Abstract
Background
The recent RV144 clinical trial showed that an ALVAC/AIDSVAX prime-boost HIV vaccine regimen may confer partial immunity in recipients and reduce transmission by 31%. Trial data suggest that efficacy may initially exceed 70% but decline over the following 3.5 years. Estimating the potential health benefits associated with a one-time vaccination campaign, as well as the projected benefits of repeat booster vaccination, may inform future HIV vaccine research and licensing decisions.
Methods
We developed a mathematical model to project the future course of the HIV epidemic in the United States under varying HIV vaccine scenarios. The model accounts for disease progression, infection transmission, antiretroviral therapy, and HIV-related morbidity and mortality. We projected HIV prevalence and incidence over time in multiple risk groups, and we estimated quality-adjusted life years (QALYs) and costs over a 10-year time horizon. We used an exponentially declining efficacy curve fit to trial data, and we assumed subsequent vaccine boosters confer similar immunity. Variations in vaccine parameters were examined in sensitivity analysis.
Results
Under existing HIV prevention and treatment efforts, an estimated 590,000 HIV infections occur over 10 years. One-time vaccination achieving 60% coverage of adults could prevent 9.8% of projected new infections over 10 years (and prevent 34% of new infections in the first year) and cost approximately $91,000/QALY gained relative to the status quo, assuming a vaccination price of $500. Targeted vaccination of high-risk groups results in net cost savings for vaccines costing less than $750. One-time vaccination of 60% of all adults coupled with three-year boosters only for men who have sex with men and injection drug users could prevent 21% of infections for $81,000/QALY gained relative to vaccination of high-risk groups only. A program attaining 90% vaccination coverage prevents 15% of new HIV cases over 10 years (and approximately 50% of infections in the first year).
Conclusions
A partially effective HIV vaccine with effectiveness similar to that observed in the RV144 trial would provide large health benefits in the United States and could meet conventionally accepted cost-effectiveness thresholds. Strategies that target high-risk groups are most efficient, but broader strategies provide greater total population health benefit.
Keywords: HIV vaccine, cost-effectiveness analysis, mathematical model
1. Introduction
Despite dramatic increases in the availability of antiretroviral therapy for HIV in resource-limited settings, approximately two new infections occur worldwide for every person placed on treatment.[1] This observation highlights the urgent need for expanded HIV prevention efforts, including the potential use of an HIV vaccine. After many years of disappointing results, several promising developments have provided more cause for optimism.[2] In 2009, a large phase III trial of an ALVAC and AIDSVAX vaccine (RV144) demonstrated modest protection from infection with HIV, with a 31% reduction among trial volunteers.[3] More recently, investigators have reported the development of broadly neutralizing antibodies, which provides potential new targets for vaccine development.[4, 5]
The modest success of the RV144 trial prompted renewed interest in understanding whether the use of a partially effective HIV vaccine would improve health outcomes sufficiently to justify its use.[6-11] In particular, although the RV144 trial showed an overall effectiveness of 31%, vaccine efficacy was substantially higher (approximately 70%) in the first year and rapidly declined over time. Given such a modestly effective vaccine, it is unknown to what extent a universal vaccination campaign, or one exclusively targeting high-risk populations, will reduce overall HIV incidence in the population.
As part of a collaboration established by the Centers for Disease Control and Prevention and the World Health Organization, we evaluated the health and economic outcomes resulting from broad use of a partially effective HIV vaccine in the United States. Our analysis assumed declining vaccine efficacy as observed in the RV144 trial. We evaluated three strategies: one-time vaccination; vaccination followed by booster vaccinations at three- or five-year intervals; and a hybrid strategy, in which only individuals with high-risk behavior received booster vaccinations. We evaluated the effect of these strategies on HIV incidence, infections averted, life expectancy, quality-adjusted life expectancy, costs, and cost-effectiveness.
2. Methods
Applying our previously published HIV vaccine model,[6] we incorporated the 2009 ALVAC/AIDSVAX randomized clinical trial results to consider the effects of such a modestly effective HIV vaccine on population health outcomes and cost-effectiveness.[3] Additional model details can be found elsewhere.[6, 12] The model was implemented in the mathematical programming language Matlab 2010b.
2.1. Study overview
We implemented a mathematical model of HIV transmission and disease progression in the adult US population aged 15 to 64 years. The key model components are (1) risk groups, (2) HIV transmission, progression, and treatment, (3) HIV vaccination, (4) initial conditions, and (5) health and economic outcomes. In our base case, we calculated outcomes over a 10-year time horizon. The Appendix contains further details about model structure, inputs, and assumptions.
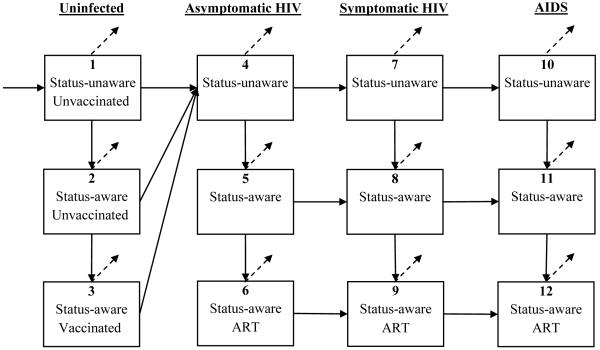
Individuals were stratified into groups based on gender and risk behavior: men who have sex with men (MSM), male and female male injection drug users (IDUs), MSM/IDUs, and male and female low-risk heterosexuals. Each group was further sub-divided into compartments based on HIV-infection status (uninfected or infected), HIV-identification status (status-aware or status-unaware), and disease and antiretroviral treatment (ART) status, if infected (Appendix Figure A-1).
After specifying a set of initial conditions for reach risk group (e.g., population size, HIV prevalence, fraction receiving ART, etc.) based on published data, we numerically simulated the projected course of the HIV epidemic over time, under the status quo of no vaccination and under alternative HIV vaccination scenarios. In our prior analyses,[6] we considered a range of values for vaccine efficacy and duration of protection; in the present study, we modeled vaccine efficacy as estimated from the ALVAC/AIDSVAX clinical trial, as well as the effects of offering a vaccine booster in the future.
2.2. Disease transmission and progression
For each population sub-group, we estimated average risk behavior, including number of homosexual, heterosexual, and needle-sharing partners, as well as condom use. The likelihood of selecting an HIV-infected partner assumes proportional mixing in the population (i.e., persons with many partners are more likely to select partners who also have many partners). We then calculated the probability of HIV transmission between HIV sero-discordant partners, adjusting for genders, HIV disease status, antiretroviral treatment status, and HIV vaccination status. Finally, we incorporated risk behaviors and transmission probabilities to estimate new HIV infections over time in each risk group. Importantly, our dynamic modeling framework allows us to estimate secondary HIV infections, or the number of future infections caused by new primary infections.
For persons who were initially HIV-infected at the model’s start or who became infected over the time horizon simulated, we accounted for HIV disease progression based on the natural history of HIV.[6, 12] By summing over the time spent in each health state and adjusting this by an appropriate quality of life factor for each person in the population, we calculated total quality-adjusted life years (QALYs). We also included the benefits of ART on reduced morbidity and mortality, as well as the effects of suppressive ART on reduced HIV transmission.
2.3. Vaccine characteristics
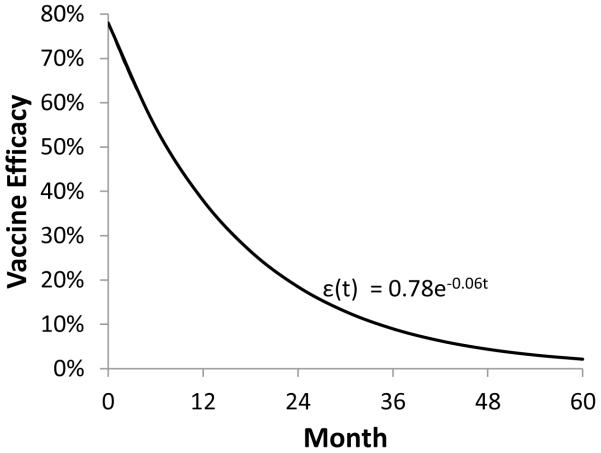
In the base case analysis, we assumed an exponentially declining vaccine efficacy, ε(t) = 0.78 e−0.06t, where t (measured in months) is time post-vaccination. The parameter ε(t) refers to the instantaneous vaccine efficacy, or the reduction in the likelihood of HIV transmission in uninfected vaccine recipients, at time t.
2.4. Intervention strategies
To assess the potential costs and benefits of alternative vaccination campaigns, we considered a one-time vaccination, as well vaccination followed by a booster vaccine every three or five years. Although not yet tested in a clinical trial, a future booster vaccine could enhance the benefits of a rapidly declining vaccine efficacy. We also considered a hybrid strategy offering one-time vaccination to all adults, and subsequent booster vaccines only to high-risk individuals (i.e., injection drug users and men who have sex with men). Although the uptake of a mass vaccination program is uncertain, we assumed a base case coverage level of 60%, where vaccination coverage refers to the fraction of eligible adults who complete a vaccination series. We also considered pessimistic (30% coverage) and optimistic (90% coverage) scenarios. To evaluate the hypothetical cost-effectiveness, we conservatively assumed that a primary vaccination series costs $500 per recipient and each subsequent booster vaccine also costs $500, but we varied this assumption in sensitivity analysis.
