Abstract
Lymphocytes from the peripheral blood of patients with prostate cancer—the most frequent (noncutaneous) tumor in men—display epigenetic aberrations (altered modes of allelic replication) characteristic of the malignant phenotype. The present study aims to determine whether replication aberrations add certainty to the suspicion of prostate cancer provided by the prostate-specific antigen (PSA) blood test. The allelic replication mode (whether synchronous or asynchronous) was exemplified for RB1 and AML1. These two genes normally exhibit a synchronous mode of allelic replication. Fluorescence in situ hybridization (FISH) replication assay was used for replication analyses. The FISH assays were applied to PHA-stimulated lymphocytes, established from peripheral blood samples of 35 men referred to biopsy due to suspected prostate cancer. Following biopsy 13 out of these 35 men were found positive for prostate malignancy. The FISH assay—showing asynchronous or synchronous RB1 and AML1 replication—was able to predict, respectively, the results of all biopsy-positive men and in 18 out of the 22 biopsy-negative ones. These measurements, distinguishing biopsy-positive from biopsy-negative men, were highly significant (P < 10−8; 100% sensitivity and 81.8% specificity). Yet, distinguishing between the two groups of men based on the PSA measurements was nonsignificant (P > 0.70). The FISH replication assay applied to peripheral blood lymphocytes of 35 men referred for biopsy significantly predicted the outcome of the pathological examination, more precisely than the serum PSA test. As such, the epigenetic alteration offers a potential noninvasive blood marker, complementary to the PSA, for a preliminary prostate cancer diagnosis.
Keywords: Asynchronous replication, Replication timing, Cancer biomarkers, PSA, Prostate cancer, FISH
Introduction
Prostate cancer is the second leading cause of cancer death in American men, following lung cancer (Jemal et al. 2008). According to the most recent data of the American Cancer Society, about one man in six will be diagnosed with prostate cancer during his lifetime and about 1 man in 35 will die of prostate cancer, which accounts for about 10% of cancer-related deaths in men (Jemal et al. 2008). Yet, more than two million men in the United States who had been diagnosed with prostate cancer at some point in their life are still alive today. This may be attributed partly to early detection achieved by the prostate-specific antigen (PSA) blood test (Hankey et al. 1999). When the PSA test was introduced [at the beginning of the “PSA Era,” reviewed in Stamey et al. (2004)], serum levels bellow to 4.0 ng/mL were considered normal, while higher values were considered abnormal and called for performing an invasive procedure, i.e., tissue biopsy, which is uncomfortable, painful, and costly (Carter 2000; Catalona et al. 2000). Unfortunately, the PSA test has certain limitations, as it appears that 10–15% of men with PSA values below 4.0 ng/mL have malignant prostate tumors, and two out of three men with elevated (>4.0 ng/mL) PSA levels are free from prostate cancer but suffer from benign prostate hyperplasia (BPH), prostatitis, or other nonmalignant prostate irregularities (Stamey et al. 2004). Accordingly, during the last few years, the PSA cutoff values are under debate (Carter 2000; Catalona et al. 2000; Smith et al. 2008). The latest recommendations of the ACS give the cutoff value of 2.5 ng/mL (Jemal et al. 2008), leading to an increase in the number of biopsies in patients with elevated PSA, resulting from nonmalignant causes (Applewhite et al. 2002; Eichler et al. 2006). In light of the low specificity of PSA, urologists and oncologists would clearly welcome novel blood markers that would differentiate biopsy-positive from biopsy-negative cases with higher specificity (Stamey et al. 2004).
Recently, it was reported that DNA replication-timing analyses applied to peripheral blood lymphocytes of cancer patients differentiate between prostate cancer subjects and BPH individuals. In brief, allelic counterparts of various “cancer genes” such as RB1, AML1, TP53, and C-MYC, which normally replicate synchronously in phytohemagglutinin (PHA)-stimulated lymphocytes, will replicate highly asynchronously in prostate cancer patients (Dotan et al. 2004, 2008).
Asynchronous replication of allelic counterparts is a characteristic epigenetic marker of monoallelically expressed genes (Goldmit and Bergman 2004). A simple method for evaluating the temporal order of allelic replication utilizes the fluorescent in situ hybridization (FISH) assay (Boggs and Chinault 1997; Dotan et al. 2008). Of major importance for this work is the fact that the temporal order (synchronous or asynchronous) of replication of two allelic counterparts is preserved even in cells in which these alleles are not expressed. Therefore, the order of allelic replication can be used as a marker of whether the alleles of a particular gene are epigenetically marked in a similar or a different mode (Ensminger and Chess 2004).
