Abstract
Naturally occurring 8-O-methylated sialic acids, including 8-O-methyl-N-acetylneuraminic acid and 8-O-methyl-N-glycolylneuraminic acid, along with 8-O-methyl-2-keto-3-deoxy-D-glycero-D-galacto-nonulosonic acid (Kdn8Me) and 8-deoxy-Kdn were synthesized from corresponding 5-O-modified six-carbon monosaccharides and pyruvate using a sialic acid aldolase cloned from Pasteurella multocida strain P-1059 (PmNanA). In addition, α2–3- and α2–6-linked sialyltrisaccharides containing Neu5Ac8Me and Kdn8Deoxy were also synthesized using a one-pot multienzyme approach. The strategy reported here provides an efficient approach to produce glycans containing various C8-modified sialic acids for biological evaluations.
Keywords: Chemoenzymatic synthesis, C8-modification, Sialic acid, Sialoside, Sialyltransferase
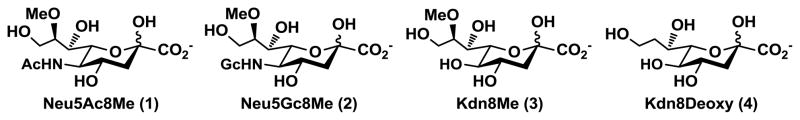
Sialic acids have been widely found in higher animals and some microorganisms. They are commonly found at the termini of the glycan chains on glycoproteins and glycolipids.1 Sialic acids constitute a structurally diverse family of nine-carbon acidic monosaccharides with more than 50 members having been identified. N-Acetylneuraminic acid (Neu5Ac), N-glycolylneuraminic acid (Neu5Gc), and 2-keto-3-deoxy-D-glycero-D-galacto-nonulosonic acid (Kdn) are the three basic forms of sialic acids which are distinguished from one another by different substituents at carbon-5.2–4 Additional modifications at different hydroxyl groups of sialic acids include O-acetylation as the most frequently occurring modification. 8-O-Methylation of sialic acids is also commonly observed. For example, 8-O-methylated sialic acids have been reported in starfish Asterias rubens as the components of gangliosides5–8 and glycoproteins.9 Several 8-O-methylated sialic acid forms observed include 8-O-methyl-N-acetylneuraminic acid (Neu5Ac8Me 1, Figure 1), 8-O-methyl-N-glycolylneuraminic acid (Neu5Gc8Me 2, Figure 1), and their O-acetylated derivatives.10–11 Neu5Ac8Me has also been found in the sperm and eggs of teleost fish,12 in human red blood cell membrane,13 and in mouse tissues.14 As 8-O-methylated sialic acids are resistant to sialidases,15 8-O-methylation of sialic acid may play important biological roles. Nevertheless, the significance of naturally occurring C8-modified sialic acid derivatives is currently unclear.
Figure 1.
Structures of C8-modified sialic acid forms.
Only a few methods have been reported for chemical synthesis of sialosides containing Neu5Ac8Me.16–18 These methods, however, are inefficient, lengthy, and tedious. In comparison, enzyme-catalyzed reactions often offer great advantages and are considered attractive and practical approaches for the synthesis of sialosides including those containing uncommon sialic acid forms. Recently, Withers et al. reported the chemical synthesis of C8-modified sialic acids and their application in the CMP-sialic acid synthetase-catalyzed synthesis of CMP-sialic acid derivatives. Campylobacter jejuni α2–3-sialyltransferase Cst-I-catalyzed formation of α2–3-linked sialyllactose containing C8-modified sialic acids was also achieved from CMP-sialic acid derivatives.19 Nevertheless, both chemical and enzymatic syntheses of sialosides containing C8-modified sialic acids so far have been limited to Neu5Ac-based structures and with α2–3-sialyl linkage.
Here we report a facile chemoenzymatic approach for preparative synthesis of Neu5Ac8Me (1), Neu5Gc8Me (2), Kdn8Me (3), and Kdn8Deoxy (4) from chemically synthesized C5-modified N-acetylmannosamine (ManNAc), N-glycolylmannosamine (ManNGc), and mannose derivatives using a sialic acid aldolase-catalyzed reaction. The use of 5-O-methyl-ManNAc and 5-deoxy-mannose as donor substrates in a one-pot three-enzyme system for preparing both α2–3- and α2–6-linked sialosides containing Neu5Ac8Me and Kdn8Deoxy are also described. These compounds are important probes for studying the importance of C8-hydroxy group at the sialic acid residue in the interaction of sialylated carbohydrates and sialic acid binding proteins.