2.5. Health outcomes
By using a dynamic HIV epidemic model, we simulated the change in population compartment sizes over time, due to persons receiving a vaccine, acquiring HIV infection, progressing to a later disease stage, initiating treatment, dying, or entering or exiting the adult population. From these projections, we calculated the number of new infections occurring per year, HIV prevalence in each risk group, overall population health benefits, which are measured in QALYs, and total vaccination and healthcare costs, under a variety of HIV vaccination scenarios. We then calculated the incremental cost-effectiveness ratio (ICER) of each vaccination program, relative to no vaccination or the next-best alternative. Our analysis was performed using a societal perspective, and costs and QALYs were discounted at 3% annually,[13] with costs given in 2009 U.S. dollars.
3. Results
With no HIV vaccination program, we estimated that approximately 590,000 new HIV infections will occur over the next 10 years, with 47% of infections occurring among MSM, 20% among IDUs, 6% among MSM/IDUs, and 27% among heterosexual populations.
3.1. One-time vaccination, waning efficacy
One-time vaccination of 60% of the adult U.S. population with an HIV vaccine that has waning efficacy could prevent 58,123 infections over 10 years, or 9.8% of the projected total (Table 1, Figure 1). In the first year following vaccination, approximately 34% of new infections are prevented, but the downstream benefits of a one-time vaccination decrease as efficacy wanes.
Table 1. Health and economic outcomes of alternative HIV vaccination strategies.
| HIV infections prevented (% of status quo) |
Incremental costs (billions)b |
Incremental life years (millions) |
Incremental QALYs (millions) |
ICER ($/QALY) relative to c |
||||
|---|---|---|---|---|---|---|---|---|
| Vaccination strategy a | Coverage | over 1 year | over 10 years | status quo | next-best | |||
| Universal one-time | 30% | 10,520 (16.9%) | 29,341 (5.0%) | $28.9 | 0.36 | 0.32 | $90,424 | Dominated |
| Universal one-time | 60% | 20,923 (33.7%) | 58,123 (9.8%) | $57.9 | 0.71 | 0.63 | $91,355 | Dominated |
| Universal one-time | 90% | 31,208 (50.3%) | 86,356 (14.6%) | $86.9 | 1.07 | 0.94 | $92,294 | Dominated |
| Universal 5-year booster | 60% | 20,923 (33.7%) | 95,933 (16.2%) | $153.7 | 1.12 | 0.99 | $155,858 | Dominated |
| Universal 3-year booster | 60% | 20,923 (33.7%) | 142,742 (24.2%) | $206.4 | 1.61 | 1.42 | $145,077 | $645,292 |
| High-risk one-time | 60% | 15,534 (24.7%) | 46,009 (7.8%) | -$0.9 | 0.56 | 0.47 | Cost-saving | Cost-saving |
| Hybrid (Universal one-time & high-risk 3-year booster) |
60% | 20,923 (33.7%) | 122,447 (20.7%) | $58.1 | 1.42 | 1.19 | $48,693 | $81,480 |
All results in this table assume an exponentially declining vaccine efficacy, ε = 0.78 e−0.06t.
Universal vaccination includes vaccination of adults aged 15 to 64.
High-risk vaccination includes only men who have sex with men and injection drug users.
One-time vaccination occurs immediately, and boosters occur at subsequent time intervals.
Hybrid strategy includes vaccination of all adults, followed by boosters only for high-risk persons.
Incremental costs, life years, and quality adjusted life years (QALYs) are relative to the status quo (no vaccination)
Incremental cost-effectiveness ratio (ICER) is relative to the status quo or the next-best alternative.
Dominated = dominated strategy (i.e., the strategy is not on the cost-effectiveness efficient frontier).
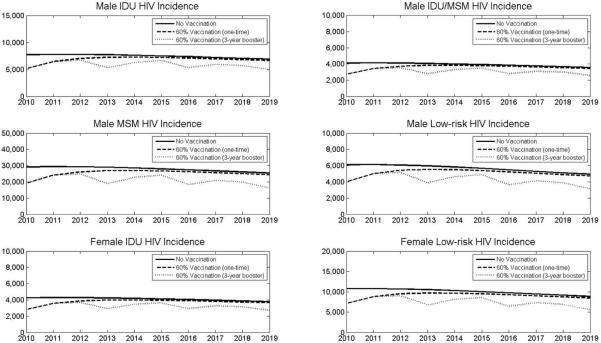
Figure 1. HIV incidence over 10 years under HIV vaccination strategies.
Projected HIV incidence in each risk group is shown assuming no vaccination (solid line), universal one-time vaccination with 60% coverage (dashed line), or universal vaccination with 60% coverage and 3-year booster (dotted line). Assumes exponentially declining vaccine efficacy. MSM = men who have sex with men; IDU = injection drug user; Low-risk = low-risk heterosexual population.
If vaccination coverage only reaches 30% of eligible persons, incidence is reduced by only 5.0% over 10 years (or 17% over one year). Conversely, an effective vaccination campaign reaching 90% of adults prevents nearly 15% of infections over 10 years. In the first year alone, this imperfect vaccine prevents more than 50% of new infections. Even after vaccine efficacy has completely waned, overall annual incidence is reduced by approximately 6% because of reduced secondary infections (i.e., persons who avoid infection in the first year cannot infect others in subsequent years).
At a vaccination cost of $500, universal HIV vaccination costs less than $95,000 per QALY gained, relative to no vaccination (Figure 2). A targeted strategy that instead vaccinates only high-risk individuals (MSM and male and female IDUs) with 60% coverage, prevents 7.8% of infections over 10 years (compared to 9.8% with universal vaccination), but requires 95% fewer vaccinations. Of note, this strategy is the most economically efficient, resulting in a net cost savings compared to no vaccination, assuming a vaccine price of $500 (Table 1, Figure 2).
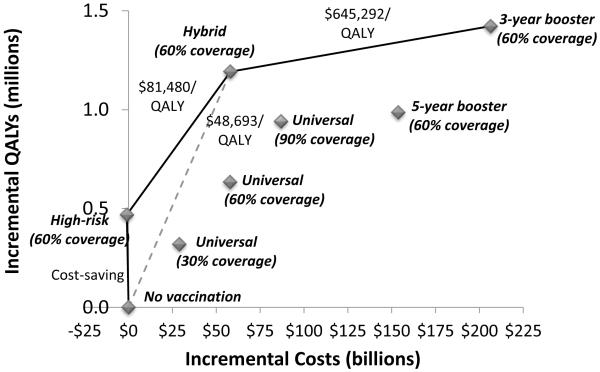
Figure 2. Cost-effectiveness of alternative HIV vaccination strategies.
The incremental costs and QALYs (discounted over 10 years) and cost-effectiveness ratios of HIV vaccination strategies are shown. Universal = one-time universal vaccination with 30%, 60%, or 90% coverage; high-risk = one-time high-risk group targeted vaccination with 60% coverage; 5-year booster = universal vaccination with 60% coverage and booster every five years; 3-year booster = universal vaccination with 60% coverage and booster every three years; hybrid = universal vaccination with 60% coverage and booster every three years for high-risk individuals only. Assumes exponentially declining vaccine efficacy and vaccination price of $500. QALY = quality adjusted life year.
3.2. Booster vaccination strategies
Offering a booster vaccine with a similar declining efficacy profile to all adults every five years improves long-term outcomes substantially: 16.2% of infections are prevented over 10 years, compared to 9.8% with no booster. Increasing the vaccine booster frequency to once every three years prevents 24% of infections over 10 years. Although one-year outcomes are identical, a frequent vaccine booster program offers more downstream benefit in the form of reduced incidence (Figure 1). However, at a vaccine price of $500 per person, these strategies cost more than $145,000 per QALY gained, compared to the status quo (Figure 2).
A more economically attractive strategy would be to offer universal vaccination to all adults initially, and a subsequent booster vaccine to high-risk persons (MSM and IDUs) only. This hybrid strategy prevents more than one-fifth of new infections over 10 years, and 34% of new infections in the first year. Compared to the cost-saving high-risk targeted vaccination, a hybrid strategy costs $81,480 per QALY gained. However, if a targeted vaccination strategy is impractical, the hybrid strategy costs less than $50,000 per QALY gained compared to no vaccination, which is significantly more cost-effective than a universal vaccine campaign with frequent boosters, assuming a vaccine price of $500 (Figure 2). Of note, this strategy adds 84% of the QALYs that a universal three-year booster strategy offers, but uses only 27% of the requisite number of vaccines.
Although booster vaccination characteristics are unknown, we assumed similar coverage levels (60% in the base case) as with primary vaccination. If multiple vaccine doses are required for boosters, lower compliance levels would attenuate infections prevented and costs.
3.3. Effect of herd immunity
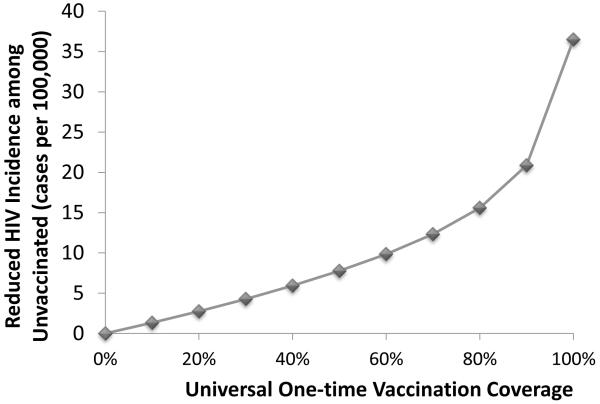
One benefit of a broad HIV vaccination campaign is herd immunity, or reduced HIV infections among the unvaccinated population. Our epidemic modeling framework enables us to estimate the extent of this positive externality (Figure 3). In the base case, one-time vaccination with 60% coverage prevents 9,450 infections among unvaccinated persons over 10 years, a reduction of 9.8 cases per 100,000 people. Greater vaccination coverage increases the protective benefit to the initially unvaccinated population and unvaccinated adolescents entering the sexually active population.
