Abstract
Although regional brain abnormalities underlying spatial working memory (SWM) deficits in schizophrenia have been identified, little is known about which brain circuits are functionally disrupted in the SWM network in schizophrenia. We investigated SWM-related interregional functional connectivity in schizophrenia using functional magnetic resonance imaging (fMRI) data collected during a memory task that required analysis of spatial information in object structure. Twelve schizophrenia patients and eleven normal control subjects participated. Patients had SWM performance deficits and deficient neural activation in various brain areas, especially in the high SWM load condition. Examination of the covariation of regional brain activations elicited by the SWM task revealed evidence of functional disconnection between prefrontal and posterior visual association areas in schizophrenia. Under low SMW load, we found reduced functional associations between dorsolateral prefrontal cortex (DLPFC) and inferior temporal cortex (ITC) in the right hemisphere in patients. Under high SWM load, we found evidence for further functional disconnection in patients, including additional reduced functional associations between left DLPFC and right visual areas, including the posterior parietal cortex (PPC), fusiform gyrus, and V1, as well as between right inferior frontal cortex and right PPC. Greater prefrontal-posterior cortical functional connectivity was associated with better SWM performance in controls, but not in patients. These results suggest that prefrontal-posterior functional connectivity associated with the maintenance and control of visual information is central to SWM, and that disruption of this functional network underlies SWM deficits in schizophrenia.
Keywords: schizophrenia, functional connectivity, spatial working memory, controlled visual processing, fMRI
1. INTRODUCTION
Impaired spatial working memory (SWM) is thought to be one of the fundamental cognitive dysfunctions in schizophrenia patients (Fleming et al., 1997; Park & Holzman, 1992; Tek et al., 2002) and a possible source of psychotic symptoms (Goghari, Sponheim, & MacDonald, 2010; Goldman-Rakic, 1994). Since SWM deficits have also been observed in schizotypal personality disorder (Park & McTigue, 1997; Roitman et al., 2000) and unaffected biological relatives of schizophrenia patients (Conklin, Curtis, Katsanis, & Iacono, 2000; Park, Holzman, & Goldman-Rakic, 1995), the SWM deficit is considered to be a candidate endophenotype for schizophrenia indicative of genetic liability to the disorder (Gottesman & Gould, 2003). SWM deficits in schizophrenia are analogous to those found in monkey and human patients with prefrontal lesions (Funahashi, Bruce, & Goldman-Rakic, 1993; Verin et al., 1993), thus providing considerable evidence that functional disruption of prefrontal cortex is integral to the disease and to SWM. Two components of working memory, those associated with executive control and maintenance, have been tied to activity in the PFC (Miller & Cohen, 2001). Neuroimaging studies have reported a failure in PFC areas associated with working memory deficits in schizophrenia (Glahn et al., 2005; Minzenberg, Laird, Thelen, Carter, & Glahn, 2009), thus supporting top-down control deficit theories of the disorder (Cohen & Servan-Schreiber, 1992; Goldman-Rakic, 1991). Specifically, recent neuroimaging studies have documented that schizophrenia is associated with PFC activation decay during maintenance (Driesen et al., 2008) and deficient dorsolateral PFC (DLPFC) activation during manipulation (i.e., executive control) of material in working memory (Cannon et al., 2005).
However, working memory is a multicomponent cognitive system where various brain regions coordinate to encode, maintain, and manipulate mental representations of stimuli (Repovs & Baddeley, 2006; Zimmer, 2008), thus a failure in any SWM network components or failure in the functional coordination between them can lead to performances deficits. The functional disconnection hypothesis of schizophrenia proposes that disruption of functional connectivity between PFC and other cortical association areas produce the symptoms of the disease (Friston, 1998). Functional connectivity has been defined as “the temporal correlation between spatially remote neurophysiological events” (Friston, 1998, p. 90). That is, if an increase (or decrease) of activity in one region is associated with an increase (or decrease) of activity in another region, these two regions may be considered functionally connected. Studies have reported functional disconnection between the PFC and superior temporal gyrus during various verbal cognitive tasks (Friston & Frith, 1995; Lawrie et al., 2002; Shergill et al., 2003), suggesting that cognitive deficits in schizophrenia result not only from disruption of local processing in PFC but also disruption of coordinated processing in distributed PFC networks.
A few studies have investigated disrupted interregional functional coordination in prefrontal networks for working memory in schizophrenia patients using N-back tasks (Kim et al., 2003; Meyer-Lindenberg et al., 2005; Schlosser et al., 2003; Tan et al., 2006). The most consistent finding is that working memory deficits in schizophrenia are associated with reduced functional coordination between PPC and lateral PFC, especially in dorsolateral (DLPFC) rather than ventrolateral PFC (VLPFC). Reduced functional connectivity between DLPFC and PPC was also observed while schizophrenia patients performed a cognitive control task requiring context processing (Yoon et al., 2008). DLPFC-PPC network is thought to be a core component of a) the task-positive network, whose activations have been observed while subjects perform various cognitive tasks (Fox & Raichle, 2007) and b) a top-down control network which initiates and adjusts cognitive control on a trial-to-trial basis (Dosenbach, Fair, Cohen, Schlaggar, & Petersen, 2008; Dosenbach et al., 2007). The functional coordination of these regions appears to reflect activity within the dorsal attentional network involved with top-down control of attention regardless of stimulus modality (Corbetta & Shulman, 2002). Therefore, findings of reduced DLPFC-PPC functional connectivity during working memory in schizophrenia patients may reflect dysfunctional top-down control and monitoring processes, rather than deficits in the processing of visual stimuli.
However, SWM also involves controlled visual processing, which may require the functional coordination of primary or associative visual areas with PFC in addition to the functional interaction in the DLPFC-PPC top-down control network. During a visual working memory task, complex stimulus information is encoded and maintained by the unique activity pattern of neurons in posterior association cortices, such as posterior parietal cortex (PPC) and inferior temporal cortex (ITC) (Zimmer, 2008), in which spatial and object information of visual stimuli are processed, respectively. Such maintenance of stimulus information is likely to be mediated in part by feedback projections from prefrontal cortex (PFC) to posterior association cortices, through reciprocal connections between these regions (Chafee & Goldman-Rakic, 2000; Miller, Erickson, & Desimone, 1996). Thus, failures in the domain-specific functional coordination between PFC and posterior visual association cortices may underlie SWM deficits in schizophrenia. On the other hand, although majority of working memory studies in schizophrenia have focused on deficient prefrontal top-down controls in schizophrenia, assuming intact early sensory and perceptual processes, findings in early visual processing deficits in schizophrenia patients have suggested contribution of perceptual deficits to visual working memory deficits in schizophrenia (Javitt, 2009). Consistent with the bottom-up hypothesis of cognitive deficits in schizophrenia, a recent neuroimaging study demonstrated that early sensory and perceptual processing deficits in occipital regions also contribute to deficits in encoding visual information and subsequent impaired performance on visual working memory tasks in schizophrenia patients (Haenschel et al., 2007). These findings further suggest that failures in visual areas or functional coordination deficits involving visual areas may also underlie SWM deficits in schizophrenia. Therefore it remains unclear what aspects of functional coordination (e.g., between primary and secondary visual association areas, between primary visual and PFC areas, or between visual association and PFC areas) are disrupted in schizophrenia and which functional disconnection most greatly contributes to SWM deficits.
In the present study, we address the question of altered functional connectivity in schizophrenia with a visual object construction (VOC) task that has been used to extensively characterize controlled visual processing at a cellular level in PPC of nonhuman primates (Chafee, Averbeck, & Crowe, 2007; Chafee, Crowe, Averbeck, & Georgopoulos, 2005; Crowe, Averbeck, & Chafee, 2008). In the VOC task, subjects view two objects presented in sequence, separated by an intervening delay period. Each object consists of a variable arrangement of identical square elements. The first object is a model, the configuration of which subjects must store in working memory. The second object is a partial copy of the preceding model, identical except that a single square element is missing. Subjects have to compare the copy object to the model stored in working memory to localize the missing square in the copy object, and then replace the missing square in order to reproduce (construct) the model configuration. SWM load is manipulated by varying the complexity of the visual objects. Successful performance of the task requires a computation that compares information provided by visual input (the configuration of the copy object) with information stored in working memory (the configuration of the model object), the result of which (the missing square differentiating the two objects) provides the basis for the correct response in the task. Unlike many prior studies that have examined cortical activation during passive maintenance of information in SWM or its resistance to distraction (Driesen, et al., 2008; Lee, Folley, Gore, & Park, 2008), the VOC task requires active computation using information stored in SWM. This allows us to examine PFC network connectivity associated with cognitive control, a top-down process by which the brain uses working memory to control computation and analysis of the sensory input to achieve a specific cognitive goal.
We hypothesized that the high demand on controlled visual processing for spatial and object information imposed by the visual stimuli in the VOC task would push network dynamics between the PFC and posterior association cortices awry in schizophrenia. Patients with schizophrenia were expected to have regional brain activation deficits associated with increased SWM loads, especially in PFC. In the analysis of interregional functional connectivity in SWM network, we hypothesized further that PFC would have deficient functional connectivity with posterior visual association areas (i.e., PPC and ITC) in patients relative to controls, and that the degree of this deficiency would scale with the degree of controlled processing. Specifically, the functional connectivity of DLPFC with visual association areas in schizophrenia patients was expected to be more reduced in high SWM load than in low SWM load. To test these hypotheses, we identified ROIs of SWM network components activated in response to SWM load, and conducted temporal correlation analyses for the all possible pairs of the components’ BOLD time series. Although whole brain network analysis such as graph theoretical or independent component analysis (ICA) approach may be utilized to assess functional connectivity, the inclusive ROI-based approach provides a better framework to test our hypothesis of functional connectivity between specific cortical areas within the SWM network. The association of the regional activation strength and the functional connectivity of these brain regions with SWM performance were also tested.
