Abstract
One of the major obstacles to the success of cancer chemotherapy is the multidrug resistance (MDR) often resulting due to the overexpression of drug efflux transporter pumps such as P-glycoprotein (P-gp). Highly efficacious third generation P-gp inhibitors, like tariquidar, have shown promising results in overcoming the MDR. However, P-gp is also expressed in normal tissues like blood brain barrier, gastrointestinal track, liver, spleen and kidney. To maximize the efficacy of P-gp inhibitor and reduce the systemic toxicity, it is important to limit the exposure of P-gp inhibitors and the anticancer drugs to normal tissues and increase their co-localization with tumor cells. In this study, we have investigated the co-delivery of the P-gp inhibitor, tariquidar, and cytotoxic drug, paclitaxel, into tumor cells to reverse the MDR using long-circulating liposomes. Tariquidar- and paclitaxel-loaded long-circulating liposomes showed significant resensitization of the resistant variant for paclitaxel, which could be correlated with an increased accumulation of paclitaxel in tumor cells. These results suggest that the co-delivery of the P-gp inhibitor, tariquidar, and the cytotoxicity inducer, paclitaxel, looks like a promising approach to overcome the MDR.
Keywords: P-glycoprotein, Tariquidar, Co-delivery, Multidrug resistance, Liposomes
1. Introduction
The efficacy of chemotherapy is often impaired by the development of the multidrug resistance (MDR), which then requires the administration of higher doses of cytotoxic drugs in the following treatments. One of the major mechanisms responsible for the acquired drug resistance is the overexpression of the P-gp, a plasma membrane glycoprotein with a molecular weight of 170,000. This MDR1 gene product belonging to the ABC (ATP binding cassettes) family is responsible for the energy-dependent efflux of a variety of drugs including anthracyclins, vinca alkaloids, and taxanes (Childs and Ling 1994; Germann 1996). This efflux results in the reduced accumulation of an anticancer drug inside cancer cells so that higher doses are required to produce an equivalent toxicity as before the overexpression of the P-gp. Thus, it appears important to inhibit the P-gp to reverse the MDR and to maintain the accumulation of anticancer drugs in cancer cells.
A broad range of compounds that inhibit P-gp has been identified. The first generation P-gp modulators, which include calcium channel blockers, antibiotics, and other miscellaneous compounds, lacked specificity and were weak inhibitors. These first generation modulators produced P-gp inhibition at relatively high doses, which resulted in unwanted pharmacological effects (Lum, Fisher et al. 1993; Raderer and Scheithauer 1993). So, the further research was carried out to discover P-gp modulators with higher specificity and increased affinity for P-gp. Thus, the second generation modulators were found, such as PSC 833(Boesch, Muller et al. 1991), GG918 (Hyafil, Vergely et al. 1993) and S9788 (Pierre, Dunn et al. 1992). Related studies with this second generation modulators did indicate the significance of the approach but also showed the requirement for even more potent and specific P-gp modulators to achieve the successful reversal of the MDR. XR9051, a diketopiperazine derivative, has also demonstrated MDR reversal at submicromolar concentration (Dale, Tuffley et al. 1998). Experiments involving molecular modeling of XR9051 to improve its potency resulted in the discovery of XR9576 (tariquidar), a potent and specific P-gp inhibitor (Roe, Folkes et al. 1999). Studies evaluating the ability of XR9576 to sensitize the variety of resistant cell lines have shown great promise to reverse the MDR caused by the P-gp overexpression (Mistry, Stewart et al. 2001). There is an increasing concern, however, for the non-specific binding of tariquidar to the P-gp present in normal tissues, such as blood brain barrier and gastrointestinal track. This non-specific binding can result in the alteration of pharmacokinetics of concomitantly taken other drugs leading to unexpected toxicity (Martin, Berridge et al. 1999).
The present study was undertaken to develop a system for co-delivery of tariquidar and paclitaxel to cancer cells in long-circulating liposomes capable of accumulating in tumors via the enhanced permeability and retention effect (Maeda 2001; Maeda, Fang et al. 2003) and to evaluate its potential to reverse the MDR. Loading efficiency of tariquidar and paclitaxel into these long-circulating liposomes was determined using HPLC technique. Rhodamine 123 (Rh123), a substrate for P-gp, is a fluorescent dye and is readily effluxed out of cells by P-gp. Possible changes in the potency of tariquidar after its incorporation into long-circulating liposomes was evaluated by the Rh123 uptake study. We have tested this formulation for its potency in reversing the MDR in drug-resistant ovarian cancer cells SKOV-3TR by determining IC50 values for paclitaxel, using the cytotoxicity assay.