Sialic acid aldolases are enzymes involved in the metabolism of sialic acids. They catalyze the aldol cleavage reaction in nature but the reaction is reversible and the enzymes can be used synthetically in the aldol addition direction for the formation of Neu5Ac from pyruvate and ManNAc. The sialic acid aldolase from Pasteurella multocida P-1059 (PmNanA) recently cloned in our lab has shown flexible substrate specificity.20 It is a more efficient enzyme than the E. coli sialic acid aldolase reported previously21 for using 5-O-methyl ManNAc as a substrate.20 Based on the extremely flexible substrate specificity of PmNanA, we hypothesize that the 5-O-methyl ManNGc, 5-O-methyl mannose, and 5-deoxy-mannose are also potential substrates for this enzyme to produce the corresponding C8-modified sialic acids 2–4.
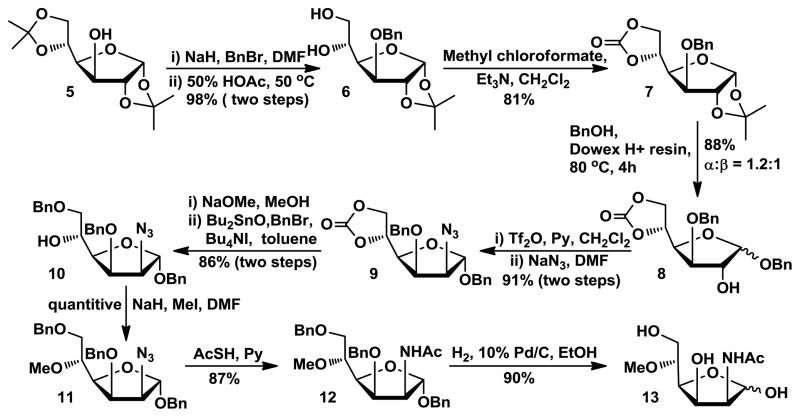
5-O-Methyl ManNAc 13 was synthesized from readily accessible and inexpensive 1,2:5,6-di-O-isopropylidene-α-D-glucopyranose (5).22 As shown in Scheme 1, after benzylation of C3-OH, the 5,6-isopropylidene protecting group was selectively removed by mild acid hydrolysis and the resulting intermediate diol 6 was treated with methyl chloroformate to produce carbonate 7.23 Treatment of 7 with benzyl alcohol in the presence of acidic ion exchange resin24 produced benzyl α- and β-anomers of furanoside 8 in 88% yield with a ratio of approximately 1.2:125, which can be separated by flash chromatography. The C2-OH of the α-anomer 8 was converted to triflate ester by treating with Tf2O. It was then reacted with NaN3 in DMF to produce 2-azido-2-deoxy-manopyranoside 9. The carbonate protecting group was removed by treating 9 with sodium methoxide in methanol. Selective 6-O-benzylation of the azido diol was then achieved by formation of a dibutylstannylene derivative followed by alkylation with benzyl bromide.26 The product 10 was treated with iodomethane and sodium hydride to produce methylation product 11 as the required key intermediate. The 2-azido group of 11 was converted to acetamido group by treating with AcSH in pyridine to produce 12.27–28 After hydrogenation in the presence of H2 and Pd/C, 5-O-methyl-ManNAc 13 was produced in 90% yield.
Scheme 1.
Synthesis of 5-O-methyl ManNAc 13.
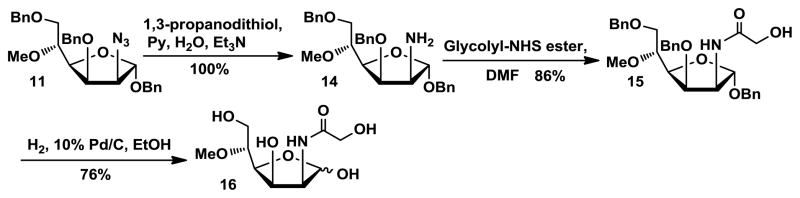
As shown in Scheme 2, to synthesize 5-O-methyl ManNGc 16, the azido group in compound 11 was reduced to an amino group in the presence of 1,3-dithiopropanol and Et3N in pyridine/H2O.28 The resulting amino group in 14 was readily converted to N-glycolyl by coupling with N-hydroxysuccinamide-activated glycolyl ester (glycolyl-NHS ester),21 leading to the formation of compound 15. Debenzylation by hydrogenolysis with H2 and Pd/C in methanol produced the desired 5-O-methyl-ManNGc 16 in 76% yield.