2. MATERIALS AND METHODS
2.1. Participants
Twelve schizophrenia patients and 11 age and gender matched non-psychiatric control subjects participated in the study. All participants were right-handed. Schizophrenia participants were recruited from the outpatient clinics of the Minneapolis VA Medical Center, community support programs for the mentally ill, and a county mental health clinic. Exclusion criteria included English as a second language, charted IQ less than 70 or a diagnosis of mental retardation, current alcohol or drug abuse, past drug dependence, a current or past central nervous system disease or condition, a medical condition or disease with likely significant central nervous system effects, history of head injury with skull fracture or loss of consciousness of greater than 20 min, and significant tardive dyskinesia as indicated by a Dyskinesia Identification System: Condensed User Scale (Sprague, Kalachnik, & Slaw, 1989) (DISCUS). Demographic and clinical characteristics of participants are provided in Table 1. The age range for participants in both groups was between 28 and 59 years. Control subjects were recruited through flier advertisement in the community. To obtain diagnostic information a trained interviewer completed the Diagnostic Interview for Genetic Studies (Nurnberger et al., 1994) with each patient. Using all available clinical information for a patient the interviewer completed the Operational Criteria for Psychotic Illness (McGuffin, Farmer, & Harvey, 1991) (OPCRIT) to derive a DSM-IV diagnosis. A doctoral-level clinical psychologist functioned as a consensus reviewer and also completed an OPCRIT for the participant. Any diagnostic disagreement between the interviewer and consensus reviewer was resolved by reviewing OPCRIT items on which ratings differed. All patients were being treated with antipsychotics at the time of testing. The average chlorpromazine equivalent for antipsychotic dosages was 788.89 mg (587.25 SD). Intelligence was measured for all participants using the Block Design and Vocabulary subtests of the Wechsler Adult Intelligent Scale, Third Edition (Wechsler, 1997). IQ was derived from the formula of Booker and Cyr (1986) using Vocabulary and Block Design subtests. From the structured interview and supplemental questions the interviewer rated the participant’s current symptomatology using the Scale for the Assessment of Negative Symptoms (Andreasen, 1981), the Scale for the Assessment of Positive Symptoms (Andreasen, 1983), and the 24-item version of the Brief Psychiatric Rating Scale (Ventura et al., 1993).
Table 1.
Demographic, clinical, treatment characteristics of the participants
| Schizophrenia Patients (N=12) | Controls (N=11) | Statisticsa | p | |
|---|---|---|---|---|
| Demographic variables
|
||||
| Age (years) | 45.4 (11.3) | 44.0 (7.9) | .35 | .732 |
| Education (years) | 14.0 (2.3) | 16.3 (1.8) | −2.70 | .014 |
| Parental education (years) | 13.9 (2.8) | 13.3 (2.1) | .54 | .595 |
| IQ estimate | 99.7 (14.6) | 110.2 (10.0) | −1.88 | .076 |
| % Male | 83 % | 73% | .38 | .538 |
|
| ||||
| Clinical variables
|
||||
| Overall symptomatologyb | 43.8 (10.7) | - | - | - |
| Psychosis symptom scorec | 1.6 (1.6) | - | - | - |
| Negative symptom scored | 1.5 (1.1) | - | - | - |
| Disorganization symptom scorese | 1.5 (1.5) | - | - | - |
| Illness duration (years) | 22 (10.15) | |||
|
| ||||
| Treatment variables
|
||||
| Medication (CPZ equivalent) | 788.9 (587.3) | - | ||
Note: Values are mean and standard deviation unless otherwise noted.
t(21) for continuous variables and χ2(1) for discrete variables.
Overall symptomatology score was computed as a total BPRS score (range: 0~168)
Psychosis symptom score was computed as the average global score for hallucinations and delusions in SAPS (range: 0~5).
Negative symptom score was computed as the average global score for alogia, affective flattening, avolition-apathy, and anhedonia-asociality in SANS (range: 0~5).
Disorganization symptom score was the global score for positive formal thought disorder in SAPS (range: 0~5).
2.2. Task and Procedure
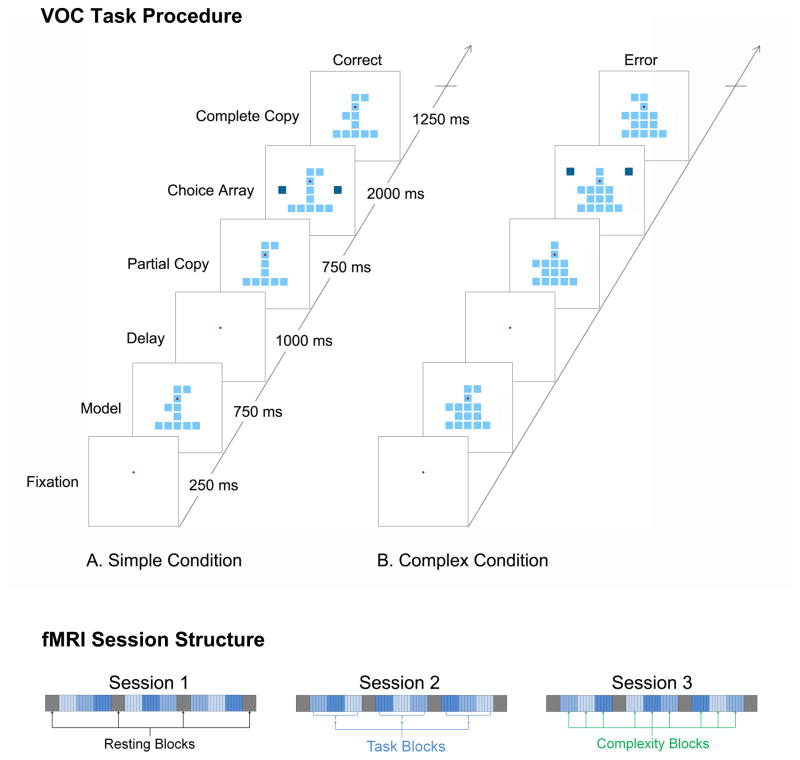
Subjects completed a VOC task as depicted in Figure 1 while fMRI data were acquired. The VOC task is based on tests of spatial cognitive function that require subjects to analyze the spatial structure of objects (Benton, 1967; Black & Strub, 1976; Driver, Baylis, Goodrich, & Rafal, 1994; Driver & Halligan, 1991; Piercy, Hecaen, & de Ajuriaguerra, 1960). In construction tasks of this type subjects attempt to produce a copy of a model object. This process requires that subjects perform a spatial analysis of the structure of the model object to determine the spatial relationships that localize components with respect to one other, a form of controlled visual processing that is reflected by the activity of single neurons in parietal cortex of monkeys performing the VOC task (Chafee, et al., 2007; Chafee, et al., 2005; Crowe, et al., 2008). In the VOC task, human subjects were instructed to fixate their eyes on a central point on the display screen. We then presented a model object that consisted of a varying spatial configuration of identical square elements (Fig. 1; ‘Model’, 750 ms) centered on the gaze fixation target. (Model objects were constructed on a 5 by 5 square grid of positions. All models included squares in the central column and base row of the grid to form an object frame in the shape of an inverted ‘T’. Different models were generated by adding a variable number of squares to the frame at random locations). Subjects had to analyze and remember the spatial configuration of the model object. After a delay period (1 s), we then presented a partial copy of the preceding model (Fig. 1; ‘Copy’, 750 ms), identical except that a single square element had been removed. The task was for the subject to compare the visible copy object to the preceding model object stored in working memory, in order to identify the location of the single missing square element that differentiated them. The activity of single neurons in parietal cortex of monkeys reflects the result of this spatial cognitive analysis, providing a signal that localizes the missing element when the copy object appears (Chafee, et al., 2005). We next presented a pair of choice squares on opposite sides of the copy object at the same vertical level as the missing square, and subjects pressed one of two response keys to indicate whether they wished to add the right or left choice square to the copy object. The selected choice square then animated inward to join the copy object automatically, producing a new configuration. If the subject selected the choice square on the same side of the object as the missing square, the addition of that choice square replaced the missing square, thereby reproducing (‘constructing’) the model configuration. If the subject selected the incorrect choice square, an erroneous configuration resulted that did not match the preceding model. A single trial lasted a total of six seconds. We varied the complexity of the model objects by varying the number of square elements added to the frame in order to test the effect of the increased SWM load on performance and neural responses. That is, model objects consisted of two, four, or six square elements added to the frame, which corresponded to the simple, intermediate, and complex conditions, respectively. These three levels of stimulus complexity allowed parametric manipulation of SWM load.
Figure 1.
Top: Visual object construction (VOC) task procedure for assessment of spatial working memory (SWM): A) simple (low load) condition with two added square elements, B) complex (high load) condition with six added square elements. Bottom: fMRI session structure.
During the fMRI sessions, participants performed three sessions of VOC trials. Each session consisted of four resting blocks of 28 seconds (during which the subjects were presented with a black visual field) interleaved with three task blocks. Each task block consisted of 18 trials (inter-stimulus interval = 6 sec), which took 108 seconds. The 18 trials in a task block consisted of 3 complexity blocks of 6 consecutive trials for each complexity level (e.g., 444444222222666666), in which the order of the complexity conditions was counterbalanced across the three sessions (see Fig 1, bottom).
