Abstract
On the basis of efficacy, opioid antagonists are classified as inverse opioid agonists (e.g. naltrexone) or neutral opioid antagonists (e.g. 6β-naltrexol). This study examined the interaction between naltrexone and 6β-naltrexol in the precipitated opioid withdrawal syndrome in morphine dependent mice. Furthermore, the possible contribution of constitutive opioid receptor activity to precipitated withdrawal was evaluated using increasing levels of morphine dependence. In the first experiment, low doses of 6β-naltrexol antagonized naltrexone precipitated withdrawal while high doses acted additively. All doses of naltrexone increased 6β-naltrexol’s potency to precipitate withdrawal. The next experiment examined changes in antagonist potency to precipitate withdrawal with increasing morphine dependence. Mice were exposed to morphine for 1-6 days and then withdrawal was precipitated. Naltrexone was more potent than 6β-naltrexol at all the time points. The ED50 of both drugs decreased at the same rate suggesting that increased dependence produced no change in constitutive opioid receptor activity. Taken together these results indicate that the functional efficacy of 6β-naltrexol is dose-dependent and that constitutive opioid receptor activity did not change as opioid dependence increased from 1-6 days.
Keywords: Naltrexone, 6β-naltrexol, Morphine, Constitutive receptor activity, Withdrawal, Dependence, Neutral opioid antagonist, Inverse opioid agonist
1. Introduction
Antagonists, in general, are classified as “inverse agonists” or “neutral antagonists”. Inverse agonists have negative efficacy and decrease constitutive receptor activity, whereas neutral antagonists have zero efficacy and bind without affecting receptor activity (Kenakin, 2001; Milligan et al., 1997; Prather, 2004). In the absence of constitutive receptor activity these two classes of antagonist are functionally indistinguishable (Kenakin, 2001; Milligan et al., 1997; Prather, 2004). Studies demonstrate that naltrexone is an inverse opioid agonist and 6β-naltrexol is a neutral opioid antagonist (Raehal et al., 2005; Sadée et al., 2005; Sirohi et al., 2009; Wang et al., 2001, 2004). There has been interest in the possibility that neutral opioid antagonists might have advantages over inverse opioid agonists in the treatment of opioid overdose (Sadée et al., 2005; Sirohi et al., 2009; Wang et al., 2001). As such, understanding the characteristics of these opioids may be of clinical importance.
Previous studies suggest that naltrexone and 6β-naltrexol posses relatively similar potency profiles to antagonize opioid (morphine, fentanyl) induced analgesia and toxicity (Sirohi et al., 2007; 2009), but that 6β-naltrexol is dramatically less potent in precipitating opioid withdrawal in opioid dependent animals (Raehal et al., 2005; Sirohi et al., 2007; 2009; Walker and Sterious, 2005; Wang et al., 2001). Thus, the neutral opioid antagonist might be useful in reversing the effects of opioids during overdose without liability to precipitate withdrawal, which could be life-threatening (van Dorp et al., 2007). These differences between naltrexone and 6β-naltrexol might be due to differential effects on constitutive opioid receptor activity, which may be involved in opioid dependence and withdrawal (Liu and Prather, 2001; Sadée et al., 2005; Wang et al., 1994; 2001; 2004). Consistent with this suggestion, constitutive opioid receptor activity has been shown to increase following exposure to opioid agonists and the development of dependence (Costa and Herz, 1989; Liu and Prather, 2001; Sadée et al., 2005; Wang et al., 1994; 2001; 2004).
In order to study the relative efficacy of naltrexone and 6β-naltrexol, we determined the effect of 6β-naltrexol pretreatment on the potency of naltrexone to precipitate withdrawal in mice and vice versa. Although 6β-naltrexol has been shown to antagonize naltrexone-induced withdrawal in rodents (Sirohi et al., 2009), there has been no study examining its effect on an inverse opioid agonist (e.g. naltrexone) across a full range of doses. It was predicted that 6β-naltrexol at low doses that do not precipitate withdrawal would inhibit the effect of naltrexone, as previously reported (Sirohi et al., 2009; Raehal et al., 2005). It was hypothesized that higher doses of 6β-naltrexol, that do precipitate withdrawal, would act additively with naltrexone; revealing a dose-dependent change in the functional efficacy of 6β-naltrexol. On the other hand, it was hypothesized that the inverse opioid agonist naltrexone, irrespective of the dose administered, would only have an additive effect on 6β-naltrexol induced opioid withdrawal. In the next experiment, the change in the relative potency of naltrexone and 6β-naltrexol to induce withdrawal with increasing dependence was determined. Studies show that the ED50 of opioid antagonists to precipitate withdrawal decreases with increasing dependence (Adams and Holtzman, 1990; Azar et al., 2003; Schulteis et al., 2005; 2009; Villarreal and Castro, 1981; Way et al., 1969). It was predicted that if constitutive opioid receptor activity develops in parallel with opioid dependence then the potency of naltrexone to precipitate withdrawal should increase more than 6β-naltrexol.
