Abstract
This review begins with the premise that an organism's life span is determined by the balance between two countervailing forces: (i) the sum of destabilizing effects, and (ii) the sum of protective longevity-assurance processes. Against this backdrop, the role of electrophiles is discussed, both as destabilizing factors and as signals that induce protective responses. Because most biological macromolecules contain nucleophilic centers, electrophiles are particularly reactive and toxic in a biological context. The majority of cellular electrophiles are generated from polyunsaturated fatty acids by a peroxidation chain reaction that is readily triggered by oxygen-centered radicals, but propagates without further input of reactive oxygen species (ROS). Thus, the formation of lipid-derived electrophiles such as 4-hydroxynon-2-enal (4-HNE) is proposed to be relatively insensitive to the level of initiating ROS, but to depend mainly on the availability of peroxidation-susceptible fatty acids. This is consistent with numerous observations that life span is inversely correlated to membrane peroxidizability, and with the hypothesis that 4-HNE may constitute the mechanistic link between high susceptibility of membrane lipids to peroxidation and shortened life span. Experimental interventions that directly alter membrane composition (and thus their peroxidizability) or modulate 4-HNE levels have the expected effects on life span, establishing that the connection is not only correlative but causal. Specific molecular mechanisms are considered, by which 4-HNE could (i) destabilize biological systems via non-targeted reactions with cellular macromolecules, and (ii) modulate signaling pathways that control longevity assurance mechanisms.
Keywords: aging, longevity assurance, reactive oxygen species, lipid peroxidation, membrane peroxidizability, polyunsaturated fatty acids, oxidative damage, electrophilic damage, electrophiles, 4-HNE, 4-hydroxynonenal, 4-hydroxynon-2-enal, aldehydes
Introduction
Aging holds a special fascination for humans, as sentient beings subject to its ravages. From the dawn of recorded history, much of human thought and culture has revolved around aging and the resulting mortality. The earliest preserved writings, such as the epic of Gilgamesh and the ancient Egyptian pyramid texts, speak of death and the longing for immortality; these topics remain preeminent in contemporary art and philosophy. Yet, the desire to comprehend and then to conquer aging, however urgent, does not necessarily promote a rational understanding of the phenomenon. The quest for the fountain of youth may have culminated in geographical discoveries, allegedly including early exploration of Florida, but failed to either restore youth or shed the slightest light on the mechanism of aging. Substantial progress in understanding the molecular biology of aging dates back less than thirty years. Even today, there is startlingly little agreement among gerontologists as to the most fundamental principles guiding the field. In fact, some theories of aging appear to be championed with a single-mindedness worthy of Ponce de León. This is especially true of mechanistic theories of aging, as opposed to evolutionary explanations – which by their nature tend to be cast in broader terms. Each mechanistic theory is typically presented with the implication, or sometimes explicit claim, that it can account for most or all of the aging process – notwithstanding the existence of equally reasonable competing theories. Such far-reaching assertions are not necessarily in contradiction. Statistically, it is possible for two factors, X and Y, to each account for 80% of an outcome; this would simply mean that factors X and Y are not fully independent of one another. Even allowing for the possibility that the various theories of aging describe highly interdependent phenomena, the broad nature of most of the explanatory claims appears to reflect primarily each author's research focus and interests. In addition, while convincing evidence has been amassed in support of multiple but distinct processes underlying aging, the gerontology community has been considerably less successful in ranking their relative importance in any particular model organism.
In the present review, I will discuss the nature of electrophilic stress and its role in aging. I hope to present compelling evidence that electrophiles are, in fact, a long-neglected causal contributor to aging, and that electrophilic stress, while initiated by an oxidative event, is distinct, and can be functionally decoupled, from oxidative stress. In focusing on these topics, I risk evoking the impression, criticized above, of an all-inclusive explanatory claim. Thus, at the outset, I would like to state most emphatically that this is not my intention. I think that present evidence justifies only a qualitative assertion that the peroxidation of membrane lipids and the electrophiles thus created are capable of modulating life span. However, other pro- and anti-aging mechanisms are certain to exist. Future research should be able to determine the quantitative contribution of electrophiles, versus unrelated processes, to aging and to longevity assurance of Caenorhabditis elegans, other model organisms, and humans.
Atherogenesis, neurodegenerative diseases, and cancer will not be addressed in this review, even though they obviously affect mammalian life span and are modulated by electrophiles. The reason for excluding the above conditions from further discussion is two-fold. First, it is a matter of definition whether diseases such as cancer are part of aging or secondary consequences of aging. Second, the model organism C. elegans, in which much of the information about electrophiles and aging was obtained, does not suffer from the above conditions [with the exception of germline tumors; refs. 1,2]. Yet, C. elegans is subject to aging. In fact, aging of C. elegans may represent a subset of aging processes that are phylogenetically conserved. This review will thus focus on these presumably universal (“public”) mechanisms.
Biological electrophiles
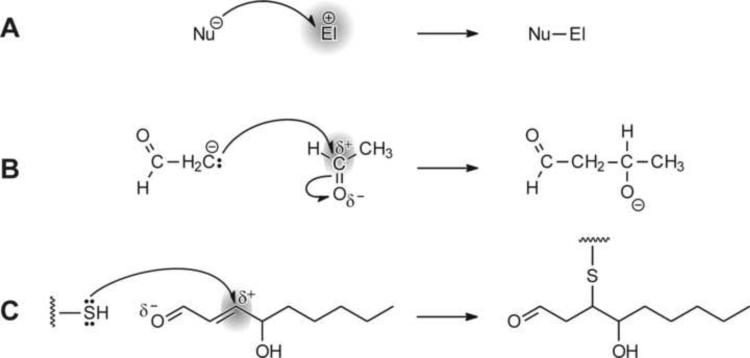
In organic chemistry, the formation of a new bond typically occurs by one of two mechanisms [ref. 3, pp. 179–180]. In a free radical-mediated reaction, each of the two reaction partners supplies one electron to the resulting bond. Alternatively, the bonding electron pair is donated by one, and accepted by the other, reaction partner (shown schematically in Fig. 1A and illustrated by specific examples in Fig. 1B and C). The molecule, or region of a molecule, that supplies the electron pair is a nucleophile; nucleophilic centers may carry a partial or full negative charge and contain non-bonding electron pairs. The reactant that accepts the electron pair is an electrophile. Electrophilic compounds (or electrophilic centers) have a relative electron deficit, for example, because of polarization of a bond that shifted electrons away from the electrophilic region, because of cationic character, or because of an incomplete valence shell. The relative reactivity of an electrophile depends not only on its own structure, but also on that of its nucleophilic reaction partner [4–6].
Fig. 1.
Examples of reactions of electrophiles with nucleophiles. Panel A: A general reaction scheme in which an electron-rich nucleophile Nu donates an electron pair to form a bond with electrophile El. Panel B: Aldol condensation of acetaldehyde, a reaction in which a carbanion, derived from acetaldehyde by action of a base, attacks an electrophilic carbon of another acetaldehyde molecule. A new carbon-carbon bond is formed in the process. Panel C: Reaction of a nucleophilic thiol group, such as in a cysteine side chain in proteins or in glutathione, with an electrophilic center on carbon 3 of 4-hydroxynon-2-enal (4-HNE). The reaction, a Michael addition, leads to the formation of a thioether. In all three panels, the electrophilic molecule or electrophilic center is highlighted by grey shading.
The majority of biological macromolecules are nucleophilic. In proteins, accessible thiol and primary amino groups constitute strongly nucleophilic centers, with histidine imidazole and tyrosine hydroxyl groups also contributing. Chemical modification of these nucleophilic sites often alters or decreases protein function, resulting in cytotoxicity. Purine and pyrimidine bases of nucleic acids contain both nucleophilic (on nitrogen and oxygen atoms) and electrophilic (on certain carbon atoms) centers [7]. Formation of covalent adducts on bases in DNA may lead to mutagenicity. The preponderance of nucleophilic sites in biologically essential macromolecules explains the susceptibility of these macromolecules to electrophilic attack. As a result, electrophiles are bioactive and, in general, detrimental.
