Abstract
Background and Aims
Impaired glucose tolerance based on 2-hr glucose levels is more predictive of future cardiovascular disease and more sensitive in detecting earlier diabetes compared to impaired fasting glucose. However, the 1-hr OGTT may be even more sensitive than the 2-hr. We assessed the relative value of 1-hr OGTT by exploring its relationship with adiposity and other measures of glucose homeostasis.
Methods and Results
Ninety four overweight/obese individuals free of diabetes and major cardiovascular conditions were included in the analyses. We adjusted for age, gender, smoking status and physical activity. One-hr OGTT showed similar partial correlations with fasting glucose and 2-hr OGTT (r= 0.60 and 0.64 respectively). Fasting glucose, fasting insulin and HOMA correlated better with 1-hr OGTT (r= 0.60, 0.47 and 0.52) than with 2-hr OGTT (r= 0.50, 0.41, and 0.45). BMI and waist circumference also showed stronger correlation with 1-hr (r= 0.31, 0.29), compared to 2-hr OGTT (r= 0.16, 0.16) or fasting glucose (r= 0.23, 0.22). Metabolic syndrome was associated similarly with 1-hr and 2-hr OGTT.
Conclusions
The 1-hr OGTT correlates well with both fasting glucose and 2-hr OGTT and shows similar or higher associations with obesity measures. The 1-hr OGTT has potential utility in epidemiologic studies.
Keywords: Type 2 diabetes, 1-hr OGTT, glucose abnormalities, abnormal glucose homeostasis, 2-hr OGTT
Introduction
Two longitudinal studies among adults have shown that 1-hr plasma glucose concentration during the Oral Glucose Tolerance Test (OGTT) performs equally or better than 2-hr plasma glucose concentration in predicting of the risk of type 2 diabetes in Mexican Americans (1) and Caucasians (2). However, few epidemiological studies routinely consider using the 1-hr OGTT, and the 2-hr OGTT is still considered the gold standard for diabetes diagnosis (3).
Type 2 diabetes is a heterogeneous condition characterized by insulin resistance and beta cell dysfunction. Although a fasting plasma glucose level of 7 mmol/L or above on 2 or more occasions is diagnostic for diabetes, a 2-hr OGTT of 11.1 mmol/L or above is a more sensitive indicator of diabetes (4). Fasting plasma glucose alone fails to diagnose approximately 30% of previously undiagnosed diabetes cases (5). OGTT is frequently needed to confirm or exclude an abnormality of glucose tolerance in asymptomatic people.
The use of OGTT in the diagnosis of diabetes has been controversial for decades. The recognition that fasting glucose levels alone missed many individuals with type 2 diabetes led to the development of multiple protocols for glucose tolerance tests with oral glucose loads ranging from 50 to 100 gm. with or without regard to body size, and testing postprandial glucose from between 30 minutes to 30 hours after the glucose load. However, because post-challenge (compared to fasting) glucose levels had poorer reproducibility, were relatively inconvenient and were significantly influenced by age, OGTT fell into relative disfavor. Accordingly, in 1997, the American Diabetes Association proposed new criteria for diabetes based on fasting glucose alone, lowering the threshold for fasting plasma glucose to 7.0 mmol/L, adding an impaired fasting glucose category (5.6 to 6.9 mmol/L), and de-emphasizing a glucose challenge test altogether (6). Current guidelines indicate that diabetes can be diagnosed with fasting and postprandial glucose (7), and glycosylated hemoglobin (HbA1c) ≥ 6.5% has been added recently as a new criteria for diagnosing diabetes (3).
However, because of the risk of misclassification of diabetes in high risk groups, the importance of OGTT has become increasingly recognized (8). In Bartnik’s study of 3362 patients without known glucometabolic abnormalities (8), 33% of patients would have been under-diagnosed and 8% over-diagnosed using fasting blood glucose alone compared to 2-hr OGTT, resulting in a total misclassification of 41%. Postprandial glucose may also be more predictive of health outcomes than fasting plasma glucose. In the Framingham offspring study, postprandial but not fasting hyperglycemia, independently predicted the occurrence of cardiovascular events (9). Another recent study showed that HbA1c outperformed fasting blood glucose as a predictor of cardiovascular disease (CVD) and had similar predictive accuracy for diabetes (10). The relative utility of different measures of glucose homeostasis may also vary across populations (11).
It is unclear whether 1-hr or 2-hr postprandial glucose measures are more predictive of any particular outcome. The 1-hr OGTT is well accepted and used mainly for pregnant women to screen for gestational diabetes (12). Women with a single abnormal glucose value of 1-hr OGTT in the late second trimester (1-hr Gestational Impaired Glucose Tolerance) had outcomes similar to those with true gestational diabetes, including increased infant birth weight and postpartum insulin resistance (13). Similarly, in a study by Jovanovic, the 1-hr postprandial glucose level during pregnancy was the best predictor of HbA1c in patients with well-controlled type 2 diabetes mellitus (14). Logistically, the 1-hour OGTT is less resource intensive than the 2-hour, both from a provider and patient perspective. If its value is equivalent to the 2-hour in identifying risk, then the 1-hour would be a more cost-effective assessment tool.