2. Methods
2.1 Subjects
Male Swiss Webster mice, weighing 26–33 g, obtained from Taconic Farms (Germantown, NY) were used throughout. Animals were housed 10 per cage with food and water available ad-libitum for at least 24 h before experimentation. All protocols were approved by the St John’s University Institutional Animal Care and Use Committee.
2.2 Procedure
The effect of 6β-naltrexol pretreatment on naltrexone potency to precipitate withdrawal jumping in morphine dependent mice was examined (see Withdrawal Jumping Assay below). Mice were implanted s.c. with a morphine pellet (25 mg) for 3 days. At the end of treatment, mice were injected s.c. with saline or 6β-naltrexol (0.5–10.0 mg/kg) and 70 minutes later, injected s.c. with naltrexone (0.00625–0.4 mg/kg) (N=4–10/dose) and immediately observed for withdrawal jumping for 15 min. Next, the effect of naltrexone pretreatment on 6β-naltrexol potency to precipitate withdrawal jumping was determined. Mice were implanted s.c. with a morphine pellet (25 mg) for 3 days. At the end of the treatment, mice were injected s.c. with saline or naltrexone (0.0125–0.05 mg/kg), immediately followed by 6β-naltrexol (0.5–20 mg/kg) (N=5/dose) and observed for withdrawal jumping for 15 min. In all cases withdrawal jumping was observed at the approximate time of peak effect for the pretreatment drug (naltrexone ≈ 5–20 min, 6β-naltrexol ≈ 60–105 min; based on preliminary studies and Sirohi et al., 2007, 2009). The doses used were based on previous studies (Sirohi et al., 2007, 2009) and preliminary experiments.
Next, the potency of naltrexone and 6β-naltrexol to precipitate withdrawal jumping was determined following morphine treatment protocols ranging from 1 to 6 days. Mice were implanted s.c. with a morphine pellet (25 mg) for 1 or 3 days. Other mice were implanted s.c. with one morphine pellet (25 mg) for 3 days and then an additional morphine pellet (25 mg) for 3 more days (total 6 days of morphine treatment). At the end of treatment, mice (N=5–30/dose) were injected s.c. with naltrexone (0.0125–2 mg/kg) or 6β-naltrexol (1.5–140 mg/kg) and immediately observed for withdrawal jumping for 15 min. A decrease in the ED50 of opioid antagonists to precipitate withdrawal is a common measure of the degree of opioid dependence (Adams and Holtzman, 1990; Azar et al., 2003; Schulteis et al., 2005; 2009; Villarreal and Castro, 1981; Way et al., 1969).
2.3 Withdrawal Jumping Assay
Mice were placed in a clear plastic container (4L) for observation of withdrawal jumping. The full observation period was video recorded and jumping was quantified for each subject at the end of the experiment. Withdrawal jumping was defined as all 4 paws leaving the bottom of the container. For quantal dose-response analysis, mice that jumped 35 or more times were defined as positive responders for withdrawal jumping. While other threshold criteria could be used, the 35 jump criterion was chosen because it yielded orderly quantal dose-response functions using both drugs. ED50s (quantal data) and EC50s (graded data) for naltrexone and 6β-naltrexol were estimated based on these data (see Data Analysis below). In most cases, 5–10 mice per dose were tested, although 4 mice were used in one experiment with the lowest dose of naltrexone. Thirty mice were tested for four 6β-naltrexol doses in part of one experiment, because the original drug lot was depleted during the study and therefore we conducted replications using the new supply.