The abnormal replication pattern of various “cancer genes” observed in blood cells of prostate cancer patients (Dotan et al. 2004, 2008) appears to be non-cancer-type specific (Amiel et al. 2000, 2001a, b; Brás et al. 2008; Dotan et al. 2000; Grinberg-Rashi et al. 2010; Korenstein-Ilan et al. 2002). Whatever the mechanism giving rise to the epigenetic replication aberrations in the blood lymphocytes, this study will check the potential of the replication timing analyses for predicting the biopsy results.
Material and methods
Study subjects
This study included 35 male, consenting urology patients, above 50 years old who had a life expectancy of at least 10 years and were referred to biopsy because of suspected prostate cancer. Digital rectal examinations (DRE) of 34 individuals (out of the 35 studied) reported negative results, only 1 appeared positive by the test. Each patient was evaluated for the blood level of the PSA. Histological examinations were carried out on tissues removed from the prostate gland by biopsy, performed according to the sextant scheme with at least 12 cores (2 from each sextant regiment) obtained from each patient. The outcome of the histological test of each patient was released at the end of the study, following completion of the FISH analyses. The histological results indicated that 13 individuals (including that with a positive DRE outcome) were prostate cancer positive (designated hereinafter “CAP”), and 22 were found to be negative for prostate cancer by the biopsy procedure [designated hereinafter “BN” (biopsy negative)].
Cell culture
Each subject, prior to the invasive biopsy procedure, donated 5 mL of peripheral blood from which cell cultures of PHA-stimulated lymphocytes were set up according to standard protocol used for routine karyotype analyses (Dotan et al. 2004). Following harvesting, cells were fixed and stored in suspension in the fixative solution (3:1 methanol to acetic acid) at −20°C until used for FISH analysis.
Probes
We tested two loci using directly labeled commercial probes obtained from Vysis (Abbott Laboratories, Abbott Park, IL, USA): (1) the RB1 probe (LSI 13; 32–190001) and (2) the AML1 probe (LSI 21; 32–190002).
In situ hybridization
We followed a standard protocol as recommended by Abbott Laboratories, with a few minor changes. Cells were dropped onto two well slides (Insitus Biotechnologies, Albuquerque, NM, USA) without any pretreatment. Five microliters of probe solution was placed on the target cell area of the slides and covered with a round 12-mm silianized cover-slip (Insitus Biotechnologies), which was then sealed with rubber cement. The slides were placed into a micro-heating system (Vysis HYBrite, Abbott Labs), programmed for 6 min denaturation at 76°C followed by 18 h hybridization at 37°C.
Post hybridization treatment
Post hybridization washes of each probe consisted of immersing the slides for 2 min in a solution of 0.4x saline sodium citrate (SSC) pH 7.0 with 0.3% NP40 (Nonidet P40 deterent) at 72°C, followed by 2 min in 2x SSC with 0.1% NP40 at room temperature in a shaking water bath. After brief drying, the cell area was exposed to anti-fade containing 4,6-diamidino-2-phenylindole (3 μg/mL; Vector Laboratories, Burlingame, CA, USA), covered with a glass cover-slip and stored at −20°C until analyzed.
Cytogenetic evaluation
Slides were analyzed blindly using an Olympus BH2 fluorescent microscope, fitted with a triple band-pass filter (Chroma Technology, Brattleboro, VT, USA). For each sample, at least 200 cells exhibiting two distinct well-defined fluorescence signals were blindly scored for each probe. The structure of each signal, either singlet (S), representing a non-replicated sequence, or doublet (D), disclosing a replicated sequence, was noted. Thus, cells appeared to carry either two asynchronous signals (SD; Fig. 1a) or synchronous signals (SS and DD; Fig. 1b, c, respectively). For each locus, the frequencies of SD cells, out of the total population of cells containing two signals (total of SD, SS, and DD cells), were noted. The frequency of SD cells was used to estimate whether a locus replicated synchronously or asynchronously: a low range of frequencies would indicate synchrony and a high range of frequencies would indicate asynchrony. Our lab experience shows that in samples of PHA-stimulated lymphocytes, following hybridization with a commercial probe, a frequency bellow to 25% is regarded low, since it characterizes normally biallelic loci (Dotan et al. 2008).
Fig. 1.