Figure 3. Effect of herd immunity.
Reduction in HIV cases among unvaccinated individuals over 10 years for varying levels of vaccination coverage is shown. Even with 100% one-time vaccination coverage, adolescents who later enter the sexually active population remain unvaccinated and thus benefit from reduced HIV transmission. Assumes exponentially declining vaccine efficacy.
3.4. Sensitivity analysis
In addition to considering variations in vaccine efficacy, we also varied key model parameters in sensitivity analysis.
3.4.1. Baseline rate of new infections
In general, if baseline HIV incidence is greater than we initially estimated, the cost-effectiveness of universal vaccination becomes more favorable. If HIV prevalence among each risk group is 25% greater than in our base case assumption, universal vaccination costs $76,000 per QALY gained, compared to $91,000 in the base case. Similarly, if the probability of HIV transmission (due to sexual contact and needle-sharing) is 25% greater, this strategy costs $64,000 per QALY gained. We also find the reverse effect: if HIV prevalence or transmission probabilities are 25% lower, then vaccination costs $117,000 or $138,000 per QALY gained, respectively. However, in all scenarios, the fraction of HIV infections prevented due to vaccination remains between 9% and 11%. These findings are generally consistent with the notion that offering HIV vaccination in higher incidence settings is more economically efficient.
3.4.2. Catch-up vaccination
In the base case, we assumed a one-time vaccination policy with 60% coverage of the adult population aged 15 to 64 years. Including annual catch-up vaccination for 15-year-olds entering the model slightly improves outcomes: 12.4% of HIV infections are prevented (compared to 9.8%), and cost-effectiveness improves to $89,000 per QALY gained (compared to $91,000).
3.4.3. Behavior disinhibition
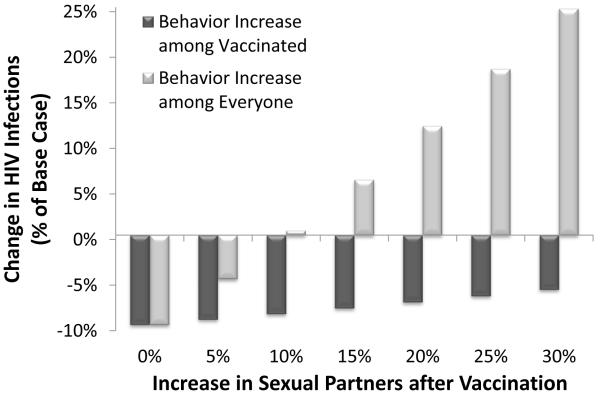
One concern with implementing a mass HIV vaccination is the possibility of behavior disinhibition post-vaccination. If only vaccinated individuals increase their number of sexual partners by 25% then infections prevented are reduced from 9.8% to 6.7% (Appendix Figure A-4). This implies that vaccinated individuals are predominantly increasing contact with other vaccinated persons, in order for sexual partnerships to balance. A worst-case scenario where vaccinated individuals increase sexual contact with only unvaccinated persons substantially worsens the epidemic: a 25% increase in sexual partners leads to an 18% increase in new infections, even with 60% vaccination coverage. This suggests that a comprehensive counseling program to reduce risky behavior must accompany a large-scale vaccination program.
3.4.4. Vaccine cost
Given that an HIV vaccine has not been licensed or manufactured, the expected price per dose is unknown. We initially assumed a conservative price of $500. At $250 per vaccination series, universal vaccination with 60% coverage costs $43,000 per QALY gained; at $100 per series, this strategy costs $21,000 per QALY gained. Adding a booster vaccine for high-risk persons (hybrid strategy) costs $21,000 or $5,000 per QALY gained with a vaccination price of $250 or $100, respectively. If a vaccination series costs only $100, then vaccination with three-year boosters for all adults should be re-evaluated, given a cost-effectiveness ratio of $125,000 compared to the hybrid strategy (or $24,000 compared to no vaccination).
3.4.5. Constant vaccine efficacy
We considered an alternative scenario where vaccine efficacy is constant over time, in case such a vaccine becomes available in the future (Figure 4). With this assumption, the relationship between vaccine efficacy and health outcomes is more mathematically straightforward than with a waning efficacy vaccine. The change in number infections occurring over the modeled time horizon is proportional to the size of the vaccinated population and vaccine efficacy:
The parameter α1 depends on background epidemic characteristics and based on our model’s results we estimated that α1 ≈ 0.003. Life years and quality-adjusted life years increase proportionately with the number of infections prevented (Figure 4a):
We estimated that α2 ≈ 0.03, suggesting that approximately 10 QALYs (discounted) are gained for each HIV infection prevented. This value reflects advances in antiretroviral therapy prolonging life expectancy and quality of life for HIV-infected persons.
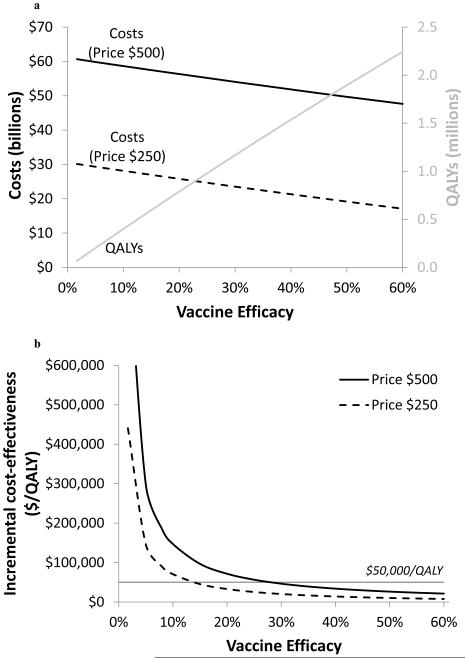
Figure 4. Sensitivity analysis of vaccine efficacy.
Sensitivity analysis of variations in constant vaccine efficacy on (a) incremental costs, QALYs, and (b) incremental cost-effectiveness ratios, assuming a vaccine price of $500 (solid line) or $250 (dashed line) is shown. Assumes universal one-time vaccination with 60% coverage, and constant vaccine efficacy with lifetime duration of protection. QALY = quality adjusted life year.
The incremental costs of vaccination include a fixed vaccination costs and a variable cost corresponding to healthcare costs saved per averted HIV infection (Figure 4a):
We estimated that the parameter α3 ≈ 180, suggesting that there are savings of approximately $60,000 (=180/0.003) per HIV infection prevented, because HIV infected persons utilize more health services but live a shorter period of time.
Finally, the incremental cost-effectiveness ratio is the incremental cost per QALY gained:
The ICER is thus proportional to 1/efficacy, implying that cost-effectiveness rapidly improves for very low efficacy vaccines up to approximately 20% efficacy (Figure 4b). With a vaccination price of $500, one-time universal vaccination with 60% coverage costs less than $50,000 per QALY gained if efficacy exceeds 28%. At a vaccine price of $250, this threshold decreases to a vaccine efficacy of 14%. As efficacy improves, the cost-effectiveness of universal vaccination continues to improve.
4. Discussion
In this model-based analysis for the United States population, we estimated the population health and economic outcomes that would result from use of an HIV vaccine with efficacy similar to that observed in the RV144 trial. Over the three years of the RV144 trial, use of the vaccine reduced new infections by 31%. However, the efficacy of the vaccine was higher during the first year and subsequently declined rapidly, and our analysis was designed to reflect this declining efficacy. Because of the short-lived efficacy, we also evaluated vaccination strategies that included booster vaccination at three- and five-year intervals. Booster vaccination was not evaluated in the RV144 trial and thus our analyses of booster vaccination are hypothetical.
Our analyses indicated that an HIV vaccine with the modest efficacy observed in the RV144 trial could provide substantial health benefit and meet generally accepted thresholds for cost-effectiveness in the United States, particularly with vaccination strategies that are targeted to high-risk groups. These findings are of particular importance because the modest efficacy observed in the RV144 trial would be considered a failure by many observers, and is certainly poor compared to vaccines that are in use for other diseases. Our analyses emphasize that even a modest and temporary reduction in new HIV infections results in important benefit at the population level.
A key finding of our study is that vaccination strategies targeted only to MSM and IDUs results in net cost savings for vaccines costing less than $750 per person, assuming exponentially declining efficacy. The expense of vaccination is more than outweighed by the savings associated with prevention of HIV infections among these high-risk individuals and their partners. The drawback of targeted strategies is that the total health benefit is not as great as with broader vaccination strategies, because fewer individuals are protected. In practice, strategies targeted to risk groups may also be challenging because individuals may not disclose risk behaviors. Targeted, risk-based strategies for identification of HIV through screening have had little success, leading to recommendations for routine screening in the United States.[14, 15] Nonetheless, vaccination of high-risk groups would be particularly economically efficient.