Scheme 2.
Synthesis of 5-O-methyl ManNGc 16.
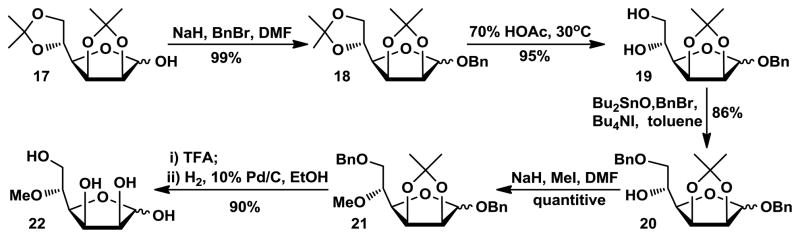
The preparation of 5-O-methyl mannose 22 is outlined in Scheme 3. Commercially available 2,3:5,6-di-O-isopropylidene-α-D-mannofuranose 17 was treated with sodium hydride and benzyl bromide to produce the corresponding benzyl α- and β-glycosides 18 in 57% and 42% yields, respectively. Partial regioselective hydrolysis of 18 was achieved at 30ºC for 20 h using 70% aqueous acetic acid to produce the desired diol 19. Selective 6-O-benzylation was achieved by formation of a dibutylstannylene derivative followed by alkylation with benzyl bromide. Product 20 was treated with iodomethane and sodium hydride to produce the methylation product 21, which was then treated with 75% TFA followed by hydrogenolytic removal of the benzyl groups to produce desired 5-O-methyl mannose 22 in 90% yield.
Scheme 3.
Synthesis of 5-O-methyl-D-mannose 22.
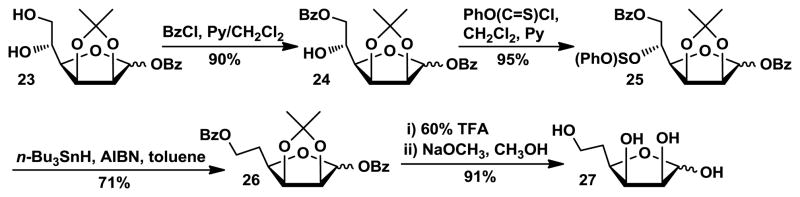
For the preparation of 5-deoxy-mannose 27 (Scheme 4), diol 23 was obtained from D-mannose in three steps.29 Regioselective benzoylation of 23 produced partially benzoated compound 24 in 90% yield. Treatment of benzoate 24 with phenyl chlorothionoformate and pyridine produced the corresponding thiocarbonyl derivative 25 in good yield (95%). Reaction of 25 with tri-n-butylstannane and AIBN provided the deoxy intermediate 26 in 71% yield. Subsequent removal of the isopropylidene group using TFA/H2O followed by debenzoylation produced the target compound 5-deoxy mannose 27 in 91% yield.
Scheme 4.
Synthesis of 5-deoxy-D-mannose 27.
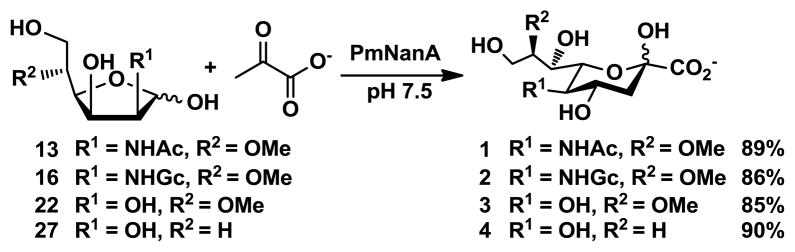
With these C-5 modified monosaccharides (13, 16, 22, 27) on hands, the substrate specificity of PmNanA was examined. Despite of several reported unsuccessful aldolase-catalyzed enzymatic reaction of 5-O-methyl ManNAc with pyruvate25 or the observation of trace amount of product,30 our results showed that all of the C5-modified monosaccharides tested can be tolerated by the recombinant PmNanA as the substrates. The PmNanA-catalyzed aldol addition of 13, 16, 22, and 27 with five equivalents of sodium pyruvate in Tris-HCl buffer (100 mM, pH 7.5) at 37ºC for 24 h followed by the combination of anion exchange and gel filtration column purifications produced corresponding sialic acids and derivatives (1, 2, 3, and 4, respectively) in excellent yields (Scheme 5). The structures of the products were confirmed by NMR and high resolution mass spectrometry (HRMS). The PmNanA, thus, is a highly efficient enzyme for synthesizing C8-modified sialic acids.
