muscarinic receptor activation and subsequent muscarinic inhibition. Further studies are needed to assess the role of the muscarinic, nicotinic and purinergic receptors in this complex interaction as well as to evaluate a possible presynaptic effect for ATP release (figure 4).
5. Conclusions
Activation of muscarinic cholinergic receptors (with carbachol or muscarine) blocks NANC and purinergic contractions of neurogenic bladder in NI and SCI rats. The carbachol-induced inhibition of the NANC contraction is more expressed in SCI bladders compared to NI bladders. Along with receptor plasticity and desensitization, this change in bladder function may involve additional, P2X-receptor independent mechanisms.
Research highlights.
Sequential activation of the cholinergic receptors while unmasking the NANC response with 4-DAMP switched the neurally evoked bladder contraction from predominantly NANC to predominantly cholinergic.
Activation of cholinergic receptors blocks NANC and purinergic contractions of SCI rat bladders.
The carbachol-induced inhibition of the NANC contraction is expressed more in SCI bladders compared to neurally intact bladders.
Along with receptor plasticity, the change in bladder function may involve P2Xindependent mechanisms.
Acknowledgments
This research is supported in part by the National Institute of Health (R01-DK-069988), the American Urological Association Foundation, and the St Louis Veterans Medical Center. We would also like to thank Dr. Nilson Salas for assistance with SCI surgery.
Abbreviations
- 4-DAMP
4-diphenylacetoxy-N-methylpiperidine methiodide
- α,β-mATP
α,β-methylene ATP
- CCh
carbachol
- NI
neurally intact
- SCI
spinal cord injury
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ambache N, Zar MA. Non-cholinergic transmission by post-ganglionic motor neurones in the mammalian bladder. The Journal of physiology. 1970;210:761–783. doi: 10.1113/jphysiol.1970.sp009240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barajas-Lopez C, Espinosa-Luna R, Zhu Y. Functional interactions between nicotinic and P2X channels in short-term cultures of guinea-pig submucosal neurons. The Journal of physiology. 1998;513(Pt 3):671–683. doi: 10.1111/j.1469-7793.1998.671ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayliss M, Wu C, Newgreen D, Mundy AR, Fry CH. A quantitative study of atropine-resistant contractile responses in human detrusor smooth muscle, from stable, unstable and obstructed bladders. J Urol, United States. 1999:1833–1839. [PubMed] [Google Scholar]
- 4.Bolego C, Pinna C, Abbracchio MP, Cattabeni F, Puglisi L. The biphasic response of rat vesical smooth muscle to ATP. British journal of pharmacology. 1995;114:1557–1562. doi: 10.1111/j.1476-5381.1995.tb14939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown EN, Galligan JJ. Muscarinic receptors couple to modulation of nicotinic ACh receptor desensitization in myenteric neurons. American journal of physiology, United States. 2003:G37–G44. doi: 10.1152/ajpgi.00053.2003. [DOI] [PubMed] [Google Scholar]
- 6.Bruggmann D, Lips KS, Pfeil U, Haberberger RV, Kummer W. Multiple nicotinic acetylcholine receptor alpha-subunits are expressed in the arterial system of the rat. Histochem Cell Biol. 2002;118:441–447. doi: 10.1007/s00418-002-0475-2. [DOI] [PubMed] [Google Scholar]
- 7.Burcher E, Zeng XP, Strigas J, Shang F, Millard RJ, Moore KH. Autoradiographic localization of tachykinin and calcitonin gene-related peptide receptors in adult urinary bladder. J Urol. 2000;163:331–337. [PubMed] [Google Scholar]
- 8.Burnstock G, Cocks T, Crowe R, Kasakov L. Purinergic innervation of the guinea-pig urinary bladder. Br J Pharmacol. 1978;63:125–138. doi: 10.1111/j.1476-5381.1978.tb07782.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caulfield MP, Birdsall NJ. International Union of Pharmacology. XVII. Classification of muscarinic acetylcholine receptors. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 10.Eglen RM, Hegde SS, Watson N. Muscarinic receptor subtypes and smooth muscle function. Pharmacol Rev. 1996;48:531–565. [PubMed] [Google Scholar]
- 11.Giuliani S, Santicioli P, Lippi A, Lecci A, Tramontana M, Maggi CA. The role of sensory neuropeptides in motor innervation of the hamster isolated urinary bladder. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:242–248. doi: 10.1007/s002100100447. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto M, Kokubun S. Contribution of P2-purinoceptors to neurogenic contraction of rat urinary bladder smooth muscle. British journal of pharmacology. 1995;115:636–640. doi: 10.1111/j.1476-5381.1995.tb14979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoyle CH. Non-adrenergic, non-cholinergic control of the urinary bladder. World J Urol. 1994;12:233–244. doi: 10.1007/BF00191202. [DOI] [PubMed] [Google Scholar]
- 14.Jinno S, Hua XY, Yaksh TL. Nicotine and acetylcholine induce release of calcitonin gene-related peptide from rat trachea. J Appl Physiol. 1994;76:1651–1656. doi: 10.1152/jappl.1994.76.4.1651. [DOI] [PubMed] [Google Scholar]