2. Materials and methods
2.1 Materials
SKOV-3 ovarian cancer cell line was obtained from ATCC (American type culture collection, Manassas, VA, USA). SKOV-3TR taxol resistant variant was a kind gift from Dr. Duan Zhenfeng (MGH, Boston, MA). SKOV-3 and SKOV-3TR were cultured in RPMI 1640 media (supplemented with 10% fetal bovine serum and 1% antibiotics as recommended by ATCC) at 37 ºC with 5% CO2 incubator in all experiments. L-α-phosphatidylcholine (egg, chicken) (EPC), 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), cholesterol (CHOL), polyethylene glycol 2000-phosphatidylethanolamine (PEG2kPE) and rhodamine 123 (Rh123) were obtained from Avanti Polar Lipids (Alabaster, AL, USA). Tariquidar was a kind gift from Dr. Susan Bates (NCI, Bathesda, MD). FITC labeled P-gp specific antibody (UIC2) was obtained from Abcam (Cambridge, MA, USA). Cell titer blue reagent was obtained from Promega (Madison, WI, USA).
2.2 Methods
2.2.1 Preparations of long-circulating liposomes
Liposomes were prepared by thin film hydration method followed by extrusion. Briefly, the lipid components, EPC, CHOL, DOTAP, along with PEG2kPE were weighed and dissolved in chloroform at the molar ratio of 64:30:6:2 (EPC:CHOL:DOTAP:PEG2kPE). Lipids dissolved in chloroform were dried under rotary evaporator to form a thin film. The film was subjected to freeze-drying to remove any trace level of organic phase on a Freezone 4.5 Freeze Dry System (Labconco, Kansas city, MO, USA). Lipid film was then hydrated for 30 minutes with the phosphate buffer solution (PBS), pH 7.4 at the final lipid concentration of 2 mg/ml. The liposomal suspension was then subjected to bath sonication for 20 min. Liposomes were allowed to rest for 15 min to overcome any structural defects. Then liposomes were extruded through 200 nm pore size polycarbonate membranes using a hand held extruder (Avanti Polar Lipids, Alabaster, AL, USA) to obtain small unilamellar vesicles.
While preparing tariquidar- and paclitaxel-loaded liposomes, drugs were added to chloroform along with lipids before preparing thin lipid film. Combined amount of equimolar quantities of paclitaxel and tariquidar was kept at 1% w/w final lipids.
2.2.2 Characterization of long-circulating liposomes
2.2.2.1 Liposome size and zeta potential
The size distribution and zeta potential of liposomes were measured using the Zetaplus (Brookhaven Instruments Corporation, Holtsville, NY, USA). Briefly, 50 μl of liposome formulation was resuspended in 1.5 ml of distilled water, and liposomes size distribution and zeta potential were analyzed using these diluted samples according to the manufacturer’s protocol.
2.2.2.2 Loading efficiency of long-circulating liposomes
Loading efficiency of long-circulating liposomes for paclitaxel and tariquidar was evaluated with the reversed phase HPLC technique using the Xbridge C18 (2.1cm×250cm) column (Waters corporation, Milford, MA). Ammonium acetate buffer (pH 4): acetonitrile mixture (40:60 wt ratio) was used as a mobile phase. The running time for each injection was kept at 8 minutes and the injection volume was kept at 50 μl. At these conditions, a standard curve was produced for paclitaxel and tariquidar using 0.5 ug/ml, 1 ug/ml, 2 ug/ml, and 4 ug/ml concentrations. For loading efficiency determination, liposomal formulations were centrifuged at 10000 rpm for 10 min to precipitate non-entrapped drugs. From the supernatant, 150 μl of liposomal formulation were taken, and liposomes were lysed with 850 μl of acetonitrile. The final solution was subjected to HPLC analysis.