In light of the controversy on the role of 1-hr vs. 2-hr OGTT in predicting outcomes and the sparse data on this topic, we sought to evaluate and compare 1-hour and 2-hour postprandial glucose correlations with other risk factors for glucose intolerance, such as adiposity measures. We were interested in 1-hour postprandial glucose because of: (1) its potential to better reflect both changes in insulin sensitivity and in insulin secretion; (2) its less burdensome testing, time wise, compared to the 2-hr OGTT; and (3) its potential, relative to 2-hr OGTT, to better predict long term outcomes. We compared the association between 1 and 2-hour OGTT with anthropometric and metabolic abnormalities to better understand the potential advantage of one testing time interval over the other.
Methods
The study was conducted in a convenience sample from the San Juan municipality among individuals free of diabetes, aged 40 to 65 years. The study was approved by the University of Puerto Rico Institutional Review Board. Overweight or obese individuals were recruited through press releases, newspaper advertisements, flyers and posters, and word of mouth. We excluded people who were pregnant, had a history of hypoglycemia, coronary heart disease, congenital heart murmurs, heart valve disease, congenital heart disease, endocarditis, stroke, rheumatic fever, and hemophilia or bleeding disorders or who were undergoing dialysis treatment, or undergoing anticoagulation therapy. Because the study also included a periodontal examination, we excluded people who had less than 4 natural teeth, needed antibiotic prophylaxis prior to dental procedures or who had braces or orthodontic appliances.
A total of 141 people responded; 27 did not meet eligibility criteria and 2 did not want to participate in the study. Of the remaining 112 potential participants who initially agreed, 11 did not show up, and 1 person could not complete the study procedures due to medical reasons. The remaining 100 participants signed written informed consents and completed all the study procedures. Five participants had a provisional diagnosis of diabetes based on the fasting glucose (≥ 6.99 mmol/L) and 2-hr OGTT (≥ and 11.1mmol/L) conducted during the study, and were excluded from the analyses. One participant who was outside the age range was excluded. The final sample for the analyses included 94 participants.
Participants were asked to fast from 10 hours prior to their appointments up to the completion of the 2-hr OGTT. After the consent was signed; blood was drawn at baseline and subsequently at 1-hr and 2-hr intervals after ingesting 296 ml Glucola (75 g of Dextrose). Fasting plasma glucose (FPG), 1-hour OGTT and 2-hour OGTT were assessed using a SIRRUS analyzer (intra-assay coefficient of variation 1.21%; inter-assay 3.06%). Fasting insulin was determined using a TOSOH analyzer (intra-assay coefficient of variation 1.49%; inter-assay 4.42%). Serum lipids from the fasting samples were processed using standard commercial procedures. Vitros direct HDL reagents and Vitros triglyceride slides were used to quantitatively measure HDL cholesterol (HDL-C) and triglyceride concentrations in serum and plasma respectively. LDL cholesterol (LDL-C) was calculated using the Friedewald equation from the triglyceride values (15). Fasting insulin was processed using standard commercially available Chemiluminescence assays in serum.
We conducted in-person interviews to collect information on socio-demographic variables, health-related habits and behavior, nutritional intake, physical activity, medical and dental history and family history of diabetes. We assessed anthropometric measures using a protocol adapted from National Health and Nutrition Examination Survey III (16). Weight and body fat mass were measured on a Tanita scale after the participants voided. Height was measured using a stadiometer. Waist and hip circumferences were measured using a Gulick 2 plus measuring tape on a horizontal plane; waist was measured around the umbilicus, and hip circumference was measured at the maximum circumference. We computed body mass index from the height and weight measures. All anthropometric measures except weight and fat mass were taken twice and a third measure was taken if the two measurements varied substantially, and the measures were averaged.
Blood pressure was measured thrice on the right arm within 1-2 minutes intervals using a protocol that followed the American Heart Association guidelines (17). Participants were classified as hypertensive if they reported a previous diagnosis of hypertension, reported taking medications for hypertension, or were classified as hypertensive from the systolic and/or diastolic blood pressure assessed during the study using the cut offs of and ≥ 85 mm Hg for diastolic (18). Participants were classified positive for hypercholesterolemia if they reported a previous diagnosis, or had high cholesterol assessed during the study using the cutoff of ≥ 6.216 (19, 20). mmol/L We computed the HOMA Insulin Resistance index (HOMA IR) from the fasting glucose and fasting insulin levels. We computed binary variables based on clinically meaningful thresholds: Fasting Plasma Glucose ≥ 5.55 mmol/L, 1-hr OGTT ≥ 8.60 mmol/L, 2-hr OGTT ≥ 7.77 mmol/L, Fasting Insulin > 105.4 pmol/L, HOMA IR Index ≥ 2.5 (19, 21, 22). Participants were classified as having high triglycerides if they reported high triglycerides, if they had triglycerides levels > 105.4 pmol/L, HOMA IR Index ≥ 2.5 nicotinic acid. Participants were classified as having low HDL if they reported low HDL, if they had HDL levels < 1.04 mmol/L for men or < 1.30 mmol/L for women or if they reported taking fibrates or nicotinic acid (18). Participants were classified as having high LDL if they reported high LDL, if they had LDL levels ≥ 3.367 mmol/L or if they reported taking statins or bile acid sequestrants (18). Pre-diabetes was defined as impaired fasting glucose (IFG: 5.55 - 6.98 mmol/L) or impaired glucose tolerance (IGT: 7.77 - 11.04 mmol/L). Cutoffs for high % fat mass were based on thresholds put forth by the World Health Organization and the National Institutes of Health, which were over 40% for women and over 27% for men (23). Finally, we also created the metabolic syndrome variable using the ATPIII definition (18) with the different components assessed as above.
