Abstract
We measured the nucleotide turnover rate of myosin in tarantula leg-muscle fibers by observing single turnovers of the fluorescent nucleotide analog, mantATP, as monitored by the decrease in fluorescence when mantATP is replaced by ATP in a chase experiment. We find a multi-exponential process, with approximately two-thirds of the myosin showing a very slow nucleotide turnover time constant, ~30 minutes. This slow-turnover state is termed the super-relaxed state (SRX). If fibers are incubated in mantADP and chased with ADP, the SRX is not seen, indicating that trinucleotide-relaxed myosins are responsible for the SRX. Phosphorylation of the myosin regulatory light chain eliminates the fraction of myosin with the very long lifetime. The data imply that the very long-lived SRX in tarantula fibers is a highly novel adaptation for energy conservation in an animal that spends extremely long periods of time in a quiescent state employing a lie-in-wait hunting strategy. The presence of the SRX measured here correlates well with the binding of myosin heads to the core of the thick filament in a structure known as the “interacting-heads motif” observed previously by electron microscopy. Both the structural array and the long-lived SRX require relaxed filaments or relaxed fibers, both are lost upon myosin phosphorylation, and both appear to be more stable in tarantula than in vertebrate skeletal or vertebrate cardiac preparations.
Keywords: super-relaxed state, SRX, tarantula, mantATP, epi-fluorescence
We have recently observed a new state in relaxed vertebrate skeletal and vertebrate cardiac muscle fibers characterized by an ATP-turnover rate that is approximately an order of magnitude smaller than normally observed in preparations of purified myosin 1; 2. We termed this state the super-relaxed state (SRX). The SRX introduces a fundamentally new sub-population among the cross-bridges in relaxed muscle. Cross-bridges in relaxed muscle are still detached from actin. However, instead of a single population as previously assumed, the cross-bridges partition into multiple subsets of populations with dramatically different ATP turnover rates.
The regulation of muscle's most obvious transition, that between active and relaxed states, is complex. In vertebrate skeletal and vertebrate cardiac muscles, regulation is achieved by the binding/sequestering of calcium bound to troponin on the thin filaments 3. In vertebrate smooth muscles, activation is myosin-based and achieved by phosphorylation of the regulatory light chain of myosin 4. In scallop muscles activation is likewise myosin-based, but produced by binding of calcium to the light chain region5. In some muscles activation is instead dual controlled by both the thin and the thick filaments. Activation in these muscles requires phosphorylation of myosin, but is also regulated by the binding of calcium to troponin on the thin filament. Tarantula leg muscle is a muscle with dual control that has been extensively characterized both biochemically and structurally 6; 7; 8; 9.
A fundamental question becomes the presence or absence of multiple relaxed states in various muscle types. Is the SRX a fundamental property of the relaxed state, or specific to some muscles? Evolutionary pressures for energy conservation would favor the former. However, other muscle-specific constraints may supersede this pressure. Here we extend our studies of the SRX to a dual-regulated muscle, tarantula leg muscle. Tarantula muscle is further of interest due to the fact that it is the system in which myosin heads were first visualized unambiguously in the thick filament 9. The two myosin heads of each molecule were seen to be bent backwards interacting with the S2 region on the surface of the thick filament. An intramolecular interaction between the actin-binding region of one head and the converter region of the other switches both heads off. The two heads form a structure resembling a “J”. The J-motif, together with the additional interactions between the heads and the core of the thick filament has been termed the “interacting-heads motif” 9; 10. The interacting-heads motif had been seen previously in two-dimensional crystals of relaxed dephosphorylated smooth muscle myosin 11. The evolutionary distance between dual-regulated tarantula leg muscle and myosin-regulated vertebrate smooth muscle is so great that the observation of a similar structural motif in both suggested the widespread distribution of this motif 12. The subsequent observation of the interacting-heads motif in scallop molecules 13, Limulus filaments 14, and in particular in the thin filament regulated vertebrate cardiac 15 and vertebrate skeletal myosin 13, further supported this conclusion.