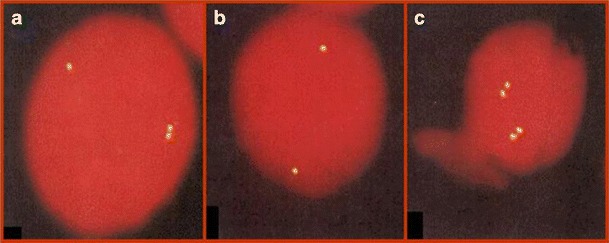
Fluorescent signals in PHA-stimulated lymphocytes at interphase following one-color FISH with the AML1 probe. a Cell with one singlet and one doublet (SD cell), which is an S-phase cell in which one allele has replicated while its partner has still to do so; b cell with two singlets (SS cell), in which neither allele has replicated; and c cell with two doublets (DD cell), in which both alleles have replicated
Statistical method
The statistical significance of the differences between two cell populations was determined using the two-tailed Student's t test (Microsoft Excel), with P < 0.01 considered to be statistical significant.
Ethical basis
Informed consent was obtained from each patient examined, and the study was approved by the institutional review board.
Results
The frequencies of SD cells for RB1 and AML1 in cell samples of all 13 patients positively diagnosed by biopsy (CAP cases) ranged between 26.0% and 40.0% for RB1 and 26.5–37.0% for AML1. At the same time, the corresponding values in 19 out of the 22 patients negatively diagnosed by biopsy (BN cases) ranged between 15.7% and 23.0% for RB1 and 13.4–26.5% for AML1. Yet, cell samples of three BN cases displayed high SD values for both genes, similar to those observed for the CAP patients—32.1%, 33.2%, and 34.0% for RB1 and 31.0%, 32.2%, and 31.0% for AML1, respectively (Fig. 2a). No statistical differences were observed within each group between the SD values for RB1 and those for AML1 (P > 0.65 for the CAP group of samples and P > 0.80 for the BN group).
Fig. 2.
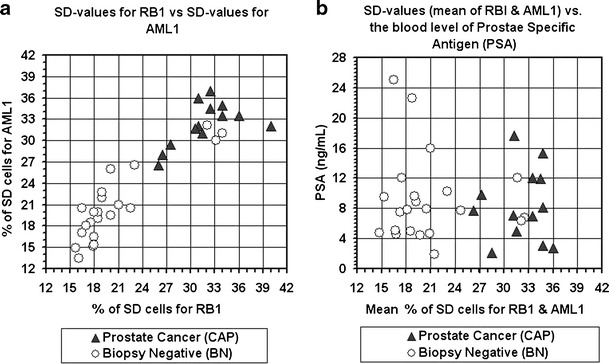
Distribution of blood samples according to their SD values (a) and SD vs. PSA values (b). CAP–samples of patients diagnosed cancer positive by the prostate biopsy examination; BN—sample of patients diagnosed cancer-negative by the prostate biopsy examination. Note the considerable high separation, between the two groups, attained by the SD values, and low separation achieved by the PSA blood levels
By assessing each patient's mean SD value of the RB1 and AML1 loci, all CAP samples ranged above 24% (100% sensitivity) while only 4 out of the 22 BN samples exhibited SD values above 24% (81.8% specificity, Fig. 2b). The differences in SD values between the two groups of patients were highly significant, P < 10−7 for RB1, P < 10−8 for AML1, and P < 10−8 for the combined RB1 and AML1 data (Fig. 3a).
Fig. 3.
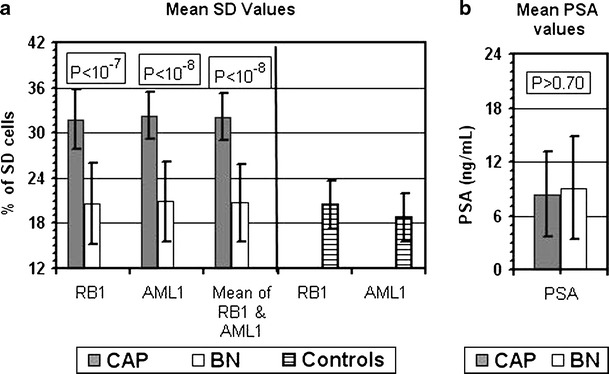
Mean and standard deviation values of SD (a) and PSA (b). The upper boxes within each frame present the level of significance (P) of the differences between the biopsy-cancer-positive group (CAP; 13 cases) and the biopsy-cancer-negative (BN; 22 cases) group for the relevant parameter. The striped (last two) bars in frame (a) present SD values reported previously for RB1 (15 cases) and for AML1 (41 cases) in blood samples of subjects without cancer (controls) [described in Korenstein-Ilan et al. (2002)]
It is worth mentioning that the SD values for RB1 and AML1 found in the BN group were similar to those observed previously [described by Korenstein-Ilan et al. (2002)] for these loci in subjects without cancer, designated controls (Fig. 3a).