The main goal of the analyses was to compare the relative utility of different glucose measures by comparing their associations with major established predictors of diabetes and abnormal glucose homeostasis. The primary analysis consisted of correlations. The distributions looked reasonably normal and the Pearson and Spearman correlation coefficients were generally similar; hence we present only the Pearson correlation coefficients. Crude and adjusted Pearson correlation coefficients were computed to quantify the associations within and between glucose homeostasis measures (FPG, 1-hr OGTT, 2-hr OGTT), body fat distribution variables (BMI, waist circumference, and fat mass by Bio-impedance analysis), lipids (HDL, LDL, and Triglycerides) and metabolic variables (fasting insulin and HOMA-IR index). Partial correlation coefficients were adjusted for age, gender, smoking and physical activity. Since the crude and adjusted partial correlation coefficients were similar, we only present the adjusted coefficients in the tables. We also present scatter plots for the main associations (1-hr OGTT with 2-hr OGTT and fasting glucose, BMI with 1-hr).
We conducted logistic regression for the binary metabolic variables. Separate models were constructed for each of the glucose homeostasis predictors with each of the metabolic factors. Multivariate analyses adjusting for age, gender, smoking and physical activity were conducted.
Results
The participants, as described in Table 1, had a mean age of 51 years, (SD = 7.3), age range 40 to 65, with 31% being male. Thirty seven percent had ever smoked; 17% were current smokers. The mean body mass index was 33.2 kg/m2; 64% were obese and 36% were overweight. The mean fasting glucose was 5.0 mmol/L and the mean fasting insulin was 105.4 pmol/L, with 42% of participants having elevated fasting insulin and 65% having elevated HOMA IR index. The participants mean 1-hr OGTT was 7.6 mmol/L and mean 2-hr OGTT was 5.9 mmol/L. Forty five percent of the participants had hypercholesterolemia, 63% had hypertension, 18% had pre-diabetes and 48% had metabolic syndrome.
Table 1. Sample description, body fat and metabolic measures.
| Mean or (%) |
(SD) or N | |
|---|---|---|
| Age (years) | 51.02 | (7.3) |
| % Males | 31 | 29 |
| Smoking Current (%) Former (%) Never (%) |
17 20 63 |
16 19 59 |
| Physical Activity Vigorous or Regular Structured (%) |
31 |
29 |
| Body Fat Measures | ||
| Body Mass Index % Overweight (25–29.9 kg/m2) % Obese (≥30 kg/m2) |
33.2 36.2 63.8 |
(6.2) 34 60 |
| Waist Circumference (cm) | 105.5 | (14.3) |
| % High Waist Circumference | 83.9 | 78 |
| Mean fat mass percent % High fat mass (> 40% for women or > 27% for men) |
40.1 25.5 |
(7.4) 24.0 |
| Metabolic Variables | ||
| Fasting glucose (mg/L) | 5.0 | (0.5) |
| % Fasting glucose ≥ 5.55 mmol/L | 13.8 | 13 |