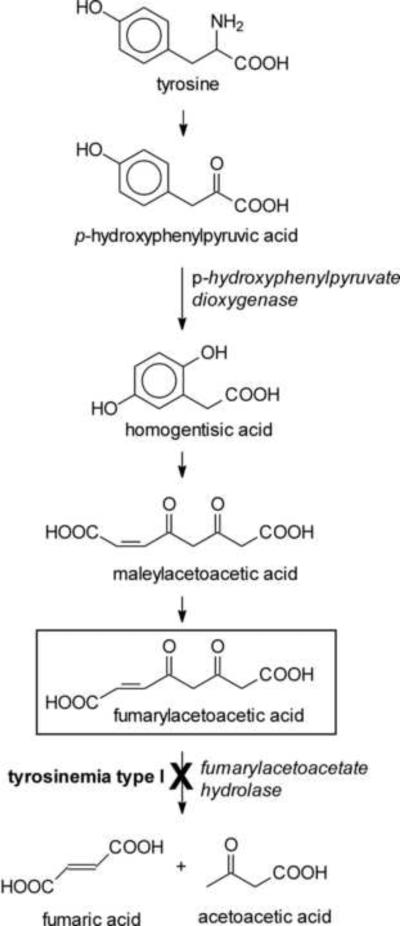
Where do biologically relevant electrophiles come from? There are two major sources of such compounds. The first is external. Xenobiotics can be present in food, especially of plant origin, can be inhaled, or can be administered on purpose, e.g., as pharmacological agents. Many xenobiotics are directly electrophilic or can be metabolically converted to electrophiles [activation of toxins or drugs; see ref. 8 for a review]. The other source of electrophiles is the cell's own metabolism. Certain intermediary metabolites are electrophilic. The catabolism of tyrosine may serve as an example. Fumarylacetoacetate (Fig. 2), an intermediate in the pathway of tyrosine breakdown, is electrophilic owing to its α,β-unsaturated carbonyl moiety. Because of the electrophilic character, fumarylacetoacetate could react with nucleophilic centers on proteins or DNA. However, these side reactions are minimized because fumarylacetoacetate is rapidly converted by fumarylacetoacetate hydrolase to fumarate and acetoacetate (Fig. 2). In the inherited disease tyrosinemia type I, in which fumarylacetoacetate hydrolase activity is deficient, fumarylacetoacetate and related compounds accumulate and exert a variety of toxic effects, both acutely and long-term [9–12]. Experimentally, silencing by RNA interference (RNAi) of fumarylacetoacetate hydrolase in the nematode C. elegans led to a range of detrimental outcomes [13], a result that is consistent with toxicity of accumulating fumarylacetoacetate. Strikingly, silencing of p-hydroxyphenylpyruvate dioxygenase, an enzyme that is upstream of fumarylacetoacetate in the catabolic pathway of tyrosine (Fig. 2), caused an extension of life span [14]. This indicates that even at its low, physiological steady-state concentrations, fumarylacetoacetate may exert a low-grade electrophilic stress which, over long periods of time, has a cumulative destabilizing effect and limits life span. Consequently, depletion of fumarylacetoacetate to sub-physiological levels could have a positive effect on longevity [13].
Fig. 2.
The pathway of tyrosine catabolism. Fumarylacetoacetic acid (boxed structure) accumulates in tyrosinemia type I, in which fumarylacetoacetate hydrolase activity is impaired. Fumarylacetoacetate is an electrophile (Michael acceptor) because it contains a double bond conjugated to a carbonyl group.
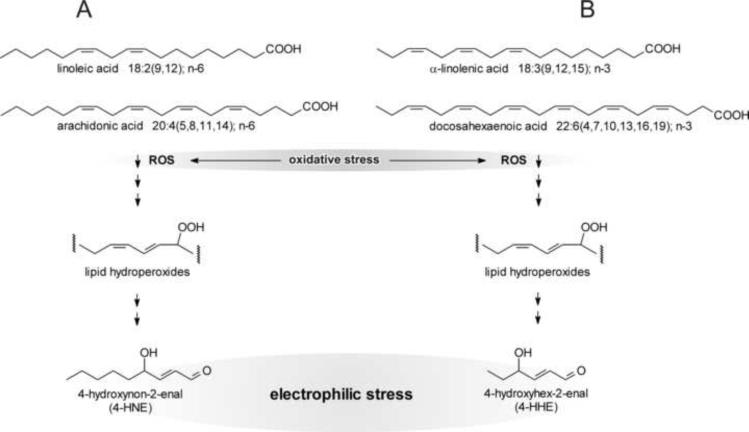
As shown by the example of fumarylacetoacetate, some intermediary metabolites are electrophilic. The effective steady-state concentration of such metabolites is typically low, either because of reaction kinetics (fast utilization by the next step in the pathway) or because of metabolite channeling [15]. However, in contrast to intermediates of metabolic pathways, many end- or, more accurately, by-products of metabolism are not tightly controlled and their levels may spike under certain conditions. By-products of metabolism that are electrophilic, and thus of interest for the present discussion, are mostly α,β-unsaturated carbonyls derived from oxidation of various cellular constituents. Although α,β-unsaturated aldehydes (IUPAC name: alk-2-enals) can result from the oxidation of sugars and amino acids [16], the most abundant such compounds are derived from oxidation of polyunsaturated fatty acids (PUFAs) [17–22]. The most extensively studied alk-2-enal, 4-hydroxynon-2-enal (4-HNE), is formed from n-6 PUFAs (Fig. 3A). Other alk-2-enals, including 4-hydroxyhex-2-enal derived from n-3 PUFAs [Fig. 3B and ref. 23], 4-oxonon-2-enal [24,25], acrolein, crotonaldehyde, malondialdehyde, and others, differ in their specific chemical and thus biological properties from 4-HNE. Nevertheless, all of the above compounds are electrophilic because of the presence of an α,β-unsaturated carbonyl function, and all can contribute to electrophilic stress. The chemical properties of 4-HNE have been characterized in great detail [26]. Unless stated otherwise, the following discussion will focus on 4-HNE because its biological properties are better understood than those of other alk-2-enals. However, it is likely that the other electrophiles have similar effects on aging, at least qualitatively.
Fig. 3.
Generation of 4-hydroxynon-2-enal (4-HNE) and 4-hydroxyhex-2-enal from PUFAs. Part A: n-6 PUFAs (the examples of linoleic and arachidonic acids are shown) are attacked by ROS, typically a hydroxyl radical ˙OH, or undergo a lipoxygenase-catalyzed reaction (not shown), to form a lipid hydroperoxide. The latter is non-enzymatically converted to end-products that include 4-HNE. Part B: n-3 PUFAs, exemplified by α-linolenic and docosahexaenoic acids, yield 4-hydroxyhex-2-enal in a reaction sequence analogous to that shown for n-6 PUFAs. These reactions convert an initial oxidative stress to electrophilic stress.
As mentioned above, the formation of electrophiles from PUFAs and other cell constituents requires an initial oxidative step. Therefore, in the following section, oxidative stress will be briefly reviewed and its relationship to electrophile generation will be discussed.
Oxidative stress and its relationship to electrophilic stress
Thermodynamically, dioxygen (O2) is an oxidant in a biological context because the O2/2H2O half-cell has a more positive electrode potential [approximately +0.8 V at pH 7, refs. 27,28] than most biologically relevant redox half-cells. This means that the oxidation by O2 of organic compounds to carbon dioxide and water will have a negative Gibbs free energy and should proceed spontaneously.
In other words, organic compounds and structures composed of them, such as our bodies and, in fact, all organisms, are thermodynamically unstable in an oxygen-containing atmosphere. We owe our existence to a kinetic barrier: oxidation of most organic compounds by O2 is exceedingly slow at physiological temperatures because of the peculiar electron occupancy pattern of the molecular orbitals in O2 [reviewed in ref. 29]. This results in a high activation energy and a slow reaction of O2 with typical C—C or C—H bonds in organic compounds. Oxidation of organic material by O2 can be accelerated if the activation energy barrier is overcome by increasing the temperature. The free energy of the reaction is then released as heat, which maintains the high temperature of the reactants and makes the combustion self-sustaining. Alternatively, the reaction rate can be increased by using catalysts to lower the activation energy barrier. This is how foodstuffs are oxidized biologically – the process is catalyzed by a series of enzymes. Even though there is a spatial and temporal separation of the removal of electrons from metabolites (oxidation of organic material) and transfer of these electrons to O2 (reduction of O2 to H2O), the overall reaction remains the same as in combustion, and the same total amount of free energy is made available. However, in biological oxidations a large fraction of this free energy is not released as heat but is captured in high-energy bonds such as those in ATP. In addition, enzyme-catalyzed reactions assure specificity with regard to which metabolites are oxidized and which are spared.
Because of the already mentioned electronic structure of the O2 molecule, its reduction typically occurs via a sequential transfer of single electrons that is mediated by transition metals. Biologically, the latter are part of prosthetic groups of enzymes. In the case of O2 reduction by the respiratory chain, the enzyme cytochrome oxidase sequentially transfers four electrons to O2 which remains protein-bound and thus sequestered until the reaction is complete. Compared with alternative oxidants, the use of O2 as the terminal electron acceptor allows for more Gibbs free energy to be generated from the oxidation of nutrients. Consequently, the evolutionary adoption of O2 as the terminal electron acceptor is thought to have made possible such fundamental biological features as multicellularity, complex nervous systems, or rapid and forceful mechanical movement necessary for behaviors such as powered flight or predation [30]. However, the benefits of aerobic metabolism carry a cost. Components of redox pathways and reactions, in particular the mitochondrial respiratory chain [31] but also others such as the microsomal cytochrome P450 system, may leak single electrons which are readily accepted by the O2 molecule, leading to the formation of the superoxide radical anion, O2˙−. This reaction is facilitated by a high partial pressure of O2 and by redox chain components that are highly reduced, thereby increasing electron availability. O2˙− is then converted, in both enzyme-catalyzed and non-enzymatic reactions, to a series of compounds collectively known as “reactive oxygen species” or ROS. It is generally accepted that the mitochondrial respiratory chain is the major generator of ROS in most animal cells [but see ref. 32 for a contrary view]. In addition to the mitochondrial respiratory chain and to microsomal cytochromes P450, there are other sources of ROS, e.g., α-ketoglutarate dehydrogenase of the mitochondrial matrix [33,34], NADPH oxidase [35,36] and nitric oxide synthase [37] in phagocytic and non-phagocytic cells, radiolytic cleavage of water, and others.