On the other hand, in the group of 35 men studied here, there was no significant difference (P > 0.70) between the two groups in the serum levels of PSA (Fig. 3b). The PSA values of the CAP patients ranged between 2.1 and 17.6 ng/mL (mean and standard deviation of 8.4 ± 4.8 ng/mL) and that of the BN subjects was 1.9–25.0 ng/mL (mean and standard deviation of 9.1 ± 5.7 ng/mL, Figs. 2b and 3b). In the group of 35 men studied here, serum PSA levels above 4 ng/mL were displayed by 10 (out of 13) CAP cases and by 21 (out of 22) BN cases (Fig. 2b).
Discussion
We investigated the level of replication synchrony of the allelic counterparts of two cancer-related genes (RB1 and AML1): RB1—the retinoblastoma tumor-suppressor gene—is the first known tumor suppressor gene which was the first such gene to exhibit epigenetic rather than genetic inactivation in a tumor suppressor gene, which was linked to an allele-specific event [reviewed in Sakai et al. (1991)]; and AML1, which is an ETS family gene involved in the multiple 8;21 translocations associated with leukemia [reviewed in Zhang and Rowley (2006)]. Normally, these two “cancer genes” exhibit a synchronous mode of allelic replication. Yet, in peripheral blood cells of prostate cancer patients, each displayed a loss of synchrony, a behavior normally observed in genes subjected to allelic specific expression (reviewed in the introduction). Here, we show that all patients tested positively by prostate biopsy displayed, in their lymphocytes, replication aberrations (the change from synchronous to asynchronous modes of replication) in both studied genes (100% specificity). At the same time, most cases in our study group (18 out of the total of 22) that were diagnosed as prostate cancer free under biopsy displayed normal synchronous replication of these genes, similar to that observed previously (Korenstein-Ilan et al. 2002) for non-cancerous controls. Yet, the four biopsy-negative cases showed asynchronous modes of replication for both genes, similar to those observed in the biopsy-positive cancer cases. Simply, these results point to a reduced specificity (∼82%). However, since prostate histopathologic examination (although the gold standard for diagnosis) has a false negative rate as high as 30% (Applewhite et al. 2002; Eichler et al. 2006), it is logical to assume that some of these asynchronous FISH-tested patients are in fact prostate cancer cases missed by the histopathologic examination. This assumption could be tested by subjecting these biopsy-negative subjects to repeat biopsies. A reversal of some of these pathological determinations would further strengthen the specificity of the FISH approach. However, if asynchronous replication cases are clearly free of prostate cancer (false positives in our study), there is a possibility that these patients may be suffering from some other malignancy, as the epigenetic aberrations we measured are also seen in the lymphocytes of patients with miscellaneous hematological malignancies (Amiel et al. 2000, 2001a, b; Korenstein-Ilan et al. 2002; Nagler et al. 2004, 2010), renal cell carcinoma (Dotan et al. 2000), and breast cancer (Grinberg-Rashi et al. 2010).
However, even if the false positives observed by the epigenetic assay are truly prostate cancer-free individuals, our assay is still promising for forecasting the biopsy results. As such, the epigenetic aberrations in peripheral blood lymphocytes offer a potential noninvasive assay to differentiate between patients that require biopsy to verify the diagnosis based on elevated PSA and those that have no need to undergo the invasive biopsy procedure. Our test is also superior to the “liquid biopsy” examination based on circulating tumor cells (CTCs) in the blood stream of cancer patients. Because CTCs are extremely rare, they require laborious and compound technologies to be captured and isolated [reviewed in Stott et al. (2010)].
Although the mechanism giving rise to the measurable epigenetic alterations in lymphocytes of cancer patients is still obscure, it appears to be highly dependent on methylation capacity (Dotan et al. 2004, 2008; Korenstein-Ilan et al. 2002; Nagler et al. 2004, 2010). This is in line with studies showing that methylation capacity of cancer-critical genes may be of use in differentiating between prostate tissues obtained from BPH subjects and those from prostate cancer patients (Costa et al. 2007; Harden et al. 2003).