Scheme 5.
PmNanA-catalyzed enzymatic synthesis of C8-modified sialic acids 1–4 (54–84 mg).
The obtained C8-modified sialic acids 1–4 were used in substrate specificity study of a recombinant N. meningitidis CMP-sialic acid synthetase (NmCSS).21 Neu5Ac8Me 1 was an excellent substrate for NmCSS, which was in consistent with the previous report for CMP-sialic acid synthetase from Neisseria.19 However, to our surprise, Neu5Gc8Me 2 and Kdn8Me 3 were not substrates for NmCSS. Interestingly, unlike Kdn8Me 3, Kdn8Dexoy 4 was able to be used by NmCSS as a good substrate. These results indicate that the activity of NmCSS is affected by certain modifications on C-8 and/or C-5 of sialic acids.
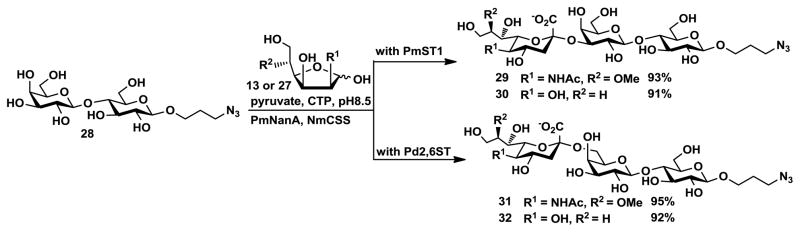
It turned out that both 5-O-methyl ManNAc 13 and 5-deoxy-D-mannose 27 can be used as sialic acid precursors for efficient one-pot three-enzyme synthesis31–33 of α2–3- and α2–6-linked sialosides containing C-8 modified sialic acids including 8-O-methyl Neu5Ac 1 and 8-deoxy-Kdn 4. As shown in Scheme 6, α2–3-linked sialosides Neu5Ac8Meα2–3LacβProN3 29 and Kdn8Deoxyα2–3LacβProN3 30 were synthesized in excellent yields (93% and 91%, respectively) from 3-azidopropyl β-D-galactopyranosyl-(1→4)-β-D-glucopyranoside (LacβProN3) 28 catalyzed by Pasteurella multocida multifunctional α2–3-sialyltransferase PmST133 in the presence of PmNanA and NmCSS. Similarly, α2–6-linked sialosides Neu5Ac8Meα2–6LacβProN3 31 and Kdn8Deoxyα2–6LacβProN3 32 were obtained highly efficiently (95% and 92% yields, respectively) catalyzed by a Photobacterium damselae α2–6-sialyltransferase (Pd2, 6ST)31,34 in the presence of PmNanA and NmCSS. All sialoside products were purified by Bio-Gel P-2 gel filtration chromatography and the structures were characterized by 1H and 13C NMR as wells as high resolution mass spectrometry (HRMS). These compounds are valuable probes for investigating, at a molecular level, the involvement of the C-8 group of sialic acids in the interaction of sialosides and sialic acid-binding proteins. The azido group at the reducing end of the synthesized sialosides can be easily reduced to form a primary amino group, which can be used to link to proteins or other molecules.35–38 Alternatively, the azido group can be directly used for efficient conjugation with molecules containing a terminal or strained alkyne group via Huisgen’s [3+2] cyclization reaction with or without the catalysis by Cu (I).39–42 The azido group can also be coupled to molecules with a functionalized phosphine via the Staudinger ligation.43
Scheme 6.