The chemistry and biology of ROS and the defenses against ROS are the topics of many excellent reviews [for example, refs. 29,38] and will not be further described here, except to point out the link between oxidative and electrophilic stress. The preceding brief discussion demonstrates that the formation of ROS is inevitable in aerobic organisms. By limiting the formation of ROS and bolstering anti-ROS defenses, oxidative stress can be minimized but not eliminated. A certain level of ROS is necessary because of its role in signaling [38,39] and in the defense against pathogens [40–42]. The continuous presence of at least some ROS in aerobes, together with the ability of certain ROS, in particular the hydroxyl radical ˙OH, to initiate lipid peroxidation, and the ubiquitous nature of PUFAs in biological membranes, indicate that the formation of electrophilic lipid peroxidation products is inevitable. In fact, excessive levels of electrophiles such as 4-HNE are often considered to be not only a direct consequence, but essentially a part of oxidative stress. A more common and moderate point of view is that oxidative stress is the primary event, whereas lipid peroxidation products are second messengers that convey to the cell information about the initiating oxidative event [16,43,44]. This formulation acknowledges the distinct chemistries and thus biological modes of action of ROS and electrophiles, but still implies that the generation of electrophiles closely mirrors the intensity of the original oxidative stress [e.g., ref. 45]. While an initial oxidative event, whether enzymatic (lipoxygenase action) or non-enzymatic (reaction with certain ROS; see below) is necessary to initiate lipid peroxidation, I will argue that under some circumstances, in particular those relevant to aging, the level of electrophilic stress can be largely uncoupled from the severity of its oxidative trigger.
Lipid peroxidation
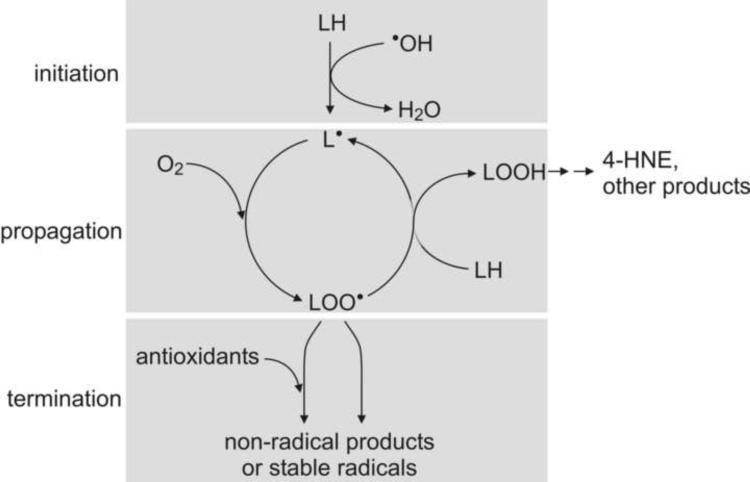
A highly simplified scheme of ROS-triggered lipid peroxidation is shown in Fig. 4 [for a stringent discussion of the topic, see refs. 21,46,47]. Briefly, the process consists of three phases. The first is initiation, in which a radical, typically ˙OH, abstracts a hydrogen from a PUFA (denoted as LH in Fig. 4) in a membrane phospholipid. The resulting carbon-centered radical L˙ then becomes part of the second phase, propagation. L˙ rapidly reacts with an O2 molecule to form an oxygen-centered radical, LOO˙. In a slower reaction, LOO˙ collides with, and abstracts a hydrogen from, another PUFA in the membrane. This converts the LOO˙ to a lipid hydroperoxide LOOH, and leaves behind a new carbon-centered radical L˙, which is ready to undergo the next propagation cycle. The ensuing chain reaction is linear (non-branched) and thus does not accelerate, but could in principle continue to produce LOOH as long as PUFA and O2 are available. However, there is a finite probability of two LOO˙ molecules colliding and recombining to a non-radical dimer. This is an example of a termination reaction, the third phase of radical-mediated lipid peroxidation. Other radicals may also react with LOO˙ and terminate lipid peroxidation, as can non-radical antioxidants which, while converting LOO˙ to LOOH, themselves give rise to radicals too stable to support lipid peroxidation. An example of the latter is α-tocopherol (vitamin E). In either case (i.e., recombination of two radicals or scavenging of LOO˙ by a non-radical species), the lipid peroxidation chain reaction is terminated.
Fig. 4.
ROS-triggered lipid peroxidation chain reaction. Initiation: A hydroxyl radical (˙OH) reacts with a PUFA, usually part of a phospholipid in a biological membrane, abstracting a hydrogen atom. In this process, ˙OH is converted to water and a carbon-centered radical is formed on the fatty acid. Propagation: Dioxygen is added to the carbon-centered radical, forming in several steps an oxygen-centered peroxyl radical LOO˙. The latter abstracts a hydrogen from another PUFA and, in the process, is converted to a fatty acid hydroperoxide (LOOH). The PUFA from which a hydrogen was abstracted gives rise to a carbon-centered radical, thus completing the reaction cycle. A single initiation can lead to multiple propagation cycles as long as dioxygen and PUFAs are available. Termination: The peroxyl radical LOO˙ can react with another LOO˙ or with another radical, resulting in non-radical end products. Alternatively, LOO˙ can react with a sacrificial radical scavenger (antioxidant) which gives rise to a stable radical that lacks the ability to abstract hydrogen from PUFA. In either case, the lipid peroxidation chain reaction is terminated.
The LOOH that accumulate during lipid peroxidation are themselves reactive oxygen species, or ROS, and can oxidize other biomolecules. However, a considerable fraction of LOOH is non-enzymatically converted to a large variety of secondary products, including electrophiles such as 4-HNE [48]. For the sake of simplicity, 4-HNE is shown as a major end-product of lipid peroxidation in Fig. 4.
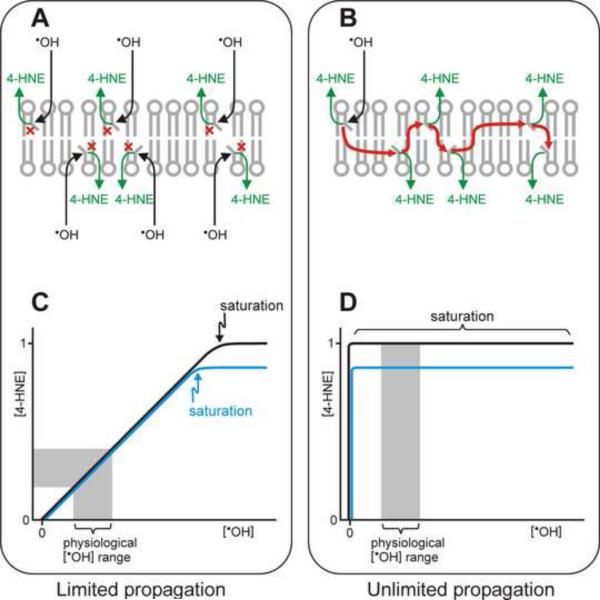
Is the amount of 4-HNE that is generated proportional to the level of oxidative stress that triggers lipid peroxidation? The answer to this question is of considerable importance to an understanding of the effects of 4-HNE on aging, as will be further elaborated in the following section. To answer this question, it is useful to consider two extreme scenarios. In one case, the propagation phase of the lipid peroxidation chain reaction is very short. If, on average, propagation were limited to only one cycle, each initiating event would produce one molecule of product (LOOH or 4-HNE), after which the reaction would terminate. This is shown schematically in Fig. 5A. Such a stoichiometric relationship between initiating ˙OH and resulting 4-HNE would yield a linear correlation between initial oxidative and resulting electrophilic stress, as schematically depicted in Fig. 5C; only a very strong burst of ˙OH, able to hit and saturate all available PUFA simultaneously, would cause the curve in Fig. 5C to level off. Changing the amount of PUFAs in the membrane would have no effect on the amount of generated electrophiles at moderate oxidative stress but would alter the potentially achievable electrophilic load (black versus blue line in Fig. 5C).
Fig. 5.
Relationship between the concentrations of initial ROS and resulting lipid peroxidation products. Panels A and B: Two theoretical extreme cases are schematically depicted in which the lipid peroxidation chain reaction terminates after a single cycle (A), or does not terminate until all PUFA substrate is used up (B). In the first case (panel A), a hydroxyl radical ˙OH attacks a PUFA (denoted by a bent fatty acyl chain in a membrane phospholipid), resulting in the formation of a lipid peroxidation product, for simplicity denoted as 4-HNE. The chain reaction is then immediately terminated (shown by the red “×” symbol). Thus, the formation of each 4-HNE molecule requires a separate attack on a PUFA by ˙OH. At the other extreme (panel B), a single initiation event starts a chain reaction (red arrows) that continues indefinitely, generating a 4-HNE molecule from each PUFA. Panels C and D depict graphically the two idealized relationships (corresponding to A and B, respectively) between the initiating ˙OH concentration and the amount of resulting 4-HNE. The black and blue lines in panels C and D denote, respectively, a higher and lower content of peroxidizable PUFA in the membrane. For rapidly terminating chain reactions (panel C, depicting results of the mechanism shown in panel A), there is a stoichiometric relationship between [˙OH] and [4-HNE] over a wide range of [˙OH], including a range that is physiologically normal (grey zone in panel C). In this range, the amount of 4-HNE formed is sensitive to the level of oxidative stress but not to the PUFA amount in the membrane, except for a very intensive oxidative burst that would deplete all PUFAs simultaneously (saturation point in panel C). Only under conditions of very high oxidative stress does the PUFA amount play a role; for example, less peroxidizable PUFA would lead to less 4-HNE formed under saturating [˙OH] (blue line). In the case of unlimited propagation of the chain reaction (panel D, illustrating results from the mechanism shown in panel B), a very small amount of ˙OH initiates a reaction that uses up all available PUFA and produces a maximal amount of 4-HNE. Thus, under these conditions, the formation of 4-HNE is a function of the content of peroxidizable PUFA in the membrane (black versus blue line in panel D) but is independent on the ˙OH concentration, including the physiological [˙OH] range (grey zone). Note that the two depicted situations are idealized extremes; an actual membrane may exhibit an intermediate behavior.