2.4 Drugs
Naltrexone HCl and morphine sulfate were obtained from Spectrum Chemicals Inc. (Gardena, CA, USA). 6β-naltrexol HCl and morphine implant pellets (25 mg morphine base) were obtained from the Research Triangle Institute (Research Triangle Park, NC) through the Research Technology Branch of NIDA. Morphine pellets were wrapped in nylon mesh before s.c. implantation. Pellets were implanted while mice were lightly anesthetized with isoflurane:oxygen (4:96) and pellets were not removed prior to testing. Drugs for injection were dissolved in 0.9% saline and doses are expressed as the free base.
2.5 Data Analysis
Quantal dose-response data from each experiment incorporating up to 6 dose-response functions were analyzed as a group using the BLISS-21 computer program (Department of Statistics, University of Edinburgh) which uses Probit analysis (Finney, 1973) to calculate ED50 values, standard errors, 95% confidence intervals and relative potency estimates. Significant differences (p < 0.05) in ED50’s and potency were based on Probit analysis. In addition, graded withdrawal data (mean jumps) were analyzed to estimate the EC50s using nonlinear regression (3 parameter dose-response function; Prism ver 5, Graphpad Software, La Jolla, CA).
3. Results
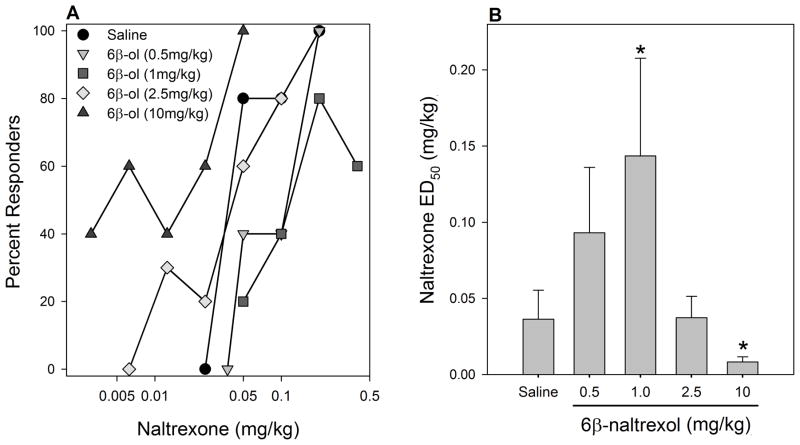
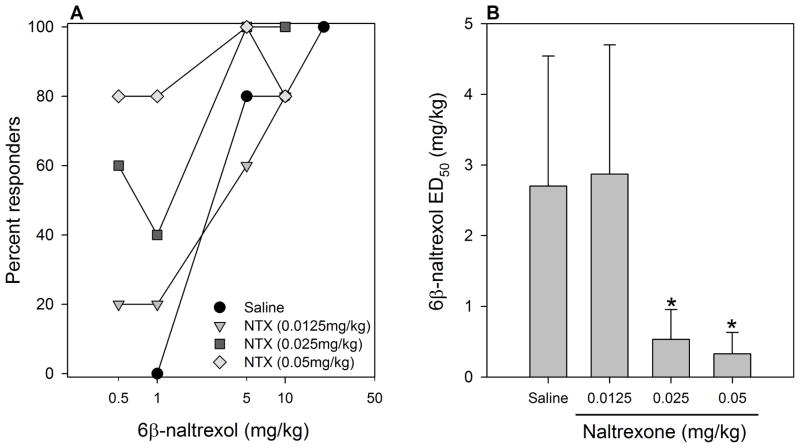
In all experiments, analysis of quantal data indicated that naltrexone was more potent that 6β-naltrexol in precipitating withdrawal (Figs 1, 2, 3), as has been reported previously (e.g., Sirohi et al. 2007; Raehal et al., 2005). Low doses of 6β-naltrexol (0.5, 1.0mg/kg) shifted the dose response function for naltrexone to the right by 2.3- and 3.5-fold, respectively, whereas a higher 6β-naltrexol dose (2.5mg/kg) did not significantly alter the potency of naltrexone (Fig. 1). The highest dose of 6β-naltrexol (10mg/kg) significantly increased the potency of naltrexone. On the other hand, naltrexone pretreatment dose dependently increased the potency (decreased ED50) of 6β-naltrexol, with the highest dose shifting the 6β-naltrexol dose response curve to the left by 8.2-fold (Fig. 2). Taken together, 6β-naltrexol displayed a dose-dependent change in functional efficacy. Increasing doses of 6β-naltrexol produced a bidirectional shift of the dose response function for naltrexone precipitated opioid withdrawal. Naltrexone’s effect on the 6β-naltrexol dose response function is unidirectional.