| 1-hr OGTT (mmol/L) | 7.6 | (2.2) |
| % 1-hr OGTT ≥ 8.60 mmol/L | 30.9 | 29 |
| 2-hr OGTT (mmol/L) | 5.9 | (1.4) |
| % 2-hr OGTT ≥ 7.77 mmol/L | 7.5 | 7 |
| Fasting insulin (pmol/L) | 105.4 | (58.4) |
| % with Fasting insulin> 105.4 pmol/L | 41.5 | 39 |
| HOMA IR index | 3.5 | (2.2) |
| % with HOMA IR index ≥ 2.5 | 64.9 | 61 |
| % Pre-diabetes (IFG or IGT) | 18.1 | 17 |
| % with Hypertension | 62.8 | 59 |
| % with Metabolic Syndrome | 47.9 | 45 |
| Serum Lipids | ||
| % with Hypercholesterolemia | 45.2 | 42 |
| HDL (mmol/L) | 1.2 | (0.3) |
| % HDL cholesterol < 1.04 mmol/L for men or < 1.30 mmol/L for women |
43.6 | 41 |
| LDL (mmol/L) | 3.4 | (0.9) |
| % LDL cholesterol ≥ 3.37 mmol/L | 54.3 | 51 |
| Triglycerides (mmol/L) | 1.6 | (1.2) |
| % Triglycerides ≥ 1.70 mmol/L | 41.5 | 39 |
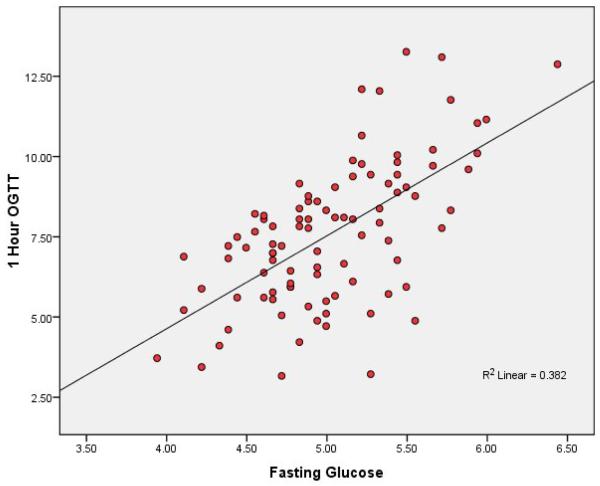
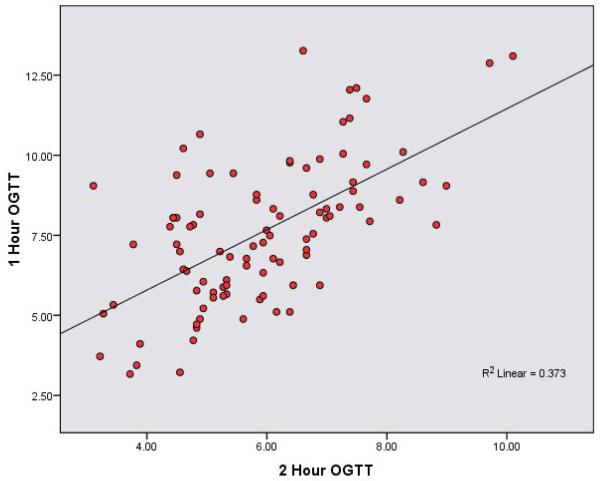
The scatter plots (figures 1 and 2) show strong consistent associations between 1-hr OGTT and FPG and 2-hr OGTT. Table 2 shows partial Pearson correlation coefficients between metabolic variables and between anthropometric measures, lipids and metabolic variables adjusted for age, gender, smoking (ever vs. never), and physical activity (none vs. moderate or active). Fasting glucose (mmol/L) had a stronger correlation with 1-hr OGTT (r = 0.60) than with 2-hr OGTT (r = 0.50). Both fasting insulin and HOMA-IR index also showed stronger correlations with 1-hr OGTT compared to 2-hr OGTT (r = 0.47 versus r = 0.41 for fasting insulin, and r = 0.52 versus r = 0.45 for HOMA IR index).
Figure 1. Scatter Plot Relating 1-hr OGTT and Fasting Plasma Glucose.
Figure 2. Scatter Plot Relating 1-hr OGTT and 2-hr OGTT.
Table 2. Partial Pearson correlation coefficients between metabolic variables, and between body fat, lipids and metabolic variables.
| Fasting glucose (mg/dL) |
1-hr OGTT (mg/dL) |
2-hr OGTT (mg/dL) |
Fasting insulin (pmol/L) |
HOMA IR index |
|
|---|---|---|---|---|---|
|
1-hr OGTT (mg/dL) |
0.60 | ||||
|
2-hr OGTT (mmol/L) |
0.50 | 0.64 | |||
|
Fasting insulin (pmol/L) |
0.44 | 0.47 | 0.41 | ||
|
HOMA IR index |
0.56 | 0.52 | 0.45 | 0.98 | |
|
Body mass index |
0.23 | 0.31 | 0.16 | 0.48 | 0.46 |
|
Waist circumference (cm) |
0.22 | 0.29 | 0.16 | 0.43 | 0.41 |
|
Body fat mass % |
0.26 | 0.34 | 0.17 | 0.44 | 0.42 |
|
HDL (mmol/L) |
−0.09 | −0.19 | −0.29 | −0.25 | −0.25 |
|
LDL (mmol/L) |
0.02 | 0.04 | −0.06 | −0.07 | −0.07 |
|
Triglycerides (mmol/L) |
0.10 | 0.07 | 0.17 | 0.21 | 0.16 |
|
Fasting insulin (pmol/L) |
0.45 | 0.47 | 0.41 | N/A | 0.98 |
|
HOMA IR index |
0.57 | 0.52 | 0.45 | 0.98 | N/A |
The correlation coefficients are adjusted for age, gender, smoking status (ever vs. never), physical activity (structured or vigorous). Bold numbers indicate correlations that are statistically significant (p < 0.05).