The other extreme scenario is depicted in Fig. 5B. Here, the rate of the termination reaction approaches zero. Therefore, even a single initiating event leads to an essentially unlimited propagation; the process would come to an end only when all available PUFAs are used up. The relationship between oxidative and electrophilic stress for this extreme case is schematically depicted in Fig. 5D. Here, a very low concentration of ROS (theoretically, one molecule of ˙OH is sufficient) generates the maximal possible electrophilic stress. The level of electrophilic stress would decrease if less PUFA were available in the membrane (blue line in Fig. 5D), regardless of the concentrations of ROS.
The idealized Gedankenexperiment shown in Fig. 5 leads to the conclusion that the length of the propagation phase of the lipid peroxidation chain reaction is the deciding factor. If propagation is limited to a single or just a few cycles, electrophilic stress is directly proportional to oxidative stress at moderate ROS levels that are within the normal physiological range (grey zone in Fig. 5C), but the amount of generated electrophiles is independent of the PUFA content of the membrane. At the other extreme, wherein the propagation phase is unlimited, the developing electrophilic stress is not affected by the level of ROS but is sensitive to the amount of available PUFAs (grey zone in Fig. 5D).
Which of the two extreme scenarios summarized above better approximates the process of lipid peroxidation in a real membrane? In principle, a full kinetic description of lipid peroxidation and determination of the rate constants of the relevant reactions would provide the answer to this question. However, the kinetics of chain reactions is complex. This is particularly true of lipid peroxidation because multiple reactions are involved. A thorough kinetic analysis of the process [47] led to the conclusion that a general analytical solution of the kinetic equations is not feasible, but that accurate approximations can be derived with reasonable simplifying assumptions. Two situations were distinguished: a single initiating event and continuous initiation over extended periods of time. The two cases differed quantitatively, but in both, termination by recombination of radicals lowered the rate but was unable to stop LOOH formation [47]. This would indicate that even a single initiating event could result in a protracted chain reaction (and 4-HNE formation) that would not stop by radical recombination until all PUFAs were exhausted. This scenario is similar to that depicted in Fig. 5B and D.
Obviously, the conclusion that a lipid peroxidation chain reaction is unstoppable once initiated should be applied with caution to real biological membranes. Such a pernicious process would be certain to damage membranes severely [e.g., refs. 45,49,50]. The fact that functional biomembranes exist indicates either that the damaged components are replaced rapidly enough to prevent deterioration, or that the lipid peroxidation chain reaction can be terminated by means other than recombination of radicals, for example, by sacrificial radical scavengers such as α-tocopherol. Thus, an actual membrane exposed to ROS conforms to neither Fig. 5C nor Fig. 5D but follows an intermediate path. Nevertheless, kinetic analysis suggests that the propagation segment of the chain reaction is not very short. Therefore, the previous conclusion can be reformulated for real biological membranes in a somewhat more qualified way: the generation of electrophilic end products from PUFAs is only weakly dependent on the level of the triggering oxidative stress; a small (“seeding”) amount of ROS is sufficient to initiate a chain reaction which then propagates without the need for further ROS. On the other hand, the formation of electrophiles is sensitive to the membrane content of PUFAs that are susceptible to peroxidation.
Oxidative stress, electrophilic stress, and aging
Conceptual advances in aging research date as far back as the first half [51] and the middle [52–54] of the twentieth century. Yet, in spite of these as well as subsequent theoretical insights and the intense research effort during the past three decades which brought the aging field into the mainstream of modern experimental biology, the causes and mechanisms of aging remain a mystery. In the literature, descriptions of aging range from radical-mediated wear-and-tear [55] to a genetically regulated process [56], a developmental program that inappropriately activates in adulthood [57], or even a deterministic genetic pathway [58,59]. Some of the disagreements may be only apparent: evolutionists, geneticists, molecular biologists, chemists, and statisticians speak their own, quite distinct languages, and their respective contributions to the field are not always sufficiently integrated. As has been eloquently and forcefully stated [60], a large part of the confusion in aging research is semantic: “regulation”, “life span” and “aging” not only mean different things to different people but are often used imprecisely. Even a short survey of the theories of aging is clearly beyond the scope of this review. However, as a backdrop for the following discussion of the role of electrophiles in aging, I will briefly summarize the conceptual framework that will be used in this article. Somewhat presumptuously, I have called this formulation the “standard theory” of aging [61] because I believe that the majority of researchers in the field subscribe to some variant of it, perhaps with different emphases on the particulars. According to the “standard theory”, aging results from an imbalance of two countervailing forces. One is destabilizing and leads to a gradual decrease in homeostasis, and thus, eventually, to death caused by loss of an essential physiological function. The opposing force is the totality of genetically controlled, evolved mechanisms that maintain the functional state of the organism. The cornerstone of this formulation is the postulate that neither one of the two forces is sufficient to explain aging: it is their interaction that is essential. In this, the conceptual framework I propose differs from theories that take the (undeniable) existence of genetic mechanisms able to modulate aging as a valid reason to reject the relevance to aging of undirected or even stochastic wear-and-tear [e.g., refs. 56,62].
What is the nature of the destabilizing forces that promote aging? Many factors contribute, including the tendency of any system that is far from equilibrium to lose its organization. The fact that living systems are able to repair damage and/or self-organize does not exempt them from the laws of thermodynamics; it simply means that organisms are open systems in which Gibbs free energy of metabolic reactions is used to maintain or increase local order. When metabolism ceases at the time of death, loss of organization and increase of entropy inevitably follow.
Another major contributor to destabilization is the intrinsic chemical reactivity, and thus susceptibility to damage, of molecules which constitute the building blocks of any organism. Metabolites can enter into side reactions or react with inappropriate macromolecular targets. The susceptibility of proteins to damage is at least partly due to their marginal folding stability [63]; relatively minor disturbances, be it physical (heat) or chemical (covalent or non-covalent modifications) may trigger unfolding and aggregation. This not only eliminates the protein's function but can initiate cytotoxicity. In fact, protein aggregation has emerged as a leading candidate for a causative factor in aging [64–66] as well as in many degenerative diseases [67,68]. DNA is chemically more stable than most proteins but damage to DNA has more severe consequences because mutations propagate. Damage to lipids compromises membrane structure and thus impacts fundamental processes such bioenergetics, signaling, and even cell integrity.
There is no agreement as to the most relevant types of reactions that could cause destabilization and aging. As already mentioned, oxidative and radical-mediated damage have been long-standing favorites [52,55,69]. Recently, the pendulum has swung the other way and these theories have been declared severely compromised or even fatally flawed [70–73 and others], perhaps prematurely. The demise of the oxidative stress theory of aging has been proclaimed on the basis of two types of evidence: the failure of many antioxidants to extend life span, and, in many cases, lack of an inverse correlation between markers of oxidative damage and longevity. To this, the Popperian reasoning was applied that even a single instance of contrary data is sufficient to invalidate a theory. However, redox reactions are complex; as has been pointed out by many authors [for example, refs. 38,74], the same compound can act as either an antioxidant or an oxidant, depending on the conditions. Most of the time, externally administered antioxidants fail to alter the intracellular redox status [75]; to be biologically active, antioxidants have to be present in the right chemical form, at the right intracellular site, and at the right time. Moreover, many of the commonly used oxidative stress markers are flawed or simplistically interpreted [38,76]; ROS have specific signaling functions in addition to causing untargeted damage [77–79]; various stresses, including oxidative, may elicit a protective hormetic response [80–83]. Given this complex network of interactions and the variety of experimental systems used, the fact that some results support and others contradict the oxidative stress theory of aging is not surprising and not necessarily fatal. Critics are likely to counter that a theory that is impossible to falsify loses its scientific standing. This is a valid point, but the conclusion should be to refine rather than summarily discard the theory. A more precise theoretical foundation and better experimental tools will demonstrate in which situations, in which tissues, and to what extent oxidative damage contributes to aging. In fact, the link between electrophiles and aging discussed in this review is an attempt at such refinement.
Although historically, oxidative and radical-mediated destabilizing chemical modifications of biomolecules have received most attention, a broader range of reactions may contribute to aging. An identification of such reactions has been attempted by a global comparison of gene expression levels in long-lived mutants (such as daf-2 hypomorphs in C. elegans) versus wild-type controls. In this approach, a correlation of longevity with the increased expression of a detoxifying enzyme indicates that the substrate of that enzyme may be causally involved in limiting life span. The microarray comparison was initially carried out in C. elegans [84,85] and later extended to Drosophila melanogaster and to mice [86]. Taken together, the results [reviewed in ref. 61] show that long life correlates most consistently with elevated expression of glutathione transferases (GSTs) and short chain dehydrogenases/reductases, i.e., enzymes which accept electrophiles as substrates. The correlation was less pronounced, or did not hold across as many species, for other detoxification enzymes such as UDP-glucuronosyltransferases (which conjugate nucleophiles) and cytochromes P450. Strikingly, there was little or no correlation with the levels of antioxidant enzymes. These results point to a possible pro-aging role of electrophiles, probably mediated by their already discussed ability to modify biologically essential macromolecules. Other toxicants, including ROS, would play a lesser role.
