Abstract
Pulmonary hypertension contributes significantly to the morbidity and mortality associated with many pediatric pulmonary and cardiac diseases. Nitric oxide, a gas molecule, is a unique pharmaceutical agent that can be inhaled and thus delivered directly to the lung. Inhaled nitric oxide was approved by the FDA in 1999 as a therapy for infants with persistent pulmonary hypertension. Since then, the use of inhaled nitric oxide has expanded to other neonatal and pediatric conditions, and our knowledge of its properties and mechanisms of action has increased tremendously. This review will discuss the physiology of nitric oxide signaling, the most common indications for its clinical use, and promising new investigations that may enhance endogenous production of nitric oxide and/or improve vascular response to it.
Introduction
The introduction of inhaled nitric oxide for the treatment of persistent pulmonary hypertension of the newborn represents a tremendous advance in neonatal intensive care. Since its initial approval in 1999, the use of inhaled NO has expanded to other conditions, and our knowledge of its properties and mechanisms of action has increased greatly. This potent and successful therapy continues to be studied intensively to better define its role in neonatal and pediatric lung and cardiac disease. In addition, there remains intense interest in possible new applications for newborns, as well as strategies that may enhance its efficacy.
Endogenous Nitric Oxide Physiology
Nitric oxide (NO) is synthesized from the terminal nitrogen of L-arginine by the enzyme nitric oxide synthase (NOS). All three NOS isoforms are expressed in the lung, and are distinguished by the regulation of their activities, as well as by specific sites and developmental patterns of expression (1). Endothelial NOS (NOS3 or eNOS) is expressed in vascular endothelial cells, and is believed to be the predominant source of NO production in the pulmonary circulation.
NOS is a complex oxidoreductase enzyme that functions physiologically as a dimer. A particularly important requirement for normal NOS function is sufficient availability of substrate (L-arginine), as well as co-factors such as heat shock protein 90, an intracellular molecular chaperone, and tetrahydrobiopterin, a bioactive form of folic acid (2). Depletion of these cofactors or inhibition of hsp90-eNOS interactions leads to “uncoupled” NOS activity, which promotes release of the oxygen radical superoxide instead of NO. In addition to substrate and cofactors, NOS expression and activity is regulated by multiple other factors, including oxygen tension, hemodynamic forces, hormonal stimuli (eg, estrogen), paracrine factors (eg, VEGF), and reactive oxygen species.
NO is a gas molecule that diffuses freely from the endothelium to the vascular smooth muscle cell. The biologic effects of NO in vascular smooth muscle are mediated primarily through activation of soluble guanylate cyclase, which promotes conversion of GTP to cGMP (Figure 1). cGMP is a critical second messenger that relaxes vascular smooth muscle through activation of cGMP-gated ion channels and activation of cGMP-dependent protein kinase (PKG). However, recent studies indicate that alternative NO signaling pathways likely also exist through reaction of NO with protein thiols to form S-nitrosothiols (SNO), which may induce vasodilation or protein modification (3).
Figure 1.
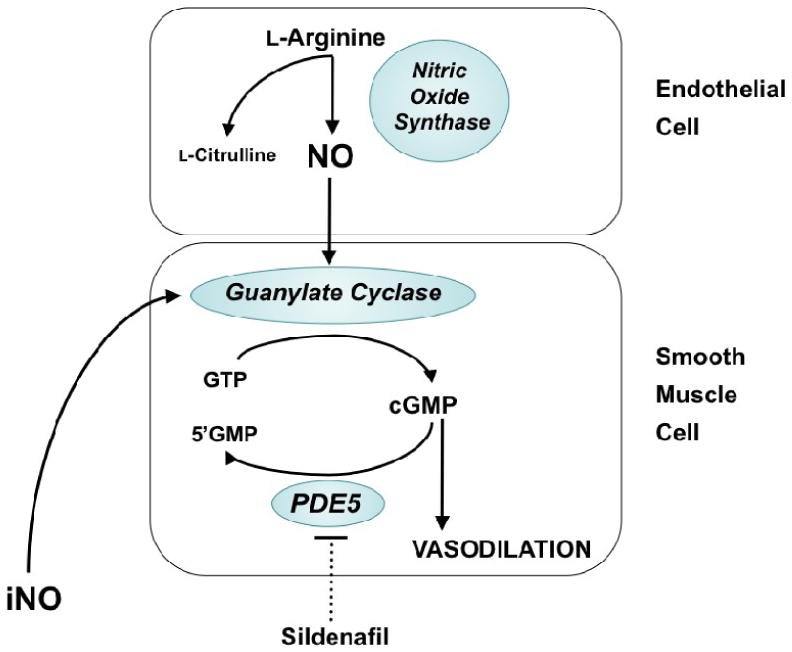
Nitric oxide (NO) signaling pathways in the regulation of pulmonary vascular tone. NO is synthesized by nitric oxide synthase (NOS) from the terminal nitrogen of L-arginine. NO from endogenous sources, or delivered as an inhaled gas, stimulates soluble guanylate cyclase (sGC) to increase intracellular cGMP, which indirectly decreases free cytosolic calcium, resulting in smooth muscle relaxation. Specific phosphodiesterases such as PDE5 hydrolyze cGMP, thus regulating the intensity and duration of its vascular effects. Inhibition of cGMP phosphodiesterases with agents such as sildenafil may enhance pulmonary vasodilation.
Phosphodiesterases serve to catalyze the breakdown of cGMP, and are therefore able to regulate the magnitude and duration of vasodilation observed in response to NO. Each family of PDEs has a distinct pattern of specificity for cAMP and/or cGMP, and each PDE isoform has specific tissue and cellular distributions (4). The prevalent PDE within the lung is the cGMP-specific PDE5, although significant amounts of PDE1, PDE3, and PDE4 are also present (5, 6). As the primary enzyme responsible for regulating cGMP, PDE5 potentially represents the most important regulator of NO-mediated vascular relaxation in the normal pulmonary vascular transition after birth.
Developmental regulation of NO physiology
Pulmonary hypertension is a normal state for the fetus that is essential to fetal survival. Because the placenta, not the lung, serves as the organ of gas exchange, most of the right ventricular output crosses the ductus arteriosus to the aorta, and only 5-10% of the combined ventricular output is directed to the pulmonary vascular bed. Pulmonary vascular constriction plays a key role in maintaining high pulmonary vascular tone during fetal life. At the same time, the fetal lung and pulmonary vasculature must prepare for the dramatic adaptation to air breathing at the time of birth.
This complex orchestration of fetal and postnatal lung development is regulated in part by important maturational changes in expression and activity of nitric oxide synthase (NOS) isoforms. Lung eNOS mRNA and protein is present in the early fetus and increases with advancing gestation and during the early postnatal period in multiple species (7, 8). This increase in lung endothelial NOS content occurs at the same time the fetal pulmonary vasculature develops the capacity to respond to endothelium-dependent vasodilator stimuli, such as oxygen and acetylcholine (9). Recent data indicate that low fetal NOS activity may be maintained during fetal life in part through increased levels of asymmetric dimethyl arginine (ADMA), an endogenously produced arginine analogue that acts as a competitive NOS inhibitor (10, 11).
The neuronal and inducible NOS isoforms have been also been identified in the fetal lung of multiple species, and appear to be primarily localized to the airway epithelium. Similar to eNOS, their expression and activity appear to be regulated in specific developmentally-driven patterns. For instance, epithelial NO production increases during the third trimester, and occurs in parallel with rises in nNOS and eNOS expression in the proximal lung (12). NO is now recognized as important to epithelial development and function during lung development, and appears to regulate critical functions such as lung liquid clearance by the respiratory epithelium (13).