The observation of the interacting -heads motif in tarantula muscle provides a further motivation for the investigation of the myosin ATPase activity in tarantula muscle fibers. Myosin heads in the interacting-heads motif have been shown to have very slow ATP turnover rates, even slower than that found for the SRX in vertebrate skeletal muscle. The ATP turnover time for monomeric smooth-muscle myosin in the interacting-heads motif is 50 minutes 16 and for scallop thick filaments is over 30 minutes 17. Although the association of the interacting-heads motif with slow nucleotide turnover and its presence in tarantula filaments suggest that nucleotide turnover in relaxed dephosphorylated tarantula fibers would also be slow, this rate had not been measured. Here we measure single ATP turnovers via epi-fluorescence of mant-nucleotides bound to bundles of skinned tarantula muscle fibers. We find a robust SRX in tarantula muscle with a significant population of cross-bridges in the longest-lived component of the SRX and with a longer lifetime than previously seen in skeletal or cardiac muscle. ATP turnover times were greater than 30 min., in the range measured in the myosin-regulated proteins or filaments discussed above. Our results further support the hypothesis that the SRX may be a fundamental property of relaxed muscle throughout the animal kingdom.
Measuring single nucleotide turnovers in skinned muscle fibers
Skinned fibers are complex preparations containing a number of enzymes that turn over ATP rapidly. In addition there is a small fraction of myosin heads that are not regulated and thus have a high ATPase activity. These effects have dominated traditional ATPase activity measurements on skinned fibers. In order to measure the very slow ATP turnover of myosin in the SRX, it is necessary to measure single nucleotide turnovers. Following protocols first established for vertebrate skeletal muscle 2, we have employed quantitative epi-fluorescence microscopy of mant-nucleotides bound to myosin to observe the SRX. MantATP has an ATPase rate similar to ATP, with mantATP fluorescence is enhanced when bound to myosin 18; 19. Small bundles of fibers were mounted in a flow cell. The fibers had been glycerinated in the absence of nucleotide, thus the preparation begins with nucleotide-free myosin. A fiber is first incubated in a relaxing solution containing the fluorescent nucleotide, mantATP, (125μM). The binding of mantATP to the fiber increases fluorescence intensity. The preparation is then chased with a relaxing solution containing 4 mM ATP. The fluorescence decays as the mant-nucleotides are released from the fiber and are replaced by ATP. Fig. 1 shows the fluorescence signal during all phases of a chase experiment. Fig. 2 (red trace) shows the signal analyzed during the chase phase. Decay occurs in several components. There is an initial component that decreases rapidly. The initial decay is compatible with rapid ATPases within the bundle (< 0.05s-1), the release of non-specifically bound nucleotides, presumably fast, and the diffusion of released nucleotides out of the fiber occurring in ~10s 20. The lifetime of the second component is much longer. This is the SRX. The decay of intensity is not due to photobleaching. This is because the fiber is only exposed to the exciting light for a brief period during data acquisition, typically 100 ms, and data were acquired at intervals of 5-30 seconds. Fluorescence decreased by only 12% during constant illumination for 36 seconds, equal to the total exposure during a two-hour epifluorescence microscopy run. The fibers were mechanically relaxed as evidenced by a lack of shortening of unrestrained fibers in relaxing solutions.
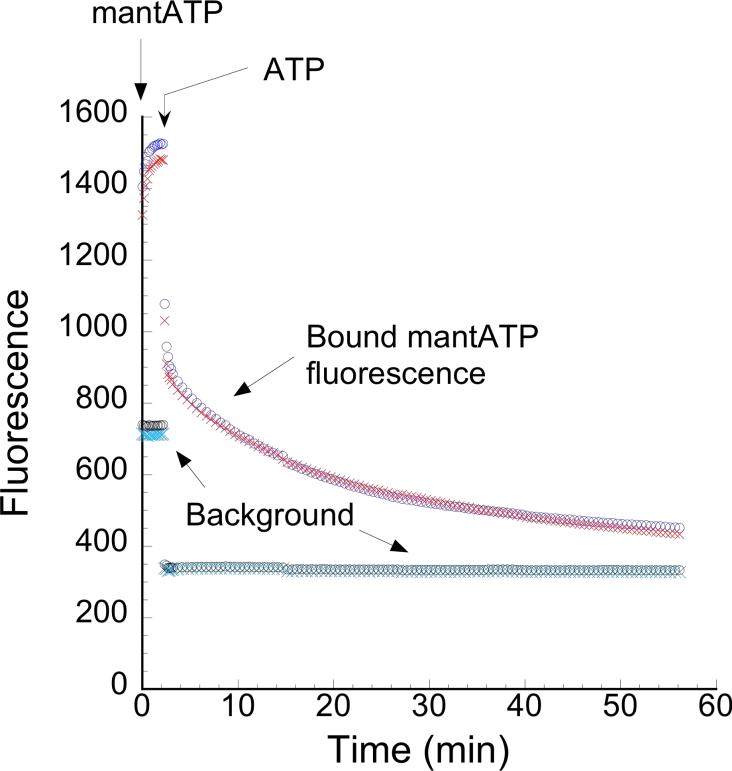
Figure 1.