The destabilizing and potentially life-shortening factors discussed so far are physical or chemical in nature: the thermodynamic tendency of highly organized systems to become disordered and, more importantly, the susceptibility of organisms to chemical disturbances. Organisms can be viewed as extremely complex and finely tuned, but ultimately chemical systems. As such, they are necessarily affected by compounds, such as electrophiles, able to react with, and alter the function of, organismal components. I have previously discussed in more details these, and other related physico-chemical pro-aging factors [61]. Are all destabilizing events that accelerate aging physico-chemical in nature? In other words, are there biologically regulated, evolved pro-aging processes? The answer to this question is a qualified yes. The principle of antagonistic pleiotropy [54] describes traits that provide a reproductive advantage early in life but increase mortality after the reproductive period. Because natural selection optimizes reproductive success, the linked detrimental late-life outcomes will be co-selected. The prominent role of TOR signaling in aging [87–89] is rooted mainly in the ability of the pathway to coordinate protective functions (see below) but may have also an antagonistic pleiotropy element. TOR signaling is essential during growth [90,91] but, if over-activated in adulthood, may cause inappropriate growth/proliferation which leads to loss of homeostasis and to aging [57,92,93]. Another example of antagonistic pleiotropy is the innate immune system, essential for protection against pathogen but likely to contribute to aging [94]. It is worth stressing that no instance is known of a genetically determined, evolved trait whose sole function is to promote aging. In all cases, the acceleration of aging is a side effect of a process that is adaptive elsewhere, for example earlier in life or in a different tissue.
The relative importance of the pro-aging factors mentioned above is hotly debated. The present review is not intended to address this issue, except for pointing out the likely contribution of electrophiles to the aging process.
According to the “standard theory” of aging, life span is determined by the interplay of two opposing forces. The destabilizing, or pro-aging, factors have been discussed above. The following section deals with processes that maintain biological homeostasis and, therefore, postpone aging.
Factors that oppose aging: longevity assurance mechanisms
Two signaling pathways are of particular relevance to aging: the insulin/insulin-like signaling (IIS) [56,95] and the target of rapamycin (TOR) [56,87–89,96] pathways. The details of these pathways are complex and include cross-talk between them and with other signaling modalities, but their overall “logic” can be summarized as follows. If favorable conditions are sensed, in particular an abundance of nutrients, signaling through IIS and TOR is increased, stimulating growth and reproduction. On the other hand, limited food availability decreases IIS and TOR signaling and turns on programs that favor somatic maintenance, low reproduction, and long life span. While these pathways are best characterized in C. elegans (and therefore, the C. elegans nomenclature will be used in the remainder of this article unless stated otherwise), the findings apply at least in part to other species such as yeast, fruit flies, mice, and probably humans.
The “disposable soma” theory of aging posits a switch, in the face of adverse conditions, from high-reproduction to a pro-maintenance state featuring high stress resistance and increased life span, permitting the organism to survive until conditions improve [97–99]. In the original formulation of the theory, the switch results from the need to optimize the allocation of limited metabolic resources. The underlying mechanism may be more complex [100]; if the switch is adaptive in the long term, it may be actuated purely in response to sensing pathways such as IIS and TOR, even if metabolic energy is not limiting. Regardless of how the switch has evolved, in the context of the present discussion the key conclusion is that, in principle, signaling pathways are possible (and, in fact, exist) that push an organism into a metabolic state associated with longevity.
As already discussed, the realization that life span is under genetic control has led some to imply that aging is regulated. There is, however, broad agreement that it is not aging but the longevity assurance processes that are genetically determined. If these processes are weak, they are not able to stem the inevitable tide of destabilizing reactions for long, resulting in a short life span. If the protective pathways are efficient and/or highly activated, loss of homeostasis is delayed and the organism is long-lived.
The details of the metabolic state conducive to long life are, of course, of central interest to gerontology. If we learn which pathways or processes extend life, we will also know which destabilizing reactions, and resulting pathologies, drive aging. Therefore, the downstream processes orchestrated by IIS and TOR signaling have received considerable attention. Unfortunately, these two signaling pathways affect a wide range of downstream processes. Thus, there is still no consensus on which destabilizing forces, in conjunction with their cognate protective mechanisms, are most relevant to aging. Moreover, it appears likely that the answer will differ at least to some extent between species [101].
What are some of the downstream longevity assurance mechanisms, and how are they regulated by IIS and TOR? Low signaling through IIS activates the transcription factor DAF-16 (FoxO in mammals) [95]. At least in C. elegans, low IIS also activates another transcription factor, SKN-1 [102]. In mammals, the regulation of Nrf2 (the ortholog of worm SKN-1) may be more complex: it has been reported that, in cultured mammalian cells, insulin-triggered high IIS activates Nrf2 [103,104]. This response, opposite to that observed in C. elegans, could be cell-specific or linked to metabolic regulation exerted by insulin, superimposed on longevity assurance elicited by the insulin growth factor. Thus, further discussion will be based on the better-understood situation in C. elegans where increased longevity is linked to low IIS which activates SKN-1 [102,105]. Active DAF-16 and SKN-1 drive the expression of a large set of detoxification and antioxidant genes. As discussed in the previous section, the contribution of oxidants to aging is disputed and may be marginal, at least in some situations. Thus, antioxidant enzymes may be more important in protection against acute oxidative stress than in assuring longevity. However, other detoxification enzymes may have a more direct protective role that counteracts aging. Strikingly, GSTs are induced in both Nrf2/SKN-1-dependent [106–109] and DAF-16-dependent [110,111] manner, although DAF-16-dependence may be indirect [112]. Given the likelihood, discussed earlier, that electrophilic compounds contribute to destabilization of biological systems, the possibility should be considered that electrophiles and the enzymes that metabolize them, such as GSTs, comprise a pair of countervailing factors relevant to aging. This hypothesis will be discussed in more detail in the following section.
Low TOR signaling activates several additional categories of responses relevant to longevity assurance [for recent reviews, see refs. 87–89]. One of these is autophagy [113]. The process enables cells to re-use amino acids and other nutrients when food is limiting. However, autophagy also clears damaged and thus potentially harmful organelles or proteins, a process likely to delay aging [114]. Another process downregulated by low TOR signaling is protein synthesis, which is attenuated at several levels, including translation initiation and the biogenesis of ribosomes. In addition to being an obvious adaptation to limited nutrient availability, lower protein synthesis also means fewer misfolded protein molecules. Given the importance of protein homeostasis (“proteostasis”) in a variety of chronic diseases and in aging [101,115–120], diminished misfolding would have an anti-aging effect. In agreement with this conclusion, (i) the upregulation by low IIS of heat shock proteins, which prevent misfolding and aggregation or repair improperly folded proteins, also delays aging [121], and (ii) translational silencing has been demonstrated in several systems to extend life [122].
While protein synthesis is generally downregulated by low TOR signaling, certain proteins are in fact overexpressed. Specifically, in yeast, inhibition of TOR signaling caused an increase in the translation of certain mitochondrially encoded components of the respiratory chain, and led to increased respiration that was linked to an extended life span [123]. Similarly, in D. melanogaster, inhibition of TOR signaling by dietary restriction triggered increased translation (but not transcription) of some nucleus-encoded components of the mitochondrial respiratory chain; this was associated with a higher enzymatic activity of the respective complexes. This elevated activity was necessary for the life span extension triggered by dietary restriction [124]. The mechanism linking elevated mitochondrial respiration to longevity is not clear. The increased capacity to synthesize ATP could support maintenance functions. Moreover, a higher sustained activity of the respiratory chain could limit ROS production: a faster flow of electrons through the chain would keep the components in a less-reduced state, and would decrease the local steady-state oxygen tension. A lower tendency of one-electron transfers (leakage) from the chain and a limited availability of O2, the acceptor for leaking electrons, would decrease ROS generation [31,123,125]. Alternatively or in addition, an elevated activity of respiratory complex I would accelerate the oxidation of NAD(P)H and increase the NAD(P)+/NAD(P)H ratio, thus activating sirtuins, as well as limiting the activity of fatty acid desaturases [126]. The latter possibility is of particular interest in the context of this review because lower desaturase activity would lead to less PUFAs in membranes, rendering the membranes more resistant to peroxidation (see section “Role of electrophiles in aging” below). Individually or in combination, greater availability of ATP, lower ROS concentrations, and/or increased NAD(P)+/NAD(P)H ratio could delay aging.
A generalization can be derived from the examples listed above. Multiple destabilizing processes exist with the potential of interfering with biological homeostasis. If such interference leads to a persistent increase in the probability of death per unit of time, then by definition it promotes aging. Some of the destabilizing factors are physico-chemical in nature and others are the consequence of pleiotropic biological processes; the two categories are not sharply delineated and their relative importance is subject to debate. For most, or perhaps all, of the destabilizing forces, a cognate biological process can be identified that mitigates the damage. As any biological process, these longevity assurance mechanisms are genetically determined and subject to natural selection. Thus, aging is neither a consequence of purely random damage accumulation, nor is it determined solely by a regulated biological process, but rather it results from the interplay of these two opposing forces.
Of the various identifiable pairs consisting of a destabilizing factor and its matched protective process, some are relevant to aging and others co-determine outcomes such as acute toxicity or chronic diseases. Finding the factors that are most pertinent to aging remains one of the greatest challenges in gerontology, not least because such knowledge carries with it the promise of anti-aging interventions. In the following section, I will present arguments in support of the hypothesis that electrophilic stress, counteracted by reactions that clear electrophiles from biological systems, are significant contributors to the aging process.