Figure 1. Effect of 6β-naltrexol pretreatment on naltrexone-precipitated withdrawal jumping after 3 days morphine treatment.
Mice were implanted s.c. with a morphine pellet (25 mg) for 3 days. At the end of treatment, mice were injected s.c. with saline or 6β-naltrexol (6β-ol) (0.5–10.0 mg/kg) and 70 minutes later, injected s.c. with naltrexone (0.00625–0.4 mg/kg) (N=4–10/dose) and immediately observed for withdrawal jumping for 15min. (A) Dose-response functions for withdrawal jumping. (B) Naltrexone ED50 (+SE) after pretreatment with saline or 6β-naltrexol.
*significantly different (p < 0.05) from the saline pretreated group.
Figure 2. Effect of naltrexone pretreatment on 6β-naltrexol-precipitated withdrawal jumping after 3 days morphine treatment.
Mice were implanted s.c. with a morphine pellet (25 mg) for 3 days. At the end of treatment, mice were injected s.c. with saline or naltrexone (NTX) (0.0125–0.05 mg/kg), followed immediately by s.c. injection of 6β-naltrexol (0.5–20 mg/kg) (N=5/dose) and observed for withdrawal jumping for 15min. (A) Dose-response functions for withdrawal jumping. (B) 6β-naltrexol ED50 (+SE) after pretreatment with saline or naltrexone.
*significantly different (p < 0.05) from the saline pretreated group.
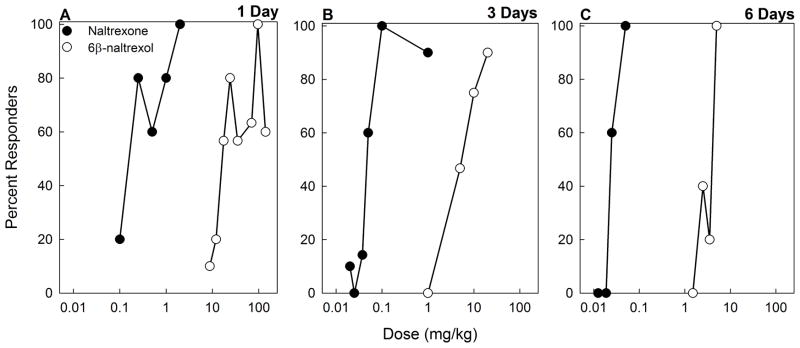
Figure 3. Withdrawal jumping induced by naltrexone and 6β-naltrexol following 1, 3 or 6 days of morphine treatment.
Mice were implanted s.c. with a morphine pellet (25 mg) for 1 (A) or 3 days (B). Other mice were implanted s.c. with one morphine pellet (25 mg) for 3 days and then an additional morphine pellet (25 mg) for 3 more days for total of 6 days of treatment (C). At the end of treatment, mice (N=5–30/dose) were injected s.c. with naltrexone (0.0125–2 mg/kg) or 6β-naltrexol (1.5–140 mg/kg) and immediately observed for withdrawal jumping for 15 min.
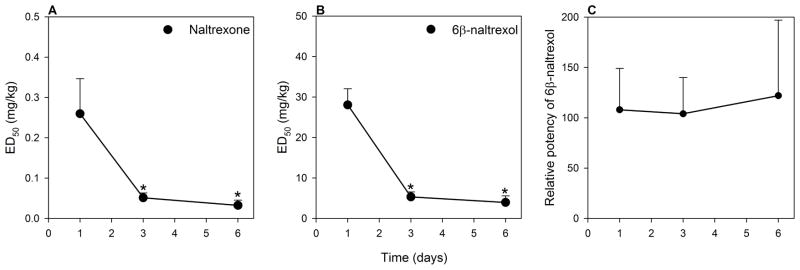
In the next experiment, naltrexone and 6β-naltrexol dose-dependently precipitated withdrawal jumping in mice treated with morphine for 1, 3 or 6 days (Fig. 3). Naltrexone was more potent than 6β-naltrexol at all the time points. The potency of naltrexone and 6β-naltrexol to precipitate withdrawal increased with an increase in the duration of morphine treatment as evidenced by the shift to the left of the dose response functions (Fig. 3) and by a decrease in ED50’s (Fig. 4A,B). However, the relative potency of naltrexone compared to 6β-naltrexol did not change (Fig. 4C). Following 1, 3 and 6 days of morphine treatment, naltrexone was approximately 100–125 times more potent than 6β-naltrexol, which is consistent with previous estimates of relative potency (Sirohi et al. 2007; 2009). Analysis of graded data yielded similar relative potency of naltrexone to 6β-naltrexol (e.g., EC50s and 95%CL for naltrexone and 6β-naltrexol following 3 days morphine treatment = 0.066mg/kg, 0.024–0.184; 6.50mg/kg, 2.31–18.27; relative potency = 98.5).