2.3. fMRI Data Acquisition
Imaging scans were carried out on a 3.0 Tesla Siemens scanner using a standard CP head coil. Subjects were supine in the magnet and faced a mirror mounted above the head coil apparatus to view stimuli projected onto a screen in the bore of the scanner behind the head coil. The subject’s head was fixed using Styrofoam cushions to minimize head motion. Functional data was acquired using a standard Echo Planar Imaging (EPI) pulse sequence with the following parameters: 35 slices, axial orientation TR (repetition time) = 2 sec, TE (echo time) = 28 ms, matrix size = 64 × 64, flip angle = 90 degree, slice thickness = 3.5 mm, FOV (field of view) = 224. Slices were positioned along the anterior commissure–posterior commissure plane with reference to a high-resolution functional image. In addition, standard T1-weighted anatomical data were collected in the same orientation, with the same center slice (240 slices; 1 mm thickness).
2.4. fMRI Data Preprocessing
Image processing and analysis was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.63, part of FSL (FMRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl). Motion correction within each scan was performed using MCFLIRT (Jenkinson, Bannister, Brady, & Smith, 2002). Pre-statistics processing was conducted, including slice-timing correction using Fourier space time-series phase-shifting; non-brain removal using BET (Smith, 2002); spatial smoothing using a Gaussian kernel of FWHM 7 mm; mean-based intensity normalization of all volumes by the same factor; and high pass temporal filtering with cut-off of 72 sec (c.f., one complexity block length = 36 sec). Independent components analysis-based blind source separation was also carried out using MELODIC (Beckmann & Smith, 2004) to detect and remove artifacts, structured physiological and movement related noise. Finally, registration to the standard MNI152 template image was carried out using FLIRT (Jenkinson, et al., 2002; Jenkinson & Smith, 2001).
2.5. Statistical Analyses
2.5.1. Behavioral Data
Accuracy (% correct) and mean reaction time (RT; ms) were calculated for in-scanner task performance and analyzed using repeated measure analysis of variance (ANOVA) with independent variables of group and complexity. All analyses were corrected for any sphericity assumption violation via Hyun-Feldt correction. Significant interaction effects and effects of an independent variable with more than 2 levels were further analyzed with follow-up t-test.
2.5.2. Whole Brain Analyses
General linear modeling (GLM) analysis of fMRI data was carried out using FEAT with local temporal autocorrelation correction. Analyses were performed in two stages: First, statistical maps were calculated for each individual, and then the values of these maps were combined for hypothesis testing. Specifically, the complexity and group effects were estimated according to the GLM at each voxel. The statistical map associated with increased SWM load was generated by the subtraction of the simple condition results from those of the complex condition, yielding areas of relatively increased BOLD signals in response to high SWM load in both groups. Given that schizophrenia patients had worse VOC task performance, variance associated with error trials was modeled by a regressor delineating timing of error trials and removed in the GLM. Using a random effects analysis of activation in schizophrenia patients and normal controls, a between-group comparison was performed to assess the significance of differences in the magnitude of these activation effects across groups. The resulting set of voxel values for each contrast constituted a statistical parametric map of the z-statistics. The minimum cluster size for brain-wise significance was determined by AFNI AlphaSim program, using Monte Carlo simulations with a Gaussian filter to compensate for spatial autocorrelation effects (Ward, 2000). Then the z-statistics images were thresholded using clusters determined by z>2.242 with brain-wise cluster significance threshold of p=.05 (two-tailed test). For interregional functional connectivity analysis in SWM network, spherical ROIs (8 mm radius) masks were defined in z-statistics map showing greater activity in complex than in simple condition in both groups using AFNI program (http://afni.nimh.nih.gov/afni). To estimate the ROIs’ SWM-related activation strengths, mean % signal changes associated with the GLM experimental paradigm representing high SWM loads (simple<complex) were estimated using featquery (part of FSL) and the spherical ROI masks.
2.5.3. Interregional Functional Connectivity Analysis
For the thirteen ROIs of SWM network defined in the whole brain analysis, BOLD response time-series were extracted from each session fMRI data using the spherical ROI masks and 3dmaskave program (http://afni.nimh.nih.gov/pub/dist/doc/program_help/3dmaskave.html), a part of AFNI, by averaging the BOLD signals of voxels within the spherical ROIs. The BOLD times-series of individual blocks in the three sessions were concatenated for the three complexity condition periods and for all task periods. Temporal autocorrelation associated with artifact variances in the BOLD time-series due to scanner gain and physiological or movement related artifacts were removed in the preprocessing described above (i.e., mean-based intensity normalization and ICA artifact removal procedure). BOLD time-series data was further preprocessed to remove another type of temporal autocorrelation associated with linear trends due to session-related signal variance using a GLM procedure (Caclin & Fonlupt, 2006). Temporal Pearson correlation coefficients between all pairs of the thirteen ROI time-series in each task condition were computed as the index of interregional functional connectivity for each individual.
To test if patients had SWM task-related functional connectivity deficits compared to controls, we tested the group by complexity interaction effect in each ROI pair using permutation tests. To control for main effects of group and complexity in the interaction, we permuted residuals of temporal correlation coefficients, which were computed by subtracting the means of group and complexity levels from the correlation coefficients in each group by complexity cell and adding the total mean to them (Anderson & ter Braak, 2003). The residuals were permuted across the cells for 10000 times and the F-statistics of the interaction was computed with the permuted residuals to generate the random distribution of the interaction F-values. The p-value of the interactions in each ROI was computed as the fraction of the F-values on the permuted data that exceeded the actual F-value.
The main effects of group and complexity on the functional connectivity in each ROI pairs were also tested by permutation tests. In these tests, permutations were restricted within the levels of the other factor to control the interaction effect. That is, the group effects were tested by shuffling the data for each complexity levels separately between groups, while the complexity effects were tested by shuffling the data for each group separately between complexity levels (Anderson & Braak, 2003). The same procedure described above was used to generate random F-value distributions of group and complexity effects and compute the p-values of the group and the complexity effects.
To investigate group differences in functional connectivity for each complexity level in each ROI pair, we also used permutation tests. The functional connectivity measures for each complexity level were permuted between groups for 10000 times to generate t-statistics distribution of random group differences, and the p-values of group effect in each ROI pair for each complexity level were computed as described above. In these statistical tests for 78 ROI pairs, false discovery rate (FDR) approach was adopted to correct the increased Type I error due to multiple comparisons for the 78 pairs of the ROIs, controlling FDR below .05 (Benjamini & Hochberg, 1995).
2.5.4. Association of the Neural Activity Indices with SWM Performances
The association of the functional connectivity and regional mean % change indices with the behavioral performance indices (i.e., accuracy and RT) was tested using Pearson correlation analyses between these indices in each group. For the analyses to test associations between the different levels of measures (i.e., neurophysiological measure vs. behavioral measure), a relatively stringent criterion p-value of .005 was adopted instead of the more conservative FDR approach1.
3. RESULTS
3.1. Task Performance
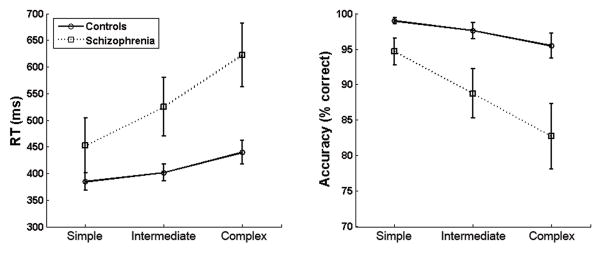
There was a main effect of complexity on the accuracy of subject responses. (F2,21=18.44, p<.001). Follow-up tests indicated that subjects had lower accuracy as stimulus complexity increased (simple vs. intermediate: t22=3.24, p=.004; intermediate vs. complex: t22=4.34, p<.001). Schizophrenia patients had lower accuracy than controls (F1,21=5.81, p<.025, Cohen’s d=1.03). An interaction of group and complexity (F2,21 =5.43, p<.014) indicated that the degree of group difference in accuracy varied depending on the complexity of the stimuli. Follow-up tests indicated that schizophrenia patients had significantly lower accuracy than controls in all three complexity conditions (simple: t22=2.21, p=.047, Cohen’s d=.91; intermediate: t22=2.40, p=.031, d=.98; complex: t22=2.57, p=.022, d=1.06), but the group difference was greatest for complex stimuli (see Figure 2).
Figure 2.
VOC task performance for schizophrenia patients and control subjects. Patients had significantly longer RTs and lower accuracy than controls, especially in the complex condition.
There was a main effect for stimulus complexity on reaction time (RT) (F2,21=13.00, p<.001). Follow-up tests indicated that subjects had longer RTs as complexity increased (simple vs. intermediate: t22=2.69, p=.013; intermediate vs. complex: t22=3.59, p=.002). There was also a main effect of group (F1,21=5.14, p=.034, d=-1.00), indicating that schizophrenia patients had longer RTs than controls. There was a trend of group by complexity interaction (F2,21=3.33, p=.066; see Figure 2). Follow-up tests indicated that patients had significantly longer RTs than control subjects for complex stimuli (t21=2.87, p=.012, d=-1.43), but the group difference in RTs was marginal for intermediate trials (t21=2.15, p=.052, d=-.88), and not present in simple trials (t21=1.27, p=.227, d=-.52).