Role of electrophiles in aging
Correlative evidence
As discussed previously (section on “Lipid peroxidation”), a case can be made for the hypothesis that the amount of lipid-derived electrophiles such as 4-HNE that form in response to oxidative stress depends strongly on the amount of peroxidizable PUFAs available in the membrane, but depends only weakly on the level of ROS that initiated the lipid peroxidation chain reaction. (The present discussion is limited to endogenously generated electrophiles, derived mostly from lipid peroxidation, as opposed to xenobiotic electrophiles such as some drugs.) From this hypothesis it can be predicted that any physiological and pathological consequences of electrophilic stress, while requiring an oxidative trigger, should correlate with the content, and especially peroxidizability, of membrane PUFAs, but may not correlate with most markers of oxidative damage. In the context of senescence, the above conclusion constitutes a departure from the standard oxidative-damage theory of aging. An initiating oxidative event is still required, but this condition is almost always satisfied: under aerobic conditions, a finite level of ROS will be present even if antioxidants are abundant. Thus, lipid peroxidation will always be initiated at a finite frequency. Whether the resulting chain reaction generates significant or negligible amounts of electrophiles, depends primarily on the PUFA content of the membrane (see Fig. 5). In this model, pro-aging activity is attributed to electrophiles rather than to ROS, and is no longer coupled to the level of the initial oxidative stress.
Is there experimental evidence that high susceptibility of membrane lipids to peroxidation inversely correlates with longevity? In fact, such evidence is available and will be reviewed below. It is, however, important to remember that correlative data do not prove causality. PUFAs could affect aging by mechanisms other than production of electrophiles, low PUFA content in membranes could be a consequence rather than a cause of longevity, or PUFA levels and aging could be causally unrelated but co-modulated by a third factor. Yet, given the previously discussed chemical properties of electrophiles in biological systems, a causal role of electrophiles in aging appears possible or even likely. This tentative conclusion has been confirmed by direct intervention studies that will be described in the following sub-section.
Birds have a generally longer life span compared with mammals of the same body mass. A study of heart mitochondria phospholipid composition of pigeons, which live in excess of 30 years, and rats, which have a life span of less than 5 years, has shown that the latter have a higher content of PUFAs and higher markers of lipid peroxidation [127]. A comparison of long-lived smaller birds (parakeet, canary) with equal-sized mice showed the same disparity in PUFA content [128]. The data were interpreted in terms of a greater resistance to lipid peroxidation in birds contributing to their longevity. Consistent with this interpretation, an inverse correlation between membrane peroxidizability and longevity was found in a group of mammalian species [129,130] and in a comparison of several species of long-lived sea birds versus short-lived fowl [131].
The maximal life span of naked mole-rats (Heterocephalus glaber) approaches 30 years, approximately nine times that of mice [132]. Therefore, comparisons of these two species are highly instructive, especially if carried out at comparable fractions of their respective life spans. Naked mole-rat membrane lipids are less susceptible to peroxidation than mouse membranes [133,134], a finding that is in agreement with the other inter-species comparisons described above. However, additional results obtained with the naked mole-rat pose unique theoretical challenges. Antioxidant enzymes such as SOD or catalase are not much different between the naked mole-rat and the mouse; glutathione peroxidase is actually much lower in the mole-rat [135]. Glutathione levels have been variably reported to be 25% lower [136] or 40% higher [119]. Consistent with these unexceptional antioxidant defenses, oxidative damage is not prevented in mole-rats. Urinary isoprostanes (a marker of whole-body oxidative stress) are higher in mole-rats than in mice [137]. Thus, naked mole-rats enjoy an exceptionally long life span and long reproductive period in spite of rather severe oxidative damage [132,138]. This apparent paradox can be resolved if it is related to the paradigm I am proposing, namely that electrophiles such as 4-HNE are relevant to aging, and that the formation of 4-HNE is largely decoupled from the initiating oxidative stress but is a function of membrane peroxidizability. The composition of naked mole-rat membranes would result in relatively low 4-HNE production, even if overall oxidative stress and oxidative damage are high. Moreover, it has been recently reported that GST activity in mole rats is 3-fold higher than in mice [supplementary data to ref. 119]. Thus, the pair consisting of a destabilizing factor (4-HNE) and the matched protective mechanism (GST) is set up very differently in the two species. In mice, the 4-HNE output is high and the disposal rate of 4-HNE is low, resulting in a high steady-state 4-HNE concentration and rapid aging. The situation is reversed in the naked mole-rat, perhaps contributing to its longevity. This interpretation suggests that lipid-derived electrophilic aldehydes are relevant to aging, probably through their ability to react with specific sites on proteins, whereas many other types of oxidative changes are well tolerated and are either neutral with respect to aging or even protective, owing to their ability to activate Nrf2 and induce the expression of detoxifying enzymes including GSTs [107,108,139].
The short-beaked echidna (Tachyglossus aculeatus) is a monotreme mammal with an unusually long life span for its size. The fatty acid composition of muscle, liver, and liver mitochondrial membranes obtained from this animal is consistent with the pattern observed in other long-lived species: the membranes were found to be low in PUFAs and thus resistant to peroxidation [140].
The inverse correlation between longevity and peroxidizability of membrane lipids extends taxonomically beyond mammals and birds. It has been found that honeybee (Apis mellifera) lipids have a consistently low PUFA content and thus low susceptibility to peroxidation in long-lived queens. Young workers resemble queens in this respect, but their PUFA content increases significantly by 1 week post-eclosion and remains high for the remainder of their life [141]. The life span of honeybee workers is typically less than a month, versus several years for a queen.
The results obtained with honeybees are particularly valuable because comparisons carried out within a single species are more persuasive than those between species where additional confounding factors may come into play. Another intra-species comparison is that of wild-derived versus laboratory mice [142]. Two strains were established from wild-caught progenitors, and were compared with a laboratory strain that was outbred to assure hybrid vigor. All mice were kept under identical, optimized laboratory conditions. The wild-derived strains were longer-lived than the laboratory strain, and had membranes less susceptible to peroxidation [142].
The correlation between membrane peroxidizability and life span appears to hold for humans. In comparison to age-matched controls, erythrocytes obtained from the offspring of nonagenarians had a lipid composition characterized by lower PUFA content but higher levels of monounsaturated fatty acids (MUFA) which are resistant to peroxidation [143].
Whereas the correlation of life span with body mass of animal species is generally high, there are several notable outliers. These include the naked mole-rat and the short-beaked echidna discussed previously, as well as humans [144]. Also, the regression line for birds is distinct from that for mammals; birds are, on average, longer-lived than same-sized mammals [144]. Both the outliers and the systematic difference between mammals and birds collapse when scaled body mass is replaced by the peroxidation index derived from the content and type of PUFA present in membranes [140,144]. The correlation between membrane peroxidizability and life span is the basis of the membrane pacemaker theory of aging [144–146].
A correlation proves neither causality nor a molecular mechanism. It is certainly plausible to propose that membranes, if subjected to peroxidation, both lose their normal function and generate potentially toxic hydroperoxides and electrophiles which spread the damage to other cell constituents, such as proteins and DNA. However, it is equally possible that species which have a long life span due to other mechanisms, evolved membranes resistant to peroxidation as a secondary adaptive trait. The direction of causality, if any, can be determined only by interventional studies in which the membrane composition, or the level of peroxidation-derived end products, is experimentally manipulated, and the ensuing effect on life span is recorded. The following sub-section addresses attempts at such interventions.
Evidence obtained through experimental interventions
The hypothesis that membrane lipid peroxidizability co-determines life span could be directly tested by experimental interventions that change membrane composition, followed by life span determinations. However, there are two major problems with this approach. One is technical. The lipid composition of membranes is under tight genetic control and fairly resistant to modifications. The finding that manipulation of dietary lipids leads to relatively limited, but still measurable changes in membrane lipid patterns [147,148] is thus fortunate. This somewhat surprising success of the dietary approach is probably attributable to an evolutionary conservation of overall properties of membranes, such as their fluidity, in preference to any particular lipid composition [“homeoviscous adaptation”, ref. 149]. Thus, within limits, the ratio of more to less peroxidizable fatty acids can shift while maintaining constant membrane fluidity. The more serious problem with experimental alterations of membrane lipid composition is that any of the interventions used, whether dietary, pharmacological, or genetic, is likely to have multiple effects. Dietary restriction may serve as an example. This intervention alters membrane fatty acid patterns, as will be discussed below, but obviously has other consequences, in particular those mediated through TOR signaling. Nevertheless, the interpretation of results is less ambiguous than in the case of inter-species comparisons, even if a final determination of causality remains elusive. Any given intervention may have a large, but still limited number of effects, thus narrowing the field of possibilities to be considered. The greater the number of different manipulations that result in both a shift to less peroxidation-susceptible membranes and concomitant life span extension, the higher the likelihood that the two are causally linked. Moreover, experimental interventions may rule out the possibility that resistant membranes evolved as secondary adaptation to long life, because interventions limited to an individual's life span obviously offer insufficient time for any role of natural selection. Thus, even though the specter of a correlation without causality is not completely banished, interventional studies are intrinsically more powerful than purely comparative approaches. Several illuminating results have been obtained using experimental interventions that alter membrane fatty acid composition, and will be discussed below. This will be followed by a description of experiments in which the levels of end products of lipid peroxidation were manipulated.