The time course of a chase experiment is shown. At time = 0, the fiber is washed in a relaxing buffer containing 125 μM mantATP. Fiber fluorescence (red and blue traces) rises rapidly and reaches a new equilibrium as the mantATP binds to the nucleotide site. At time = 1 min., the fiber is chased with a wash in 4 mM ATP. Fiber fluorescence then decreases as ATP replaces mantATP at the nucleotide site. The background fluorescence is shown in cyan and black. The microscope system allows simultaneous monitoring of the fluorescence intensity of mant-nucleotides in four regions in the field. Two regions are placed on the fiber (red and blue traces). The other two regions (black and cyan traces) are placed to record the background. The background signal is averaged and subtracted from the individual observed fiber fluorescences for fluorescence signal analysis.
Figure 2.
A comparison of the fluorescence decay for three conditions is shown: (1) incubation in 125μM mantATP chased by 4mM ATP (red); (2) incubation in 125μM mantADP chased by 4mM ADP (blue); (3) incubation in 125μM mantATP and 4 mM ATP chased by 4mM ATP (green). The experiment for 125μM mantATP chased by 4mM ATP was recorded and fit out to 3000s. Only the initial 800s are shown to facilitate comparison with the other two conditions. Parameters of the fits: (1) P1=0.42, T1=15s, P2=0.15, T2=221s, P3=0.40, T3=1730; (2) P1=0.55, T1=6s, P2=0.26, T2=21.4s, P3=0.10, T3=164s; (3) P1=0.77, T1=12.4s, P3=0.15, T3=63s. Data for case (2) were well fit (χ2 = 0.000015) using only a two-exponential fit. Only for case (1) is there a significant SRX component (P3) to the fluorescence decay.
Fitting the data to a multi-exponential function
Data for the ATP chase of mantATP-incubated fibers were modeled using a three-exponential fit. Previously two-exponential fits were adequate for vertebrate skeletal and cardiac muscle. We were able to obtain reasonable fits for tarantula muscle with a two-exponential fit. However, the χ2 deviations for the fits were a factor of four less with three-exponentials, and this option was employed. The improved fit was most obvious in the part of the fluorescence trace in the “knee” of the trace in the transition from the rapidly decaying initial transient into the long-lived SRX. The three-exponential fit gave a slightly increased lifetime for the longest-lived component when compared to the two-exponential fit. We cannot rule out the possibility that even more than three exponential processes are involved in tarantula muscle, and additional parameters always give better fits to data.
Average values for the relative fractions of cross-bridges in the three components and their lifetimes are given in Table 1. For the ATP-chase experiment described above, approximately 34% of the total fluorescence is in the most rapidly decaying component. The intermediate component comprises 17% of the total fluorescence with a lifetime of 278s. The largest component, comprising 46% of total fluorescence, has the longest time constant, 1995s, or slightly longer than 33 min. The fibers, described above, are mechanically relaxed. Although they are held on the coverslip by a small drop of grease on either end and thus are not prevented from shortening if they generate force, they do not shorten.
Table 1.
Summary of kinetic constants for the SRX in tarantula muscle.
| Incubationa | Chase | P1b | T1 (sec) | P2 | T2 (sec) | P3 | T3 (sec) | Nc |
|---|---|---|---|---|---|---|---|---|
| mantATP | ATP + ML-7 | 0.34 ± .02 | 23± 3 | 0.17 ± .02 | 278 ± 31 | 0.48 ± .03 | 1995 ± 231 | 22 |
| mantADP | ADP | 0.55 ± .03 | 6 ± 1 | 0.26 ± .02 | 27 ± 3 | 0.11 ± .03 | 193 ± 26 | 11 |
| mantATP+ATPd | ATP | 0.79 ± .02 | 9 ± 4 | 0.11 ± .02 | 54 ± 19 | 6 | ||
| ~Pe mantATP | ATP | 0.48 ± .03 | 15 ± 2 | 0.22 ± .03 | 34 ± 5 | 0.23 ± .02 | 319 ± 29 | 13 |
The first 2 columns give the nucleotides in the initial incubation and the chase solutions.