Vaccination strategies that include all of the adult population offer greater total health benefits than do targeted strategies. The hybrid strategy we evaluated (vaccination of 60% of all adults followed by booster vaccination every three years for high-risk groups only) is particularly attractive, as it confers 84% of the total benefit of the most effective strategy (vaccination of all adults every three years) but requires only 28% of the incremental expenditures (Figure 2). This strategy prevents 21% of new HIV infections over a 10-year period. As shown in Figure 2, the hybrid strategy is both more effective and less expensive than is universal vaccination of all risk groups at 60% or even 90% coverage, or with five-year boosters.
The total health benefit in the population is determined by the efficacy and duration of protection, and the proportion of the population that is vaccinated. Even a low efficacy vaccine provides some benefit to the unvaccinated population through herd immunity. Duration of protection is also a key determinant of population benefit. Our analyses indicate that the total benefit from vaccination could double with booster vaccination at three-year intervals if the booster provides the same protection as the original vaccine. How often vaccination should be done depends on the rapidity of the decline in efficacy, thus indicating the importance of assessing the duration of protection in vaccine clinical trials.
A vaccine that confers constant immunity over the 10-year simulated time horizon remains cost-effective for even low efficacies. For less than $50,000 per QALY gained, a conservative cost-effectiveness threshold, universal vaccination (with 60% coverage) could prevent over 10,000 HIV infections per year if efficacy exceeds 28% and the vaccination series costs $500. At $250 per vaccination, a low 14%-efficacy vaccine results in such a favorable cost-effectiveness ratio.
Our analysis has several limitations. The actual efficacy of an HIV vaccine is, of course, unknown. Our analysis reflects the available data from the RV144 trial, and a vaccine with lower efficacy is unlikely to be used. Thus, our analysis likely provides a lower bound on the health benefit from an HIV vaccine. Likewise, the cost of an HIV vaccine is unknown. Our base-case assumption of a cost of $500 for the vaccination series is plausible given the price of vaccines currently on the market, but the price will have a direct bearing on the cost-effectiveness of the vaccine. Our model simplifies the complex process of sexual partnership formation and dissolution (see Appendix). In addition, we do not stratify the population into multiple age groups, so we cannot evaluate vaccination strategies that target specific age groups. Because HIV incidence varies with age, such analyses would be useful. Finally, we do not assess other HIV prevention interventions such as pre-exposure prophylaxis. In practice, use of multiple prevention interventions simultaneously may be the most useful approach, and analysis of such portfolios would be an important extension of our analyses.
In conclusion, a partially effective HIV vaccine with effectiveness similar to that observed in the RV144 trial would provide large health benefits in the United States and could meet conventionally accepted thresholds for cost-effectiveness. Strategies that target high-risk groups are most efficient, but broader strategies provide greater total population health benefit. More realistic estimates of the population health and economic outcomes from an HIV vaccine will depend on a more detailed understanding of the efficacy, duration of protection, and cost of the vaccine.
Acknowledgements
This work was supported by a grant from the National Institute on Drug Abuse, United States National Institutes of Health (R-01-DA-15612) and the United States Department of Veterans Affairs. Each author reports no conflict of interest or any external financial support that is relevant to this study.
Role of the Funding Source Our funding sources had no role in the design of the study, in the collection, analysis, and interpretation of data, in the writing of the manuscript, and in the decision to submit the manuscript for publication.
Appendix
Technical Appendix
We extended a previously published model of HIV transmission, and additional model details can be found elsewhere.[6, 12] Parameters used in the mathematical model are given in Table A-3.
1. Risk groups
By subdividing the adult population aged 15 to 64 into risk stratifications (Table A-1), we accounted for variations in baseline demographic characteristics (population size, HIV prevalence, mortality rate, population entry and exit rates), sexual behavior (annual number of sexual partners, condom use), drug using behavior (annual number of drug injections, frequency of needle-sharing), and HIV transmission probabilities (male to male, male to female, female to male).
Table A-1.
Population risk groups and modes of HIV transmission.
| Male |
Female |
||||||
|---|---|---|---|---|---|---|---|
| MSM | MSM/IDU | IDU | Other | IDU | Other | ||
| Male | MSM | Homosexual | Homosexual | Heterosexual | Heterosexual | ||
| MSM/IDU | Homosexual | Homosexual Needle-sharing |
Needle-sharing | Heterosexual Needle-sharing |
Heterosexual | ||
| IDU | Needle-sharing | Needle-sharing | Heterosexual Needle-sharing |
Heterosexual | |||
| Other | Heterosexual | Heterosexual | |||||
| Female | IDU | Heterosexual | Heterosexual Needle-sharing |
Heterosexual Needle-sharing |
Heterosexual | Needle-sharing | |
| Other | Heterosexual | Heterosexual | Heterosexual | Heterosexual | |||
MSM = men who have sex with men; IDU = injection drug user; Other = low-risk general population.
2. HIV transmission, progression, and treatment
We next sub-divided each risk group based HIV infection status, disease stage, screening status, antiretroviral treatment status, and preventive vaccination status (Figure A-1). We modeled HIV transmission as a binomial process, where individuals in compartment i select partners in compartment j with probability p, where p depends on the number of people in compartment j as well as the number of partners each person in j has. This is known as proportional mixing. If a susceptible person selects an HIV-infected partner, the probability of transmission depends on both genders, the mode of transmission, disease stage, antiretroviral treatment status, and vaccination status (for the uninfected partner only). Individuals in compartment i then repeat this partnership selection process n times per year, for each type of partnership (heterosexual or homosexual). HIV transmission via injection drug use was modeled in a similar manner, except the number of injections, needle-sharing frequency, and the appropriate transmission probabilities are substituted.
Upon becoming HIV infected, individuals progress through a continuum of disease states, ultimately ending in death. At various intervals, individuals may be screened for HIV or initiate an antiretroviral treatment regimen, which further adjusts the probability of infecting others (either through a reduced viral load due to suppressive ART, or through fewer sexual partners following HIV screening). We estimated disease progression rates and disease-related mortality based on a model of the natural history of HIV.[16]
Figure A-1. Schematic model diagram.
The boxes represent disease-stage compartments and the arrows represent transitions between compartments. Individuals enter into the unvaccinated population and may die or exit the population at various disease stages (dashed arrows).
3. HIV vaccination
We considered alternative HIV vaccination strategies that vary the target population (universal, high-risk targeted), coverage level (30%, 60%, 90%), and frequency of a booster vaccine (no booster, every 3 years, every 5 years). We assumed an exponentially declining vaccine efficacy, ε(t) = 0.78 e−0.06t, where t (measured in months) is time post-vaccination, based on the RV144 trial results (Figure A-2).[3] This function implies that efficacy is initially 78% at the time of vaccination, 38% at 12 months post-vaccination, 19% at 24 months, and 9% at 36 months. We included the effects of a preventive vaccine by adjusting the appropriate transmission probabilities by 1-ε(t).
Most of the efficacy of such a vaccine wanes within three to four years post-vaccination. Hence, we considered a hypothetical scenario where a vaccine booster enhances vaccine efficacy up to the original levels. As future trial data on the feasibility of a booster vaccine become available, we will update this assumption.
We also explored an alternative scenario in which constant vaccine efficacy, ε, is conferred over the duration of the study (10 years). We modeled how variations in constant efficacy and vaccination coverage levels affect the projected health outcomes (Figure A-5). In our prior published study, we considered simultaneous variations in vaccine efficacy and duration of protection.[6]
Figure A-2.
Exponentially declining vaccine efficacy curve.
4. Model calibration
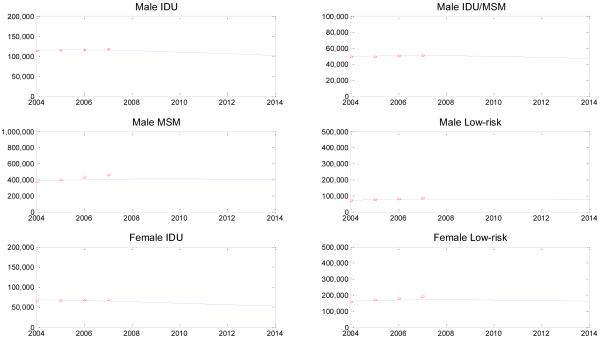
We parameterized our model using the best available demographic, epidemiologic, and behavioral data from published literature (Table A-3). Additionally, we calibrated the model to past estimates of the number of people living with HIV (Figure A-3), which suggests that our model approximates observed data reasonably well. Additionally, we compared our model’s projected HIV incidence over the next 10 years with the most recent estimates from 2006 (Table A-2). Here we find that our model’s projections of HIV transmission among MSM and heterosexuals are very close to the CDC’s estimates, and together these categories account for 75-85% of all new cases. Our model projects more HIV infections occurring among injection drug users over the next 10 years, which may be due to misestimates in our parameter values (e.g., overestimate of transmission probabilities, underestimate of fraction of IDUs receiving antiretroviral therapy) or underdiagnosis of HIV infection in these risk groups.
Figure A-3. Number of people living with HIV, 2004-2014.
The model’s projections are shown by the solid line, and CDC estimates from 2004 to 2007 are shown by the red asterisks. The CDC estimates are calculated based on the total number of people living with HIV and the percent of cases due to each transmission mode.[17]
Table A-2.