3.2. SWM-related Brain Regions
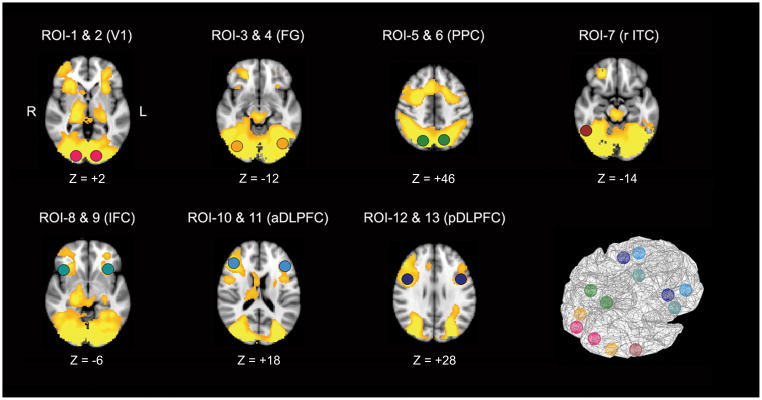
In response to high SWM load (complex > simple), both groups of subjects had increased activations of a network that included the PFC, posterior visual association areas, primary visual cortex, and subcortical areas such as thalamus, and cerebellum. From this activation map, 13 cortical areas were identified as components of the SWM neural network, which have been known to have roles in working memory and controlled visual processing. These cortical regions include bilateral DLPFC, bilateral IFC, bilateral PPC, right ITC, bilateral fusiform gyrus (FG) and bilateral primary visual cortex (V1; see Table 2 and Figure 3).
Table 2.
Regions demonstrating significantly greater activity in complex trials than in simple trails in both groups.
| Brain Regions | Brodman Area | Talairach Coordinates (mm)
|
z-stats | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R Anterior DLPFC | BA 46 | 44 | 32 | 18 | 3.91 |
| L Anterior DLPFC | BA 46 | −42 | 28 | 20 | 3.23 |
| R Posterior DLPFC | BA 9 | 44 | 4 | 28 | 4.15 |
| L Posterior DLPFC | BA 9 | −42 | 4 | 28 | 3.76 |
| R IFC | BA 47 | 34 | 20 | −6 | 3.70 |
| L IFC | BA 47 | −38 | 18 | −6 | 3.51 |
| R PPC | BA 7 | 18 | −70 | 48 | 4.45 |
| L PPC | BA 7 | −14 | −70 | 44 | 4.01 |
| R ITC | BA 20 | 52 | −56 | −14 | 4.58 |
| R FG | BA 37 | 34 | −78 | −12 | 4.36 |
| L FG | BA 37 | −40 | −68 | −14 | 4.38 |
| R V1 | BA 17 | 18 | −92 | 2 | 4.09 |
| L V1 | BA 17 | −14 | −94 | 0 | 4.28 |
Abbreviations: L: left, R: right, DLPFC: dorsolateral prefrontal cortex, FG: fusiform gyrus, IFC: inferior frontal cortex, ITC: inferior temporal cortex, PPC: posterior parietal cortex, V1: primary visual cortex
Figure 3.
Regions demonstrating significantly greater activity in complex trials than in simple trails in both groups (R: right, L: left).
3.3. Group Difference in SWM-related Regional Activations
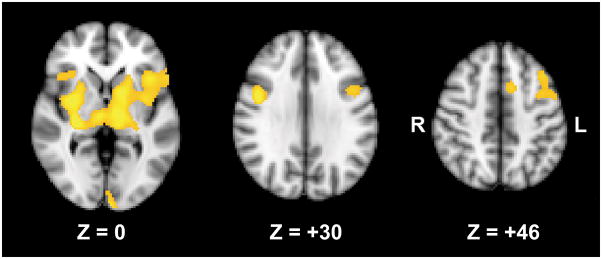
In the group comparison of regional activation in each complexity level, no group difference was found. Instead, there was a significant group by complexity interaction, where a significant group difference was observed in the comparison of regional activations in response to high SWM load (complex > simple). In this contrast, schizophrenia patients had reduced activations in bilateral DLPFC (BA 9), bilateral IFC (BA 47), left insula (BA 13), left pre-motor cortex (BA 6), left primary visual cortex, bilateral cerebellum, bilateral thalamus, bilateral putamen, and right hippocampus (see Table 3 and Figure 4).
Table 3.
Regions demonstrating significant group by difficulty interactions (controls > patients in complex > simple contrast).
| Brain Regions | Brodman Area | Talairach Coordinates (mm)
|
z-stats | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R Posterior DLPFC | BA 9 | 46 | 4 | 30 | 3.00 |
| L Posterior DLPFC | BA 9 | −40 | 12 | 36 | 2.58 |
| R IFC | BA 47 | 36 | 24 | −4 | 2.50 |
| L IFC | BA 47 | −42 | 18 | −2 | 2.85 |
| L Insula | BA 13 | −36 | 4 | 18 | 2.66 |
| L SMA | BA 6 | −12 | 10 | 50 | 2.70 |
| L V1 | BA 17 | −10 | −96 | −14 | 3.27 |
| R Cerebellum | 38 | −70 | −28 | 2.89 | |
| L Cerebellum | −34 | −58 | −34 | 2.88 | |
| R Thalamus | 6 | −16 | −2 | 2.96 | |
| L Thalamus | −12 | −14 | 2 | 3.12 | |
| R Putamen | 32 | −18 | −6 | 3.34 | |
| L Putamen | −14 | 6 | −2 | 3.02 | |
| R Hippocampus | −32 | −22 | −6 | 3.17 | |
Abbreviations: SMA: supplementary motor area
Figure 4.
Regions demonstrating significant group by complexity interactions (controls > patients for complex – simple contrast). The bottom numbers indicates the z coordinates in the Talairach space.
3.4. Group, Complexity, and Group by Complexity Interaction Effects in Functional Connectivity Measures
Regardless of complexity levels, schizophrenia patients had significantly lower functional connectivity than controls in 37 ROI pairs, after controlling FDR below .05 (10 ROI pairs between visual areas; 10 ROI pairs between prefrontal areas, and 17 ROI pairs between prefrontal and visual areas). Although functional connectivity between the ROIs tended to slightly increase as stimulus complexity increased (i.e., simple < intermediate < complex), no significant complexity effect was found in the functional connectivity between any of the pairs of ROIs. There were trends of group by complexity interaction effects in 6 prefrontal-visual ROI pairs (right IFC ~ left FG: p=.037; left IFC ~ right PPC: p=.025; left anterior DLPFC ~ left PPC: p=.047; right anterior DLPFC ~ right PPC: p=.026; right posterior DLPFC ~ left PPC: p=.048; left posterior DLPFC ~ right PPC: p=.020) and one between-prefrontal ROI pair (right posterior DLPFC ~ left anterior DLPFC: p=.033)2, suggesting that patients had tendencies to have more prefrontal-visual functional connectivity deficits in these ROI pairs as SWM load increased.
3.5. Group Difference in Functional Connectivity in Each Complexity Level
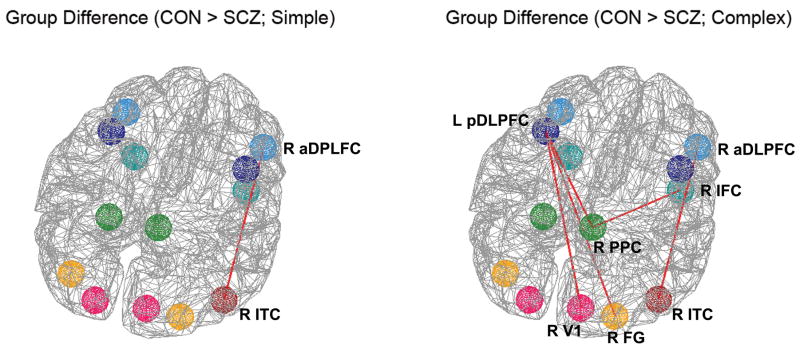
In the simple condition, schizophrenia patients had significantly reduced functional connectivity between the right anterior DLPFC and the right ITC (see Table 4 and Figure 5, left). When patients constructed more complex objects, significantly reduced temporal correlation in BOLD signal relative to controls was detected in additional pairs of areas in prefrontal and posterior cortex (Figure 5, right). Specifically, in the complex condition, significantly reduced temporal correlations in the patient group were detected between the ROI pairs of the right anterior DLPFC and the right ITC, left posterior DLPFC and right V1, left posterior DLPFC and right FG, left posterior DLPFC and right PPC, and right IFC and right PPC (see Table 4 and Figure 5, right). These results indicated that schizophrenia patients had reduced functional connections of the posterior sensory association areas with PFC areas, in response to complex model stimuli. Thus the degree of prefrontal-visual functional disconnection evident in the patient group relative to the control group was more profound when more controlled visual processing was required.
Table 4.
Group differences in temporal correlations of the fMRI time-series between the ROIs.
| Simple | Complex | ||||
|---|---|---|---|---|---|
| Means (SD) | p | Means (SD) | p | ||
| R ITC (BA 20) ~ R aDLPFC (BA 46) | CON | .71 (.24) | .0005 | .71 (.23) | .0004 |
| SCZ | .18 (.35) | .15 (.37) | |||
|
| |||||
| R V1 (BA 17) ~ L pDLPFC (BA 9) | CON | .53 (.18) | .0028 | .52 (.12) | .0004 |
| SCZ | .27 (.19) | .22 (.24) | |||
|
| |||||
| R FG (BA 37) ~ L pDLPFC (BA 9) | CON | .55 (.23) | .0065 | .54 (.15) | .0010 |
| SCZ | .30 (.18) | .26 (.22) | |||
|
| |||||
| R PPC (BA 7) ~ L pDLPFC (BA 9) | CON | .60 (.22) | .0376 | .67 (.12) | .0014 |
| SCZ | .39 (.25) | .31 (.34) | |||
|
| |||||
| R PPC (BA 7) ~ R IFC (BA 47) | CON | .56 (.26) | .0064 | .57 (.15) | .0010 |
| SCZ | .22 (.28) | .21 (.33) | |||
Figure 5.