There was a stronger and significant correlation between body mass index and 1-hr OGTT (r = 0.31) compared with 2-hr OGTT, which was not significant. Waist circumference was significantly correlated with 1-hr OGTT (r = 0.29), but not with 2-hr OGTT. Body fat % was also significantly correlated with 1-hr OGTT (r = 0.34), but there was no significant correlation with 2-hr OGTT. Fasting insulin was more strongly correlated with 1-hr OGTT (r = 0.47) than with 2-hr OGTT (r = 0.41). Similarly, HOMA had a stronger correlation with 1-hr OGTT than with 2-hr OGTT (r = 0.52 and r = 0.45, respectively). On the other hand, the inverse correlation of HDL was significant with the 2-hr OGTT (r = −0.29) but not with 1-hr OGTT.
Table 3 shows the results of multivariate logistic regression analysis relating continuous glucose homeostasis measures with metabolic variables. Only HOMA Index and fasting insulin had a significant association with hypertension with an OR for a one unit increment of HOMA of 1.48 (95% CI: 1.06 - 2.06) and for insulin of 1.02 (95% CI: 1.00 - 1.03). Metabolic syndrome was significantly associated with 1-hr OGTT, 2-hr OGTT, fasting insulin and HOMA (OR = 1.50, OR = 1.48, OR = 1.02, and OR = 2.07 respectively). Although FPG is one of the components of metabolic syndrome, when both FPG and 1-hr OGTT were put simultaneously in the same model, only 1-hr OGTT remained significant. HOMA is strongly associated with metabolic syndrome in both models with and without 1-hr OGTT, and the 1-hr OGTT remained significant even in models that included HOMA (data not shown). 1-hr OGTT was significantly associated with fasting insulin and HOMA index and pre-diabetes (OR = 1.75, 1.02 and 1.95, respectively).
Table 3.
Logistic Regression relating glucose metabolism with metabolic variables
| Exposure | Outcome | Multivariate1 | |
|---|---|---|---|
| Odds ratio | 95% CI | ||
| Fasting glucose (mmol/L) | Hypertension2 | 2.15 | (0.78 - 5.89) |
| 1-hr OGTT (mmol/L) | Hypertension | 1.25 | (1.00 - 1.56) |
| 2-hr OGTT (mmol/L) | Hypertension | 1.15 | (0.83 - 1.59) |
| Fasting insulin (pmol/L) | Hypertension | 1.02 | (1.00 - 1.03) |
| HOMA IR index | Hypertension | 1.48 | (1.06 - 2.06) |
| Fasting glucose (mmol/L) | Hypercholesterolemia3 | 1.22 | (0.46 -3.21) |
| 1-hr OGTT (mmol/L) | Hypercholesterolemia | 1.20 | (0.97 - 1.49) |
| 2-hr OGTT (mmol/L) | Hypercholesterolemia | 1.17 | (0.84 - 1.61) |
| Fasting insulin (pmol/L) | Hypercholesterolemia | 1.00 | (1.00 - 1.01) |
| HOMA IR index | Hypercholesterolemia | 1.09 | (0.89 - 1.35) |
| 1-hr OGTT(mmol/L) | Pre-diabetes4 | 1.75 | (1.28 - 2.39) |
| 2-hr OGTT (mmol/L) | Pre-diabetes | 3.97 | (2.03 - 7.78) |
| Fasting insulin (pmol/L) | Pre-diabetes | 1.02 | (1.01 - 1.03) |
| HOMA IR index | Pre-diabetes | 1.95 | (1.35 - 2.83) |
| Fasting glucose (mmol/L) | Metabolic syndrome5 | 4.62 | (1.62 - 13.2) |
| 1-hr OGTT (mmol/L) | Metabolic syndrome | 1.50 | (1.18 - 1.91) |
| 2-hr OGTT (mmol/L) | Metabolic syndrome | 1.48 | (1.06 - 2.05) |
| Fasting insulin (pmol/L) | Metabolic syndrome | 1.02 | (1.01 - 1.04) |
| HOMA IR index | Metabolic syndrome | 2.07 | (1.45 - 2.97) |
Adjusted for age, sex, smoking (never vs. ever) and physical activity (structured or vigorous)
Hypertension = Have systolic BP ≥130 or diastolic BP ≥ 85 mm Hg or self-report of high BP or self-report of taking medication for high BP
Hypercholesterolemia = Have total cholesterol ≥ 240mg/dL OR self-report of high cholesterol or self-report of taking medication for high cholesterol
Pre-diabetes = Have fasting plasma glucose (Impaired FPG) ≥ 100mg/dL or have 2hr-glucose tolerance test ≥ 140mg/dL (Impaired GT)
Metabolic syndrome = Have 3 out of 5 of the following criteria (ATPIII definition)
Abdominal obesity (waist circumference): men >102 cm; women > 88 cm
Triglycerides ≥ 150mg/dL
HDL Cholesterol: men < 40 mg/dL; women <50mg/dL
Hypertension = Have systolic BP ≥130 or diastolic BP ≥ 85 mm Hg or self-report of high
BP or self-report of taking medication for high BP
Fasting Plasma glucose ≥ 100mg/dL
Among people who had elevated 1-hr OGTT (>8.60 mmol/L), 45% were classified as having pre-diabetes using FPG and/or 2-hr OGTT. Among people with normal 1-hr OGTT (24), 94% did not have pre-diabetes. The 1-hr OGTT correctly classified 79% of people without pre-diabetes. However, among those classified with pre-diabetes, the 1-hr OGTT only correctly classified 76%. This data is not presented in the tables.