Annual HIV incidence with model (average over 10 years) and CDC estimates.
| Transmission Category | CDC Estimate[18] (2006) |
Model Projection (2009-2019) |
|---|---|---|
| Men who have sex with men (MSM) | 28,700 (53%) | 27,904 (47%) |
| Injection drug users (IDU) | 6,600 (12%) | 11,466 (20%) |
| MSM/IDU | 2,100 (4%) | 3,925 (7%) |
| Heterosexual | 16,800 (31%) | 15,687 (27%) |
| Total | 56,300 (100%) | 59,082 (100%) |
5. Initial conditions
Our mathematical model is characterized by a set of nonlinear differential equations, which estimate the size of each model compartment over time. After instantiating the model with a set of initial conditions using 2009 data, we numerically solved the system using the mathematical programming language Matlab 2010b. Initial population sizes and HIV prevalence levels are given in Table A-3.
6. Health and economic outcomes
We applied the model under various vaccination strategies to estimate future HIV infections, HIV prevalence over time, quality-adjusted life years (QALYs) in the population, costs, and incremental cost-effectiveness ratios (ICERs). QALYs were calculated by applying a quality of life factor to each health state and integrating the total time spent in each health state. Similarly, HIV-related health costs were computed and added to the overall cost of the vaccination program. All costs and QALYs were discounted to the present at 3% annually.[13] Finally, we calculated the ICERs of each vaccination strategy relative to the status quo and next-best alternative.
7. Additional sensitivity analyses
7.1. Behavior disinhibition
We expanded the sensitivity analysis on behavior disinhibition following vaccination. The extent of the effect on the epidemic depends on assumptions about assortative or random sexual mixing in the population (Figure A-4). A best-case scenario is that vaccinated individuals only increase sexual contact with other vaccinated individuals. In this case, behavior disinhibition has only a modest adverse effect on the epidemic because most of the additional partnerships are between vaccine-protected individuals (dark grey bars). On the other hand, if increased behavior disinhibition prompts unvaccinated persons to increase their partnerships, then the epidemic would substantially worsen (light grey bars).
Figure A-4. Change in HIV infections with increased behavior disinhibition.
Change in HIV infections over 10 years relative to the status quo (590,852 new infections) is shown for varying levels of behavior disinhibition among vaccinated individuals only (dark grey) or all adults (light grey). In the base case, a declining efficacy vaccine with 60% coverage of all adults prevents 9.8% of new infections.
7.2. Constant vaccine efficacy
We extended our analysis on constant vaccine efficacy to explore the relationship between efficacy, vaccination coverage levels, and HIV infections prevented. A 31% (constant) efficacy vaccine, similar to the average efficacy observed in the RV144 trial, could prevent more than 20% of new infections over 10 years, assuming 60% vaccination coverage is achieved (Figure A-5). Increasing (or decreasing) vaccine efficacy or vaccination coverage prevents more (or fewer) infections, with the relative gain in infections prevented similar for both parameters.
Given a low-efficacy vaccine, coverage needs to be increased substantially to attain a small increase in infections prevented. For example, with a 20% efficacy vaccine, coverage must increase from 60% to nearly 90% to prevent an additional 5% of infections. However, with a highly effective 80% efficacy vaccine, coverage only needs to increase from 60% to 69% to achieve the same gain in infections averted. Hence, use of a low-efficacy vaccine necessitates expanding vaccination coverage as much as possible.
Figure A-5. Sensitivity analysis of vaccine efficacy coverage levels on infections prevented.
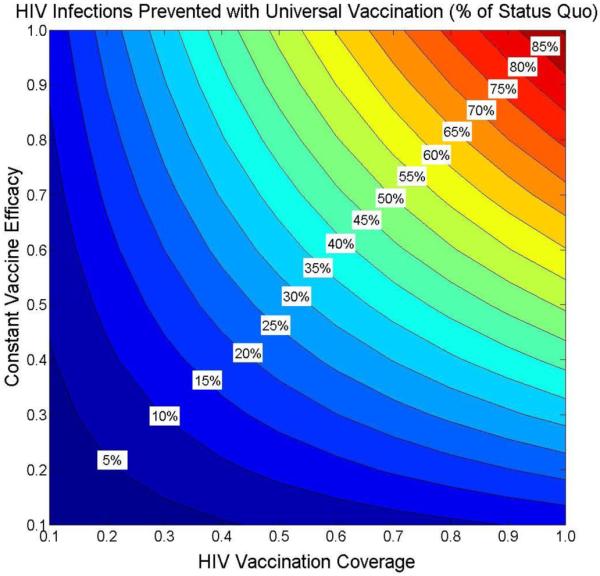
Fraction of HIV infections prevented over 10 years relative to the status quo (590,852 new infections) is shown for varying levels of vaccination coverage (x-axis) and constant vaccine efficacy (y-axis). Assumes constant vaccine efficacy with lifetime duration of protection. In the base case, a 31%-efficacy vaccine with 60% coverage of all adults prevents 20.6% of new infections.
Table A-3.
Summary of key model parameters.
| Parameter | Value | Range | Source |
|---|---|---|---|
| Demographics | |||
| Total population (15 to 64 years) | |||
| Male IDU | 1,000,000 | 0.5-1.5 million | Calculated [19-23] |
| Male MSM | 4,057,194 | 3-6 million | Calculated [19-21, 24] |
| Male IDU/MSM | 300,000 | 200,000-500,000 | Calculated [17, 19-25] |
| Male Other | 96,022,652 | 95-100 million | Calculated [19-21] |
| Female IDU | 450,000 | 300,00-600,000 | Calculated [19-23] |
| Female Other | 101,632,781 | 100-105 million | Calculated [19-21] |
| HIV prevalence | |||
| Male IDU | 12.9% | 10-20% | Calculated [19-23] |
| Male MSM | 12.6% | 5-20% | Calculated [19-21, 24] |
| Male IDU/MSM | 18.8% | 15-30% | Calculated [19-25] |
| Male Other | 0.10% | 0.05-0.25% | Calculated [19-21] |
| Female IDU | 17.3% | 15-30% | Calculated [19-23] |
| Female Other | 0.22% | 0.10-0.40% | Calculated [19-21] |
| Annual mortality rate | |||
| Male | 0.0041 | 0.002-0.005 | [26] |
| Female | 0.0024 | 0.002-0.005 | [26] |
| IDU (excess) | 0.025 | 0-0.05 | [27] |
| Annual maturation rate | |||
| Male | 0.0111 | 0.01-0.02 | [21] |
| Female | 0.0122 | 0.01-0.02 | [21] |
| Annual entry rate | |||
| Male | 0.0227 | 0.01-0.05 | [21] |
| Female | 0.0213 | 0.01-0.05 | [21] |
| Sexual Transmission | |||
| Transmission probability per partnership | |||
| Heterosexual (FHIV+→MHIV−) | |||
| Asymptomatic HIV | 0.02 | 0.01-0.04 | [28-36] |
| Symptomatic HIV | 0.03 | 0.01-0.04 | [28-36] |
| AIDS | 0.05 | 0.03-0.06 | [28-36] |
| Heterosexual (MHIV+→FHIV−) | |||
| Asymptomatic HIV | 0.03 | 0.02-0.05 | [28-36] |
| Symptomatic HIV | 0.04 | 0.02-0.05 | [28-36] |
| AIDS | 0.08 | 0.05-0.10 | [28-36] |
| Homosexual (MHIV+→MHIV−) | |||
| Asymptomatic HIV | 0.04 | 0.03-0.06 | [32, 37-39] |
| Symptomatic HIV | 0.05 | 0.03-0.06 | [32, 37-39] |
| AIDS | 0.10 | 0.08-0.15 | [32, 37-39] |
| Annual number of same-sex partners | |||
| Male MSM | 3.0 | 2.0-5.0 | [40-43] |
| Male IDU/MSM | 3.0 | 2.0-5.0 | [40, 41, 43] |
| Condom usage with same-sex partners | |||
| Male MSM | 40% | 30-60% | [25, 40-43] |
| Male IDU/MSM | 40% | 30-50% | [43] |
| Annual number of opposite-sex partners | |||
| Male IDU | 3.0 | 2.0-5.0 | [44] |
| Male MSM | 0.1 | 0-1.0 | [42] |
| Male IDU/MSM | 0.1 | 0-1.0 | [45] |
| Male Other | 1.1 | 0.5-2.0 | [42, 46-49] |
| Female IDU | 3.5 | 2.0-5.0 | [50] |
| Female Other | 1.1 | 0.5-2.0 | [46, 47, 49, 51] |
| Condom usage with opposite-sex partners | |||
| Male IDU | 25% | 15-35% | [25, 45] |
| Male MSM | 30% | 20-50% | [25, 43] |
| Male IDU/MSM | 30% | 30-50% | [43, 45] |
| Male Other | 20% | 10-40% | [46] |
| Female IDU | 25% | 20-50% | [44, 50] |
| Female Other | 20% | 10-40% | [46] |
| Reduction in heterosexual HIV transmission due to male circumcision |
50% | 48-60% | [52-54] |
| Injection Drug Use Transmission | |||
| Transmission probability per shared injection | |||
| Asymptomatic HIV | 0.002 | 0.001-0.005 | [27, 55, 56] |
| Symptomatic HIV | 0.003 | 0.001-0.005 | [27, 55, 56] |
| AIDS | 0.003 | 0.001-0.005 | [27, 55, 56] |
| Average injections per year | 200 | 100-500 | [27, 57] |
| Fraction of injections that are shared | 20% | 10-40% | [27, 43, 44, 58] |
| HIV Screening | |||
| Fraction of population tested in past 12 months | |||
| High-risk individuals | 23% | 10-30% | [20] |
| Low-risk individuals | 10% | 5-20% | [20] |
| Annual probability of symptom-based case finding | |||
| HIV | 10% | 0-30% | [16] |
| AIDS | 20% | 10-60% | [16] |
| Reduction in sexual behavior among HIV+ identified individuals due to screening |
20% | 0-50% | [59-62] |
| Antiretroviral Therapy (ART) | |||