As already mentioned, dietary restriction may change the fatty acid composition of membranes in rats and in mice. In general, the percentage of highly peroxidizable PUFAs has been reported to increase with age in membranes of ad libitum-fed animals, but this trend was attenuated or abrogated by dietary restriction [150–152]. It should be, however, noted that conflicting results have been reported [153]; the reasons for the discrepancy remain unknown. Peroxidation-resistant membranes, if formed, may contribute to longevity, although clearly this one factor does not explain the entire life span gain seen in dietary restriction.
Studies of humans are more difficult to control than those of laboratory animals, but are of obvious interest, especially because some aging mechanisms are species-specific (“private”) and may apply only to humans [e.g., ref. 101] or only to particular non-human model organisms. In a rare study involving humans, older Italian subjects were followed for 8.5 years. A self-reported diet high in MUFAs from olive oil (Mediterranean diet) correlated with a lower all-cause mortality. In contrast, subjects ingesting a higher proportion of PUFAs had a (marginally) elevated mortality [154]. A MUFA-rich diet also protected against cognitive decline [155]. These studies have inevitable limitations, especially with regard to possible mechanisms. For example, it is not known whether the dietary fatty acids altered cellular biomembrane composition, and whether lipid peroxidation contributed to the observed effects. Nevertheless, it appears that a MUFA-rich diet is protective, while ingestion of PUFAs, in particular the highly peroxidizable n-3 PUFAs, may be a risk factor in terms of aging, in spite of its benefits in other areas [156–158]. In fact, the protection against a variety of human diseases afforded by increased intake of n-3 PUFAs may not reflect an absolute requirement for large amounts of n-3 PUFAs. Instead, diets rich in n-3 PUFAs could rectify the severe PUFA imbalance (high n-6/n-3 ratio) characteristic of the human food supply since the advent of industrial agriculture [159–161].
Disruption in mice of adenylyl cyclase type 5 [an isoform highly expressed in the heart, ref. 162] results in significant longevity (extension of median life span by 32%), along with health benefits such as protection from cardiomyopathy and attenuation of age-related loss of bone quality; cardiomyocytes and fibroblasts from the knockout animals are resistant to oxidative stress [163]. At least some of the protective effects, as well as the life span extension, were attributed to elevated ERK signaling [163]. In order to study further the applicable mechanism of longevity, other authors established a mouse model in which adenylyl cyclase disruption was mimicked by administration of atenolol, a cardioselective β1 adrenergic receptor antagonist [164]. As expected, treatment with atenolol for 2 weeks activated ERK signaling. While no decrease in mitochondrial ROS production was observed in the treated animals, administration of atenolol caused a dramatic shift in heart fatty acid composition. Saturated fatty acids and MUFAs were significantly increased in the atenolol group, whereas PUFAs, and in particular the highly peroxidizable n-3 PUFAs, were sharply decreased [164]. Of other, potentially longevity-related parameters, the expression of respiratory complexes I and IV was elevated. There was no indication that addition of atenolol to drinking water triggers dietary restriction. Assuming that disruption of adenylyl cyclase type 5 and atenolol administration are equivalent in terms of their longevity effects (which remains to be established, as no life span determination was done in atenolol-treated mice), either the shift in heart fatty acid composition or the increased expression of respiratory chain complexes could, by mechanisms described in the previous section, contribute to the observed life span extension.
The current record for enhanced longevity caused by a single-gene mutation in a multicellular animal is the remarkable 10-fold life span extension in C. elegans in which the phosphatidylinositol 3-kinase (PI3K) gene has been disrupted [165]. Further studies of this very long-lived mutant established a pronounced shift in its fatty acid composition as compared with an isogenic wild-type strain [161]. The strain comparison was then expanded to include additional mutants with life spans ranging from that of the wild-type to the PI3K mutant which lives ten times longer. Regression analysis of the full set of mutants revealed that PUFA content decreases, and MUFA and short-chain fatty acid content increase, with increasing life span. More abundant shorter-chain fatty acids in the long-lived strains may be an adaptation to maintain membrane fluidity in the face of declining PUFA content, although elevated MUFAs could also accomplish this. The peroxidizability index derived from fatty acid types and amounts showed a strong inverse correlation with the log(10) of life span (Pearson correlation coefficient R = −0.85, P <0.002). Analysis of transcript levels demonstrated that the observed shift in fatty acid composition between wild-type and the very long-lived mutant could be explained by lower expression of several fatty acid elongases, lower expression of a desaturase responsible for PUFA synthesis, and higher expression of desaturases involved in MUFA synthesis.
The data presented above are suggestive of a role of lipid peroxidizability in the determination of life span, but do not prove such a role. It is conceivable that all analyzed mutations independently modulate life span and fatty acid anabolic enzymes. To establish causality, the expression of the candidate enzymes was silenced by RNAi. In the wild-type genetic background, i.e., in the absence of any life-extending mutations, silencing of fatty acid elongases or of a desaturase involved in PUFA synthesis resulted in a longer life span. This result is highly significant in that it proves causation. It should be noted that the longevity gain in the RNAi experiments was smaller than in the very long-lived PI3K mutant which blocks IIS and attenuates several other signaling pathways [166]. This indicates that IIS orchestrates the expression of a large set of longevity assurance genes, of which the fatty acid elongases and desaturases constitute only a subset. Interventions that target such subset of IIS “effector” genes are expected to have smaller contribution to longevity than targeting of IIS itself [see ref. 61 for further discussion of this topic]. Additional work will be needed to define the relative importance for aging of lipid peroxidizability, versus other factors that destabilize biological homeostasis. However, the results summarized above establish unequivocally that the susceptibility of lipids to peroxidation not only correlates with, but has a causal role in, aging.
How does a susceptibility of membrane lipids to peroxidation translate into either a deterioration of the biological system that drives aging, or into an attenuation of longevity assurance processes that would otherwise delay aging? Several possibilities could be considered. All assume that peroxidation actually takes place, a reasonable proposition in light of the previous discussion that a small “seed” amount of ROS is sufficient to initiate a chain reaction whose propagation will depend on the availability of peroxidizable PUFAs in the membrane. Such lipid peroxidation reaction could be biologically detrimental for a number of reasons [167]. One is a change of intrinsic membrane properties that renders the membrane non-functional. For example, increased membrane permeability could collapse ion gradients and affect regulatory and ATP-generating processes, changes in fluidity could compromise protein function, and shifts in composition may alter lipid rafts. Another destabilizing consequence of lipid peroxidation is the generation of organic hydroperoxides and radicals (Fig. 4) which could then spread the damage to other cell constituents, within and outside the membrane. Finally, as already discussed, lipid hydroperoxides can give rise to electrophilic aldehydes able to modify macromolecules, including functionally essential proteins, both in the affected and in adjacent cells. Relative to most ROS, aldehydes such as 4-HNE have a long biological half-life [168] and higher chemical selectivity. These properties make 4-HNE and related α,β-unsaturated carbonyls attractive candidates for mediators of either destabilizing damage or targeted modulation of signaling. For these reasons, experimental interventions that alter the steady-state levels of 4-HNE were used to study the effects of this compound on life span.
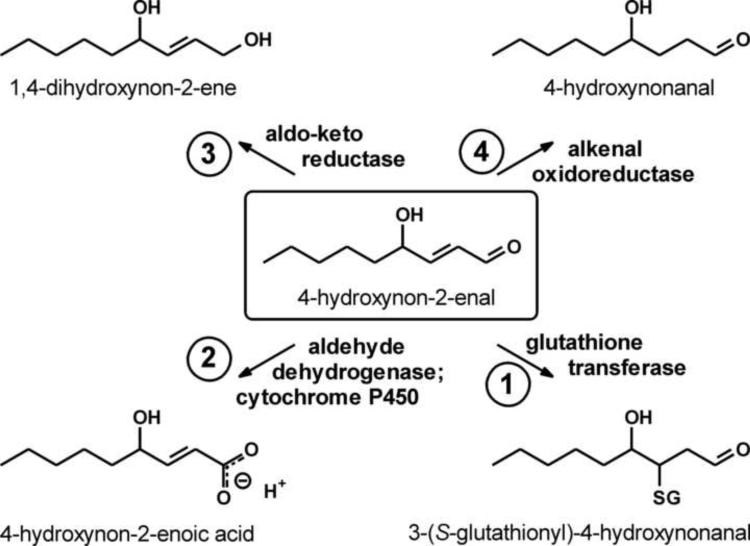
The formation of 4-HNE starts with lipid peroxidation which can be either chemical, typically by a reaction with a hydroxyl radical (Fig. 3), or enzymatic [by lipoxygenase-catalyzed reaction, reviewed in refs. 19,169]. The resulting lipid hydroperoxides give rise to 4-HNE in a non-enzymatic reaction [19,170]. Therefore, the formation of 4-HNE is likely to reflect primarily the prevalence of PUFAs in a membrane, or the membrane's peroxidizability, and is biologically not regulated other than by shifts in PUFA content. (This statement is obviously oversimplified, as it neglects important factors such as chain-breaking oxidants, but is nevertheless useful as a first approximation.) If the formation of 4-HNE is not biologically regulated, the steady-state level of the compound will be determined by its disposal. There are four major initial reactions by which 4-HNE can be metabolized (Fig. 6) [171–173]: conjugation with glutathione, or Michael addition, catalyzed by GSTs; oxidation of the aldehyde group by aldehyde dehydrogenases or by cytochromes P450; reduction of the aldehyde group by aldo-keto reductases; and reduction of the double bond by alkenal oxidoreductase. Experimental modulation of any of these activities will change the steady-state level of 4-HNE in a tissue.