2.2.3 P-glycoprotein expression
SKOV-3 cells and SKOV-3TR cells were allowed to grow in T12.5 until 80% confluency. Cells were then detached from the flask using the mechanical cell scraper and centrifuged to get a cell pellet. The cell pellet was resuspended in PBS (pH 7.4). Cells were counted and adjusted to get 300,000 cells/100 μl of PBS. Cells were then treated with 5 μl of FITC-labeled antibody against P-glycoprotein (UIC2). Cells were incubated for 1 hr on ice according to the manufacturer’s protocol. After the incubation, cells were centrifuged and washed twice with ice-cold PBS and analyzed using FACS for the green fluorescence intensity (FL1). Untreated cells were taken as controls for both cell lines.
2.2.4 Rh123 uptake
SKOV-3 cells and SKOV-3TR cells were seeded in T75 flask at a density of 500,000 cells/flask. Cells were then allowed to grow until 80% confluency. Cells were then trypsinized and centrifuged to get a cell pellet. The cell pellet was than resuspended in complete RPMI1640 media to achieve 106 cells/ml. Cells were then treated with tariquidar alone or with tariquidar-loaded long circulating liposomes at 100 nM final concentration of tariquidar for 1 hr at 37 ºC. After 1 hr of treatment, cells were incubated with Rh123 at 0.2 μg/ml for 1 hr. Cells treated only with Rh123 were taken as control. Cells were than centrifuged and washed with ice cold PBS, pH 7.4. Cells were fixed with 4% formalin and kept on ice until further analysis. Cells were analyzed using FACS for Rh123 uptake.
2.2.5 Multidrug resistance reversal by cytotoxicity assay
SKOV-3 cells and SKOV-3TR cells were seeded in 96-well plates at a density of 3000 cells/well 24 hrs before treatment. After 24 hrs, cells were treated with tariquidar, tariquidar-loaded liposomes, empty liposomes, paclitaxel-loaded liposomes, and tariquidar-paclitaxel-co-loaded liposomes at various concentrations. Cells were then allowed to grow for 48 hrs. After the desired period of incubation, cells were washed with fresh media. Cytotoxicity was measured using Cell Titer Blue assay according to the manufacturer’s protocol. Briefly, washed cells were incubated for 2 hrs with 100 μl of fresh media containing 20 μl of Cell Titer Blue reagent. After the incubation, plates were analyzed for the fluorescent signal at 560 nm excitation and 590 nm emission wavelength.
2.3 Results
2.3.1 Preparation and characterization of long circulating liposomes
We have prepared tariquidar- and paclitaxel-loaded long-circulating liposomes using thin film hydration method. All liposomal formulations demonstrated a uniform size distribution ranging from 180 to 200 nm (Table 1). Zeta potential values of all the liposomal preparations were ranging from 23 to 32 due to the presence of the positively charged DOTAP (Table 1). Upon the addition of tariquidar and/or paclitaxel to the formulation, there is no significant change in the liposome size distribution or zeta potential.
Table 1.
Size and zeta potenatial of different liposomal formulations.
| Liposomal formulation | Lipid composition | Molar ratio | Size** | Zeta potential** |
|---|---|---|---|---|
| Empty Liposomes | EPC:CHOL:DOTAP:PEG2kPE | 64:30:6:2 | 188.3±1.5 | 32.01 ± 0.86 |
| XR liposomes* | EPC:CHOL:DOTAP:PEG2kPE | 64:30:6:2 | 196.2±2.3 | 25.69 ± 0.44 |
| PCL liposomes* | EPC:CHOL:DOTAP:PEG2kPE | 64:30:6:2 | 194.3±1.8 | 23.68 ± 1.71 |
| XRPCL liposomes* | EPC:CHOL:DOTAP:PEG2kPE | 64:30:6:2 | 194.1±1.2 | 23.56 ± 0.82 |
XR liposomes – tariquidar-loaded liposomes, PCL liposomes – paclitaxel-loaded liposomes, XRPCL liposomes – tariquidar- and paclitaxel-loaded liposomes.
The values are for 5 measurements of the same formulations.