One-pot three-enzyme synthesis of α2–3- and α2–6-linked sialosides containing Neu5Ac8Me and Kdn8Deoxy (54–82 mg). PmNanA, Pasteurella multocida sialic acid aldolase;20 NmCSS, N. meningitidis CMP-sialic acid synthetase;21 PmST1, Pasteurella multocida multifunctional α2–3-sialyltransferase;33 Pd2,6ST, Photobacterium damselae α2–6-sialyltransferase.31,34
In conclusion, we report here a convenient and efficient sialic acid aldolase-catalyzed enzymatic approach for producing C8-modified sialic acids. Using chemically synthesized 5-O-methylated monosaccharides and pyruvate, natural occurring 8-O-methylated sialic acids Neu5Ac8Me and Neu5Gc8Me as well as non-natural sialic acids Kdn8Me and Kdn8Deoxy were synthesized in excellent yields using a promiscuous recombinant Pasteurella multocida P-1059 sialic acid aldolase (PmNanA). In addition, α2–3- and α2–6-linked sialosides containing Neu5Ac8Me and Kdn8Deoxy were also synthesized using an efficient one-pot three-enzyme system. The obtained 8-O-methylated sialic acids and sialosides are important probes for understanding the significance of O-methyl modification of sialic acids in nature.
Supplementary Material
Acknowledgments
This work was supported by the Beckman Young Investigator Award from the Arnold and Mabel Beckman Foundation and the National Institutes of Health Grant R01GM076360.
Footnotes
Supplementary data (experimental procedures and characterization of compounds) associated with this article can be found, in the online version.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Varki A. Nature. 2007;446:1023. doi: 10.1038/nature05816. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Varki A. ACS Chem Biol. 2010;5:163. doi: 10.1021/cb900266r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Varki A. FASEB J. 1997;11:248. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- 4.Schauer R. Zoology (Jena) 2004;107:49. doi: 10.1016/j.zool.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Sugita M. J Biochem. 1979;86:289. doi: 10.1093/oxfordjournals.jbchem.a132526. [DOI] [PubMed] [Google Scholar]
- 6.Kochetkov NK, Smirnova GP, Glukhoded IS. Biochim Biophys Acta. 1982;712:650. [Google Scholar]
- 7.Smirnova GP, Kochetkov NK, Sadovskaya VL. Biochim Biophys Acta. 1987;920:47. doi: 10.1016/0005-2760(87)90309-2. [DOI] [PubMed] [Google Scholar]
- 8.Muralikrishna G, Reuter G, Peter-Katalinic J, Egge H, Hanisch FG, Siebert HC, Schauer R. Carbohydr Res. 1992;236:321. doi: 10.1016/0008-6215(92)85025-u. [DOI] [PubMed] [Google Scholar]
- 9.Defreese A, Shaw L, Reuter G, Schauer R. Glycoconjugate J. 1993;10:330. [Google Scholar]
- 10.Bergwerff AA, Hulleman SH, Kamerling JP, Vliegenthart JF, Shaw L, Reuter G, Schauer R. Biochimie. 1992;74:25. doi: 10.1016/0300-9084(92)90181-d. [DOI] [PubMed] [Google Scholar]
- 11.Zanetta JP, Srinivasan V, Schauer R. Biochimie. 2006;88:171. doi: 10.1016/j.biochi.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 12.Varki A. Glycobiology. 1992;2:25. doi: 10.1093/glycob/2.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bulai T, Bratosin D, Pons A, Montreuil J, Zanetta JP. FEBS Lett. 2003;534:185. doi: 10.1016/s0014-5793(02)03838-3. [DOI] [PubMed] [Google Scholar]
- 14.Rinninger A, Richet C, Pons A, Kohla G, Schauer R, Bauer HC, Zanetta JP, Vlasak R. Glycoconjugate J. 2006;23:73. doi: 10.1007/s10719-006-5439-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kelm A, Shaw L, Schauer R, Reuter G. Eur J Biochem. 1998;251:874. doi: 10.1046/j.1432-1327.1998.2510874.x. [DOI] [PubMed] [Google Scholar]
- 16.Dufner G, Schworer R, Muller B, Schmidt RR. Eur J Org Chem. 2000;1467 [Google Scholar]