Discussion
This study suggests that 1-hr OGTT is superior to 2-hr OGTT in identifying those with the highest metabolic risk in this population. Indeed, abnormalities in 1-hr OGTT may represent a more severe metabolic perturbation, characterized by greater glycemia, lower insulin sensitivity, and markedly reduced β-cell function.
Elevated glucose levels in type 2 diabetes occur because of decreased muscle uptake of glucose, increased hepatic glucose production, and decreased first phase insulin release. Elevated glucose levels are cytotoxic; thus hyperglycemia may be responsible, in part, for an additional decline in beta-cell function, which leads to lower insulin production. The effects of hyperglycemia also include reduced response to stimulus to secrete insulin and a gradual depletion of insulin stores. Although these differences may vary across populations and individuals, impaired fasting glucose appears to be relatively more indicative of insulin resistance and endogenous glucose production, whereas the 2-hr OGTT appears to be relatively more indicative of disturbances in insulin production (beta cell function) (24). The stronger correlation of the 1-hr OGTT with adiposity in our study suggests that the 1-hr OGTT better reflects the reduced insulin uptake in skeletal muscle known to be closely associated with obesity than what is reflected by the 2-hr OGTT. However, the correlation of 1-hr OGTT with both BMI and waist circumference, while higher than the parallel associations with 2-hr OGTT, is only moderately strong. Nonetheless, the 1-hr OGTT may reflect a more balanced combination of several homeostatic defects, by simultaneously manifesting the effects of insulin production and insulin resistance.
The percentage of people with insulin resistance (HOMA IR ≥ 2.5) is lower (90%) among the 29 people with high 1-hr PG (≥ 8.6 mmol/L) compared to 100% among the 7 people with 2hr-PG (≥ 7.77 mmol/L). This is expected because high 2-hr OGTT is less common and indicates more severe glucose abnormalities than high 1-hr OGTT. In spite of this, the partial correlation is higher between HOMA-IR and continuous variable for 1-hr OGTT (r=0.52) compared to the correlation between HOMA-IR and 2hr OGTT (r=0.45).
A cross-sectional analysis by Bardini et al. showed that elevated 1-hr plasma glucose in subjects with normal glucose tolerance and pre-diabetes was significantly associated with inflammatory markers, blood lipids, and insulin resistance (25). One cohort study showed that elevated 1-hour glucose levels might predict mortality, but no comparison was made to 2-hour glucose levels (26). In addition, two longitudinal studies, the San Antonio Heart Study (1) and the Botnia Study (2), have shown that the 1-hr plasma glucose concentration during the OGTT correlates better with indices of insulin secretion and insulin resistance compared to the 2-hr OGTT or the FPG concentrations. In both studies, the predictive power of 1-hr plasma glucose concentration, using the area under the receiver-operating curve (ROC), was significantly greater compared with both the fasting and 2-hr plasma glucose concentrations. In addition, both studies showed that participants with a 1-hr plasma glucose ≥155 mg/dl (or 8.60 for future incidence of diabetes than those with a 1-hr plasma glucose <155 mg/dl (or 8.60 mmol/L). In another recent cohort study from the same group, an increase in 1-hour OGTT in the normal range was associated with an increase in the incidence of type 2 diabetes. After controlling for 1-hour plasma glucose concentration, the increase in FPG concentration was not associated with an increase in the incidence of type 2 diabetes (27). A recent cross sectional study showed that high values of 1-hour OGTT may identify a condition between normal and impaired glucose tolerance, characterized by greater insulin resistance, reduced beta-cell glucose sensitivity, and reduced beta-cell rate sensitivity (28). A major finding of our study is the stronger correlation of the 1-hr OGTT with adiposity, suggesting it superior role in identifying the insulin resistance in skeletal muscle associated with obesity. The results of our study add evidence to studies indicating that the 1-hr OGTT may have greater utility than 2-hr OGTT.
There is limited information on the utility of different measures in predicting future cardiovascular risk. Studies show that 2-hr OGTT is better than fasting plasma glucose in predicting CVD risk (29). A recent study among adults free of diabetes, found that in comparison with fasting glucose, HbA1c was equally predictive of risk of diabetes and more strongly associated with risks of cardiovascular disease and death from any cause (10). However, no studies have compared 1-hr OGTT with HbA1c.