2.3.2 Loading efficiency of long circulating liposomes
Drug loading efficiency was determined using the HPLC technique. Standard curves were obtained for tariquidar and paclitaxel at 0.5 ug/ml, 1ug/ml, 2 ug/ml and 4 ug/ml. The resulting standard curves had the correlation coefficient of more than 0.999. Then the liposomal solution containing tariquidar and/or paclitaxel equivalent to 2 ug/ml was lysed in acetonitrile:water. The resulting solution was subjected to HPLC analysis to determine the loading efficiency for all formulations (Table 2).
Table 2.
Loading efficiency of long circulating liposomes for tariquidar and paclitaxel.
| Liposomal formulations | Loadingefficiency for tariquidar (%) | Loadingefficiency for paclitaxel (%) |
|---|---|---|
| Empty Liposomes | - | - |
| XR liposomes | 68% | - |
| PCL liposomes | - | 71.76% |
| XRPCL liposomes | 70.14% | 70.11% |
2.3.3 P-glycoprotein expression
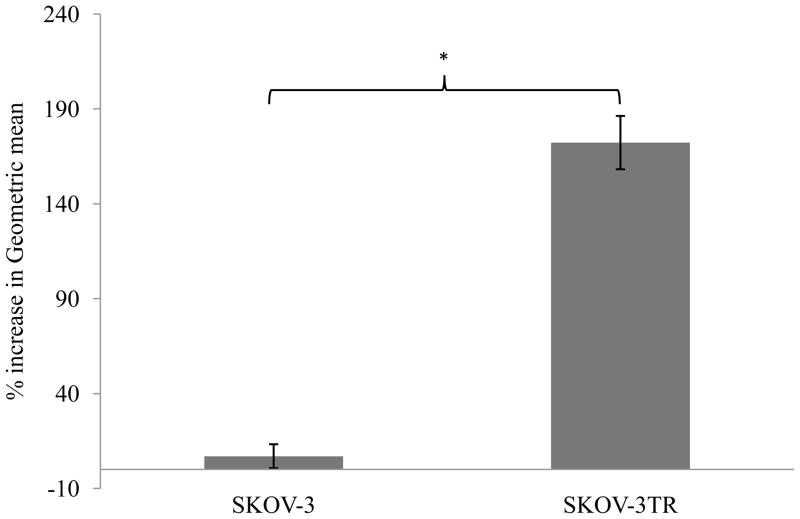
SKOV-3 and SKOV-3TR cells were evaluated for the expression of the P-gp on the cellular surface. Using FITC-labeled P-gp-specific antibody (UIC2), P-gp expression level was determined by FACS analysis. The FACS analysis showed a 7% increase in the green fluorescence signal (indicated by fl1 channel in FACS) in FITC-labeled antibody treated SKOV-3 cells compared to untreated SKOV-3 cells. In case of SKOV-3TR cells, however, FACS analysis showed 173% increase in green fluorescence signal in FITC-labeled antibody-treated cells compared to untreated cells. The results showed significantly higher expression of P-gp in SKOV-3TR cells compared to SKOV-3 cells (Figure 1), which renders this pair a good model to evaluate P-gp inhibitors.
Figure 1.
P-gp expression in SKOV-3 and SKOV-3TR cells as shown by FACS analysis. *P<0.005.