At the time of birth, dramatic cardiopulmonary events facilitate the transition to gas exchange by the lung. These include a rapid fall in pulmonary vascular resistance and pulmonary artery pressure, and a 10-fold rise in pulmonary blood flow. Birth-related stimuli, such as ventilation, increasing oxygen tension, and increasing shear stress rapidly increase NOS activity and NO production. Acute or chronic inhibition of NOS in fetal lambs produces pulmonary hypertension following delivery, indicating the fundamental importance of endogenous NO-cGMP signaling in the normal pulmonary vascular transition (14, 15).
Not surprisingly, the enzymes responsible for the downstream vascular responses to NO are also developmentally regulated. Soluble guanylate cyclase, which produces cGMP in response to NO activation, is expressed and active by late gestation (16). Interestingly, the developmental patterns of PDE5 expression are not as clearly defined as for NOS and sGC. For instance, in rats, PDE5 expression and activity steadily increase through the end of gestation, peak shortly after birth, and then drop dramatically into adulthood (17). In contrast, in neonatal lambs, PDE5 activity and expression appear to acutely decrease at birth, and then rise again at 4 to 7 days of life (18).
Role of NO in Pediatric Pulmonary Disease
Persistent Pulmonary Hypertension (PPHN)
Neonatal respiratory failure affects 2% of all live births, and is responsible for over one third of all neonatal mortality. Pulmonary hypertension complicates the course of more than 10% of all neonates with respiratory failure, and the most severe PPHN has been estimated to occur in 1 of every 500 term infants (19). Even with appropriate therapy, the mortality for moderate-severe PPHN remains just under 10%, and up to 25% of surviving infants are at risk for long-term neurodevelopmental disability (20, 21).
PPHN occurs in association with a diverse group of illnesses, but generally occurs in the following conditions: 1) the abnormally constricted pulmonary vasculature due to lung parenchymal diseases associated with alveolar hypoxia, such as meconium aspiration syndrome, respiratory distress syndrome, or pneumonia; 2) the lung with normal parenchyma and remodeled pulmonary vasculature, also known as idiopathic PPHN; or 3) the hypoplastic vasculature as seen in congenital diaphragmatic hernia. While “pure” idiopathic pulmonary hypertension occurs in the minority (∼20-25%) of infants with PPHN, severe cases are almost certainly affected by both parenchymal and vascular disease. Severe, early pulmonary vascular disease is believed to be the result of antenatal events that lead to significant pulmonary vascular remodeling in utero (22).
Because of the inherent difficulties in studying the pulmonary vasculature in the fetal and neonatal period, much of our knowledge about altered NO-cGMP signaling in PPHN has been derived from animal models. Ligation or constriction of the ductus arteriosus in the fetal lamb results in rapid antenatal remodeling of the pulmonary vasculature, which has been extensively studied in the neonatal lamb. At birth, ductal ligation lambs exhibit the increased fetal pulmonary artery pressure, severe hypoxemia, and high mortality characteristic of severe human PPHN. Decreased expression of eNOS mRNA and protein, as well as diminished eNOS activity have been documented in pulmonary vascular tissue from this model (23, 24). In addition, expression and activity of vascular soluble guanylate cyclase is diminished (25), and activity of PDE5 is increased (26-29), both of which promote decreased cGMP concentrations (30). Others have used small and large animal models of chronic alveolar hypoxia after birth to induce pulmonary vascular dysfunction and remodeling. For instance, piglets exposed to 10 days of hypoxia exhibit impaired nitric oxide production due to eNOS dysfunction (31).
Clinical treatment of PPHN with inhaled nitric oxide (iNO) represents one of the most significant advances in neonatal intensive care. Inhaled NO is well suited for this task because it is a rapid and potent vasodilator that can be delivered as inhalation therapy to airspaces approximating the pulmonary vascular bed. NO that reaches the blood stream binds avidly to hemoglobin, which further localizes its effect to the lung vasculature. Inhaled NO is believed to also have a ‘micro-selective effect’, and may improve ventilation/perfusion matching by redirecting blood from poorly aerated, diseased lung regions to better-aerated distal air spaces. In contrast, intravenous dilators such as prostacyclin, tolazoline, and sodium nitroprusside often produce non-selective effects, leading to systemic hypotension, worsening ventilation/perfusion matching, and impaired oxygenation. There are relatively few contraindications to iNO, which mainly consist of congenital heart disease with left ventricular outflow tract obstruction, such as interrupted aortic arch, critical aortic stenosis, hypoplastic left heart syndrome, or severe left ventricular dysfunction. These children are likely to have pulmonary venous hypertension due to high left atrial pressures, and without correction of the underlying abnormality, use of iNO may aggravate pulmonary edema.
While large well-designed studies paved the way to FDA approval of iNO (summarized in Table 1), it is equally important to note that iNO did not reduce the mortality, length of hospitalization, or the risk of significant neurodevelopmental impairment associated with PPHN. These observations indicate that the clinical problem of PPHN and respiratory failure is much more complex than a simple deficiency in NO production. Further, one of these multicenter trials studied the potential benefit of beginning iNO at a milder or earlier point in the disease course (for an oxygenation index of 15-25). Early use of iNO did not decrease the incidence of ECMO and/or death, improve other patient outcomes, or reduce the incidence of severe neurodevelopmental impairment (32, 33).
Table 1.
Summary of the large, multicenter randomized trials of inhaled NO in term newborns with hypoxemic respiratory failure and/or PPHN, showing the effect of iNO on ECMO utilization and mortality. Table adapted from (85), * denotes significant reduction (p < 0.05).
Respiratory failure of prematurity
Considerable evidence from preclinical models indicates that impaired production of endogenous NO contributes to the pathogenesis of bronchopulmonary dysplasia (BPD), a common and serious complication of infants born with extreme prematurity. Multiple studies have demonstrated that lung eNOS expression is decreased in premature animal models of BPD (34, 35). Recent work also indicated that intervention with 14 days of NO inhalation in preterm baboons improved lung compliance and lung volume, as well as lung growth and architecture (36).
These findings have led to a substantial effort to investigate the clinical role of iNO in preterm infants. Early case reports of NO inhalation in premature newborns with severe respiratory failure demonstrated improvement in pulmonary hypertension, accompanied by marked improvement in oxygenation. However, these early reports also raised speculation about potential adverse effects such as intracranial hemorrhage, based in part on laboratory and clinical studies in adults suggesting that high doses of iNO (30-40 ppm) prolong bleeding times.
Five large randomized, masked clinical trials focusing on iNO administration in preterm infants have now presented their short-term outcomes. It is important to note that these studies focused primarily on preterm infants requiring mechanical ventilation, and further study will be needed to address the potential role for iNO in preterm infants managed with noninvasive modalities such as CPAP or nasal cannulas. However, the data thus far represent data from more than 2800 enrolled infants, and include a wide range of patient populations, disease severity, and treatment strategies. Three of the trials used an early, protective strategy, randomizing infants to receive placebo gas or iNO for 7-21 days (37-39). The smallest, single center trial showed that iNO significantly improved survival without BPD (37), but this finding was not confirmed in the two larger, multicenter trials. All three trials will continue to report neurodevelopmental outcomes through at least age 5.