P1, P2 and P3 are the magnitudes and T1 T2 and T3 are the lifetimes for the first, second and third phases of the three-exponential function that was fit to the data; see Eq. 1, Methods.
Errors are standard errors of the mean for Nc observations.
These data were fit adequately by a two-exponential fit.
~P signifies that the fibers were phosphorylated.
We note that for the ATP chase of bound mantATP in dephosphorylated fibers (line 1, Table 1), the T2 and T3 components are both longer than seen in the SRX of vertebrate cardiac and vertebrate skeletal fibers. Thus we consider them both to be SRX-like components, but of varying lifetime. The important observation is that the precise number of exponentials involved in the fit does not alter our fundamental conclusion that there is a large fraction of cross-bridges in relaxed tarantula muscle with slow to very slow nucleotide-turnover rates. Nomenclature becomes a question. In part because of correlations between components with different ATPase activities and structural states, discussed below, we choose to refer to only the longest-lived state (P3-T3 component, Eq. 1) as the SRX. We will refer to the shorter-lived state, time constant 250-300s, as the short-SRX, although it is still longer than the SRX seen in vertebrate skeletal or cardiac muscle. In addition there is a much shorter lived state, which has a time constant close to that measured for purified tarantula myosin of 25s 7.
The slow components of fluorescence decay are due to nucleotides bound to ATP-specific sites
Competitive inhibition of mantATP binding to specific sites on the fiber was studied by inclusion of 4mM ATP in the initial incubation and eliminated 72% of initial binding of mantATP, and also eliminated the slow component seen in a subsequent chase with ATP (green trace, Fig. 2, Table 1). This result shows that the two slow components in the decay of fluorescence are due to ATP-specific sites. This observation also allows the normalization of the components to the number of ATP-specific sites. Approximately 67% of the fluorescent nucleotides bound to ATP-specific sites in the fiber are released in the slowest component of the decay of fluorescence. With the further assumption that myosin forms the vast majority of the ATP-specific sites, more than one-half of the myosins are in the component with the longest time constant, with another 24% in a component that, although faster, still has a slow time constant relative to that expected for purified proteins. Thus the long-lived SRX state in tarantula muscle is both more significantly populated and has a longer lifetime than previously observed in vertebrate skeletal muscle (230s) 2 and cardiac muscle (144s) 1.
The SRX is not present in rigor-ADP fibers
Several experiments showed that the slow component of fluorescence change is only seen when the fiber is relaxed in the initial incubation. When the fiber is first incubated in mantADP and chased with ADP the slow decay of fluorescence is eliminated (blue trace, Fig. 2 and Table 1). This observation shows the need for a relaxed fiber in the incubation in order to observe the slow component of fluorescence decay. Myosin is the only enzyme in the fiber whose activity is known to be regulated by the state of fiber activity. Thus these observations also further support the hypothesis that the slow nucleotide turnover arises from nucleotides bound to myosin.
The effect of phosphorylation of the myosin regulatory light chain
The effect of phosphorylation of the myosin RLC on nucleotide turnover in the tarantula fibers was measured. Phosphorylation was achieved in situ during glycerination by inclusion of ATP and phosphatase inhibitors in the glycerol solutions, as described in Methods. Phosphorylation was checked by IEF gel electrophoresis, see Fig. 3. In dephosphorylated fibers the regulatory light chain runs as two bands, as previously observed by Craig and co-workers using urea gel electrophoresis 7; 8. These investigators showed that the two bands represented unphosphorylated and singly phosphorylated regulatory light chains. In the phosphorylated preparations, the upper band is almost entirely gone, and a third band appears at a more acidic position. Again the pattern is almost identical to that obtained previously, with the two bands being identified with singly and doubly phosphorylated regulatory light chains 7; 8. Previous work has shown that the phosphorylation procedure used here results in phosphorylation of the RLC without phosphorylation of additional proteins21. We conclude that our normal preparation produces fibers with a mixture of unphosphorylated and singly phosphorylated RLCs. Our in situ phosphorylation procedure produces fibers with a mixture of singly and doubly phosphorylated RLCs. This additional level of phosphorylation also resulted in the elimination of the exponential component with the very long time constant. A major component decays rapidly, with time constants of 36 seconds or less (Fig. 4; Table 1, T1 and T2 components, ~P mantATP chased by ATP). A second component, amounting to 25% of total fluorescence, decays with a time constant of approximately 300 seconds. Thus compared to dephosphorylated fibers, the longest lived component in phosphorylated tarantula fibers has a lifetime that is decreased by a factor of 6, with only half as many cross-bridges in the longest-lived state (Table 1). The effect of myosin phosphorylation on the longest-lived state again supports the conclusion that it arises from nucleotides bound to myosin.