Finally, replication timing measurements in PHA-stimulated lymphocytes provide potential blood indicators that would go along with PSA testing in identifying patients who should undergo biopsy to verify their cancer status. Such a combination could reduce the need for biopsies. Although the replication assay was carried out on a limited number of subjects, the obtained results clearly indicate the need of expanding the protocol to include a much larger group of suspected prostate cancer patients.
Acknowledgments
The authors would like to thank the consenting patients. We are also grateful to Cohi Shevi and Amit Barak for their assistance and help in collection and coding the blood samples.
Conflict of interest
MM, AD, and LA are cofounders of Allelis Diagnostics Ltd. All other authors declare that they have no competing interests.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Amiel A, Kitay-Cohen Y, Fejgin MD, Lishner M. Replication status as a marker for predisposition for lymphoma in patients with chronic hepatitis C with and without cryoglobulinemia. Exp Hematol. 2000;28:156–160. doi: 10.1016/S0301-472X(99)00140-X. [DOI] [PubMed] [Google Scholar]
- Amiel A, Elis A, Blumenthal D, Gaber E, Fejgin MD, Dubinsky R, Lishner M. Modified order of allelic replication in lymphoma patients at different disease stages. Cancer Genet Cytogenet. 2001;125:156–160. doi: 10.1016/S0165-4608(00)00381-2. [DOI] [PubMed] [Google Scholar]
- Amiel A, Elis A, Sherker S, Gaber E, Manor Y, Fejgin MD. The influence of cytogenetic aberrations on gene replication in chronic lymphocytic leukemia patients. Cancer Genet Cytogenet. 2001;125:81–86. doi: 10.1016/S0165-4608(00)00373-3. [DOI] [PubMed] [Google Scholar]
- Applewhite JC, Matagla BR, McCullough D. Results of the 5 region prostate biopsy method: the repeat biopsy population. J Urol. 2002;168:500–503. doi: 10.1016/S0022-5347(05)64667-8. [DOI] [PubMed] [Google Scholar]
- Boggs BA, Chinault AC. Analysis of DNA replication by fluorescence in situ hybridization. Methods. 1997;13:259–270. doi: 10.1006/meth.1997.0525. [DOI] [PubMed] [Google Scholar]
- Brás A, Cotrim CZ, Vasconcelos I, Mexia J, Onard AL, Sanzhar I, Akhmatullina N, Rueff J. Asynchronous DNA replication detected by fluorescence in situ hybridisation as a possible indicator of genetic damage in human lymphocytes. Oncol Rep. 2008;19:369–375. doi: 10.3892/or.19.2.369. [DOI] [PubMed] [Google Scholar]
- Carter HB. A PSA threshold of 4.0 ng/mL for early detection of prostate cancer: the only rational approach for men 50 years old and older. Urology. 2000;55:796–799. doi: 10.1016/S0090-4295(00)00517-3. [DOI] [PubMed] [Google Scholar]
- Catalona W, Ramos CG, Carvalhal GF, Yan Y. Lowering PSA cutoffs to enhance detection of curable prostate cancer. Urology. 2000;55:791–795. doi: 10.1016/S0090-4295(99)00602-0. [DOI] [PubMed] [Google Scholar]
- Costa VL, Henrique R, Jeronimo C. Epigenetic markers for molecular detection of prostate cancer. Dis Markers. 2007;23:31–41. doi: 10.1155/2007/356742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotan ZA, Dotan A, Litmanovitch T, Ramon J, Avivi L. Modification in the inherent mode of allelic replication in lymphocytes of patients suffering from renal cell carcinoma: a novel genetic alteration associated with malignancy. Gene Chromosome Canc. 2000;27:270–277. doi: 10.1002/(SICI)1098-2264(200003)27:3<270::AID-GCC7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Dotan ZA, Dotan A, Ramon J, Avivi L. Altered mode of allelic replication accompanied by aneuploidy in peripheral blood lymphocytes of prostate cancer patients. Int J Cancer. 2004;111:60–66. doi: 10.1002/ijc.20237. [DOI] [PubMed] [Google Scholar]