The trial conducted by Van Meurs et al (40) also focused on an early enrollment strategy, but only included infants with very severe respiratory failure (mean oxygenation index of 22-23). The mean duration of exposure for the iNO group was 76 hours, the shortest of all the clinical trials. In this much sicker population of neonates, iNO did not reduce death or BPD. Further, post hoc analysis of the 316 infants with birth weights of <750 grams suggested the worrisome finding that iNO was associated with an increased risk of death and brain injury (Grade 3 or 4 IVH or PVL). Interpretation of these data has been somewhat difficult as this study did not require a screening head ultrasound, enrolled only infants with very severe respiratory failure, and provided the shortest duration of iNO treatment. However, these findings suggest it is prudent to avoid iNO use in infants < 750 gm with severe hypoxemic respiratory failure due to parenchymal lung disease until more is known.
Ballard et al (41) studied the effects of iNO in older preterm infants still requiring mechanical ventilation, thus targeting a population at particularly high risk for BPD. In this trial, iNO was begun later (7-21 days), given at a higher dose (20 ppm, followed by stepwise weaning), and delivered for a longer time period (24 days). iNO administration resulted in a 23% improvement in survival without BPD at 36 weeks corrected gestational age. Post hoc analysis indicated that age at study entry could be an important factor, in that inhaled NO started between 7 and 14 days resulted in an 77% increase in survival without BPD, while there was no benefit if iNO was started after 15 days. A follow-up study through one year of age showed that infants randomized to iNO were significantly less likely to require home oxygen, or use bronchodilators, steroids, or diuretics after NICU discharge (42). These findings raise important questions about the timing and potential reversibility of lung injury, as well as whether higher doses or longer exposures to iNO are needed to support lung development.
Despite the large number of babies enrolled in these studies, clear recommendations for the use of iNO in specific populations of preterm infants have not yet emerged. Individual patient data meta-analysis will soon be available to help determine whether certain patient or treatment characteristics predict benefit from iNO in premature infants (43).
Pediatric primary and secondary pulmonary hypertension
Outside of the NICU setting, the most common clinical use of iNO is for the infant or child with congenital heart disease with associated pulmonary hypertension. Postoperatively, iNO is frequently used to prevent or treat pulmonary hypertensive crises, improve oxygenation, and increase cardiac output. Pulmonary hypertension commonly develops in infants with congenital heart lesions associated with pulmonary overcirculation, such as truncus arteriosus or atrioventricular canal. If the heart lesion is not corrected, medial and intimal thickening occur in the pulmonary vasculature over time, which eventually leads to luminal obliteration. Acute life-threatening exacerbation of pulmonary hypertension following cardiopulmonary bypass is a serious clinical problem even in very young infants. In addition, children undergoing cavopulmonary connections for single ventricle lesions require low pulmonary vascular resistance for surgical success. A number of small single-center studies indicate that iNO can attenuate pulmonary hypertension in at-risk postoperative patients, reduce the number of pulmonary hypertension crises, and shorten time on mechanical ventilation (44). In one larger randomized trial of pediatric patients, iNO (20 ppm) significantly decreased PVR and pulmonary hypertensive crises, and shortened the time to extubation readiness (45).
Animal models that closely mimic the conditions associated with congenital heart disease have been developed, which involve placing large aorto-pulmonary shunts in the late gestation fetal lamb (46). Shunted lambs are initially asymptomatic, and gradually develop clinical findings of pulmonary overcirculation and heart failure over a period of 6-8 weeks that are similar to those observed in human infants. The shunt lamb model allows for examination of vascular dysfunction over time, and allows for the testing of clinical strategies that prevent and reverse vascular remodeling. Substantial data indicates that abnormalities of NOS expression and Hsp90 interactions result in NOS dysfunction and decreased NO production in the pulmonary vasculature (47). More recently, mitochondrial dysfunction and increased oxidant stress have been linked to the development of pulmonary hypertension in this model (48), and these findings will likely lead to novel approaches such as L-carnitine supplementation to maintain normal eNOS-HSP90 interactions, NO signaling, and endothelial function.
Inhaled NO has also emerged as an important tool for testing acute pulmonary vascular reactivity during cardiac catheterization. Inhaled NO has several advantages over other pulmonary vasodilators in this setting, including rapid onset of action, and few potential adverse effects such as systemic hypotension. For instance, Balzer et al reported that preoperative evaluation with inhaled nitric oxide in combination with oxygen allowed for identification of a greater number of appropriate candidates for corrective cardiac surgery or transplantation (49). Others have shown that response to iNO during evaluation for pulmonary hypertension provides a safe, effective tool for predicting response to other agents such as calcium channel blockers and sildenafil.
While iNO would theoretically benefit children with chronic primary or secondary pulmonary hypertension, its application in this setting has been limited by the lack of practical home-based continuous delivery devices, and the risk for rebound pulmonary hypertension if it is inadvertently discontinued.
Pediatric respiratory failure
In initial clinical studies of adult and pediatric patients with severe acute respiratory distress syndrome (ARDS), inhaled NO produced selective pulmonary vasodilation and improved systemic oxygenation (50). However, subsequent randomized controlled trials have not shown a beneficial effect on mortality or duration of mechanical ventilation in pediatric or adult patients (51, 52). While a transient improvement in oxygenation was observed in most of these studies, it has been speculated that since most patients dying from acute respiratory distress syndrome suffer from multiple organ systems failure, lung-selective therapy such as inhaled NO may not improve the overall survival rate (53).
These are disappointing results, and likely indicate that the problem of pediatric respiratory failure involves much more than vascular dysfunction, and that there is still much to be learned about the role of NO in primary lung injury, as well as ventilator-induced lung injury (VILI) (54). In a large randomized controlled trial of lower- vs. higher-tidal volume ventilation, urinary nitrate levels were preserved to a higher degree in patients treated with a protective low tidal volume ventilator strategy when compared with patients treated with a higher tidal volumes (55). This suggests that preserved NO production may be a biomarker for better outcomes in acute lung injury, and that protective ventilator strategies could work in part through preserving NO production. However, the problem is clearly complex, and even relatively straightforward animal model studies show conflicting results. For instance, NOS3 deficient mice have been shown to be either protected from lung injury (56), or conversely more susceptible to VILI (57). The reasons for these conflicting findings are not clear, although some have suggested that the protective effects of NOS3 deficiency might be due to reduced production of superoxide through uncoupled function of the enzyme, as described below (54, 57).
Future Directions
As our understanding of nitric oxide signaling evolves, strategies that will enhance responses to endogenous and exogenous NO are steadily emerging. Several of these promising therapeutic approaches have already reached the point of clinical testing.
Enhancement of endogenous NOS activity
Tetrahydrobiopterin (BH4) is an important cofactor for NOS, and is essential for its dimerization and synthesis of NO. Without BH4, NOS becomes ‘uncoupled’ and contributes to oxidant stress through synthesis of superoxide anion. The rate-determining step for BH4 is its synthesis by GTP-cyclohydrolase (GTP-CH1). For instance, mice with impaired GTP-CH1 activity exhibit reduced lung BH4 levels and spontaneous development of vascular remodeling and pulmonary hypertension. Conversely, congenital overexpression of GTP-CH1 in vascular endothelium protects mice from hypoxic PH (58). In a piglets with pulmonary hypertension induced by chronic hypoxia, combined therapy with BH4 and a superoxide dismutase mimetic restores endothelial function (59). Additional recent evidence indicates that GTP-CH1 may be a redox sensitive enzyme, and that antioxidant treatment may restore NOS activity in part by restoring GTP-CH1 expression and BH4 synthesis (60). Others have proposed that oxidative stress may reduce BH4 activity by conversion to dihydrobiopterin (61). Sapropterin hydrochloride, a synthetic form of BH4, was recently approved for treatment of patients with phenylketonuria. This observation could lead to clinical testing in children with pulmonary hypertension.