Figure 3.
Isoelectric focusing gel of tarantula fibers showing the change in isoelectric focusing point of the myosin RLC between the normal preparation and that obtained using phosphatase inhibitors in the glycerol solutions. Lanes 1 and 2 are from the phosphorylated preparation. Lanes 3 and 4 are from the normal preparation. Both preparations had been stored in rigor/glycerol solution for 2 weeks. The bands are identified by their isoelectric point, which is similar to that of vertebrate skeletal myosin light chains, and by the shift occurring in the phosphorylated preparation. The three bands are identified as unphosphorylated RLC, singly and doubly RLC by comparison with the gels of Hidalgo et. al.8.
Figure 4.
The fluorescence intensity during the chase phase of the single nucleotide turnover experiments in dephosphorylated (●) and phosphorylated (○) tarantula fiber bundles muscle. The data for dephosphorylated fibers are from Fig. 2. The fiber bundles were incubated in mantATP and chased by ATP. As can be seen the component of fluorescence decay with the very long time constant was eliminated in the phosphorylated fibers. Fit to the data for phosphorylated fibers: P1= 0.44, T1=15s, P2=0.26, T2= 38s, P3=0.20, T3=262s.
Role of the SRX in muscle function
The SRX is a mechanism for down-regulating the ATPase activity of relaxed muscle. We have previously shown a SRX in relaxed vertebrate skeletal muscle 2. Here we have extended the observations to an invertebrate skeletal muscle, tarantula leg muscle. There are several dramatic differences between tarantula fibers and rabbit vertebrate skeletal fibers. The lifetime of the SRX in tarantula fibers is about 10-times longer than in rabbit skeletal fibers and the magnitude of the initial rapid decrease is a factor of 2 less in the tarantula fibers. There is a second component, the short-SRX, that has an intermediate time constant, 250-300s, and a smaller population, although it is not clear whether this represents a distinct state or a continuum of time constants. Although not well resolved in the unphosphorylated fibers, there is a state that we call the normal relaxed state, which has an ATP turnover time that is similar to that of purified myosin, time constant ~25s 7. This state is more obvious in the phosphorylated fibers, where the initial rapid phase is more prominent.
We have now looked at four different muscle types - fast and slow vertebrate skeletal muscle, vertebrate cardiac muscle and tarantula leg muscle. In all cases we have found the SRX. However, there are significant differences that correlate with function. In all situations the SRX allows for energy conservation in the relaxed state. However, in vertebrate skeletal muscle, partial activation rapidly and completely eliminates the SRX2 allowing for the rapid recruitment of active cross-bridges in the vertebrate skeletal system. In vertebrate cardiac fibers, myosin heads in the SRX remain there during partial activation. This facilitates the graded muscle activation of the vertebrate cardiac system, and allows for the SRX to serve as a cardio-protective mechanism during hypoxia or stress 1. In tarantula muscle, the SRX has an especially long lifetime for the relaxed state (> 30 min.). This is an ideal adaptation for energy conservation in an animal with a lie-in-wait hunting strategy, spending extraordinarily long quiescent periods (days) in a burrow waiting for prey to pass within easy reach. Just as in vertebrate skeletal muscle, the myosin heads in the normal relaxed state in tarantula muscle will consume more energy leading to greater thermogenesis. It is possible that the transition out of the SRX and into the normal relaxed state is used in tarantula to raise body temperature, preparing for activity or counteracting extreme cold.
A picture of the SRX is emerging in which it is a widely distributed state in the animal kingdom, independent of the myosin regulatory system employed. Crucially, however, the SRX appears optimized for totally different functions in different muscle systems. Additionally, the correlations noted here between the SRX and the ordered helical array seen in tarantula and other thick filament EM reconstructions provides a framework for further studies of a potential structural basis for the SRX.