| Fraction starting ART at CD4=350 cells/mm3 | 50% | 25-75% | Assumed [63, 64] |
| Annual ART entry rate if CD4<350 cells/mm3 | 0.05 | 0-0.10 | Assumed [64] |
| Reduction in injection infectivity due to ART | 50% | 25-75% | [16, 65] |
| Reduction in sexual infectivity due to ART | 90% | 50-99% | [35, 66-71] |
| Circumcision | |||
| Fraction of males circumcised | 70% | 50-80% | [72] |
| Reduction in HIV transmission due to circumcision |
50% | 48-60% | [53, 54] |
| Quality-of-life Multipliers | |||
| Uninfected | 1.0 | --- | [73] |
| Asymptomatic HIV – Unaware | 0.91 | 0.85-0.95 | [74-77] |
| Asymptomatic HIV – Aware (Year 1) | 0.84 | 0.85-0.95 | [16, 74-77] |
| Asymptomatic HIV – Aware (Years 2+) | 0.89 | 0.85-0.95 | [16, 74-77] |
| Symptomatic HIV – Unaware | 0.79 | 0.70-0.80 | [74-77] |
| Symptomatic HIV – Aware | 0.72 | 0.70-0.80 | [74-77] |
| Symptomatic HIV – Treated with ART | 0.83 | 0.82-0.87 | [74-77] |
| AIDS – Unaware | 0.72 | 0.60-0.75 | [74-77] |
| AIDS – Aware | 0.72 | 0.60-0.75 | [74-77] |
| AIDS – Treated with ART | 0.82 | 0.82-0.87 | [74-77] |
| IDU (multiplier)* | 0.90 | 0.80-1.0 | [27, 65] |
| Costs (2009 USD) | |||
| Annual HIV-related healthcare costs | |||
| Asymptomatic HIV – Untreated | $4,125 | $3,000-$6,000 | [78, 79] |
| Symptomatic HIV – Untreated | $6,925 | $5,000-$9,000 | [78, 79] |
| Symptomatic HIV – Treated with ART | $6,174 | $5,000-$7,000 | [78, 79] |
| AIDS – Untreated | $21,838 | $15,000-$25,000 | [78-80] |
| AIDS – Treated with ART | $9,938 | $6,000-$17,000 | [16, 79] |
| Annual non-HIV-related healthcare costs | $7,576 | $5,000-$8,000 | [81] |
| Annual cost of ART | $15,571 | $12,000-$18,000 | [16, 79, 80, 82] |
| Cost of HIV ELISA antibody test | $12 | $10-20 | [83] |
| Cost of confirmatory Western Blot test | $19 | $10-50 | [83] |
| Cost of behavior counseling | $60 | $50-$100 | [16, 82, 84] |
| Annual cost of ancillary IDU services | $2,500 | $1,000-$4,000 | [27] |
| Annual discount rate | 3% | 0-5% | [13] |
Quality of life for all injection drug users is multiplied by this quantity.
ART = antiretroviral therapy; ELISA = enzyme-linked immunosorbent assay;
MSM = men who have sex with men; IDU = injection drug user;
FHIV+→MHIV− = HIV transmission between HIV+ female partner and HIV− male partner;
MHIV+→FHIV− = HIV transmission between HIV+ male partner and HIV− female partner;
MHIV+→MHIV− = HIV transmission between HIV+ male partner and HIV− male partner;
USD = United States dollars.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Joint United Nations Programme on HIV/AIDS (UNAIDS) 2010 Report on the Global AIDS Epidemic. 2010. [Google Scholar]
- [2].Wayne CK, Berkley SF. The renaissance in HIV vaccine development--future directions. N Engl J Med. 2010 Jul 29;363(5):e7. doi: 10.1056/NEJMp1007629. [DOI] [PubMed] [Google Scholar]
- [3].Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009 Dec 3;361(23):2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- [4].Wu X, Yang ZY, Li Y, Hogerkorp CM, Schief WR, Seaman MS, et al. Rational design of envelope identifies broadly neutralizing human monoclonal antibodies to HIV-1. Science. 2010 Aug 13;329(5993):856–61. doi: 10.1126/science.1187659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhou T, Georgiev I, Wu X, Yang ZY, Dai K, Finzi A, et al. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science. 2010 Aug 13;329(5993):811–7. doi: 10.1126/science.1192819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Long EF, Brandeau ML, Owens DK. Potential population health outcomes and expenditures of HIV vaccination strategies in the United States. Vaccine. 2009;27(39):5402–10. doi: 10.1016/j.vaccine.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Andersson KM, Owens DK, Vardas E, Gray GE, McIntyre JA, Paltiel AD. Predicting the Impact of a Partially Effective HIV Vaccine and Subsequent Risk Behavior Change on the Heterosexual HIV Epidemic in Low-and Middle-Income Countries: A South African Example. J Acquir Immune Defic Syndr. 2007;46(1):78–90. doi: 10.1097/QAI.0b013e31812506fd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Owens DK, Edwards DM, Cavallaro JF, Shachter RD. The cost effectiveness of partially effective HIV vaccines. In: Brandeau ML, Sainfort F, Pierskalla WP, editors. Operations Research and Health Care: A Handbook of Methods and Applications. Kluwer Academic Publishers; 2004. pp. 403–18. [Google Scholar]
- [9].Owens DK, Edwards DM, Shachter RD. Costs and benefits of imperfect HIV vaccines: Implications for vaccine development and use. In: Kaplan EH, Brookmeyer R, editors. Quantitative Evaluation of HIV Prevention Programs. Yale University Press; 2001. pp. 143–71. [Google Scholar]
- [10].Owens DK, Edwards DM, Shachter RD. Population effects of preventive and therapeutic HIV vaccines in early- and late-stage epidemics. AIDS. 1998 Jun 18;12(9):1057–66. [PubMed] [Google Scholar]
- [11].Edwards DM, Shachter RD, Owens DK. A dynamic HIV-transmission model for evaluating the costs and benefits of vaccine programs. Interfaces. 1998;28(3):144–66. [Google Scholar]
- [12].Long EF, Brandeau ML, Owens DK. The cost-effectiveness and population outcomes of expanded HIV screening and antiretroviral treatment in the United States. Ann Intern Med. 2010;153:778–89. doi: 10.1059/0003-4819-153-12-201012210-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Gold MR, Siegel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. Oxford University Press; New York: 1996. [Google Scholar]
- [14].Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006 Sep 22;55(RR-14):1–17. [PubMed] [Google Scholar]
- [15].Qaseem A, Snow V, Shekelle P, Hopkins R, Jr., Owens DK. Screening for HIV in health care settings: a guidance statement from the American College of Physicians and HIV Medicine Association. Ann Intern Med. 2009 Jan 20;150(2):125–31. doi: 10.7326/0003-4819-150-2-200901200-00300. [DOI] [PubMed] [Google Scholar]
- [16].Sanders GD, Bayoumi AM, Sundaram V, Bilir SP, Neukermans CP, Rydzak CE, et al. Cost-effectiveness of screening for HIV in the era of highly active antiretroviral therapy. N Engl J Med. 2005 Feb 10;352(6):570–85. doi: 10.1056/NEJMsa042657. [DOI] [PubMed] [Google Scholar]
- [17].Centers for Disease Control and Prevention (CDC) HIV/AIDS Surveillance Report. 2007;19 [cited January 10, 2011]; Available from: http://www.cdc.gov/hiv/surveillance/resources/reports/2007report/
- [18].Hall HI, Song R, Rhodes P, Prejean J, An Q, Lee LM, et al. Estimation of HIV incidence in the United States. JAMA. 2008;300(5):520–9. doi: 10.1001/jama.300.5.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Joint United Nations Programme on HIV/AIDS (UNAIDS) 2008 Report on the Global AIDS Epidemic. 2008. [Google Scholar]
- [20].Centers for Disease Control and Prevention (CDC) Estimates of New HIV Infections in the United States. 2008 [cited January 10, 2011]; Available from: http://www.cdc.gov/hiv/topics/surveillance/resources/factsheets/incidence.htm.
- [21].CensusScope United States Age Distribution. 2000 [cited January 10, 2011]; Available from: http://www.censusscope.org/us/chart_age.html.
- [22].Evans JL, Hahn JA, Page-Shafer K, Lum PJ, Stein ES, Davidson PJ, et al. Gender differences in sexual and injection risk behavior among active young injection drug users in San Francisco (the UFO Study) J Urban Health. 2003 Mar;80(1):137–46. doi: 10.1093/jurban/jtg137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Friedman SR, Tempalski B, Cooper H, Perlis T, Keem M, Friedman R, et al. Estimating numbers of injecting drug users in metropolitan areas for structural analyses of community vulnerability and for assessing relative degrees of service provision for injecting drug users. J Urban Health. 2004 Sep;81(3):377–400. doi: 10.1093/jurban/jth125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Centers for Disease Control and Prevention (CDC) HIV and AIDS among Gay and Bisexual Men. 2010 [cited January 10, 2011]; Available from: http://www.cdc.gov/nchhstp/newsroom/docs/FastFacts-MSM-FINAL508COMP.pdf.