Sufficient availability of the substrate l-arginine is also important for eNOS activity, and recent data from human infants suggest that sufficient synthesis of l-arginine is necessary for optimal NOS function during the neonatal period (62). Exogenous l-arginine supplementation enhances NOS activity in vitro, but has been less successful when attempted in vivo. However, l-arginine can be endogenously synthesized from l-citrulline by a recycling pathway consisting of two enzymes, argininosuccinate synthase (AS) and argininosuccinate lyase (AL). Recent studies indicate that providing exogenous l-citrulline may reverse NOS dysfunction in animal models of neonatal pulmonary hypertension (63). l-citrulline has also been used in several patient populations with some success. For example, in children undergoing cardiopulmonary bypass at risk for development of postoperative pulmonary hypertension, oral supplementation with l-citrulline increased both plasma citrulline and arginine levels, and plasma citrulline levels greater than 37 μM/L appeared to protect against pulmonary hypertension (64). Intravenous l-citrulline has been shown to be safe and well tolerated in children undergoing cardiopulmonary bypass (65), and clinical trials are underway.
Modulation of oxidant stress
There is mounting evidence that oxidant stress plays an important role in the pathogenesis of PPHN, and reactive oxygen species (ROS) such as superoxide and hydrogen peroxide likely diminish the effects of NO through direct and indirect mechanisms. For instance, superoxide and hydrogen peroxide production is increased in the smooth muscle and adventitia of pulmonary arteries from lambs with chronic intrauterine pulmonary hypertension (66-68), as well as in young piglets exposed to chronic hypoxia (69). Possible sources for elevated concentrations of reactive oxygen species include increased expression and activity of NADPH oxidases, ‘uncoupled’ eNOS activity as described above, as well as reduced superoxide dismutase (SOD) activity (66, 68). Once present in the lung, elevated concentrations of ROS can promote vascular smooth muscle cell proliferation in PPHN, and alter abnormal vascular reactivity through mechanisms that blunt cGMP accumulation (Figure 2).
Figure 2.
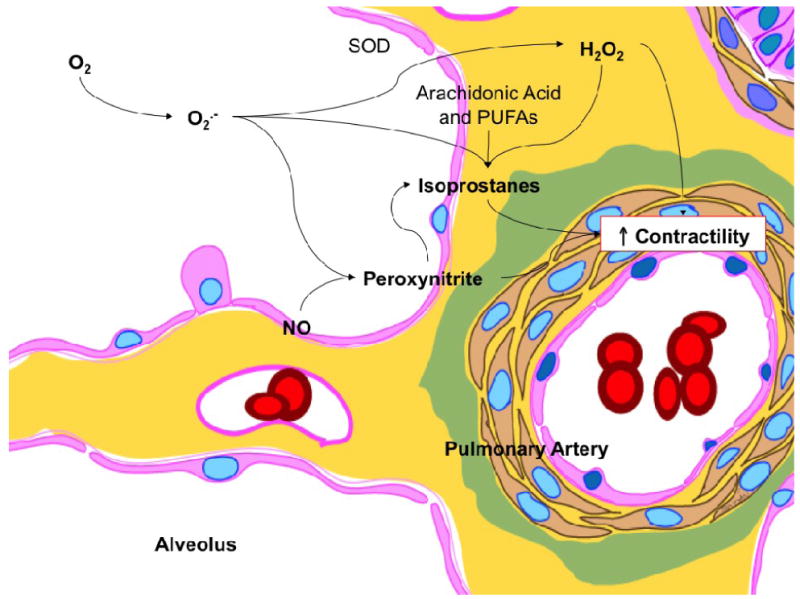
Proposed role for reactive oxygen species and their metabolites in mediating increased pulmonary arterial contractility following exposure to 100% oxygen. Exposure to high oxygen concentrations results in formation of superoxide anions (O2·-). Superoxide anions combine with nitric oxide (NO) to form peroxynitrite, a potent pulmonary vasoconstrictor. Superoxide dismutase (SOD) enzyme converts superoxide anions to hydrogen peroxide (H2O2), also a pulmonary vasoconstrictor. When membrane lipids (arachidonic acid and poly unsaturated fatty acids, PUFA) are exposed to reactive oxygen species such as superoxide anions and hydrogen peroxide or peroxynitrite, a variety of isoprostanes are formed. Isoprostanes are known constrictors of pulmonary arteries. From (90), with permission.
Inhaled NO is usually delivered with high concentrations of oxygen. While hyperoxic ventilation continues to be a mainstay in the treatment of PPHN, we know surprisingly little about what oxygen concentrations will maximize benefits and minimize risk. Recent evidence suggests that increasing oxygen concentration beyond greater than 50-60% will not lead to greater pulmonary vasodilation in the normal or remodeled pulmonary circulation (70, 71). The extreme hyperoxia routinely used in PPHN management may in fact be toxic to the developing lung by the formation of ROS (60, 68, 72). Superoxide may react with arachadonic acid to increase concentrations of isoprostanes, and may also combine with NO to form peroxynitrite (73). Both are potent oxidants with the potential to produce vasoconstriction, cytotoxicity, and damage to surfactant proteins and lipids, and peroxynitrite can directly induce NOS uncoupling. New data indicate that even brief (30 minute) periods of exposure to 100% O2 are sufficient to increase reactivity of pulmonary vessels in normal lambs (70, 74), diminish the response of the pulmonary vasculature to endogenous and exogenous nitric oxide (70), and increase activity of cGMP-specific phosphodiesterases (72).
If reactive oxygen species promote vasoconstriction, scavengers of reactive oxygen species such as superoxide dismutase (SOD) and/or catalase may augment responsiveness to iNO and promote pulmonary vasodilation. In lambs with pulmonary hypertension, a single dose of recombinant human SOD (rhSOD) given by intratracheal delivery was found to dilate the pulmonary circulation and enhance responsiveness to inhaled NO (75), as well as improve oxygenation over a 24-hour period to a degree that was similar to iNO (73). Further, rhSOD treatment appeared to block formation of oxidants such as peroxynitrite and isoprostanes, and restore activity of endogenous nitric oxide synthase (60). Thus, use of antioxidants may have multiple beneficial effects: scavenging superoxide may increase the availability of both endogenous and inhaled NO, and may also reduce oxidative stress and limit lung injury (76).
Modulation of cGMP signaling
Alterations in downstream signaling mechanisms in pulmonary vascular smooth muscle cells may also lead to inadequate vascular responses to NO. Nitric oxide stimulates soluble guanylate cyclase to increase cGMP, which is the central and critical second messenger that regulates contractility of the smooth muscle cell by modulating the activity of cGMP-dependent kinases, phosphodiesterases, and ion channels. Therefore, multiple critical points in the pathway downstream from NO production serve as attractive targets for manipulating cellular cGMP concentrations. For example, expression and activity of soluble guanylate cyclase are decreased in the abnormally remodeled pulmonary vessels of lambs with chronic intrauterine hypertension, which could potentially diminish responses to both endogenous and exogenous NO (77). This finding would indicate that new compounds that directly stimulate sGC at a NO-independent but heme-dependent site may be helpful, a hypothesis that appears to be promising in pre-clinical testing (78).