- 15.Kennedy C, Tasker PN, Gallacher G, Westfall TD. Identification of atropine- and P2X1 receptor antagonist-resistant, neurogenic contractions of the urinary bladder. J Neurosci, United States. 2007:845–851. doi: 10.1523/JNEUROSCI.3115-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai HH, Smith CP, Munoz A, Boone TB, Szigeti GP, Somogyi GT. Activation of cholinergic receptors blocks non-adrenergic non-cholinergic contractions in the rat urinary bladder. Brain Research Bulletin. 2008;77:420–426. doi: 10.1016/j.brainresbull.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McMurray G, Dass N, Brading AF. Purinoceptor subtypes mediating contraction and relaxation of marmoset urinary bladder smooth muscle. British journal of pharmacology. 1998;123:1579–1586. doi: 10.1038/sj.bjp.0701774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Munoz A, Gangitano DA, Smith CP, Boone TB, Somogyi GT. Removal of urothelium affects bladder contractility and release of ATP but not release of NO in rat urinary bladder. BMC urology, BMC Urol. 2010:10. doi: 10.1186/1471-2490-10-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitahara K, Kittel A, Liang SD, Vizi ES. A1-receptor-mediated effect of adenosine on the release of acetylcholine from the myenteric plexus: role and localization of ecto-ATPase and 5'- nucleotidase. Neuroscience, England. 1995:159–168. doi: 10.1016/0306-4522(94)00585-s. [DOI] [PubMed] [Google Scholar]
- 20.Salas NA, Somogyi GT, Gangitano DA, Boone TB, Smith CP. Receptor activated bladder and spinal ATP release in neurally intact and chronic spinal cord injured rats. Neurochem Int. 2007;50:345–350. doi: 10.1016/j.neuint.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Searl TJ, Redman RS, Silinsky EM. Mutual occlusion of P2X ATP receptors and nicotinic receptors on sympathetic neurons of the guinea-pig. The Journal of physiology. 1998;510(Pt 3):783–791. doi: 10.1111/j.1469-7793.1998.783bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sneddon P, McLees A. Purinergic and cholinergic contractions in adult and neonatal rabbit bladder. Eur J Pharmacol, Netherlands. 1992:7–12. doi: 10.1016/0014-2999(92)90088-l. [DOI] [PubMed] [Google Scholar]
- 23.Somogyi GT, Tanowitz M, de Groat WC. M1 muscarinic receptor-mediated facilitation of acetylcholine release in the rat urinary bladder. The Journal of physiology. 1994;480(Pt 1):81–89. doi: 10.1113/jphysiol.1994.sp020342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Somogyi GT, Yokoyama T, Szell EA, Smith CP, de Groat WC, Huard J, Chancellor MB. Effect of cryoinjury on the contractile parameters of bladder strips: a model of impaired detrusor contractility. Brain Res Bull. 2002;59:23–28. doi: 10.1016/s0361-9230(02)00833-x. [DOI] [PubMed] [Google Scholar]
- 25.Somogyi GT, Zernova GV, Yoshiyama M, Rocha JN, Smith CP, de Groat WC. Change in muscarinic modulation of transmitter release in the rat urinary bladder after spinal cord injury. Neurochem Int. 2003;43:73–77. doi: 10.1016/s0197-0186(02)00193-6. [DOI] [PubMed] [Google Scholar]
- 26.Somogyi GT, Zernova GV, Yoshiyama M, Yamamoto T, de Groat WC. Frequency dependence of muscarinic facilitation of transmitter release in urinary bladder strips from neurally intact or chronic spinal cord transected rats. Br J Pharmacol. 1998;125:241–246. doi: 10.1038/sj.bjp.0702041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Suzuki H, Kokubun S. Subtypes of purinoceptors in rat and dog urinary bladder smooth muscles. British journal of pharmacology. 1994;112:117–122. doi: 10.1111/j.1476-5381.1994.tb13039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong YC, Hung YC, Shinozuka K, Kunitomo M, Cheng JT. Evidence of adenosine 5'- triphosphate release from nerve and P2x-purinoceptor mediated contraction during electrical stimulation of rat urinary bladder smooth muscle. J Urol. 1997;158:1973–1977. doi: 10.1016/s0022-5347(01)64196-x. [DOI] [PubMed] [Google Scholar]
- 29.Vizzard MA. Alterations in neuropeptide expression in lumbosacral bladder pathways following chronic cystitis. J Chem Neuroanat. 2001;21:125–138. doi: 10.1016/s0891-0618(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 30.Wibberley A, Chen Z, Hu E, Hieble JP, Westfall TD. Expression and functional role of Rho-kinase in rat urinary bladder smooth muscle. British journal of pharmacology. 2003;138:757–766. doi: 10.1038/sj.bjp.0705109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoshida M, Homma Y, Inadome A, Yono M, Seshita H, Miyamoto Y, Murakami S, Kawabe K, Ueda S. Age-related changes in cholinergic and purinergic neurotransmission in human isolated bladder smooth muscles. Exp Gerontol, England. 2001:99–109. doi: 10.1016/s0531-5565(00)00175-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Douglas KL, Jin H, Eldaif BM, Nassar R, Fraser MO, Dolber PC. Sprouting of substance P-expressing primary afferent central terminals and spinal micturition reflex NK1 receptor dependence after spinal cord injury. Am J Physiol Regul Integr Comp Physiol, United States. 2008:R2084–R2096. doi: 10.1152/ajpregu.90653.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, Galligan JJ. Non-additive interaction between nicotinic cholinergic and P2X purine receptors in guinea-pig enteric neurons in culture. The Journal of physiology. 1998;513(Pt 3):685–697. doi: 10.1111/j.1469-7793.1998.685ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]