- 17.Hanashima S, Ishikawa D, Akai S, Sato K. Carbohydr Res. 2009;344:747. doi: 10.1016/j.carres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Khorlin AY, Privalov Im. Carbohydr Res. 1970;13:373. [Google Scholar]
- 19.Morley TJ, Withers SG. J Am Chem Soc. 2010;132:9430. doi: 10.1021/ja102644a. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Yu H, Cao H, Lau K, Muthana S, Tiwari VK, Son B, Chen X. Appl Microbiol Biotechnol. 2008;79:963. doi: 10.1007/s00253-008-1506-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu H, Yu H, Karpel R, Chen X. Bioorg Med Chem. 2004;12:6427. doi: 10.1016/j.bmc.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 22.Einhorn C, Luche JL. Carbohydr Res. 1986;155:258. [Google Scholar]
- 23.Shing TKM, Zhong YL. Tetrahedron. 2001;57:1573. [Google Scholar]
- 24.Fleet GWJ, Smith PW. Tetrahedron Lett. 1985;26:1469. [Google Scholar]
- 25.Auge C, David S, Gautheron C, Malleron A, Cavaye B. New J Chem. 1988;12:733. [Google Scholar]
- 26.Bai Y, Lowary TL. J Org Chem. 2006;71:9672. doi: 10.1021/jo061821a. [DOI] [PubMed] [Google Scholar]
- 27.Rosen T, Lico IM, Chu DTW. J Org Chem. 1988;53:1580. [Google Scholar]
- 28.Yang Y, Li Y, Yu B. J Am Chem Soc. 2009;131:12076. doi: 10.1021/ja9055245. [DOI] [PubMed] [Google Scholar]
- 29.Suhara Y, Ono K, Yoshida A, Fujishima T, Saito N, Honzawa S, Kishimoto S, Sugiura T, Waku K, Takayama H, Kittaka A. Heterocycles. 2004;62:423. [Google Scholar]
- 30.Augé C, Gautheron C, David S, Malleron A, Cavaye B, Bouxom B. Tetrahedron. 1990;46:201. [Google Scholar]
- 31.Yu H, Huang S, Chokhawala H, Sun M, Zheng H, Chen X. Angew Chem Int Ed Engl. 2006;45:3938. doi: 10.1002/anie.200600572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu H, Chokhawala HA, Huang S, Chen X. Nat Protoc. 2006;1:2485. doi: 10.1038/nprot.2006.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu H, Chokhawala H, Karpel R, Wu B, Zhang J, Zhang Y, Jia Q, Chen X. J Am Chem Soc. 2005;127:17618. doi: 10.1021/ja0561690. [DOI] [PubMed] [Google Scholar]
- 34.Sun M, Li Y, Chokhawala HA, Henning R, Chen X. Biotechnol Lett. 2008;30:671. doi: 10.1007/s10529-007-9588-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu H, Chokhawala HA, Varki A, Chen X. Org Biomol Chem. 2007;5:2458. doi: 10.1039/b706507h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chokhawala HA, Huang S, Lau K, Yu H, Cheng J, Thon V, Hurtado-Ziola N, Guerrero JA, Varki A, Chen X. ACS Chem Biol. 2008;3:567. doi: 10.1021/cb800127n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blixt O, Head S, Mondala T, Scanlan C, Huflejt ME, Alvarez R, Bryan MC, Fazio F, Calarese D, Stevens J, Razi N, Stevens DJ, Skehel JJ, van Die I, Burton DR, Wilson IA, Cummings R, Bovin N, Wong CH, Paulson JC. Proc Natl Acad Sci U S A. 2004;101:17033. doi: 10.1073/pnas.0407902101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liao HY, Hsu CH, Wang SC, Liang CH, Yen HY, Su CY, Chen CH, Jan JT, Ren CT, Cheng TJ, Wu CY, Wong CHJ. Am Chem Soc. 2010;132:14849. doi: 10.1021/ja104657b. [DOI] [PubMed] [Google Scholar]
- 39.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem Int Ed Engl. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 40.Tornoe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 41.Agard NJ, Prescher JA, Bertozzi CR. J Am Chem Soc. 2004;126:15046. doi: 10.1021/ja044996f. [DOI] [PubMed] [Google Scholar]
- 42.Sanders BC, Friscourt F, Ledin PA, Mbua NE, Arumugam S, Guo J, Boltje TJ, Popik VV, Boons GJ. J Am Chem Soc. 2011;133:949. doi: 10.1021/ja1081519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saxon E, Bertozzi CR. Science. 2000;287:2007. doi: 10.1126/science.287.5460.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.