Recent evidence indicates that the low cGMP concentrations associated with PPHN may also be associated with increased activity of cGMP-specific phosphodiesterases (PDE5). Inhibition of PDE5 activity would be expected to increase cGMP concentrations, dilate the pulmonary vasculature, and/or increase the efficacy of iNO. In lambs with experimental pulmonary hypertension, both enteric and aerosolized sildenafil have been shown to dilate the pulmonary vasculature and augment the pulmonary vascular response to iNO (79, 80). Intravenous sildenafil was found to be a selective pulmonary vasodilator with efficacy equivalent to iNO in a piglet model of meconium aspiration. When combined with iNO, sildenafil enhanced the reduction in pulmonary vascular resistance, but also worsened systemic hypotension and oxygenation (81, 82), raising concerns about its selectivity for the pulmonary circulation Data are now emerging on the use of sildenafil in clinical populations of critically ill infants and children. A recent report demonstrated that enteric sildenafil improved oxygenation and survival in human infants with PPHN compared to placebo (83). Findings from a pilot study of intravenous sildenafil in newborns with pulmonary hypertension indicate that it was generally well tolerated, with improvements in oxygenation noted in the cohorts that received higher infusion doses (84). This study also noted that seven infants received sildenafil prior to initiation of iNO, and showed similar improvements in oxygenation (Figure 3). Of these, six survived to discharge without need for additional therapy with iNO or ECMO, which suggests that inhibition of PDE5 activity may independently decrease pulmonary vascular resistance and improve oxygenation in human infants with PPHN.
Figure 3.
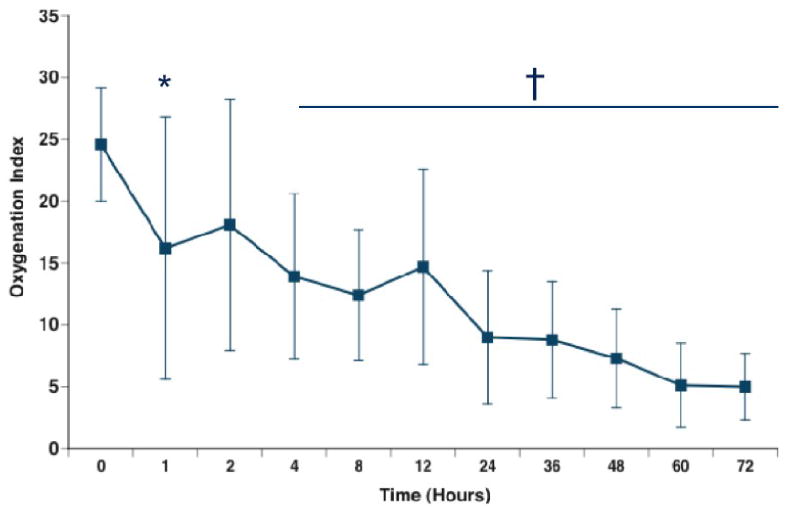
Response to sildenafil infusion without iNO. Seven infants were enrolled before the need for iNO. OI was improved by 1 hour (24.6±4.6 to 16.1±9.9; *P=0.0502), with significant and sustained improvement by 4 hours after the initiation of sildenafil (14.7±6.4, p=0.0088). From (91), with permission.
Summary
Inhaled NO is now well established as a highly effective pulmonary vasodilator for newborn infants with PPHN or hypoxemic respiratory failure. Because of this tremendous success, the use of iNO has rapidly expanded into other diseases that affect infants and children. Similarly, our understanding of the role and mechanism of action of NO in pediatric and cardiac disease continues to steadily increase. Current and future research in areas such as enhancement of eNOS activity, inhibition of cGMP phosphodiesterases and reduction of oxidant stress will undoubtedly produce new strategies that will amplify the vascular effects of both exogenous and endogenous NO.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shaul PW. Regulation of endothelial nitric oxide synthase: location, location, location. Annu Rev Physiol. 2002;64:749–74. doi: 10.1146/annurev.physiol.64.081501.155952. [DOI] [PubMed] [Google Scholar]
- 2.Landmesser U, Dikalov S, Price SR, McCann L, Fukai T, Holland SM, et al. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J Clin Invest. 2003;111(8):1201–9. doi: 10.1172/JCI14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gaston BM, Carver J, Doctor A, Palmer LA. S-nitrosylation signaling in cell biology. Mol Interv. 2003;3(5):253–63. doi: 10.1124/mi.3.5.253. [DOI] [PubMed] [Google Scholar]
- 4.Soderling SH, Beavo JA. Regulation of cAMP and cGMP signaling: new phosphodiesterases and new functions. Curr Opin Cell Biol. 2000;12(2):174–9. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 5.Maclean MR, Johnston ED, McCulloch KM, Pooley L, Houslay MD, Sweeney G. Phosphodiesterase isoforms in the pulmonary arterial circulation of the rat: changes in pulmonary hypertension. J Pharmacol Exp Ther. 1997;283(2):619–24. [PubMed] [Google Scholar]
- 6.Pauvert O, Salvail D, Rousseau E, Lugnier C, Marthan R, Savineau JP. Characterisation of cyclic nucleotide phosphodiesterase isoforms in the media layer of the main pulmonary artery. Biochem Pharmacol. 2002;63(9):1763–72. doi: 10.1016/s0006-2952(02)00919-x. [DOI] [PubMed] [Google Scholar]
- 7.North AJ, Star RA, Brannon TS, Ujiie K, Wells LB, Lowenstein CJ, et al. Nitric oxide synthase type I and type III gene expression are developmentally regulated in rat lung. Am J Physiol Lung Cell Mol Physiol. 1994;266:L635–L641. doi: 10.1152/ajplung.1994.266.6.L635. [DOI] [PubMed] [Google Scholar]
- 8.Parker TA, Le Cras TD, Kinsella JP, Abman SH. Developmental changes in endothelial nitric oxide synthase expression and activity in ovine fetal lung. Am J Physiol Lung Cell Mol Physiol. 2000;278:L202–L208. doi: 10.1152/ajplung.2000.278.1.L202. [DOI] [PubMed] [Google Scholar]
- 9.Tiktinsky MH, Morin FC., III Increasing oxygen tension dilates fetal pulmonary circulation via endothelium-derived relaxing factor. Am J Physiol Heart Circ Physiol. 1993;265:H376–H380. doi: 10.1152/ajpheart.1993.265.1.H376. [DOI] [PubMed] [Google Scholar]
- 10.Pierce CM, Krywawych S, Petros AJ. Asymmetric dimethyl arginine and symmetric dimethyl arginine levels in infants with persistent pulmonary hypertension of the newborn. Pediatr Crit Care Med. 2004;5(6):517–20. doi: 10.1097/01.PCC.0000144715.03515.55. [DOI] [PubMed] [Google Scholar]
- 11.Arrigoni FI, Vallance P, Haworth SG, Leiper JM. Metabolism of asymmetric dimethylarginines is regulated in the lung developmentally and with pulmonary hypertension induced by hypobaric hypoxia. Circulation. 2003;107(8):1195–201. doi: 10.1161/01.cir.0000051466.00227.13. [DOI] [PubMed] [Google Scholar]
- 12.Shaul PW, Afshar S, Gibson LL, Sherman TS, Kerecman JD, Grubb PH, et al. Developmental changes in nitric oxide synthase isoform expression and nitric oxide production in fetal baboon lung. Am J Physiol Lung Cell Mol Physiol. 2002;283(6):L1192–9. doi: 10.1152/ajplung.00112.2002. Epub 2002 Jul 07. [DOI] [PubMed] [Google Scholar]
- 13.Cummings JJ, Wang H. Nitric oxide decreases lung liquid production via guanosine 3′,5′-cyclic monophosphate. Am J Physiol Lung Cell Mol Physiol. 2001;280:L923–L929. doi: 10.1152/ajplung.2001.280.5.L923. [DOI] [PubMed] [Google Scholar]