Correlations with structural studies
A number of correlations between structural studies and nucleotide turnover rates show that the myosin heads with slow turnover rates are bound to the core of the thick filament in the interacting-heads motif. Here we use epi-fluorescence to show a very slow nucleotide turnover in a muscle for which there is high-resolution structural information. The fluorescence assay agrees with the structural studies. The observation of a higher population and longer time constants agrees with the qualitative observation that the stability of the ordered array is greater in tarantula than in vertebrate skeletal or vertebrate cardiac muscle 9; 12; 13; 15. Both the slow components and the ordered array are absent if the fibers or filaments are not in a relaxed state7; 22; 23.
We note that all the results shown in the figures here were obtained from fibers that were stored in glycerol at -20°C. The structural work discussed above was obtained from filaments purified from skinned fibers, which had never been in a glycerol solution. We also obtained some data from skinned fibers that were not stored in glycerol, which were identical to those obtained from the fibers stored in glycerol. These data were included in the average values reported in Table I.
Phosphorylation of the RLC disorders the array 7; 8 and also eliminates the population with the very slow time constant. The simplest interpretation of this result is that the myosin heads in the slowest SRX are also in the interacting-heads motif. Within the uncertainties in the data, with 67% of the myosin heads in the longest SRX and approximately 41% unphosphorylated by densitometric analysis of the gels, a further conclusion could be that these heads are unphosphorylated. In the phosphorylated sample, approximately 35% of the myosin heads have a time constant of 320s, while the remaining 65% have a time constant of 36s or less. Gel analysis indicates 42% singly phosphorylated and 42% doubly phosphorylated heads. It is plausible that doubly phosphorylated myosin heads have this rapid ATP turnover rate, and are the disordered heads seen in EM. However, the phosphorylation level of the short SRX, time constant 250-300s, is not easily defined by the data, as there is not a simple correlation between the population of this state and phosphorylation. The structure of this state is also not defined. It is possible that these myosins have one head bound to the core of the thick filament and one head disordered. However, there may not be a one-to-one correspondence between level of phosphorylation and either ATPase rates or structural states. An alternate model is that there is cooperativity within the thick filament structure with the overall level of phosphorylation of the filament favoring or destabilizing various structural states of the heads.
Summary
We find that myosin has at least three states in relaxed tarantula muscle fibers, one with a very long time constant, 30 minutes, one with an intermediate time constant, 250-300 seconds, and one with a faster time constant, <30 seconds. The correlations between the SRX observed in these studies and the properties of the ordered helical array seen in EM reconstructions suggest structural relationships between the two. The state with the longest time constant appears to correlate with myosin heads that are “parked” on the core of the thick filament in EM reconstructions, where they do not interact with actin. The state with the shortest time constant, <35s, is probably disordered and able to rapidly interact with actin upon binding of calcium to the thin-filament regulatory proteins. The structure and function of the intermediate state is uncertain, but probably, while conserving energy, is able to make a faster transition to active states than can the SRX with the longest time constant.
Methods
Fiber preparation
Tarantulas (Brachypelma) were euthanized by CO2 inhalation. The muscle was chemically skinned following modifications of the procedures of 7. The first large leg segment adjacent to the body was isolated and the bristles shaved off with a scalpel blade. Large windows were cut in two opposing sides of the cuticle along the length of the leg segment exposing the underlying muscle, while leaving the muscle attachments to the exoskeleton intact. The muscle tissue in the leg segment was then skinned by immersing in a buffer containing 100 mM NaCl, 8 mM MgCl2, 5 mM EGTA, 10 mM sodium phosphate, 3 mM sodium azide, 5 mM ATP, 1 mM DTT, 0.1% saponin, pH 7.0. The fibers were gently agitated on ice for 24 hr. with three changes of buffer. If not to be used within 2 days, the fibers were then exchanged into a buffer containing 0.24M KOAc, 80 mM MOPS, 10 mM EGTA, 10 mM Mg(OAc)2, diluted 50%/50% (v/v) with glycerol, pH 7.0, equilibrated with gentle agitation for 36 hr. on ice and then stored at -20°C for up to six weeks. Myosin light chains were phosphorylated in situ by including 4 mM ATP and the phosphatase inhibitors, 20 mM NaF and 20 mM KH2PO4/K2HPO4 in the rigor/glycerol solution used for fiber storage 21. We have previously observed in vertebrate skeletal muscle that inclusion of the higher concentration of PO43- and F- ions inhibit phosphatase activity, producing phosphorylated fibers 21. Phosphorylation was achieved by storage for one week. The extent of myosin light chain phosphorylation was determined using isoelectric focusing gel electrophoresis as described previously 21. The gels were stained with the protein dye, Ruby Red.