2.3.4 Rh123 uptake study
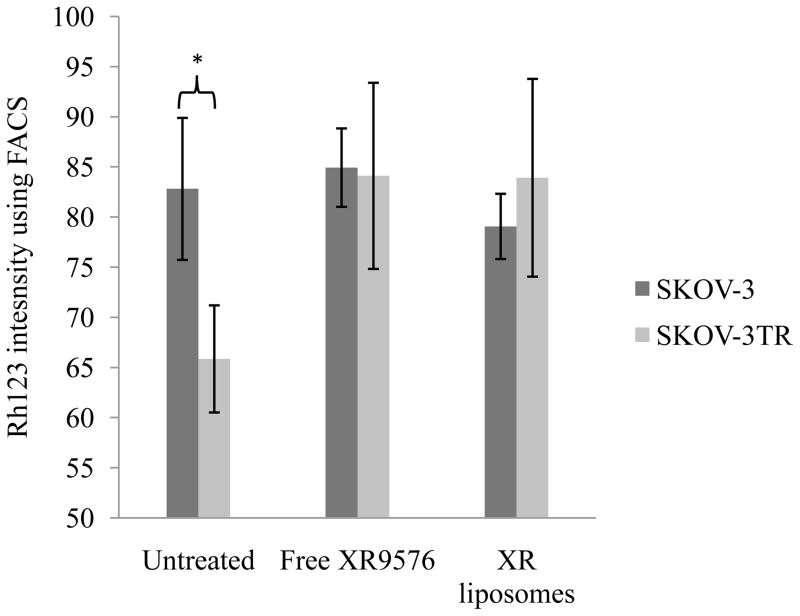
SKOV-3 and SKOV-3TR cells were treated with free tariquidar and XR-liposomes (tariquidar-loaded liposomes) at the final concentration of tariquidar at 100 nm for 30 min. Untreated cells were taken as control. This initial treatment was followed by the incubation of cells with Rh123 at the final concentration of 200 ng/ml. As seen in the figure 2, Rh123 uptake was similar in free tariquidar and tariquidar-loaded liposomes treated cells, while among the untreated cells, SKOV-3 cells demonstrated much higher uptake of Rh123. Thus, in SKOV-3TR cells with higher P-gp expression level, Rh123 uptake was significantly increased in cells treated with both, free tariquidar and tariquidar-loaded liposomes compared to control. Rh123 uptake was similar for SKOV-3TR cells treated with tariquidar alone and tariquidar-loaded liposomes, which demonstrate that tariquidar retains its efficacy upon incorporation into liposomes.
Figure 2.
Rh123 uptake study in SKOV-3 and SKOV-3TR cells. The cells were incubated with free tariquidar and tariquidar-loaded liposomes for 30 minutes at the final concentration of 100 nm of tariquidar. After 30 minutes, cells were incubated with 200 ng/ml of Rh123 for 1 hr before analysing for Rh123 uptake using FACS. Cells incubated only with Rh123 were taken as control. Error bars indicate mean ± S.D. *p < 0.05
2.3.5 Multidrug resistance reversal
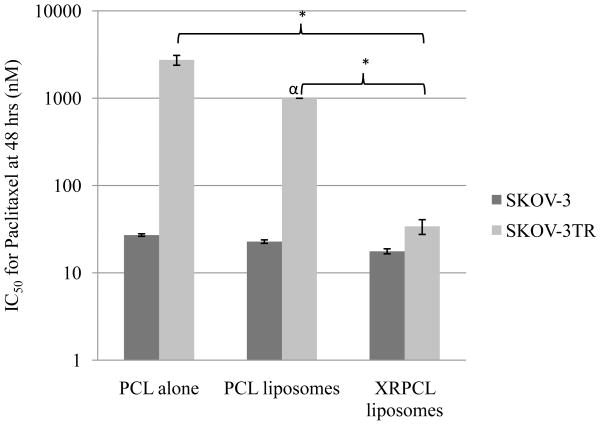
Next, SKOV-3 and SKOV-3TR cells were treated with different formulations of paclitaxel at various doses to determine the IC50 value for paclitaxel. Tariquidar alone, empty liposomes and tariquidar-loaded liposomes did not show any inherent toxicity (<20% cell death) even at doses as high as 200 nm of tariquidar. The IC50 values for paclitaxel were 27.11 nM and 2743 nM in SKOV-3 and SKOV-3TR cells, respectively, when cells were treated with paclitaxel alone (Figure 3). Thus, a 100-fold higher dose of paclitaxel is required to produce an equivalent toxicity in SKOV-3TR cells compared to SKOV-3 cells. However, the IC50 values for paclitaxel were 17.68 nM and 34 nM in SKOV-3 cells and SKOV-3TR cells, respectively, when cells were treated with tariquidar- and paclitaxel-co-loaded liposomes (Figure 3). These results indicate that a similar dose of paclitaxel produces an equivalent toxicity in both cell lines upon simultaneous delivery of tariquidar and paclitaxel in long-circulating liposomes, demonstrating the reversal of the MDR by the liposomal tariquidar.
Figure 3.
IC50 for paclitaxel in SKOV-3 and SKOV-3TR cells. Cells were incubated in 96-well plate 24 hrs before the treatment at 3000 cells/well. Cells were than treated with different formulations at various concentrations. Concentrations represents tariquidar and paclitaxel concentration. Error bars indicate mean ± S.D. * p<0.05. X-axis is shown in logarithmic scale. α IC50 for paclitaxel-loaded liposomes in SKOV-3TR cells was not achieved with dose as high as 1000 nM of paclitaxel.