- 14.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990;259:H1921–H1927. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- 15.Fineman JR, Wong J, Morin FC, III, Wild LM, Soifer SJ. Chronic nitric oxide inhibition in utero produces persistent pulmonary hypertension in newborn lambs. J Clin Invest. 1994;93:2675–2683. doi: 10.1172/JCI117281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bloch KD, Filippov G, Sanchez LS, Nakane M, delaMonte SM. Pulmonary soluble guanylate cyclase, a nitric oxide receptor, is increased during the perinatal period. Am J Physiol Lung Cell Mol Physiol. 1997;272:L400–L406. doi: 10.1152/ajplung.1997.272.3.L400. Lung Cell Mol Physiol. [DOI] [PubMed] [Google Scholar]
- 17.Sanchez LS, Del La Monte SM, Filippov G, Jones RC, Zapol WM, Bloch KD. Cyclic-GMP-binding, cyclic-GMP-specific phosphodiesterase gene expression is regulated during rat pulmonary development. Pediatr Res. 1998;43:163–168. doi: 10.1203/00006450-199802000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Hanson KA, Burns F, Rybalkin SD, Miller JW, Beavo J, Clarke WR. Developmental changes in lung cGMP phosphodiesterase-5 activity, protein, and message. Am J Respir Crit Care Med. 1998;158:279–288. doi: 10.1164/ajrccm.158.1.9711042. [DOI] [PubMed] [Google Scholar]
- 19.Walsh-Sukys MC, Tyson JE, Wright LL, Bauer CR, Korones SB, Stevenson DK, et al. Persistent pulmonary hypertension of the newborn in the era before nitric oxide: practice variation and outcomes. Pediatr. 2000;105:14–20. doi: 10.1542/peds.105.1.14. [DOI] [PubMed] [Google Scholar]
- 20.Steinhorn RH. Neonatal pulmonary hypertension. Pediatr Crit Care Med. 2010;11(2 Suppl):S79–84. doi: 10.1097/PCC.0b013e3181c76cdc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steinhorn RH. Nitric oxide and beyond: new insights and therapies for pulmonary hypertension. J Perinatol. 2008;28 3:S67–71. doi: 10.1038/jp.2008.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Geggel R, Reid LM. The structural basis for PPHN. Clin Perinatol. 1984;11:525–549. [PubMed] [Google Scholar]
- 23.Villanueva ME, Zaher FM, Svinarich DM, Konduri GG. Decreased gene expression of endothelial nitric oxide synthase in newborns with persistent pulmonary hypertension. Pediatr Res. 1998;44:338–343. doi: 10.1203/00006450-199809000-00012. [DOI] [PubMed] [Google Scholar]
- 24.Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC., 3rd Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 1997;272:L1005–L1012. doi: 10.1152/ajplung.1997.272.5.L1005. [DOI] [PubMed] [Google Scholar]
- 25.Steinhorn RH, Russell JA, Morin FC., 3rd Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 1995;268:H1483–H1489. doi: 10.1152/ajpheart.1995.268.4.H1483. [DOI] [PubMed] [Google Scholar]
- 26.Hanson KA, Ziegler JW, Rybalkin SD, Miller JW, Abman SH, Clarke WR. Chronic pulmonary hypertension increases fetal lung cGMP phosphodiesterase activity. Am J Physiol. 1998;275(5 Pt 1):L931–41. doi: 10.1152/ajplung.1998.275.5.L931. [DOI] [PubMed] [Google Scholar]
- 27.Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino SF, Davis JM, et al. Superoxide dismutase and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010 doi: 10.1152/ajplung.00309.2009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farrow KN, Lakshminrusimha S, Czech L, Groh BS, Gugino SF, Davis JM, et al. Superoxide dismutase and inhaled nitric oxide normalize phosphodiesterase 5 expression and activity in neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2010;299(1):L109–16. doi: 10.1152/ajplung.00309.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farrow KN, Wedgwood S, Lee KJ, Czech L, Gugino SF, Lakshminrusimha S, et al. Mitochondrial oxidant stress increases PDE5 activity in persistent pulmonary hypertension of the newborn. Respir Physiol Neurobiol. 2010 doi: 10.1016/j.resp.2010.08.018. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tzao C, Nickerson PA, Russell JA, Gugino SF, Steinhorn RH. Pulmonary hypertension alters soluble guanylate cyclase activity and expression in pulmonary arteries isolated from fetal lambs. Pediatr Pulmonol. 2001;31(2):97–105. doi: 10.1002/1099-0496(200102)31:2<97::aid-ppul1016>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 31.Fike CD, Aschner JL, Zhang J, Kaplowitz M. Impaired NO signaling in small pulmonary arteries of chronically hypoxic newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2004;286:L1244–L1254. doi: 10.1152/ajplung.00345.2003. [DOI] [PubMed] [Google Scholar]
- 32.Konduri GG, Solimani A, Sokol GM, Singer J, Ehrenkranz RA, Singhal N, et al. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics. 2004;113:559–564. doi: 10.1542/peds.113.3.559. [DOI] [PubMed] [Google Scholar]
- 33.Konduri GG, Vohr B, Robertson C, Sokol GM, Solimano A, Singer J, et al. Early inhaled nitric oxide therapy for term and near-term newborn infants with hypoxic respiratory failure: neurodevelopmental follow-up. J Pediatr. 2007;150(3):235–40. 240.e1. doi: 10.1016/j.jpeds.2006.11.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacRitchie AN, Albertine KH, Sun J, Lei PS, Jensen SC, Freestone AA, et al. Reduced endothelial nitric oxide synthase in lungs of chronically ventilated preterm lambs. Am J Physiol Lung Cell Mol Physiol. 2001;281(4):L1011–20. doi: 10.1152/ajplung.2001.281.4.L1011. [DOI] [PubMed] [Google Scholar]
- 35.Afshar S, Gibson LL, Yuhanna IS, Sherman TS, Kerecman JD, Grubb PH, et al. Pulmonary NO synthase expression is attenuated in a fetal baboon model of chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2003;284(5):L749–58. doi: 10.1152/ajplung.00334.2002. [DOI] [PubMed] [Google Scholar]
- 36.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2005;288(3):L450–9. doi: 10.1152/ajplung.00347.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349(22):2099–107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 38.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, et al. Early Inhaled Nitric Oxide Therapy in Premature Newborns with Respiratory Failure. N Engl J Med. 2006;355(4):354–364. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 39.Mercier J, Hummler H, Durrmeyer H, Sanchez-Luna M, Carnielli V, Field D, et al. Inhaled nitric oxide (iNO) for the prevention of bronchopulmonary dysplasia in preterm babies (EUNO): a randomised controlled trial. Lancet. 2010;376:346–354. doi: 10.1016/S0140-6736(10)60664-2. [DOI] [PubMed] [Google Scholar]