Group differences in the fMRI time-series temporal correlations in simple (left) and complex (right) conditions. Red lines indicate interregional functional connectivity where group differences were found. In the simple condition, patients had reduced temporal correlations between R aDLPFC and R ITC, while they had reduced temporal correlations in more prefrontal-visual ROI pairs in the complex condition, including L pDLPFC ~ R PPC, L pDLPFC ~ R FG, L pDLPFC ~ R V1, R aDLPFC ~ R ITC, and R IFC ~ R PPC.
3.6. Association of the fMRI Indices with Behavioral Performances
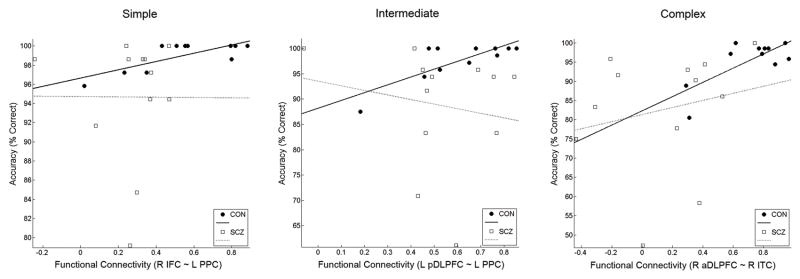
In correlation analyses of mean % signal changes in response to high SWM load in these regions with behavioral performance indices, no brain regions exhibited significant correlations in either group. In a similar analysis applied to the functional connectivity indices, control subjects exhibited a significant positive correlation of accuracy with the functional connectivity indices between left PPC and right IFC (r=.82, uncorrected p=.002) and between left PPC and left posterior DLPFC (r=.80, uncorrected p=.003) on intermediate condition trials. In addition, control subjects also exhibited positive correlations between accuracy and the functional connectivity index between left PPC and right IFC on simple trials (r=.77, uncorrected p=.006), and the functional connectivity index between right anterior DLPFC and right ITC on complex trials (r=.74, uncorrected p=.009). In contrast, schizophrenia patients did not have any significant association between the SWM performance and functional connectivity indices (see Figure 6). Thus, the strength of functional connectivity between regions appeared more predictive of successful performance than did activity within any given region in normal control subjects.
Figure 6.
Scatter plot of functional connectivity indices (temporal correlations) of the pair of right IFC and left PPC versus accuracy in simple trials (left), that of the pair of left posterior DLPFC (BA 9) and left PPC versus accuracy in intermediate trials (middle), and that of the pair of right anterior DLPFC (BA 46) and right ITC (right). Healthy control subjects had significant and tendency of positive correlations (simple: r=.77, p=.006; intermediate: r=.80, p=.003; complex: r=.74, p=.009), while schizophrenia patients did not.
3.7. Medication Effect
We tested if medications had an effect on findings by conducting a correlation analysis between CPZ-equivalent scores representing medication dosages and all fMRI and behavioral measures. It was found that CPZ-equivalent scores were negatively correlated with reaction time only for complex trials (r=-.68, p=.044), but not with accuracy measures. The regional activation strength measures were not significantly correlated with the CPZ-equivalent scores. Finally, correlation analysis of the functional connectivity measures with the CPZ-equivalent scores indicated that four functional connectivity measures between visual areas (right PPC ~ left FG, left PPC ~ left FG, left FG ~ left V1, and left PPC ~ left V1) for complex trials had significant negative correlation with CPZ equivalent (p<.005). Given that none of the prefrontal-visual functional connectivity measures where deficits were observed in patients were associated with the medication dosage, it seems that the main findings were not due to medication.
4. DISCUSSION
4.1. Summary of the Findings
In this study, we characterized changes in brain activation in schizophrenia during a task that required subjects to use spatial information stored in working memory. We parametrically manipulated the load on working memory and controlled visual processing by varying the complexity of the objects shown. We found that patients with schizophrenia relative to control subjects exhibited worse behavioral performance (Fig. 2) and reduced neural activation in a number of areas including prefrontal cortex in response to SWM load (Fig. 4). We also observed reduced functional connectivity between prefrontal and posterior visual cortical regions (Fig. 5). The prefrontal-posterior functional connectivity deficits tended to be more profound as SWM load increased, and a greater number of prefrontal-posterior functional connectivity deficits was found in complex trials compared to simple trials. These results provided evidence that SWM deficits in schizophrenia involve reduced prefrontal functional connectivity with visual association areas. Neither regional activation reduction nor functional connectivity deficit within posterior visual areas was observed, suggesting that failures in posterior visual systems might be not responsible for SWM deficits in schizophrenia. Interestingly, activities of posterior visual association cortices were uncorrelated with prefrontal activities but not reduced overall in schizophrenia. Specifically, there was no significant group by complexity interaction observed in posterior visual association cortex – BOLD signal in PPC and ITC scaled positively and equivalently with object complexity in both control and patient groups. That provides evidence that functional disconnection with PFC, more than failure to activate PPC and ITC during the construction task, was responsible for the deficits in controlled visual processing we observed. We consider these changes in cortical activation and functional connectivity and their relation to cognitive dysfunction in schizophrenia in greater detail below.
During the VOC task, many brain regions showed activations in both groups. Broad regions in visual cortex including bilateral V1, the main components of the ventral and dorsal visual pathways, such as the FG, ITC, and PPC, and PFC regions including bilateral DLPFC (BA 9 and 46) and IFC (BA 47) showed increased activations in response to higher load of encoding, maintenance, and retrieval or computation of spatial information on complex trials. Schizophrenia patients had SWM performance deficits in terms of both accuracy and RT. The significant group by task complexity interaction in behavioral performance indicated that patients had more profound SWM deficits when the task load was increased. A significant group by complexity interaction was also found in the functional imaging data. Although no group difference was found in brain activation within each level of SWM complexity, multiple brain regions showed group differences in the task complexity contrast (simple<complex), including frontal cortices (bilateral DLPFC [BA 9], bilateral IFC [BA 47], and bilateral SMA [BA 6]), primary visual cortex (left V1 [BA 17]), left insula [BA 13], subcortical regions (bilateral thalamus, left putamen, and right hippocampus), and bilateral cerebellum. Functional connectivity analyses of fMRI data indicated that schizophrenia patients had functional connectivity deficits between prefrontal and visual association areas during SWM performance. More pairs of prefrontal and visual cortical areas exhibited reduced functional connectivity on complex trials compared to simple trials and prefrontal-visual functional connectivity tended to be reduced in patients as SWM load increased, suggesting SWM-related prefrontal-visual functional connectivity deficits in schizophrenia. The fMRI functional connectivity indices between posterior visual association and prefrontal areas showed positive correlation with behavioral performance in control subjects but not in schizophrenia patients.
4.2. Distributed SWM Network
Thirteen cortical ROIs exhibited increased activation in both groups when complex relative to simple model and copy objects were shown, presumably to meet the increased demand for controlled visual processing, maintenance, and strategic retrieval of object information required. These areas have been reported to be activated during working memory or controlled visual processing in other studies; including bilateral DLPFC (BA9/46; D’Esposito, Postle, & Rypma, 2000; Funahashi, et al., 1993; Miller, et al., 1996; Petrides, 1996; Rowe, Toni, Josephs, Frackowiak, & Passingham, 2000), bilateral IFC (BA47; Badre & Wagner, 2007; Petrides, 1996), bilateral PPC (BA7; Andersen, 1997; Chafee, et al., 2005; Champod & Petrides, 2007), right ITC (BA20; Desimone, 1996; Woloszyn & Sheinberg, 2009), bilateral FG (BA37; Mainy et al., 2007; Postle, Druzgal, & D’Esposito, 2003), and bilateral primary visual cortex (BA17; D’Esposito, Cooney, Gazzaley, Gibbs, & Postle, 2006).
DLPFC has been inextricably associated with working memory processes in which information held online is monitored and controlled. These monitoring and control processes, in contrast to simple maintenance processes, have been proposed to be the main functional role of DLPFC (D’Esposito, et al., 2000; Petrides, 1996; Rowe, et al., 2000), consistent with the idea that this area serves as the ‘central executive’ proposed by Baddeley (1992). IFC (BA 47) or VLPFC have alternatively been associated with controlled retrieval of information during working memory (Petrides, 1996). Badre and Wagner (2007) further specified the function of VLPFC, in mediating the cognitive control of memory. In their scheme, the anterior VLPFC (BA 47; inferior frontal gyrus pars orbitalis) mediates top-down retrieval whereas the mid-VLPFC (BA 45; inferior frontal gyrus pars triangularis) mediates selection of goal-relevant over irrelevant information, defined in relation to goal representations in DLPFC.