3. Conclusion
Simultaneous incorporation of the P-gp inhibitor, tariquidar, and the cytotoxic drug, paclitaxel, in long-circulating liposomes resulted in the formulation with uniform size distribution and high incorporation efficacy with no apparent reduction in tariquidar potency towards P-gp. A simultaneous delivery of tariquidar and paclitaxel by long-circulating liposomes resulted in greater cytotoxicity in SKOV-3TR cells at a paclitaxel dose that was ineffective in the absence of tariquidar demonstrating a significant reversal of the MDR towards paclitaxel.
Acknowledgments
We thank Dr. Susan Bates (NCI, Bethesda, MD) for providing tariquidar. We thank Dr. Duan Zhenfeng (MGH, Boston, MA) for providing SKOV-3TR cell line. This study was supported by the NIH grants, RO1CA128486 and RO1CA121838, to Dr. Vladimir P. Torchilin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boesch D, Muller K, et al. Restoration of daunomycin retention in multidrug-resistant P388 cells by submicromolar concentrations of SDZ PSC 833, a nonimmunosuppressive cyclosporin derivative. Exp Cell Res. 1991;196(1):26–32. doi: 10.1016/0014-4827(91)90452-z. [DOI] [PubMed] [Google Scholar]
- Childs S, Ling V. The MDR superfamily of genes and its biological implications. Important Adv Oncol. 1994:21–36. [PubMed] [Google Scholar]
- Dale IL, Tuffley W, et al. Reversal of P-glycoprotein-mediated multidrug resistance by XR9051, a novel diketopiperazine derivative. Br J Cancer. 1998;78(7):885–92. doi: 10.1038/bjc.1998.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germann UA. P-glycoprotein--a mediator of multidrug resistance in tumour cells. Eur J Cancer. 1996;32A(6):927–44. doi: 10.1016/0959-8049(96)00057-3. [DOI] [PubMed] [Google Scholar]
- Hyafil F, Vergely C, et al. In vitro and in vivo reversal of multidrug resistance by GF120918, an acridonecarboxamide derivative. Cancer Res. 1993;53(19):4595–602. [PubMed] [Google Scholar]
- Lum BL, Fisher GA, et al. Clinical trials of modulation of multidrug resistance. Pharmacokinetic and pharmacodynamic considerations. Cancer. 1993;72(11 Suppl):3502–14. doi: 10.1002/1097-0142(19931201)72:11+<3502::aid-cncr2820721618>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Adv Enzyme Regul. 2001;41:189–207. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- Maeda H, Fang J, et al. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. Int Immunopharmacol. 2003;3(3):319–28. doi: 10.1016/S1567-5769(02)00271-0. [DOI] [PubMed] [Google Scholar]
- Martin C, Berridge G, et al. The molecular interaction of the high affinity reversal agent XR9576 with P-glycoprotein. Br J Pharmacol. 1999;128(2):403–11. doi: 10.1038/sj.bjp.0702807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistry P, Stewart AJ, et al. In vitro and in vivo reversal of P-glycoprotein-mediated multidrug resistance by a novel potent modulator, XR9576. Cancer Res. 2001;61(2):749–58. [PubMed] [Google Scholar]
- Pierre A, Dunn TA, et al. In vitro and in vivo circumvention of multidrug resistance by Servier 9788, a novel triazinoaminopiperidine derivative. Invest New Drugs. 1992;10(3):137–48. doi: 10.1007/BF00877238. [DOI] [PubMed] [Google Scholar]
- Raderer M, Scheithauer W. Clinical trials of agents that reverse multidrug resistance. A literature review. Cancer. 1993;72(12):3553–63. doi: 10.1002/1097-0142(19931215)72:12<3553::aid-cncr2820721203>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Roe M, Folkes A, et al. Reversal of P-glycoprotein mediated multidrug resistance by novel anthranilamide derivatives. Bioorg Med Chem Lett. 1999;9(4):595–600. doi: 10.1016/s0960-894x(99)00030-x. [DOI] [PubMed] [Google Scholar]