Both 1-hr and 2-hr OGTT will be influenced by gastric emptying time; therefore, some of the physiologic distinctiveness between the 1-hr and 2-hr OGTT will be clouded by individual differences in gastric emptying. Limitations of this study include the small sample size, restriction to overweight and obese individuals, and the unavailability of HbA1c measures and additional insulin measures at other time points. We cannot directly evaluate whether 1-hr OGTT better reflects insulin sensitivity and secretion than 2-hr OGTT in the absence of a direct measure of insulin sensitivity and secretion such as clamp or intravenous glucose tolerance test data. In addition, the cross-sectional nature of this study does not allow us to evaluate longitudinal differences in long term outcomes between the 1-hr and 2-hr OGTT over time.
The 1-hr OGTT shows stronger associations with major established predictors of diabetes and abnormal glucose homeostasis compared to the 2-hr OGTT in this Hispanic population. The 1-hr OGTT is less burdensome and shows promise for use in large scale epidemiological studies. Large prospective studies will be needed to elucidate the advantages and disadvantages of 1-hr OGTT compared to 2-hr OGTT and HbA1c in screening and diagnosis of type 2 diabetes and in evaluating its predictive use in epidemiologic studies.
Acknowledgements
The authors would like to acknowledge the SOALS team (Dr. Enrique Santiago, Dr. Mauricio Montero, Dr. Pedro Hernandez, Ms. Sasha Martínez, Mr. José L. Vergara, Mr. Francisco Muñoz, Mr. Gustavo Sanchez, Mr. Kristina Poventud, Ms. Jennifer Colon, Mr. Reinaldo Deliz, Ms. Jhezanuel Goncalves, Ms. Lumarie Cuadrado, Dr. Maribel Campos and Dr. Alberto Carrera for their help with the study. This investigation was supported by National Institutes of Health Grants K24DE16884.
Abbreviations used
- OGTT
Oral Glucose Tolerance Test
- FPG
Fasting Plasma Glucose
- hr
Hour
- HbA1c
Glycosylated Hemoglobin
- CVD
Cardiovascular Disease
- HDL
High Density Lipoprotein
- LDL
Low Density Lipoprotein
- IR
Insulin Resistance
- BMI
Body Mass Index
- ATP
Adult Treatment Panel
- IFG
Impaired Fasting Glucose
- IGT
Impaired Glucose Tolerance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- 1.Abdul-Ghani MA, Abdul-Ghani T, Ali N, Defronzo RA. One-hour plasma glucose concentration and the metabolic syndrome identify subjects at high risk for future type 2 diabetes. Diabetes Care. 2008 Aug;31(8):1650–5. doi: 10.2337/dc08-0225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting versus postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia Study. Diabetes Care. 2009 Feb;32(2):281–6. doi: 10.2337/dc08-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010 Mar;33(3):676–82. doi: 10.2337/dc09-1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Genuth S, Alberti KG, Bennett P, Buse J, Defronzo R, Kahn R, et al. Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care. 2003 Nov;26(11):3160–7. doi: 10.2337/diacare.26.11.3160. [DOI] [PubMed] [Google Scholar]
- 5.WHO. IDF . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia: report of a WHO/IDF Consultant. World Health Organization; Geneva: 2006. [Google Scholar]
- 6.Kilpatrick ES, Bloomgarden ZT, Zimmet PZ. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes: response to the International Expert Committee. Diabetes Care. 2009 Dec;32(12):e159. doi: 10.2337/dc09-1231. author reply e60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silva N, Dutzan N, Hernandez M, Dezerega A, Rivera O, Aguillon JC, et al. Characterization of progressive periodontal lesions in chronic periodontitis patients: levels of chemokines, cytokines, matrix metalloproteinase-13, periodontal pathogens and inflammatory cells. J Clin Periodontol. 2008 Mar;35(3):206–14. doi: 10.1111/j.1600-051X.2007.01190.x. [DOI] [PubMed] [Google Scholar]
- 8.Bartnik M, Ryden L, Malmberg K, Ohrvik J, Pyorala K, Standl E, et al. Oral glucose tolerance test is needed for appropriate classification of glucose regulation in patients with coronary artery disease: a report from the Euro Heart Survey on Diabetes and the Heart. Heart. 2007 Jan;93(1):72–7. doi: 10.1136/hrt.2005.086975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meigs JB, Nathan DM, D’Agostino RB, Sr., Wilson PW. Fasting and postchallenge glycemia and cardiovascular disease risk: the Framingham Offspring Study. Diabetes Care. 2002 Oct;25(10):1845–50. doi: 10.2337/diacare.25.10.1845. [DOI] [PubMed] [Google Scholar]