The increased activity in primary visual and visual association areas for high SWM load indicated that controlled visual processing of more complex visual stimuli recruited additional resources in visual processing systems. The increased activations of broad regions of primary visual cortex in complex trials compared to simple trials might be also indicative of short-term maintenance of information by a posterior cortical mechanism (D’Esposito, et al., 2006). The increased activations in posterior visual association areas, including PPC, ITC, and FG, for high SWM loading suggests increased neural activation was required to mediate spatial processing of the more complex model objects displayed. That may include the activation of neurons in parietal cortex that are involved in the spatial analysis of object structure that is required by the construction task (Chafee, et al., 2007; Chafee, et al., 2005; Crowe, et al., 2008). PPC is specialized to process primarily the spatial parameters of visual input (Andersen, 1997) and it plays a central role in the allocation of attention in space (Corbetta, 1998; Donner et al., 2000). PPC is also thought to provide visuospatial input to SWM mechanisms in PFC via its projections forward to this area (Chafee & Goldman-Rakic, 2000) and to be involved as well in manipulating spatial information held in working memory (Champod & Petrides, 2007). As shown in the previous primate cell recording study using the same VOC task where neurons in PPC were activated to code spatial information of missing squares (Chafee, et al., 2005), PPC activation in this task is thought to reflect controlled visual processing to compute spatial information rather than simple control of attention. On the other hand, in our whole brain analysis, other association areas in ventral visual pathway including ITC and FG were also found to be activated. Neurons in ITC and FG have been known to process the form or identity of visual objects (Desimone, Albright, Gross, & Bruce, 1984; Gross, 1992; Tsunoda, Yamane, Nishizaki, & Tanifuji, 2001). The increased ITC and FG activities for complex trials suggested that subjects processed not only spatial information pertaining to the objects such as the relation of parts to each other (Chafee, et al., 2007; Chafee, et al., 2005; Crowe, et al., 2008), but also form information relating to the overall shape of the objects. Taken as a whole, the increased activations we observed in distributed prefrontal networks engaging posterior visual association cortices were likely to reflect the increased controlled visual processing load imposed by increasing object complexity.
4.3. Functional Connectivity Deficits in Schizophrenia Patients
Reduced functional connectivity between right DLPFC (BA46) and right ITC was found both in simple and complex trials in schizophrenia patients, suggesting that schizophrenia patients had deficient cortical communication between prefrontal and posterior visual association cortices, which might underlie poor performance of patients even in simple trials. Patients had reduced functional connectivity in more prefrontal-posterior ROI pairs in complex trials, including those of left DLPFC (BA 9) with right visual areas (V1, FG, and PPC), and between right IFC and right PPC. Non-human primate and human neuroimaging studies have provided evidence of strong interactions between lateral PFC and posterior sensory association areas during visual working memory functions (Chafee & Goldman-Rakic, 2000; Zimmer, 2008). When nonhuman primates made delayed saccades to target locations stored in SWM, a remarkable congruence in the type and pattern of single neuron activity was found in the PFC and the PPC areas (Chafee & Goldman-Rakic, 1998, 2000). This congruence could be disrupted by reversibly inactivating either PFC or PPC (Chafee & Goldman-Rakic, 2000), indicating that neural representations of space were generated at a network level in part by virtue of a physiological interaction taking place between parietal and prefrontal cortex during SWM performance.
The present finding that PFC is functionally disconnected from PPC in schizophrenia is generally consistent with previous findings of abnormal PFC-PPC connectivity in schizophrenia during working memory performance (Kim et al., 2003; Tan et al., 2006). However, in the present study we extended the finding of central executive control deficits due to DLPFC-PPC disconnection and related disruption in prefrontal network dynamics in schizophrenia to controlled visual processing, showing that disruptions in multiple functional connections between prefrontal and posterior visual association cortical areas contributed to deficits in SWM, where its severity tended to scale with controlled visual processing demand. In the VOC task, working memory must be engaged not only to retain information, but also to use it in goal-directed computation (as working memory is required to localize the missing square in the copy object). The hierarchical organization theory of primate lateral prefrontal cortex addressed the differential role of DLPFC and VLPFC (or inferior frontal cortex [IFC], the homologous area of VLPFC in humans) in working memory, where DLPFC engages in executive control while VLPFC is involved in mnemonic process to maintain and retrieve information in working memory (Koechlin, Ody, & Kouneiher, 2003; Petrides, 1996). Therefore, functional disconnection between DLPFC and ITC/PPC might represent a failure to effectively utilize information stored in working memory to compute the location of the missing square (monitoring of stored information), while those between IFC and PPC might represent poor mnemonic processing of model configuration (maintenance or controlled retrieval). Greater functional disconnection on complex trials was found in patients relative to control subjects mainly between left DLPFC and right visual cortical areas. This significant group difference indicates that whereas control subjects utilized greater functional connectivity of left DLPFC with right visual areas on complex trials, patients failed to do so.
Functional connectivity between posterior visual association cortices (i.e., PPC and ITC) and prefrontal cortices (i.e., DLPFC and IFC) was highly correlated with SWM performance in healthy control subjects, indicating the behavioral significance of functional integration in prefrontal-visual cortical networks for SWM. Functional connectivity between different prefrontal and posterior visual association areas showed different correlations with performance as a function of object complexity (see Figure 6). That is, functional connectivity of IFC with PPC was predictive of accuracy on simple and intermediate trials, while that of posterior DLPFC (BA 9) with PPC and that of anterior DLPFC (BA 46) with ITC were predictive of accuracy on intermediate and complex trials, respectively. These correlation patterns might indicate that functional coordination of IFC with PPC was crucial to support a simple mnemonic process applied to relatively simple objects, while the functional coordination of DLPFC with PPC and ITC was crucial for monitoring and manipulating object and spatial information when more complex objects were shown. The null finding of that functional connectivity was not correlated with SWM performance in patients suggests that the lower level of accuracy patients were able to achieve did not depend on functional integration of prefrontal-visual networks.
A supplementary analysis was conducted to investigate which functional measure is more predictive of SWM performance and whether the predictability of neural activity indices is different between groups. The correlations of regional activation strength indices with SWM performances and those of interregional functional connectivity indices were compared in the two groups3. The results indicated that the functional connectivity indices were generally more predictive of SWM performance in controls, but not in schizophrenia patients. In patients, the isolated regional activation strength indices had slightly higher predictability of SWM performance than the interregional functional connectivity indices, suggesting that the schizophrenia patients were less dependent on the functional connectivity for SWM performances. Therefore, other neural mechanisms (e.g., isolated regional activations rather than interregional coordination) might be recruited to compensate for a deficient prefrontal-visual functional coordination in patients.
Given findings of frontotemporal and frontoparietal structural connectivity deficits in diffusion tensor imaging (DTI) study of schizophrenia (Burns et al., 2003; Kubicki et al., 2007), the findings of functional disconnectivity in prefrontal-visual asscoation cortical areas in this study might be partly due to the deficient structural connectivity between the areas. However, as the pattern of functional disconnection tended to vary with task load, it is not likely to reflect anatomical abnormalities only.
4.4. Limitations
The present study had some limitations in that small samples were used, thus our statistical power to detect more subtle group difference may have been limited. In addition, only a limited number of cortical regions were investigated for network dynamics related to SWM. Given the wide spread activations over subcortical and cerebellar areas during SWM task performances, complete functional connectivity analysis including the whole brain may discern additional deficits in neural network dynamic in schizophrenia patients. Finally, the current analyses addressed potential performance-related confounds in the initial whole-brain analysis using covariance techniques (Ebmeier, Lawrie, Blackwood, Johnstone, & Goodwin, 1995; Gur & Gur, 1995). This approach ameliorated any group differences driven by inaccurate trials rather than underlying functional differences; however this approach was not possible in the correlation analysis using time-series. Yet, these correlations were only calculated between regions identified in the earlier analysis, and are therefore unlikely to be spurious.
4.5. Conclusions
Recent neuroimaging studies have provided information about underlying neural mechanism of SWM deficits in schizophrenia. However, isolated regional brain deficits have limitations in explaining how brain networks fail in schizophrenia to establish the dynamic interactions between cortical areas that are likely to mediate successful SWM performance. The present study demonstrated that distributed prefrontal networks engaging both dorsal and ventral visual processing systems were more strongly activated when working memory controlled the visual spatial processing of increasingly complex objects. Although SWM-related mean signal changes in isolated brain regions failed to predict task performance, functional connectivity between prefrontal and posterior visual association areas did predict performance in control subjects, suggesting that functional integration in PFC networks was necessary to mediate the controlled visual processing required. In addition, compared to trials in which simple objects were shown, greater functional disconnection in prefrontal-visual networks became apparent when patients processed more complex objects, a condition in which patients also exhibited more profound behavioral impairment. These results indicate that disruption in the flow of information within prefrontal-visual networks might have a more profound effect on controlled visual processing that is crucial for successful SWM task performance than regional PFC failure. Future studies combining functional and structural connectivity measures may further clarify the pathophysiology associated with the working memory and controlled processing deficits in schizophrenia.
Research Highlights.
Spatial working memory brain dynamics was investigated in schizophrenia patients.
Patients had functional disconnections in prefrontal-visual association areas.
The functional disconnection was severer in complex than in simple stimulus trials.
The functional connectivity was predictive of task performance in controls.
Isolated regional activation was more predictive of task performance in patients.
Acknowledgments
This work was supported by grants from the National Institutes of Mental Health (5R24MH069675 and RO1MH77779), and by grants from the Department of Veterans Affairs Medical Research Service to Dr. Scott Sponheim, as well as by the Mental Health Patient Service Line at the Veterans Affairs Medical Center, Minneapolis Minnesota. We are grateful to the participants and also for the efforts of Rachel Force, John J Stanwyck, Amy Silberschmidt, JoAn Laes, and James N Porter.