Epi-fluorescence observations
Single or bundles of fibers (50-90 μm in diameter) were mounted in the flow cell described in 2. Sarcomere lengths varied from 4.5-5 μm, although rarely, sarcomere lengths of 2.8-3.2 μm were observed. Sarcomere length did not influence results. Fibers were incubated in rigor buffer containing 120mM KOAc, 5mM Mg(OAc)2, 5mM EGTA, 2.5mM K2HPO4, 2.5mM KH2PO4 and 50mM 3-(N-morpholino)propanesulfonic acid, 2 mM DTT, pH 6.8, with or without added mant-nucleotides (125 μM) or nucleotide. Temperature was 26 ± 1°C. For dephosphorylated fibers, 1 μM myosin light chain kinase inhibitor ML-7 was added. Although we have no direct evidence that MLCK is active in our preparations, nor that ML-7 inhibits it, ML-7 was included as we found slightly, ~10% longer time constants when it was included in the ATP chase solution. The solution was then exchanged for another buffer, again, with or without mant-nucleotide or nucleotide as appropriate for the given experiment. The change in mant-nucleotide fluorescence as a function of time was observed using a Nikon Eclipse Ti-E 300 inverted microscope with a Coolsnap HQ2 camera using a DAPI filter set (Chroma #89000) and Sutter DG4 arc lamp for excitation. The average fluorescence intensities within small rectangular areas, 15-30μm × 20-40μm, were measured and fiber fluorescence was determined by subtracting the background intensity from the fiber intensity. Images were obtained every 10-30s using a 100ms exposure time to minimize photobleaching. For longer runs, image acquisition was decreased to as long as every 2 min. Longer exposure times and more frequent image acquisition did not change the results indicating that photobleaching was not impacting our conclusions. Additional details of fluorescence protocols are in 1; 2. The fluorescence decay, F, as a function of time, t, following a chase of bound mant-nucleotide was modeled using a three-exponential fit
| (1) |
where P1, P2, and P3 are the relative fractions of myosin in three different states, and T1, T2, T3 are the lifetimes. Fitting was done using a nonlinear least-squares algorithm in KaleidaGraph v3.6 (Synergy Software). The fit also defined 95% confidence limits.
Highlights.
We have observed a super-relaxed (SRX) state in resting tarantula leg muscle.
The SRX is characterized by an extremely slow ATP turnover rate (33 min.)
Phosphorylation of the myosin RLC dramatically decreases the SRX lifetime (5 min.).
We have observed the SRX in 4 muscle types implying it is a fundamental property of the relaxed state.
The SRX appears related to the ordered helical array of myosin heads seen in tarantula muscle EM.
ACKNOWLEDGMENTS
Data for this study were acquired at the Nikon Imaging Center at UCSF/QB3. The authors would like to thank Dr. Kurt Thorn and Ms. Alice Myo Thwin for their generous help in using the microscopes. The authors would like to thank Drs. Roger Craig and Raul Padron for helpful comments on the manuscript. This work was supported by NIH grants AR053720 (E.P., N.N.), HL32145 (R.C., N.N.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations: SRX, super-relaxed state; mantATP/ADP, 2'-/3'-O-(N'-methylanthraniloyl)adenosine-5'-O-triphosphate/diphosphate; RLC, myosin regulatory light chain; ML-7, myosin light chain kinase inhibitor, (5-iodonaphthalene-1-sulfonyl homopiperazine); MLCK, myosin light chain kinase
REFERENCES
- 1.Hooijman P, Stewart MA, Cooke R. A new state of cardiac myosin with very slow ATP turnover: a potential cardioprotective mechanism in the heart. Biophys J. 2011;100:1969–1976. doi: 10.1016/j.bpj.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stewart MA, Franks-Skiba K, Chen S, Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci U S A. 2010;107:430–435. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- 4.Adelstein RS, Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–56. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- 5.Kendrick-Jones J, Lehman W, Szent-Gyorgyi AG. Regulation in molluscan muscles. J Mol Biol. 1970;54:313–26. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- 6.Zhu J, Sun Y, Zhao FQ, Yu J, Craig R, Hu S. Analysis of tarantula skeletal muscle protein sequences and identification of transcriptional isoforms. BMC Genomics. 2009;10:117. doi: 10.1186/1471-2164-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Craig R, Padron R, Kendrick-Jones J. Structural changes accompanying phosphorylation of tarantula muscle myosin filaments. J Cell Biol. 1987;105:1319–27. doi: 10.1083/jcb.105.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hidalgo C, Craig R, Ikebe M, Padron R. Mechanism of phosphorylation of the regulatory light chain of myosin from tarantula striated muscle. J Muscle Res Cell Motil. 2001;22:51–9. doi: 10.1023/a:1010388103354. [DOI] [PubMed] [Google Scholar]
- 9.Woodhead JL, Zhao FQ, Craig R, Egelman EH, Alamo L, Padron R. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436:1195–9. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- 10.Alamo L, Wriggers W, Pinto A, Bartoli F, Salazar L, Zhao FQ, Craig R, Padron R. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J Mol Biol. 2008;384:780–97. doi: 10.1016/j.jmb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wendt T, Taylor D, Trybus KM, Taylor K. Three-dimensional image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc Natl Acad Sci U S A. 2001;98:4361–6. doi: 10.1073/pnas.071051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Craig R, Woodhead JL. Structure and function of myosin filaments. Curr Opin Struct Biol. 2006;16:204–12. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Jung HS, Komatsu S, Ikebe M, Craig R. Head-head and head-tail interaction: a general mechanism for switching off myosin II activity in cells. Mol Biol Cell. 2008;19:3234–42. doi: 10.1091/mbc.E08-02-0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao FQ, Craig R, Woodhead JL. Head-head interaction characterizes the relaxed state of Limulus muscle myosin filaments. J Mol Biol. 2009;385:423–31. doi: 10.1016/j.jmb.2008.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zoghbi ME, Woodhead JL, Moss RL, Craig R. Three-dimensional structure of vertebrate cardiac muscle myosin filaments. Proc Natl Acad Sci U S A. 2008;105:2386–90. doi: 10.1073/pnas.0708912105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cross RA, Jackson AP, Citi S, Kendrick-Jones J, Bagshaw CR. Active site trapping of nucleotide by smooth and non-muscle myosins. J Mol Biol. 1988;203:173–81. doi: 10.1016/0022-2836(88)90100-3. [DOI] [PubMed] [Google Scholar]
- 17.Vibert P, Craig R. Structural changes that occur in scallop myosin filaments upon activation. J Cell Biol. 1985;101:830–7. doi: 10.1083/jcb.101.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cremo CR, Neuron JM, Yount RG. Interaction of myosin subfragment 1 with fluorescent ribose-modified nucleotides. A comparison of vanadate trapping and SH1-SH2 cross-linking. Biochemistry. 1990;29:3309–19. doi: 10.1021/bi00465a023. [DOI] [PubMed] [Google Scholar]
- 19.Woodward SK, Eccleston JF, Geeves MA. Kinetics of the interaction of 2'(3')-O-(N-methylanthraniloyl)-ATP with myosin subfragment 1 and actomyosin subfragment 1: characterization of two acto-S1-ADP complexes. Biochemistry. 1991;30:422–30. doi: 10.1021/bi00216a017. [DOI] [PubMed] [Google Scholar]
- 20.Cooke R, Pate E. The effects of ADP and phosphate on the contraction of muscle fibers. Biophys J. 1985;48:789–98. doi: 10.1016/S0006-3495(85)83837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karatzaferi C, Franks-Skiba K, Cooke R. Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am J Physiol Regul Integr Comp Physiol. 2008;294:R948–55. doi: 10.1152/ajpregu.00541.2007. [DOI] [PubMed] [Google Scholar]
- 22.Craig R, Alamo L, Padron R. Structure of the myosin filaments of relaxed and rigor vertebrate striated muscle studied by rapid freezing electron microscopy. J Mol Biol. 1992;228:474–87. doi: 10.1016/0022-2836(92)90836-9. [DOI] [PubMed] [Google Scholar]
- 23.Padron R, Craig R. Disorder induced in nonoverlap myosin cross-bridges by loss of adenosine triphosphate. Biophys J. 1989;56:927–33. doi: 10.1016/S0006-3495(89)82738-9. [DOI] [PMC free article] [PubMed] [Google Scholar]