Figure 4. Comparison of naltrexone and 6β-naltrexol to precipitate withdrawal following 1, 3 and 6 days of morphine treatment.
The ED50’s (+ SE) for naltrexone (A) and 6β-naltrexol (B) to precipitate withdrawal jumping for 1, 3 or 6 days of morphine treatment. (C) Relative potency (+ SE) of 6β-naltrexol compared to naltrexone (6β-naltrexol ED50/naltrexone ED50) for 1, 3 or 6 days morphine treatment.
*significantly different (p < 0.05) from the ED50 of 1 day morphine treatment group.
4. Discussion
The present study examined the interaction between naltrexone and 6β-naltrexol to precipitate opioid withdrawal in mice and the relative potency of naltrexone and 6β-naltrexol to precipitate withdrawal as opioid dependence increases. These studies indicate that the functional efficacy of 6β-naltrexol varies with dose, but that the relative potency of these antagonists does not change with increasing opioid dependence.
In the first experiment 6β-naltrexol had a dose-dependent effect on naltrexone precipitated withdrawal. Specifically, at low doses 6β-naltrexol shifted the naltrexone dose response function to the right; implying that 6β-naltrexol antagonized naltrexone precipitated withdrawal, as reported previously (Raehal et al., 2005; Sirohi et al., 2007; 2009). However, at the highest dose, 6β-naltrexol produced an additive effect on naltrexone precipitated withdrawal, suggesting a functional role similar to that of naltrexone. Conversely, naltrexone acted additively with 6β-naltrexol irrespective of the dose used. These results can be accommodated by proposing that precipitated opioid withdrawal in opioid dependent mice may be mediated by two mechanisms: displacement of agonist which releases downstream compensatory processes (e.g., cAMP; Avidor-Reiss et al., 1995; Sharma et al., 1975) and by decreases in constitutive opioid receptor activity (Raehal et al., 2005; Sirohi et al., 2007; 2009; Walker and Sterious, 2005; Wang et al., 2001). In this scenario, 6β-naltrexol functions as a neutral antagonist and precipitated withdrawal is a result of displacement from the receptor only. On the other hand, naltrexone would be predicted to engage both mechanisms. We propose that the single mechanism activated by 6β-naltrexol requires a relatively high dose to displace morphine from a large percentage of receptors and produce withdrawal. Thus, 6β-naltrexol acts to antagonize naltrexone-induced withdrawal until a dose sufficient to displace enough agonist from the receptor is reached, at which point it now acts additively. On the other hand, naltrexone activates both of these proposed mechanisms and produces an additive effect on 6β-naltrexol at all doses. Overall, these data demonstrate the novel finding that the functional efficacy of 6β-naltrexol when combined with naltrexone is dose-dependent and confirm that 6β-naltrexol is a lower efficacy opioid antagonist relative to naltrexone (Raehal et al., 2005; Sadée et al., 2005; Sirohi et al., 2009; Wang et al., 2001, 2004).
In the next experiment we examined changes in the relative potency of naltrexone and 6β-naltrexol to precipitate withdrawal with increases in opioid dependence. Opioid dependence was increased from 1–6 days as evidenced by the decrease in ED50 for both 6β-naltrexol and naltrexone. If increased opioid dependence co-varies with increased constitutive opioid receptor activity, changes in potency to precipitate withdrawal for the two antagonists should differ; with the potency of the inverse opioid agonist increasing more. Although 6β-naltrexol was less potent than naltrexone in precipitating withdrawal at each time point, there was no difference in terms of the rate of increase in potency.