- Dotan ZA, Dotan A, Ramon J, Avivi L. Aberrant allele-specific replication, independent of parental origin, in blood cells of cancer patients. BMC Cancer. 2008 doi: 10.1186/1471-2407-8-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichler K, Hempel S, Wilby J, Myers L, Bachmann LM, Kleijnen J. Diagnostic value of systematic biopsy methods in the investigation of prostate cancer: a systematic review. J Urol. 2006;175:1605–1612. doi: 10.1016/S0022-5347(05)00957-2. [DOI] [PubMed] [Google Scholar]
- Ensminger AW, Chess A. Coordinated replication timing of monoallelically expressed genes along human autosomes. Hum Mol Genet. 2004;13:651–658. doi: 10.1093/hmg/ddh062. [DOI] [PubMed] [Google Scholar]
- Goldmit M, Bergman Y. Monoallelic gene expression: a repertoire of recurrent themes. Immunol Rev. 2004;200:197–214. doi: 10.1111/j.0105-2896.2004.00158.x. [DOI] [PubMed] [Google Scholar]
- Grinberg-Rashi H, Cytron S, Gelman-Kohan Z, Litmanovitch T, Avivi L. Replication timing aberrations and aneuploidy in peripheral blood lymphocytes of breast cancer patients. Neoplasia. 2010;12:668–674. doi: 10.1593/neo.10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankey BF, Feuer EJ, Clegg LX, Ries LA, Merrill RM, Kaplan RS. Cancer surveillance series: interpreting trends in prostate cancer—part I: evidence of the effects of screening in recent prostate cancer incidence, mortality, and survival rates. J Natl Cancer Inst. 1999;91:1017–1024. doi: 10.1093/jnci/91.12.1017. [DOI] [PubMed] [Google Scholar]
- Harden SV, Sanderson H, Goodman SN, Sidransky D. Quantitative GSTP1 methylation and the detection of prostate adenocarcinoma in sextant biopsies. J Natl Cancer Inst. 2003;95:1634–1637. doi: 10.1093/jnci/djg082. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Korenstein-Ilan A, Amiel A, Lalezari S, Lishner M, Avivi L. Allele-specific replication associated with aneuploidy in blood cells of patients with hematologic malignancies. Cancer Genet Cytogenet. 2002;139:97–103. doi: 10.1016/S0165-4608(02)00610-6. [DOI] [PubMed] [Google Scholar]
- Nagler A, Korenstein-Ilan A, Amiel A, Avivi L. Granulocyte colony stimulating factor generates epigenetic and genetic alterations in lymphocytes of normal volunteer donors of stem cells. Exp Hematol. 2004;32:122–130. doi: 10.1016/j.exphem.2003.09.007. [DOI] [PubMed] [Google Scholar]
- Nagler A, Cytron S, Mashevich M, Korenstein-Ilan A, Avivi L. The aberrant asynchronous replication—characterizing lymphocytes of patients with hematological malignancies—is erased following stem cell transplantation. BMC Cancer. 2010 doi: 10.1186/1471-2407-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai T, Toguchida J, Ohtani N, Yandell DW, Rapaport JM, Dryja TP. Allele-specific hypermethylation of the retinoblastoma tumor-suppressor gene. Am J Hum Genet. 1991;48:880–888. [PMC free article] [PubMed] [Google Scholar]
- Smith RA, Cokkinides V, Brawley OW. Cancer screening in the United States, 2008: a review of current American Cancer Society guidelines and cancer screening issues. CA Cancer J Clin. 2008;58:161–179. doi: 10.3322/CA.2007.0017. [DOI] [PubMed] [Google Scholar]
- Stamey TA, Caldwell M, McNeal JE, Nolley R, Hemenez M, Downs J. The prostate specific antigen era in the United States is over for prostate cancer: what happened in the last 20 years? J Urol. 2004;172:1297–1301. doi: 10.1097/01.ju.0000139993.51181.5d. [DOI] [PubMed] [Google Scholar]
- Stott SL, Lee RJ, Nagrath S, Yu M, Miyamoto DT, Ulkus L, Inserra EJ, Ulman M, Springer S, Nakamura Z, Moore AL, Tsukrov DI, Kempner ME, Dahl DM, Wu C-L, Iafrate AJ, Smith MR, Tompkins RG, Sequist LV, Toner M, Haber DA, Maheswaran S. Isolation and characterization of circulating tumor cells from patients with localize and metastatic prostate cancer. Sci Transl Med. 2010;2:25ra23. doi: 10.1126/scitranslmed.3000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Rowley JD. Chromatin structural elements and chromosomal translocations in leukemia. DNA Repair (Amst) 2006;5:1282–1297. doi: 10.1016/j.dnarep.2006.05.020. [DOI] [PubMed] [Google Scholar]