- [25].Rietmeijer CA, Wolitski RJ, Fishbein M, Corby NH, Cohn DL. Sex hustling, injection drug use, and non-gay identification by men who have sex with men. Associations with high-risk sexual behaviors and condom use. Sex Transm Dis. 1998 Aug;25(7):353–60. doi: 10.1097/00007435-199808000-00006. [DOI] [PubMed] [Google Scholar]
- [26].Arias E, Rostron BL, Tejada-Vera B. United States Life Tables, 2005. Natl Vital Stat Rep. 2010;58(10) [PubMed] [Google Scholar]
- [27].Zaric GS, Barnett PG, Brandeau ML. HIV transmission and the cost-effectiveness of methadone maintenance. Am J Public Health. 2000 Jul;90(7):1100–11. doi: 10.2105/ajph.90.7.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Abbas UL, Anderson RM, Mellors JW. Potential impact of antiretroviral therapy on HIV-1 transmission and AIDS mortality in resource-limited settings. J Acquir Immune Defic Syndr. 2006 Apr 15;41(5):632–41. doi: 10.1097/01.qai.0000194234.31078.bf. [DOI] [PubMed] [Google Scholar]
- [29].Downs AM, de Vincenzi I, European Study Group in Heterosexual Transmission of HIV Probability of heterosexual transmission of HIV: relationship to the number of unprotected sexual contacts. J Acquir Immune Defic Syndr Hum Retrovirol. 1996 Apr;11:388–95. doi: 10.1097/00042560-199604010-00010. [DOI] [PubMed] [Google Scholar]
- [30].Hollingsworth TD, Anderson RM, Fraser C. HIV-1 transmission, by stage of infection. J Infect Dis. 2008 Sep;198:687–93. doi: 10.1086/590501. [DOI] [PubMed] [Google Scholar]
- [31].Kaplan EH. Modeling HIV infectivity: must sex acts be counted? J Acquir Immune Defic Syndr. 1990;3:55–61. [PubMed] [Google Scholar]
- [32].Mastro TD, de Vincenzi I. Probabilities of sexual HIV-1 transmission. AIDS. 1996;10(Suppl A):75–82. doi: 10.1097/00002030-199601001-00011. [DOI] [PubMed] [Google Scholar]
- [33].Nicolosi A, Correa Leite ML, Musicco M, Arici C, Gavazzeni G, Lazzarin A, Italian Study Group on HIV Heterosexual Transmission The efficiency of male-to-female and female-to-male sexual transmission of the human immunodeficiency virus: a study of 730 stable couples. Epidemiology. 1994 Nov;5:570–5. doi: 10.1097/00001648-199411000-00003. [DOI] [PubMed] [Google Scholar]
- [34].Padian NS, Shiboski SC, Glass SO, Vittinghoff E. Heterosexual transmission of human immunodeficiency virus (HIV) in northern California: results from a ten-year study. Am J Epidemiol. 1997 Aug;146:350–7. doi: 10.1093/oxfordjournals.aje.a009276. [DOI] [PubMed] [Google Scholar]
- [35].Quinn TC, Wawer MJ, Sewankambo N, Serwadda D, Li C, Wabwire-Mangen F, et al. Rakai Project Study Group Viral load and heterosexual transmission of human immunodeficiency virus type 1. N Engl J Med. 2000 Mar 30;342(13):921–9. doi: 10.1056/NEJM200003303421303. [DOI] [PubMed] [Google Scholar]
- [36].Wawer MJ, Gray RH, Sewankambo NK, Serwadda D, Li X, Laeyendecker O, et al. Rates of HIV-1 transmission per coital act, by stage of HIV-1 infection, in Rakai, Uganda. J Infect Dis. 2005;191:1403–9. doi: 10.1086/429411. [DOI] [PubMed] [Google Scholar]
- [37].Caceres CF, van Griensven GJ. Male homosexual transmission of HIV-1. AIDS. 1994 Aug;8:1051–61. doi: 10.1097/00002030-199408000-00004. [DOI] [PubMed] [Google Scholar]
- [38].Jacquez JA, Koopman JS, Simon CP, Longini IM. Role of the primary infection in epidemics of HIV infection in gay cohorts. J Acquir Immune Defic Syndr. 1994 Nov;7:1169–84. [PubMed] [Google Scholar]
- [39].Vittinghoff E, Douglas J, Judson F, McKirnan D, MacQueen K, Buchbinder SP. Per-contact risk of human immunodeficiency virus transmission between male sexual partners. Am J Epidemiol. 1999 Aug;150:306–11. doi: 10.1093/oxfordjournals.aje.a010003. [DOI] [PubMed] [Google Scholar]
- [40].Harawa NT, Greenland S, Bingham TA, Johnson DF, Cochran SD, Cunningham WE, et al. Associations of race/ethnicity with HIV prevalence and HIV-related behaviors among young men who have sex with men in 7 urban centers in the United States. J Acquir Immune Defic Syndr. 2004 Apr 15;35(5):526–36. doi: 10.1097/00126334-200404150-00011. [DOI] [PubMed] [Google Scholar]
- [41].MacKellar DA, Valleroy LA, Behel S, Secura GM, Bingham T, Celentano DD, et al. Unintentional HIV exposures from young men who have sex with men who disclose being HIV-negative. AIDS. 2006 Aug 1;20(12):1637–44. doi: 10.1097/01.aids.0000238410.67700.d1. [DOI] [PubMed] [Google Scholar]
- [42].Pathela P, Hajat A, Schillinger J, Blank S, Sell R, Mostashari F. Discordance between sexual behavior and self-reported sexual identity: a population-based survey of New York City men. Ann Intern Med. 2006 Sep 19;145(6):416–25. doi: 10.7326/0003-4819-145-6-200609190-00005. [DOI] [PubMed] [Google Scholar]
- [43].Kral AH, Lorvick J, Ciccarone D, Wenger L, Gee L, Martinez A, et al. HIV prevalence and risk behaviors among men who have sex with men and inject drugs in San Francisco. J Urban Health. 2005 Mar;82(1 Suppl 1):i43–50. doi: 10.1093/jurban/jti023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Spittal PM, Craib KJ, Wood E, Laliberte N, Li K, Tyndall MW, et al. Risk factors for elevated HIV incidence rates among female injection drug users in Vancouver. CMAJ. 2002 Apr 2;166(7):894–9. [PMC free article] [PubMed] [Google Scholar]
- [45].Bacon O, Lum P, Hahn J, Evans J, Davidson P, Moss A, et al. Commercial sex work and risk of HIV infection among young drug-injecting men who have sex with men in San Francisco. Sex Transm Dis. 2006 Apr;33(4):228–34. doi: 10.1097/01.olq.0000204914.91923.ad. [DOI] [PubMed] [Google Scholar]
- [46].Davis JO, Smith TW. General Social Surveys (GSS), 1972-2008. National Opinion Research Center; Chicago: 2009. [Google Scholar]
- [47].Fryar CD, Hirsch R, Porter KS, Kottiri B, Brody DJ, Louis T. Drug use and sexual behaviors reported by adults: United States, 1999-2002. Adv Data. 2007 Jun;28(384):1–14. [PubMed] [Google Scholar]
- [48].Fenton KA, Korovessis C, Johnson AM, McCadden A, McManus S, Wellings K, et al. Sexual behaviour in Britain: reported sexually transmitted infections and prevalent genital Chlamydia trachomatis infection. Lancet. 2001 Dec 1;358(9296):1851–4. doi: 10.1016/S0140-6736(01)06886-6. [DOI] [PubMed] [Google Scholar]
- [49].Brisson M, Boily MC, Masse BR, Adrien A, Leaune V. Highlights of the sexual activity of the heterosexual population in the province of Quebec. Sex Transm Infect. 1999 Oct;75(5):296–9. doi: 10.1136/sti.75.5.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Tyndall MW, Patrick D, Spittal P, Li K, O’Shaughnessy MV, Schechter MT. Risky sexual behaviours among injection drugs users with high HIV prevalence: implications for STD control. Sex Transm Infect. 2002 Apr;78(Suppl 1):i170–5. doi: 10.1136/sti.78.suppl_1.i170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Johnson AM, Mercer CH, Erens B, Copas AJ, McManus S, Wellings K, et al. Sexual behaviour in Britain: partnerships, practices, and HIV risk behaviours. Lancet. 2001 Dec 1;358(9296):1835–42. doi: 10.1016/S0140-6736(01)06883-0. [DOI] [PubMed] [Google Scholar]
- [52].Siegfried N, Muller M, Deeks JJ, Volmink J. Male circumcision for prevention of heterosexual acquisition of HIV in men. Cochrane Database Syst Rev. 2009;(2):CD003362. doi: 10.1002/14651858.CD003362.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Auvert B, Taljaard D, Lagarde E, Sobngwi-Tambekou J, Sitta R, Puren A. Randomized, controlled intervention trial of male circumcision for reduction of HIV infection risk: the ANRS 1265 Trial. PLoS Med. 2005 Nov;2(11):e298. doi: 10.1371/journal.pmed.0020298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Desai K, Boily MC, Garnett GP, Masse BR, Moses S, Bailey RC. The role of sexually transmitted infections in male circumcision effectiveness against HIV--insights from clinical trial simulation. Emerg Themes Epidemiol. 2006;3:19. doi: 10.1186/1742-7622-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wall SD, Olcott EW, Gerberding JL. AIDS risk and risk reduction in the radiology department. AJR Am J Roentgenol. 1991 Nov;157(5):911–7. doi: 10.2214/ajr.157.5.1927808. [DOI] [PubMed] [Google Scholar]