These results raise the possibility that there was no increase in constitutive opioid receptor activity with increased opioid dependence over 1 to 6 days morphine treatment. This suggests that constitutive opioid receptor activity may develop rapidly, perhaps very soon following opioid agonist exposure. This suggestion is consistent with a previous report using an operant withdrawal model (Schulteis et al., 2009) that found that the relative potency of naloxone (inverse opioid agonist) and 6α-naloxol (neutral opioid antagonist) did not change in rats injected once or twice with morphine; whereas in non-dependent rats, the potency to disrupt operant performance for naloxone increased ≈ 80-fold and that for 6a-naloxol increased ≈ 6-fold following a single morphine injection. The possibility of rapid development of constitutive activity might be important to directly study in future experiments.
Taken together, these data indicate that 6β-naltrexol produces dose-dependent changes in functional efficacy as demonstrated by its effect on naltrexone potency to precipitate withdrawal. The functional effects of 6β-naltrexol on naltrexone reveal the protean nature of “antagonizing” opioid withdrawal with opioids that differ dramatically in efficacy. The results also confirm previous suggestions (Raehal et al., 2005; Sadée et al., 2005; Sirohi et al., 2007, 2009; Wang et al., 2001, 2004) that 6β-naltrexol is a relatively lower efficacy opioid antagonist compared to naltrexone. The second experiment addressed the notion that constitutive opioid receptor signaling may play a role in opioid dependence. These data indicate that there was no change in constitutive opioid receptor activity with increased opioid dependence, although this does not rule out very rapid development of constitutive opioid receptor activity during the early stages of agonist exposure. In summary, these results extend our understanding of the functional properties of 6β-naltrexol and raise questions about the contribution of constitutive receptor signaling in a whole animal model of opioid dependence.
Research highlights.
We examined the efficacy of naltrexone and 6β-naltrexol to precipitate opioid withdrawal.
6β-naltrexol’s functional efficacy is dose-dependent.
We evaluated role of constitutive opioid receptor activity in opioid withdrawal.
Constitutive opioid receptor activity may not increase concurrently with opioid dependence.
Acknowledgments
This project was supported in part by the National Institute on Drug Abuse [DA 19959] to BCY.
These data represent a portion of a thesis presented by the first author (DN) in partial fulfillment of the requirements for the Master of Science degree in Pharmaceutical Sciences, College of Pharmacy and Allied Health Professions, St. John’s University. Some of the results for Experiment 2 comprise a small portion of data from a thesis presented by the second author (SS) in partial fulfillment of the requirements for the Ph.D. degree in Pharmaceutical Sciences, College of Pharmacy and Allied Health Professions, St. John’s University. We would like to thank Shruti Jaswal for her comments and suggestions on the manuscript. Our continued thanks to Dr. M. T. Turnock for numerous discussions and support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JU, Holtzman SG. Pharmacologic characterization of the sensitization to the rate-decreasing effects of naltrexone induced by acute opioid pretreatment in rats. J Pharmacol Exp Ther. 1990;253:483–89. [PubMed] [Google Scholar]
- Avidor-Reiss T, Bayewitch M, Levy R, Matus-Leibovitch N, Nevo I, Vogel Z. Adenylylcyclase supersensitization in μ-opioid receptor-transfected Chinese hamster ovary cells following chronic opioid treatment. J Biol Chem. 1995;270:29732–8. doi: 10.1074/jbc.270.50.29732. [DOI] [PubMed] [Google Scholar]
- Azar MR, Jones BC, Schulteis G. Conditioned place aversion is a highly sensitive index of acute opioid dependence and withdrawal. Psychopharmacology (Berl) 2003;170:42–50. doi: 10.1007/s00213-003-1514-y. [DOI] [PubMed] [Google Scholar]
- Costa T, Herz A. Antagonists with negative intrinsic activity at δ opioid receptors coupled to GTP-binding proteins. Proc Natl Acad Sci USA. 1989;86:7321–5. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney DJ. Probit analysis. 3. London: Cambridge University Press; 1973. [Google Scholar]