- 40.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med. 2005;353(1):13–22. doi: 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 41.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled Nitric Oxide in Preterm Infants Undergoing Mechanical Ventilation. N Engl J Med. 2006;355(4):343–353. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 42.Hibbs AM, Walsh MC, Martin RJ, Truog WE, Lorch SA, Alessandrini E, et al. One-year respiratory outcomes of preterm infants enrolled in the Nitric Oxide (to prevent) Chronic Lung Disease trial. J Pediatr. 2008;153(4):525–9. doi: 10.1016/j.jpeds.2008.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Askie LM, Ballard RA, Cutter G, Dani C, Elbourne D, Field D, et al. Inhaled Nitric Oxide in preterm infants: a systematic review and individual patient data meta-analysis. BMC Pediatr. 2010;10:15. doi: 10.1186/1471-2431-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barr FE, Macrae D. Inhaled nitric oxide and related therapies. Pediatr Crit Care Med. 2010;11(2 Suppl):S30–6. doi: 10.1097/PCC.0b013e3181c76b42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller OI, Tang SF, Keech A, Pigott NB, Beller E, Celermajer DS. Inhaled nitric oxide and prevention of pulmonary hypertension after congenital heart surgery: a randomised double-blind study. Lancet. 2000;356(9240):1464–9. doi: 10.1016/S0140-6736(00)02869-5. [DOI] [PubMed] [Google Scholar]
- 46.Reddy VM, Meyrick B, Wong J, Khoor A, Liddicoat JR, Hanley FL, et al. In utero placement of aortopulmonary shunts. Circulation. 1995;92:606–613. doi: 10.1161/01.cir.92.3.606. [DOI] [PubMed] [Google Scholar]
- 47.Steinhorn RH, Russell JA, Lakshminrusimha S, Gugino SF, Black SM, Fineman JR. Altered endothelium-dependent relaxations in lambs with high pulmonary blood flow and pulmonary hypertension. Am J Physiol Heart Circ Physiol. 2001;280(1):H311–7. doi: 10.1152/ajpheart.2001.280.1.H311. [DOI] [PubMed] [Google Scholar]
- 48.Sharma S, Sud N, Wiseman DA, Carter AL, Kumar S, Hou Y, et al. Altered carnitine homeostasis is associated with decreased mitochondrial function and altered nitric oxide signaling in lambs with pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;294(1):L46–56. doi: 10.1152/ajplung.00247.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Balzer DT, Kort HW, Day RW, Corneli HM, Kovalchin JP, Cannon BC, et al. Inhaled Nitric Oxide as a Preoperative Test (INOP Test I): the INOP Test Study Group. Circulation. 2002;106(12 Suppl 1):I76–81. [PubMed] [Google Scholar]
- 50.Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 51.Dobyns EL, Cornfield DN, Anas NG, Fortenberry JD, Tasker RC, Lynch A, et al. Multicenter randomized controlled trial of the effects of inhaled nitric oxide therapy on gas exchange in children with acute hypoxemic respiratory failure. J Pediatr. 1999;134(4):406–12. doi: 10.1016/s0022-3476(99)70196-4. [DOI] [PubMed] [Google Scholar]
- 52.Sokol J, Jacobs SE, Bohn D. Inhaled nitric oxide for acute hypoxemic respiratory failure in children and adults. Cochrane Database Syst Rev. 2003;(1):CD002787. doi: 10.1002/14651858.CD002787. [DOI] [PubMed] [Google Scholar]
- 53.Coggins MP, Bloch KD. Nitric oxide in the pulmonary vasculature. Arterioscler Thromb Vasc Biol. 2007;27(9):1877–85. doi: 10.1161/ATVBAHA.107.142943. [DOI] [PubMed] [Google Scholar]
- 54.Ware LB, Summar M. Understanding the role of NOS-3 in ventilator-induced lung injury: don't take NO for an answer. Am J Physiol Lung Cell Mol Physiol. 2010;299(2):L147–9. doi: 10.1152/ajplung.00179.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McClintock DE, Ware LB, Eisner MD, Wickersham N, Thompson BT, Matthay MA. Higher urine nitric oxide is associated with improved outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2007;175(3):256–62. doi: 10.1164/rccm.200607-947OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Peng XQ, Damarla M, Skirball J, Nonas S, Wang XY, Han EJ, et al. Protective role of PI3-kinase/Akt/eNOS signaling in mechanical stress through inhibition of p38 mitogen-activated protein kinase in mouse lung. Acta Pharmacol Sin. 2010;31(2):175–83. doi: 10.1038/aps.2009.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vaporidi K, Francis RC, Bloch KD, Zapol WM. Nitric oxide synthase 3 contributes to ventilator-induced lung injury. Am J Physiol Lung Cell Mol Physiol. 2010;299(2):L150–9. doi: 10.1152/ajplung.00341.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Khoo JP, Zhao L, Alp NJ, Bendall JK, Nicoli T, Rockett K, et al. Pivotal role for endothelial tetrahydrobiopterin in pulmonary hypertension. Circulation. 2005;111(16):2126–33. doi: 10.1161/01.CIR.0000162470.26840.89. [DOI] [PubMed] [Google Scholar]
- 59.Nandi M, Leiper J, Arrigoni F, Hislop A, Vallance P, Haworth S. Developmental regulation of GTP-CH1 in the porcine lung and its relationship to pulmonary vascular relaxation. 2006;59:767–772. doi: 10.1203/01.pdr.0000219301.19958.a0. [DOI] [PubMed] [Google Scholar]
- 60.Farrow KN, Lakshminrusimha S, Reda WJ, Wedgwood S, Czech L, Gugino SF, et al. Superoxide dismutase restores eNOS expression and function in resistance pulmonary arteries from neonatal lambs with persistent pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295(6):L979–87. doi: 10.1152/ajplung.90238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mata-Greenwood E, Jenkins C, Farrow KN, Konduri GG, Russell JA, Lakshminrusimha S, et al. eNOS function is developmentally regulated: uncoupling of eNOS occurs postnatally. Am J Physiol Lung Cell Mol Physiol. 2006;290(2):L232–41. doi: 10.1152/ajplung.00393.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pearson DL, Dawling S, Walsh WF, Haines JL, Christman BW, Bazyk A, et al. Neonatal pulmonary hypertension--urea-cycle intermediates, nitric oxide production, and carbamoyl-phosphate synthetase function. N Engl J Med. 2001;344(24):1832–8. doi: 10.1056/NEJM200106143442404. [DOI] [PubMed] [Google Scholar]
- 63.Ananthakrishnan M, Barr FE, Summar ML, Smith HA, Kaplowitz M, Cunningham G, et al. L-Citrulline ameliorates chronic hypoxia-induced pulmonary hypertension in newborn piglets. Am J Physiol Lung Cell Mol Physiol. 2009;297:L506–L511. doi: 10.1152/ajplung.00017.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smith HA, Canter JA, Christian KG, Drinkwater DC, Scholl FG, Christman BW, et al. Nitric oxide precursors and congenital heart surgery: a randomized controlled trial of oral citrulline. J Thorac Cardiovasc Surg. 2006;132:56–65. doi: 10.1016/j.jtcvs.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 65.Barr FE, Tirona RG, Taylor MB, Rice G, Arnold J, Cunningham G, et al. Pharmacokinetics and safety of intravenously administered citrulline in children undergoing congenital heart surgery:potential therapy for postoperative pulmonary hypertension. J Thorac Cardiovasc Surg. 2007;134:319–326. doi: 10.1016/j.jtcvs.2007.02.043. [DOI] [PubMed] [Google Scholar]