- 10.Selvin E, Steffes MW, Zhu H, Matsushita K, Wagenknecht L, Pankow J, et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults. N Engl J Med. Mar 4;362(9):800–11. doi: 10.1056/NEJMoa0908359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edelstein SL, Knowler WC, Bain RP, Andres R, Barrett-Connor EL, Dowse GK, et al. Predictors of progression from impaired glucose tolerance to NIDDM: an analysis of six prospective studies. Diabetes. 1997 Apr;46(4):701–10. doi: 10.2337/diab.46.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buhling KJ, Winkel T, Wolf C, Kurzidim B, Mahmoudi M, Wohlfarth K, et al. Optimal timing for postprandial glucose measurement in pregnant women with diabetes and a non-diabetic pregnant population evaluated by the Continuous Glucose Monitoring System (CGMS) J Perinat Med. 2005;33(2):125–31. doi: 10.1515/JPM.2005.024. [DOI] [PubMed] [Google Scholar]
- 13.Retnakaran R, Qi Y, Sermer M, Connelly PW, Zinman B, Hanley AJ. Isolated hyperglycemia at 1 hour on oral glucose tolerance test in pregnancy resembles gestational diabetes mellitus in predicting postpartum metabolic dysfunction. Diabetes Care. 2008 Jul;31(7):1275–81. doi: 10.2337/dc08-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jovanovic L. Rationale of prevention and treatment of postprandial glucose-mediated toxicity. Endocrinologist. 1999;9:87–92. [Google Scholar]
- 15.Warnick GR, Knopp RH, Fitzpatrick V, Branson L. Estimating low-density lipoprotein cholesterol by the Friedewald equation is adequate for classifying patients on the basis of nationally recommended cutpoints. Clin Chem. 1990 Jan;36(1):15–9. [PubMed] [Google Scholar]
- 16.CDC . National Center for Health Statistics. Third National Health and Nutrition Examination Survey Anthropometric Procedures Video: Centers for Disease Control and Prevention. [Google Scholar]
- 17.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, et al. Human blood pressure determination by sphygmomanometry. Circulation. 1993 Nov;88(5 Pt 1):2460–70. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 18.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006 Jan;21(1):1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 19.Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002 Dec 17;106(25):3143–421. [PubMed] [Google Scholar]
- 20.Pekkanen J, Linn S, Heiss G, Suchindran CM, Leon A, Rifkind BM, et al. Ten-year mortality from cardiovascular disease in relation to cholesterol level among men with and without preexisting cardiovascular disease. N Engl J Med. 1990 Jun 14;322(24):1700–7. doi: 10.1056/NEJM199006143222403. [DOI] [PubMed] [Google Scholar]
- 21.Madeira IR, Carvalho CN, Gazolla FM, de Matos HJ, Borges MA, Bordallo MA. Cut-off point for Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) index established from Receiver Operating Characteristic (ROC) curve in the detection of metabolic syndrome in overweight pre-pubertal children. Arq Bras Endocrinol Metabol. 2008 Dec;52(9):1466–73. doi: 10.1590/s0004-27302008000900010. [DOI] [PubMed] [Google Scholar]
- 22.Kesavalu L, Chandrasekar B, Ebersole JL. In vivo induction of proinflammatory cytokines in mouse tissue by Porphyromonas gingivalis and Actinobacillus actinomycetemcomitans. Oral Microbiol Immunol. 2002 Jun;17(3):177–80. doi: 10.1034/j.1399-302x.2002.170307.x. [DOI] [PubMed] [Google Scholar]
- 23.Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000 Sep;72(3):694–701. doi: 10.1093/ajcn/72.3.694. [DOI] [PubMed] [Google Scholar]
- 24.Hanefeld M, Koehler C, Fuecker K, Henkel E, Schaper F, Temelkova-Kurktschiev T. Insulin secretion and insulin sensitivity pattern is different in isolated impaired glucose tolerance and impaired fasting glucose: the risk factor in Impaired Glucose Tolerance for Atherosclerosis and Diabetes study. Diabetes Care. 2003 Mar;26(3):868–74. doi: 10.2337/diacare.26.3.868. [DOI] [PubMed] [Google Scholar]
- 25.Bardini G, Dicembrini I, Cresci B, Rotella CM. Inflammation markers and metabolic characteristics of subjects with 1-h plasma glucose levels. Diabetes Care. Feb;33(2):411–3. doi: 10.2337/dc09-1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meisinger C, Wolke G, Brasche S, Strube G, Heinrich J. Postload plasma glucose and 30-year mortality among nondiabetic middle-aged men from the general population: the ERFORT Study. Ann Epidemiol. 2006 Jul;16(7):534–9. doi: 10.1016/j.annepidem.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Abdul-Ghani MA, Stern MP, Lyssenko V, Tuomi T, Groop L, Defronzo RA. Minimal contribution of fasting hyperglycemia to the incidence of type 2 diabetes in subjects with normal 2-h plasma glucose. Diabetes Care. Mar;33(3):557–61. doi: 10.2337/dc09-1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manco M, Panunzi S, Macfarlane DP, Golay A, Melander O, Konrad T, et al. One-hour plasma glucose identifies insulin resistance and beta-cell dysfunction in individuals with normal glucose tolerance: cross-sectional data from the Relationship between Insulin Sensitivity and Cardiovascular Risk (RISC) study. Diabetes Care. Sep;33(9):2090–7. doi: 10.2337/dc09-2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrett-Connor E. The oral glucose tolerance test, revisited. Eur Heart J. 2002 Aug;23(16):1229–31. doi: 10.1053/euhj.2002.3243. [DOI] [PubMed] [Google Scholar]