- Kenakin T. Inverse, protean, and ligand-selective agonism: matters of receptor conformation. FASEB J. 2001;15:598–611. doi: 10.1096/fj.00-0438rev. [DOI] [PubMed] [Google Scholar]
- Liu JG, Prather PL. Chronic exposure to μ-opioid agonists produces constitutive activation of μ-opioid receptors in direct proportion to the efficacy of the agonist used for pretreatment. Mol Pharmacol. 2001;60:53–62. doi: 10.1124/mol.60.1.53. [DOI] [PubMed] [Google Scholar]
- Milligan G, MacEwan DJ, Mercouris M, Mullaney I. Inverse agonism at adrenergic and opioid receptors: studies with wild type and constitutively active mutant receptors. Receptors Channels. 1997;5:209–13. [PubMed] [Google Scholar]
- Prather PL. Inverse agonists: tools to reveal ligand-specific conformations of G protein-coupled receptors. Sci STKE. 2004;2004:pe1. doi: 10.1126/stke.2152004pe1. [DOI] [PubMed] [Google Scholar]
- Raehal KM, Lowery JJ, Bhamidipati CM, Paolino RM, Blair JR, Wang D, Sadée W, Bilsky EJ. In vivo characterization of 6β-naltrexol, an opioid ligand with less inverse agonist activity compared with naltrexone and naloxone in opioid-dependent mice. J Pharmacol Exp Ther. 2005;313:1150–62. doi: 10.1124/jpet.104.082966. [DOI] [PubMed] [Google Scholar]
- Sadée W, Wang D, Bilsky EJ. Basal opioid receptor activity, neutral antagonists, and therapeutic opportunities. Life Sci. 2005;76:1427–37. doi: 10.1016/j.lfs.2004.10.024. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Liu J, Amitai N, Tzeng S. Context- and cue-conditioned potentiation of acute morphine dependence and withdrawal. Pharmacol Biochem Behav. 2005;82:82–9. doi: 10.1016/j.pbb.2005.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulteis G, Chiang D, Archer C. Relative potency of the opioid antagonists naloxone and 6-alpha-naloxol to precipitate withdrawal from acute morphine dependence varies with time post-antagonist. Pharmacol Biochem Behav. 2009;92:157–63. doi: 10.1016/j.pbb.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Klee WA, Nirenberg M. Dual regulation of adenylate cyclase accounts for narcotic dependence and tolerance. Proc Natl Acad Sci USA. 1975;72:3092–6. doi: 10.1073/pnas.72.8.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirohi S, Kumar P, Yoburn BC. μ-opioid receptor up-regulation and functional supersensitivity are independent of antagonist efficacy. J Pharmacol Exp Ther. 2007;323:701–7. doi: 10.1124/jpet.107.127019. [DOI] [PubMed] [Google Scholar]
- Sirohi S, Dighe SV, Madia PA, Yoburn BC. The relative potency of inverse opioid agonists and a neutral opioid antagonist in precipitated withdrawal and antagonism of analgesia and toxicity. J Pharmacol Exp Ther. 2009;330:513–9. doi: 10.1124/jpet.109.152678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dorp EL, Yassen A, Dahan A. Naloxone treatment in opioid addiction: the risks and benefits. Expert Opin Drug Saf. 2007;6:125–32. doi: 10.1517/14740338.6.2.125. [DOI] [PubMed] [Google Scholar]
- Villarreal JE, Castro A. A reformulation of the dual-action model of opioid dependence: opioid-specific neuronal kindling [proceedings] Psychopharmacol Bull. 1981;17:56–8. [PubMed] [Google Scholar]
- Walker EA, Sterious SN. Opioid antagonists differ according to negative intrinsic efficacy in a mouse model of acute dependence. Br J Pharmacol. 2005;145:975–83. doi: 10.1038/sj.bjp.0706247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Bilsky EJ, Porreca F, Sadée W. Constitutive μ opioid receptor activation as a regulatory mechanism underlying narcotic tolerance and dependence. Life Sci. 1994;54:PL339–50. doi: 10.1016/0024-3205(94)90022-1. [DOI] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Bilsky EJ, Sadée W. Inverse agonists and neutral antagonists at μ opioid receptor (MOR): possible role of basal receptor signaling in narcotic dependence. J Neurochem. 2001;77:1590–1600. doi: 10.1046/j.1471-4159.2001.00362.x. [DOI] [PubMed] [Google Scholar]
- Wang D, Raehal KM, Lin ET, Lowery JJ, Kieffer BL, Bilsky EJ, Sadée W. Basal signaling activity of mu opioid receptor in mouse brain: role in narcotic dependence. J Pharmacol Exp Ther. 2004;308:512–20. doi: 10.1124/jpet.103.054049. [DOI] [PubMed] [Google Scholar]
- Way EL, Loh HH, Shen FH. Simultaneous quantitative assessment of morphine tolerance and physical dependence. J Pharmacol Exp Ther. 1969;167:1–8. [PubMed] [Google Scholar]