- 66.Brennan LA, Steinhorn RH, Wedgwood S, Mata-Greenwood E, Roark EA, Russell JA, et al. Increased superoxide generation Is associated with pulmonary hypertension in fetal lambs. A role for NADPH oxidase. Circ Res. 2003;92:683–691. doi: 10.1161/01.RES.0000063424.28903.BB. [DOI] [PubMed] [Google Scholar]
- 67.Wedgwood S, Steinhorn RH, Bunderson M, Wilham J, Lakshminrusimha S, Brennan LA, et al. Increased hydrogen peroxide downregulates soluble guanylate cyclase in the lungs of lambs with persistent pulmonary hypertension of the newborn. Am J Physiol Lung Cell Mol Physiol. 2005;289(4):L660–6. doi: 10.1152/ajplung.00369.2004. Epub 2005 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wedgwood S, Lakshminrusimha S, Fukai T, Russell JA, Schumacker PT, Steinhorn RH. Hydrogen peroxide regulates extracellular superoxide dismutase activity and expression in neonatal pulmonary hypertension. Antioxid Redox Signal. 2010 doi: 10.1089/ars.2010.3630. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fike CD, Slaughter JC, Kaplowitz MR, Zhang Y, Aschner JL. Reactive oxygen species from NADPH oxidase contribute to altered pulmonary vascular responses in piglets with chronic hypoxia-induced pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2008;295(5):L881–8. doi: 10.1152/ajplung.00047.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF, et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatr Res. 2007;62:313–318. doi: 10.1203/PDR.0b013e3180db29fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lakshminrusimha S, Swartz DD, Gugino SF, Ma CX, Wynn KA, Ryan RM, et al. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatr Res. 2009;66(5):539–44. doi: 10.1203/PDR.0b013e3181bab0c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Farrow KN, Groh BS, Schumacker PT, Lakshminrusimha S, Czech L, Gugino SF, et al. Hyperoxia increases phosphodiesterase 5 expression and activity in ovine fetal pulmonary artery smooth muscle cells. Circ Res. 2008;102(2):226–33. doi: 10.1161/CIRCRESAHA.107.161463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lakshminrusimha S, Russell JA, Wedgwood S, Gugino SF, Kazzaz JA, Davis JM, et al. Superoxide dismutase improves oxygenation and reduces oxidation in neonatal pulmonary hypertension. Am J Respir Crit Care Med. 2006;174(12):1370–7. doi: 10.1164/rccm.200605-676OC. Epub 2006 Sep 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, 3rd, et al. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. 2006;59(1):137–41. doi: 10.1203/01.pdr.0000191136.69142.8c. Epub 2005 Dec 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, Davis JM. Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension. Am Rev Resp Crit Care Med. 2001;164:834–839. doi: 10.1164/ajrccm.164.5.2010104. [DOI] [PubMed] [Google Scholar]
- 76.Firth AL, Yuan JX. Bringing down the ROS: a new therapeutic approach for PPHN. Am J Physiol Lung Cell Mol Physiol. 2008;295(6):L976–8. doi: 10.1152/ajplung.90515.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Steinhorn RH, Russell JA, Morin FC., III Disruption of cyclic GMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol Heart Circ Physiol. 1995;268:H1483–H1489. doi: 10.1152/ajpheart.1995.268.4.H1483. [DOI] [PubMed] [Google Scholar]
- 78.Deruelle P, Balasubramaniam V, Kunig AM, Seedorf GJ, Markham NE, Abman SH. BAY 41-2272, a direct activator of soluble guanylate cyclase, reduces right ventricular hypertrophy and prevents pulmonary vascular remodeling during chronic hypoxia in neonatal rats. Biol Neonate. 2006;90(2):135–44. doi: 10.1159/000092518. Epub 2006 Mar 19. [DOI] [PubMed] [Google Scholar]
- 79.Weimann J, Ullrich R, Hromi J, Fujino Y, Clark MWH, Bloch KD, et al. Sildenafil is a pulmonary vasodilator in awake lambs with acute pulmonary hypertension. Anesthesiology. 2000;92:1702–1712. doi: 10.1097/00000542-200006000-00030. [DOI] [PubMed] [Google Scholar]
- 80.Ichinose F, Erana-Garcia J, Hromi J, Raveh Y, Jones R, Krim L, et al. Nebulized sildenafil is a selective pulmonary vasodilator in lambs with acute pulmonary hypertension. Crit Care Med. 2001;29:1000–1005. doi: 10.1097/00003246-200105000-00024. [DOI] [PubMed] [Google Scholar]
- 81.Shekerdemian L, Ravn H, Penny D. Intravenous sildenafil lowers pulmonary vascular resistance in a model of neonatal pulmonary hypertension. Am J Resp Crit Care Med. 2002;165:1098–2002. doi: 10.1164/ajrccm.165.8.2107097. [DOI] [PubMed] [Google Scholar]
- 82.Shekerdemian LS, Ravn HB, Penny DJ. Interaction between inhaled nitric oxide and intravenous sildenafil in a porcine model of meconium aspiration syndrome. Pediatr Res. 2004;55(3):413–8. doi: 10.1203/01.PDR.0000112033.81970.C2. Epub 2004 Jan 7. [DOI] [PubMed] [Google Scholar]
- 83.Baquero H, Soliz A, Neira F, Venegas ME, Sola A. Oral sildenafil in infants with persistent pulmonary hypertension of the newborn: a pilot randomized blinded study. Pediatrics. 2006;117(4):1077–83. doi: 10.1542/peds.2005-0523. [DOI] [PubMed] [Google Scholar]
- 84.Steinhorn RH, Kinsella JP, Butrous G, Dilleen M, Oakes M, Wessel DL. Open-Label, Multicentre, Pharmacokinetic Study of IV Sildenafil in the Treatment of Neonates With Persistent Pulmonary Hypertension of the Newborn (PPHN) Circulation. 2007;116:II–614. [Google Scholar]
- 85.Konduri GG. New approaches for persistent pulmonary hypertension of newborn. Clin Perinatol. 2004;31(3):591–611. doi: 10.1016/j.clp.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 86.Neonatal Inhaled Nitric Oxide Study Group. Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. New Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 87.Roberts JD, Fineman J, Morin FC, III, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. New Engl J Med. 1997;336:605–610. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 88.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. N Engl J Med. 2000;342:469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 89.Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: A randomized, double-masked, placebo-controlled, dose-response, multicenter study. Pediatrics. 1998;101:325–334. doi: 10.1542/peds.101.3.325. [DOI] [PubMed] [Google Scholar]
- 90.Lakshminrusimha S, Russell JA, Steinhorn RH, Ryan RM, Gugino SF, Morin FC, 3rd, et al. Pulmonary arterial contractility in neonatal lambs increases with 100% oxygen resuscitation. Pediatr Res. 2006;59(1):137–41. doi: 10.1203/01.pdr.0000191136.69142.8c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Konduri GG, Kim UO. Advances in the diagnosis and management of persistent pulmonary hypertension of the newborn. Pediatr Clin North Am. 2009;56(3):579–600. doi: 10.1016/j.pcl.2009.04.004. Table of Contents. [DOI] [PMC free article] [PubMed] [Google Scholar]