- [56].Kaplan EH, Heimer R. A model-based estimate of HIV infectivity via needle sharing. J Acquir Immune Defic Syndr. 1992;5(11):1116–8. [PubMed] [Google Scholar]
- [57].Taylor A, Hutchinson S, Lingappa J, Wadd S, Ahmed S, Gruer L, et al. Severe illness and death among injecting drug users in Scotland: a case-control study. Epidemiol Infect. 2005 Apr;133(2):193–204. doi: 10.1017/s0950268804003504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Harris ZK. Efficient allocation of resources to prevent HIV infection among injection drug users: the Prevention Point Philadelphia (PPP) needle exchange program. Health Econ. 2006 Feb;15(2):147–58. doi: 10.1002/hec.1021. [DOI] [PubMed] [Google Scholar]
- [59].The National Institute of Mental Health (NIMH) Multisite HIV Prevention Trial Group The NIMH Multisite HIV Prevention Trial: reducing HIV sexual risk behavior. Science. 1998 Jun 19;280(5371):1889–94. doi: 10.1126/science.280.5371.1889. [DOI] [PubMed] [Google Scholar]
- [60].Kamb ML, Fishbein M, Douglas JM, Jr., Rhodes F, Rogers J, Bolan G, et al. Project RESPECT Study Group Efficacy of risk-reduction counseling to prevent human immunodeficiency virus and sexually transmitted diseases: a randomized controlled trial. JAMA. 1998 Oct 7;280(13):1161–7. doi: 10.1001/jama.280.13.1161. [DOI] [PubMed] [Google Scholar]
- [61].Cleary PD, Van Devanter N, Rogers TF, Singer E, Shipton-Levy R, Steilen M, et al. Behavior changes after notification of HIV infection. Am J Public Health. 1991 Dec;81(12):1586–90. doi: 10.2105/ajph.81.12.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Higgins DL, Galavotti C, O’Reilly KR, Schnell DJ, Moore M, Rugg DL, et al. Evidence for the effects of HIV antibody counseling and testing on risk behaviors. JAMA. 1991 Nov 6;266(17):2419–29. [PubMed] [Google Scholar]
- [63].Centers for Disease Control and Prevention (CDC) CDC’s HIV Prevention Progress in the United States. 2010 [cited August 1, 2010]; Available from: http://www.cdc.gov/hiv/resources/factsheets/PDF/cdcprev.pdf.
- [64].Teshale EH, Kamimoto L, Harris N, Li J, Wang H, McKenna MT. Estimated Number of HIV-infected Persons Eligible for and Receiving HIV Antiretroviral Therapy, 2003--United States; 12th Conference on Retroviruses and Opportunistic Infections; Boston, Massachusetts. 2005 February 22-25; 2005. Abstract #167. [Google Scholar]
- [65].Long EF, Brandeau ML, Galvin CM, Vinichenko T, Tole SP, Schwartz A, et al. Effectiveness and cost-effectiveness of strategies to expand antiretroviral therapy in St. Petersburg, Russia. AIDS. 2006 Nov 14;20(17):2207–15. doi: 10.1097/QAD.0b013e328010c7d0. [DOI] [PubMed] [Google Scholar]
- [66].Vernazza P, Hirschel B, Bernasconi E, Flepp M. HIV transmission under highly active antiretroviral therapy. Lancet. 2008 Nov 22;372(9652):1806–7. doi: 10.1016/S0140-6736(08)61753-5. author reply 7. [DOI] [PubMed] [Google Scholar]
- [67].Castilla J, Del Romero J, Hernando V, Marincovich B, Garcia S, Rodriguez C. Effectiveness of highly active antiretroviral therapy in reducing heterosexual transmission of HIV. J Acquir Immune Defic Syndr. 2005 Sep 1;40(1):96–101. doi: 10.1097/01.qai.0000157389.78374.45. [DOI] [PubMed] [Google Scholar]
- [68].Porco TC, Martin JN, Page-Shafer KA, Cheng A, Charlebois E, Grant RM, et al. Decline in HIV infectivity following the introduction of highly active antiretroviral therapy. AIDS. 2004 Jan 2;18(1):81–8. doi: 10.1097/01.aids.0000096872.36052.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Vernazza PL, Troiani L, Flepp MJ, Cone RW, Schock J, Roth F, et al. Potent antiretroviral treatment of HIV-infection results in suppression of the seminal shedding of HIV. The Swiss HIV Cohort Study. AIDS. 2000 Jan 28;14(2):117–21. doi: 10.1097/00002030-200001280-00006. [DOI] [PubMed] [Google Scholar]
- [70].Zhang H, Dornadula G, Beumont M, Livornese L, Jr., Van Uitert B, Henning K, et al. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N Engl J Med. 1998 Dec 17;339(25):1803–9. doi: 10.1056/NEJM199812173392502. [DOI] [PubMed] [Google Scholar]
- [71].Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004 Jul 14;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- [72].O’Donnell H. The United States Circumcision Century. 2001 [cited January 10, 2011]; Available from: http://www.boystoo.com/history/statistics.htm.
- [73].Fryback DG, Dasbach EJ, Klein R, Klein BE, Dorn N, Peterson K, et al. The Beaver Dam Health Outcomes Study: initial catalog of health-state quality factors. Med Decis Making. 1993 Apr-Jun;13(2):89–102. doi: 10.1177/0272989X9301300202. [DOI] [PubMed] [Google Scholar]
- [74].Holtgrave DR, Pinkerton SD. Updates of cost of illness and quality of life estimates for use in economic evaluations of HIV prevention programs. J Acquir Immune Defic Syndr Hum Retrovirol. 1997 Sep 1;16(1):54–62. doi: 10.1097/00042560-199709010-00009. [DOI] [PubMed] [Google Scholar]
- [75].Honiden S, Sundaram V, Nease RF, Holodniy M, Lazzeroni LC, Zolopa A, et al. The effect of diagnosis with HIV infection on health-related quality of Life. Qual Life Res. 2006 Feb;15(1):69–82. doi: 10.1007/s11136-005-8485-x. [DOI] [PubMed] [Google Scholar]
- [76].Schackman BR, Goldie SJ, Freedberg KA, Losina E, Brazier J, Weinstein MC. Comparison of health state utilities using community and patient preference weights derived from a survey of patients with HIV/AIDS. Med Decis Making. 2002;22:27–38. doi: 10.1177/0272989X0202200103. [DOI] [PubMed] [Google Scholar]
- [77].Tengs TO, Lin TH. A meta-analysis of utility estimates for HIV/AIDS. Med Decis Making. 2002 Nov-Dec;22(6):475–81. doi: 10.1177/0272989X02238300. [DOI] [PubMed] [Google Scholar]
- [78].Bozzette SA, Joyce G, McCaffrey DF, Leibowitz AA, Morton SC, Berry SH, et al. Expenditures for the care of HIV-infected patients in the era of highly active antiretroviral therapy. N Engl J Med. 2001 Mar 15;344(11):817–23. doi: 10.1056/NEJM200103153441107. [DOI] [PubMed] [Google Scholar]
- [79].Schackman BR, Gebo KA, Walensky RP, Losina E, Muccio T, Sax PE, et al. The lifetime cost of current human immunodeficiency virus care in the United States. Med Care. 2006 Nov;44(11):990–7. doi: 10.1097/01.mlr.0000228021.89490.2a. [DOI] [PubMed] [Google Scholar]
- [80].Hutchinson AB, Farnham PG, Dean HD, Ekwueme DU, del Rio C, Kamimoto L, et al. The economic burden of HIV in the United States in the era of highly active antiretroviral therapy: evidence of continuing racial and ethnic differences. J Acquir Immune Defic Syndr. 2006 Dec 1;43(4):451–7. doi: 10.1097/01.qai.0000243090.32866.4e. [DOI] [PubMed] [Google Scholar]
- [81].World Health Organization (WHO) Global Health Observatory. 2009 [cited August 1, 2010]; Available from: http://www.who.int/gho/en/
- [82].Paltiel AD, Weinstein MC, Kimmel AD, Seage GR, 3rd, Losina E, Zhang H, et al. Expanded screening for HIV in the United States--an analysis of cost-effectiveness. N Engl J Med. 2005 Feb 10;352(6):586–95. doi: 10.1056/NEJMsa042088. [DOI] [PubMed] [Google Scholar]
- [83].Centers for Medicaid and Medicare Services . Medicaid Fee Schedule. 2009. [Google Scholar]
- [84].Paltiel AD, Walensky RP, Schackman BR, Seage GR, 3rd, Mercincavage LM, Weinstein MC, et al. Expanded HIV screening in the United States: effect on clinical outcomes, HIV transmission, and costs. Ann Intern Med. 2006 Dec 5;145(11):797–806. doi: 10.7326/0003-4819-145-11-200612050-00004. [DOI] [PubMed] [Google Scholar]