Footnotes
In terms of the corrections of statistical significance in the tests of multiple functional connectivity indices, we tried to minimize both Type I and Type II errors by choosing FDR and a stringent p-value. Although the assumption of independency of tests in FDR (Benjamini & Hochberg, 1995) is not relevant to the multiple tests of the functional connectivity measures, in which the correlation coefficients of the ROI pairs were highly correlated to each other, we choose the conservative FDR to identify truly meaningful group difference among the many pairs of brain regions with minimal probability of Type I error. On the other hand, to minimize Type II error we choose the less conservative but still stringent criterion p-value of .005 for the multiple association tests between the measures with different levels (i.e., neurophysiological and behavioral measures), where very high correlations are rarely observed. However, it should be noted that the criterion p-value of .005 is arbitrary and might allow some Type I error.
These findings were considered to be in a trend level given that they were significant in a conventional criterion p-value of .05, but not significant in FDR correction.
Given that each ROI has multiple functional connectivity indices with the other 12 ROIs and the functional connectivity of each ROI greatly varied across the pair ROIs, the maximum temporal correlations of each ROI with the other ROIs were computed as the index of functional connectivity of each ROI for this analysis.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andersen RA. Multimodal integration for the representation of space in the posterior parietal cortex. Philos Trans R Soc Lond B Biol Sci. 1997;352(1360):1421–1428. doi: 10.1098/rstb.1997.0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson MJ, ter Braak CJF. Permutation tests for multi-factorial analysis of variance. Journal of Statistical Computation and Simulation. 2003;73(2):85–113. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City: University of Iowa; 1981. [Google Scholar]
- Andreasen NC. Scale for the Assessment of Positive Symptoms (SAPS) Iowa City: University of Iowa; 1983. [Google Scholar]
- Baddeley A. Working memory. Science. 1992;255(5044):556–559. doi: 10.1126/science.1736359. [DOI] [PubMed] [Google Scholar]
- Badre D, Wagner AD. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Beckmann CF, Smith SM. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans Med Imaging. 2004;23(2):137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Benjamini Yoav, Hochberg Yosef. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Statist Soc B. 1995;57(1):289–300. [Google Scholar]
- Benton AL. Constructional apraxia and the minor hemisphere. Confin Neurol. 1967;29(1):1–16. [PubMed] [Google Scholar]
- Black FW, Strub RL. Constructional apraxia in patients with discrete missile wounds of the brain. Cortex. 1976;12(3):212–220. doi: 10.1016/s0010-9452(76)80002-0. [DOI] [PubMed] [Google Scholar]
- Booker BH, Cyr JJ. Tables for clinicians to use to convert WAIS-R short forms. Journal of Clinical Psychology. 1986;42:983. [Google Scholar]
- Burns J, Job D, Bastin ME, Whalley H, Macgillivray T, Johnstone EC, Lawrie SM. Structural disconnectivity in schizophrenia: a diffusion tensor magnetic resonance imaging study. Br J Psychiatry. 2003;182:439–443. [PubMed] [Google Scholar]
- Caclin A, Fonlupt P. Effect of initial fMRI data modeling on the connectivity reported between brain areas. Neuroimage. 2006;33(2):515–521. doi: 10.1016/j.neuroimage.2006.07.019. [DOI] [PubMed] [Google Scholar]
- Cannon TD, Glahn DC, Kim J, Van Erp TG, Karlsgodt K, Cohen MS, Shirinyan D. Dorsolateral prefrontal cortex activity during maintenance and manipulation of information in working memory in patients with schizophrenia. Arch Gen Psychiatry. 2005;62(10):1071–1080. doi: 10.1001/archpsyc.62.10.1071. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Averbeck BB, Crowe DA. Representing spatial relationships in posterior parietal cortex: single neurons code object-referenced position. Cereb Cortex. 2007;17(12):2914–2932. doi: 10.1093/cercor/bhm017. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Crowe DA, Averbeck BB, Georgopoulos AP. Neural correlates of spatial judgement during object construction in parietal cortex. Cereb Cortex. 2005;15(9):1393–1413. doi: 10.1093/cercor/bhi021. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Matching patterns of activity in primate prefrontal area 8a and parietal area 7ip neurons during a spatial working memory task. J Neurophysiol. 1998;79(6):2919–2940. doi: 10.1152/jn.1998.79.6.2919. [DOI] [PubMed] [Google Scholar]
- Chafee MV, Goldman-Rakic PS. Inactivation of parietal and prefrontal cortex reveals interdependence of neural activity during memory-guided saccades. J Neurophysiol. 2000;83(3):1550–1566. doi: 10.1152/jn.2000.83.3.1550. [DOI] [PubMed] [Google Scholar]
- Champod AS, Petrides M. Dissociable roles of the posterior parietal and the prefrontal cortex in manipulation and monitoring processes. Proc Natl Acad Sci U S A. 2007;104(37):14837–14842. doi: 10.1073/pnas.0607101104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Servan-Schreiber D. Context, cortex, and dopamine: a connectionist approach to behavior and biology in schizophrenia. Psychol Rev. 1992;99(1):45–77. doi: 10.1037/0033-295x.99.1.45. [DOI] [PubMed] [Google Scholar]
- Conklin HM, Curtis CE, Katsanis J, Iacono WG. Verbal working memory impairment in schizophrenia patients and their first-degree relatives: evidence from the digit span task. Am J Psychiatry. 2000;157(2):275–277. doi: 10.1176/appi.ajp.157.2.275. [DOI] [PubMed] [Google Scholar]
- Corbetta M. Frontoparietal cortical networks for directing attention and the eye to visual locations: identical, independent, or overlapping neural systems? Proc Natl Acad Sci U S A. 1998;95(3):831–838. doi: 10.1073/pnas.95.3.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crowe DA, Averbeck BB, Chafee MV. Neural ensemble decoding reveals a correlate of viewer- to object-centered spatial transformation in monkey parietal cortex. J Neurosci. 2008;28(20):5218–5228. doi: 10.1523/JNEUROSCI.5105-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Esposito M, Cooney JW, Gazzaley A, Gibbs SE, Postle BR. Is the prefrontal cortex necessary for delay task performance? Evidence from lesion and FMRI data. J Int Neuropsychol Soc. 2006;12(2):248–260. doi: 10.1017/S1355617706060322. [DOI] [PubMed] [Google Scholar]
- D’Esposito M, Postle BR, Rypma B. Prefrontal cortical contributions to working memory: evidence from event-related fMRI studies. Exp Brain Res. 2000;133(1):3–11. doi: 10.1007/s002210000395. [DOI] [PubMed] [Google Scholar]
- Desimone R. Neural mechanisms for visual memory and their role in attention. Proc Natl Acad Sci U S A. 1996;93(24):13494–13499. doi: 10.1073/pnas.93.24.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desimone R, Albright TD, Gross CG, Bruce C. Stimulus-selective properties of inferior temporal neurons in the macaque. J Neurosci. 1984;4(8):2051–2062. doi: 10.1523/JNEUROSCI.04-08-02051.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner T, Kettermann A, Diesch E, Ostendorf F, Villringer A, Brandt SA. Involvement of the human frontal eye field and multiple parietal areas in covert visual selection during conjunction search. Eur J Neurosci. 2000;12(9):3407–3414. doi: 10.1046/j.1460-9568.2000.00223.x. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Cohen AL, Schlaggar BL, Petersen SE. A dual-networks architecture of top-down control. Trends Cogn Sci. 2008;12(3):99–105. doi: 10.1016/j.tics.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proc Natl Acad Sci U S A. 2007;104(26):11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driesen NR, Leung HC, Calhoun VD, Constable RT, Gueorguieva R, Hoffman R, Krystal JH. Impairment of working memory maintenance and response in schizophrenia: functional magnetic resonance imaging evidence. Biol Psychiatry. 2008;64(12):1026–1034. doi: 10.1016/j.biopsych.2008.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J, Baylis GC, Goodrich SJ, Rafal RD. Axis-based neglect of visual shapes. Neuropsychologia. 1994;32(11):1353–1365. doi: 10.1016/0028-3932(94)00068-9. [DOI] [PubMed] [Google Scholar]
- Driver J, Halligan PW. Can visual neglect operate in object-centred co-ordinates?: An affirmative single case study. Cogn Neuropsychol. 1991;8:475–496. [Google Scholar]
- Ebmeier KP, Lawrie SM, Blackwood DHR, Johnstone EC, Goodwin GM. Hypofrontality revisited: a high resolution single photon emission computer tomography study in schizophrenia. Journal of Neurology, Neurosurgery and Psychiatry. 1995;58:452–456. doi: 10.1136/jnnp.58.4.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming K, Goldberg TE, Binks S, Randolph C, Gold JM, Weinberger DR. Visuospatial working memory in patients with schizophrenia. Biol Psychiatry. 1997;41(1):43–49. doi: 10.1016/s0006-3223(96)00263-6. [DOI] [PubMed] [Google Scholar]
- Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- Friston KJ. The disconnection hypothesis. Schizophr Res. 1998;30(2):115–125. doi: 10.1016/s0920-9964(97)00140-0. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD. Schizophrenia: a disconnection syndrome? Clin Neurosci. 1995;3(2):89–97. [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Dorsolateral prefrontal lesions and oculomotor delayed-response performance: evidence for mnemonic “scotomas”. J Neurosci. 1993;13(4):1479–1497. doi: 10.1523/JNEUROSCI.13-04-01479.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn DC, Ragland JD, Abramoff A, Barrett J, Laird AR, Bearden CE, Velligan DI. Beyond hypofrontality: a quantitative meta-analysis of functional neuroimaging studies of working memory in schizophrenia. Hum Brain Mapp. 2005;25(1):60–69. doi: 10.1002/hbm.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goghari VM, Sponheim SR, MacDonald AW., 3rd The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci Biobehav Rev. 2010;34(3):468–486. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Prefrontal cortical dysfunction in schizophrenia: The relevance of working memory. In: Carroll BJ, Barrett JE, editors. Psychopathology and the brain. New York: Raven Press; 1991. pp. 1–23. [Google Scholar]
- Goldman-Rakic PS. Working memory dysfunction in schizophrenia. J Neuropsychiatry Clin Neurosci. 1994;6(4):348–357. doi: 10.1176/jnp.6.4.348. [DOI] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160(4):636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gross CG. Representation of visual stimuli in inferior temporal cortex. Philosophical Transactions of the Royal Society of London - Series B: Biological Sciences. 1992;335(1273):3–10. doi: 10.1098/rstb.1992.0001. [DOI] [PubMed] [Google Scholar]
- Gur RC, Gur RE. Hypofrontality in schizophrenia: RIP. Lancet. 1995;345:1338–1340. doi: 10.1016/s0140-6736(95)92591-0. [DOI] [PubMed] [Google Scholar]
- Haenschel C, Bittner RA, Haertling F, Rotarska-Jagiela A, Maurer K, Singer W, Linden DE. Contribution of impaired early-stage visual processing to working memory dysfunction in adolescents with schizophrenia: a study with event-related potentials and functional magnetic resonance imaging. Arch Gen Psychiatry. 2007;64(11):1229–1240. doi: 10.1001/archpsyc.64.11.1229. [DOI] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5(2):143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Kwon JS, Park HJ, Youn T, Kang DH, Kim MS, Lee MC. Functional disconnection between the prefrontal and parietal cortices during working memory processing in schizophrenia: a[15(O)]H2O PET study. Am J Psychiatry. 2003;160(5):919–923. doi: 10.1176/appi.ajp.160.5.919. [DOI] [PubMed] [Google Scholar]
- Koechlin E, Ody C, Kouneiher F. The architecture of cognitive control in the human prefrontal cortex. Science. 2003;302(5648):1181–1185. doi: 10.1126/science.1088545. [DOI] [PubMed] [Google Scholar]
- Kubicki M, McCarley R, Westin CF, Park HJ, Maier S, Kikinis R, Shenton ME. A review of diffusion tensor imaging studies in schizophrenia. J Psychiatr Res. 2007;41(1–2):15–30. doi: 10.1016/j.jpsychires.2005.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrie SM, Buechel C, Whalley HC, Frith CD, Friston KJ, Johnstone EC. Reduced frontotemporal functional connectivity in schizophrenia associated with auditory hallucinations. Biol Psychiatry. 2002;51(12):1008–1011. doi: 10.1016/s0006-3223(02)01316-1. [DOI] [PubMed] [Google Scholar]
- Lee J, Folley BS, Gore J, Park S. Origins of spatial working memory deficits in schizophrenia: an event-related FMRI and near-infrared spectroscopy study. PLoS One. 2008;3(3):e1760. doi: 10.1371/journal.pone.0001760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mainy N, Kahane P, Minotti L, Hoffmann D, Bertrand O, Lachaux JP. Neural correlates of consolidation in working memory. Hum Brain Mapp. 2007;28(3):183–193. doi: 10.1002/hbm.20264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuffin P, Farmer A, Harvey I. A polydiagnostic application of operational criteria in studies of psychotic illness. Development and reliability of the OPCRIT system. Arch Gen Psychiatry. 1991;48(8):764–770. doi: 10.1001/archpsyc.1991.01810320088015. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Miller EK, Erickson CA, Desimone R. Neural mechanisms of visual working memory in prefrontal cortex of the macaque. J Neurosci. 1996;16(16):5154–5167. doi: 10.1523/JNEUROSCI.16-16-05154.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Laird AR, Thelen S, Carter CS, Glahn DC. Meta-analysis of 41 functional neuroimaging studies of executive function in schizophrenia. Arch Gen Psychiatry. 2009;66(8):811–822. doi: 10.1001/archgenpsychiatry.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS. Schizophrenics show spatial working memory deficits. Arch Gen Psychiatry. 1992;49(12):975–982. doi: 10.1001/archpsyc.1992.01820120063009. [DOI] [PubMed] [Google Scholar]
- Park S, Holzman PS, Goldman-Rakic PS. Spatial working memory deficits in the relatives of schizophrenic patients. Arch Gen Psychiatry. 1995;52(10):821–828. doi: 10.1001/archpsyc.1995.03950220031007. [DOI] [PubMed] [Google Scholar]
- Park S, McTigue K. Working memory and the syndromes of schizotypal personality. Schizophr Res. 1997;26(2–3):213–220. doi: 10.1016/s0920-9964(97)00051-0. [DOI] [PubMed] [Google Scholar]
- Petrides M. Specialized systems for the processing of mnemonic information within the primate frontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1455–1461. doi: 10.1098/rstb.1996.0130. [DOI] [PubMed] [Google Scholar]
- Piercy M, Hecaen H, de Ajuriaguerra J. Constructional apraxia associated with unilateral cerebral lesions - left and right sided cases compared. Brain. 1960;83:225–242. doi: 10.1093/brain/83.2.225. [DOI] [PubMed] [Google Scholar]
- Postle BR, Druzgal TJ, D’Esposito M. Seeking the neural substrates of visual working memory storage. Cortex. 2003;39(4–5):927–946. doi: 10.1016/s0010-9452(08)70871-2. [DOI] [PubMed] [Google Scholar]
- Repovs G, Baddeley A. The multi-component model of working memory: explorations in experimental cognitive psychology. Neuroscience. 2006;139(1):5–21. doi: 10.1016/j.neuroscience.2005.12.061. [DOI] [PubMed] [Google Scholar]
- Roitman SE, Mitropoulou V, Keefe RS, Silverman JM, Serby M, Harvey PD, Siever LJ. Visuospatial working memory in schizotypal personality disorder patients. Schizophr Res. 2000;41(3):447–455. doi: 10.1016/s0920-9964(99)00085-7. [DOI] [PubMed] [Google Scholar]
- Rowe JB, Toni I, Josephs O, Frackowiak RS, Passingham RE. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288(5471):1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Shergill SS, Brammer MJ, Fukuda R, Williams SC, Murray RM, McGuire PK. Engagement of brain areas implicated in processing inner speech in people with auditory hallucinations. Br J Psychiatry. 2003;182:525–531. doi: 10.1192/bjp.182.6.525. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague RL, Kalachnik JE, Slaw KM. Psychometric properties of the Dyskinesia Identification System: Condensed User Scale (DISCUS) Ment Retard. 1989;27(3):141–148. [PubMed] [Google Scholar]
- Tan HY, Sust S, Buckholtz JW, Mattay VS, Meyer-Lindenberg A, Egan MF, Callicott JH. Dysfunctional prefrontal regional specialization and compensation in schizophrenia. Am J Psychiatry. 2006;163(11):1969–1977. doi: 10.1176/ajp.2006.163.11.1969. [DOI] [PubMed] [Google Scholar]
- Tek C, Gold J, Blaxton T, Wilk C, McMahon RP, Buchanan RW. Visual perceptual and working memory impairments in schizophrenia. Arch Gen Psychiatry. 2002;59(2):146–153. doi: 10.1001/archpsyc.59.2.146. [DOI] [PubMed] [Google Scholar]
- Tsunoda K, Yamane Y, Nishizaki M, Tanifuji M. Complex objects are represented in macaque inferotemporal cortex by the combination of feature columns. Nat Neurosci. 2001;4(8):832–838. doi: 10.1038/90547. [DOI] [PubMed] [Google Scholar]
- Ventura J, Lukoff D, Nuechterlein KH, Liberman RP, Green MF, Shaner A. Manual for the expanded Brief Psychiatric Rating Scale. International Journal of Methods in Psychiatric Research. 1993;3:221–224. [Google Scholar]
- Verin M, Partiot A, Pillon B, Malapani C, Agid Y, Dubois B. Delayed response tasks and prefrontal lesions in man--evidence for self generated patterns of behaviour with poor environmental modulation. Neuropsychologia. 1993;31(12):1379–1396. doi: 10.1016/0028-3932(93)90105-9. [DOI] [PubMed] [Google Scholar]
- Ward BD. AlphaSim program documentation for AFNI. Milwakee: Medical College of Wisconsin; 2000. [Accessed May 11, 2011.]. Simultaneous Inference for fMRI Data. Available at: http://psyphz.psych.wisc.edu/web/afni/manuals/AlphaSim.pdf. Retrieved from http://psyphz.psych.wisc.edu/web/afni/manuals/AlphaSim.pdf. [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale. 3. New York: Psychological; 1997. [Google Scholar]
- Woloszyn L, Sheinberg DL. Neural dynamics in inferior temporal cortex during a visual working memory task. J Neurosci. 2009;29(17):5494–5507. doi: 10.1523/JNEUROSCI.5785-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon JH, Minzenberg MJ, Ursu S, Ryan Walter BS, Wendelken C, Ragland JD, Carter CS. Association of dorsolateral prefrontal cortex dysfunction with disrupted coordinated brain activity in schizophrenia: relationship with impaired cognition, behavioral disorganization, and global function. Am J Psychiatry. 2008;165(8):1006–1014. doi: 10.1176/appi.ajp.2008.07060945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer HD. Visual and spatial working memory: from boxes to networks. Neurosci Biobehav Rev. 2008;32(8):1373–1395. doi: 10.1016/j.neubiorev.2008.05.016. [DOI] [PubMed] [Google Scholar]