Fig. 6.
Modes of 4-HNE metabolism. Biological elimination of 4-HNE can proceed via four primary reactions: (1) conjugation with glutathione, (2) oxidation of the aldehyde group, (3) reduction of the aldehyde group, and/or (4) reduction of the double bond. Secondary reactions and/or transport usually follow the primary reactions.
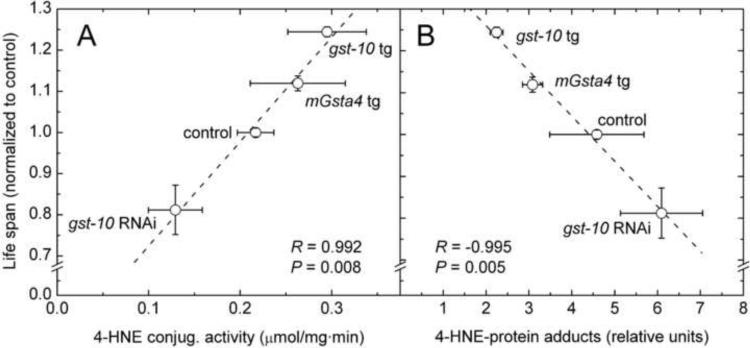
Among the more than forty GSTs of C. elegans, including five with a significant contribution to the whole-organism capacity to conjugate 4-HNE [174], the gst-10 gene product has been most extensively studied in the context of aging. Silencing of gst-10 expression by RNAi decreases the glutathione conjugating activity in worm lysates and, as expected, increases the level of 4-HNE-protein adducts. These biochemical changes are accompanied by a decrease of life span [110]. Transgenic expression, using the gst-10 promoter, either of gst-10 itself or of murine mGsta4 (another GST with high activity toward 4-HNE) has the opposite effect, namely higher enzyme activity, less 4-HNE modification of proteins, and proportionately extended life span [175]. When the results from both sets of experiments are combined, a dose-response curve can be derived. Life span is highly correlated with 4-HNE-conjugating activity (Fig. 7A) and is inversely correlated with the amount of 4-HNE adducts (Fig. 7B), which reflect the 4-HNE concentration in tissues.
Fig. 7.
Correlation of C. elegans life span with the overall capacity of the organism to conjugate 4-HNE (panel A) and with the amount of 4-HNE-protein adducts (panel B). The points represent experimental interventions in which the expression of the endogenous gst-10 gene was silenced by RNA interference (gst-10 RNAi), the expression of the same gene was increased by transgenic overexpression (gst-10 tg), or the murine mGsta4 gene was transgenically expressed using the gst-10 promoter (mGsta4 tg). The Pearson correlation coefficients R and the associated P values are given in each panel. Based on data from refs. [110,174,175].
It is important to note that the results summarized in Fig. 7B not only demonstrate an inverse correlation of the lipid peroxidation end product 4-HNE with longevity, but strongly suggest causation: because the experimental intervention that was used directly affected 4-HNE levels, the life span changes are probably caused by 4-HNE. Causation is plausible but not proven because the two GSTs that were used, even though one was from C. elegans and the other from mouse, could theoretically share some unknown life-extending activity. In such case, the ability of both enzymes to conjugate 4-HNE would be coincidental. While difficult to rule out with certainty, such possibility can be made progressively less likely by using multiple independent methods of modulating 4-HNE levels. If all such interventions yield longevity gains inversely related to 4-HNE levels, it becomes increasingly difficult to argue that they all share some unknown property, other than the ability to modulate 4-HNE, that affects life span. To pursue this approach, worms were treated with three chemical 4-HNE scavengers [176–182]: carnosine, hydralazine, or histidine hydrazide. All three scavenger compounds extended C. elegans life span (unpublished results), with hydralazine being the most effective. Each of the chemical 4-HNE scavengers that were used has additional pharmacological and/or biochemical properties; for example, hydralazine is an antihypertensive agent and carnosine is an antioxidant. However, the effect on life span was similar for the three compounds and was analogous to the life-extending phenotype of gst-10-overexpressing worms [175], indicating that the longevity gain was in fact mediated by decreased 4-HNE. This conclusion was confirmed by complementary results showing that RNAi silencing of individual aldehyde dehydrogenase or aldo-keto reductase genes resulted in life span shortening that was directly proportional to the metabolic capacity for 4-HNE attributable to any given gene product (unpublished data). Taken together, these results show that elevation of 4-HNE exerts a negative, and depletion of 4-HNE, a positive effect on longevity, at least within the limits of the genetic and pharmacological interventions that were used.
As discussed previously, an increased peroxidizability of membranes is causally linked to a shorter adult life span in C. elegans. Given that 4-HNE is directly derived from the peroxidation of PUFA, the finding that 4-HNE also curtails life strongly implies that at least part of the negative longevity impact of membrane peroxidizability is mediated by 4-HNE.
A determination of the life span of mGsta4 null mice [183] illuminates several important points related to 4-HNE and aging. The gene mGsta4 encodes mGSTA4-4, a GST with the highest catalytic efficiency for 4-HNE among murine GSTs [184,185]. Disruption of that gene is expected to raise tissue levels of 4-HNE, a prediction that was verified experimentally in the mGsta4 null mouse in 129/sv genetic background [186]; this strain was used to characterize phenotypes linked to 4-HNE [186,187]. In parallel, the knockout was also backcrossed into the C57BL genetic background. This new mGsta4 null line was found to have substantially upregulated antioxidant and anti-electrophile detoxifying enzymes, probably via activation of Nrf2 [183]. The bolstered defense mechanisms bring down the tissue concentration of 4-HNE to near-control values [187]. Therefore, no longevity-reducing effect of 4-HNE would be expected in the knockout in the C57BL background – and none was in fact observed. Actually, this knockout had a slightly (approximately 10%) extended life span [183], probably because the additional protective mechanisms are just sufficient to abrogate any increase in 4-HNE levels but overcompensate in terms of longevity. This initially unexpected result illustrates the need to verify whether any given experimental intervention has the predicted effect on the physiological mechanism being targeted. This point has been forcefully made by Halliwell and co-workers in relation to oxidative stress, where the majority of externally administered antioxidants failed to alter the intracellular redox status either in humans [reviewed in ref. 188] or in C. elegans [75]. The caveat applies equally to genetic manipulations. Electrophilic stress was present in the mGsta4 knockout in one but not another genetic background, affecting the interpretation of results. Obviously, a life span determination of mGsta4 null mice in the 129/sv background would be of interest.
Possible mechanisms by which electrophiles affect aging
The fledgling status of the field limits the discussion of molecular mechanisms mostly to speculations, even if based on solid chemical and biological characterization of electrophiles, especially 4-HNE, in areas other than aging. Two major types of (partially overlapping) mechanisms can be considered: (i) toxicity as a destabilizing, pro-aging factor, and (ii) modulation of signaling pathways which could weaken longevity assurance mechanisms or, perhaps, trigger pro-aging damage via pleiotropic processes. Some of these possibilities will be discussed in this section.
When 4-HNE and related aldehydes were initially discovered in biological material by Esterbauer and co-workers [189,190], they were considered to be toxic and detrimental end-products of lipid peroxidation. This view is still represented in the literature, and is supported by experiments in which high (supraphysiological) concentrations of 4-HNE are used. Under such conditions, any selectivity of the Michael adduct formation would be lost because many, or most, of the 4-HNE-reactive nucleophilic centers in proteins would tend to be saturated. Such indiscriminate modification of proteins would be cytotoxic. Further destabilization would result from reaction with aminophospholipids and nucleic acids. The global concentration of 4-HNE (averaged over the total volume of a cell or a tissue) may only rarely, if ever, reach levels necessary for untargeted toxicity. Thus, such a mechanism could play a role in isolated acute toxic events but be less relevant to aging. However, 4-HNE partitions preferentially into membranes [191] because of its high octanol/water partitioning coefficient, estimated to be 22 or 71 [refs. 192,193, respectively] (see also ref. [194] for a different model of 4-HNE partitioning). The local concentration of 4-HNE in the membrane core could exceed 300 μM [195,196]. Thus, 4-HNE not only forms within or near membranes, but tends to concentrate there. Prolonged exposure of integral membrane proteins and, perhaps, of aminophospholipids to high local 4-HNE concentrations could conceivably cause untargeted damage directly related to the electrophilicity of the compound [26,197], and accelerate aging.
The concept of “specificity of the third kind” has been formulated for ROS and reactive nitrogen species [198] but applies equally to reactive electrophiles such as 4-HNE. In contrast to the first (unique macromolecular interactions) and second (interactions of small ligands with a limited number of macromolecules) types of specificity, the “third kind” refers to covalent modifications of multiple target macromolecules by reactive compounds such as ROS. Such modifications usually do not trigger a distinctive signaling of their own but may modulate existing signaling or metabolic pathways and reactions [198]. It is in this context that 4-HNE has been recognized as a signaling molecule [20,43,44,199–203, and others]. Long lists have been assembled of 4-HNE targets and of possible functional consequences of adduct formation (for examples, see the above references). Of these, which are the processes that are relevant to aging and longevity? This question is difficult to answer. 4-HNE can form adducts on cysteines, lysines, histidines [26] and, in rare cases, arginines [204]. The intrinsic reactivities of the above amino acid side chains differ from one another and are further modified by the microenvironment [26]. Thus, at a low (physiological) 4-HNE concentration, only the most reactive nucleophilic groups will be modified. Still, adducts will form on multiple proteins, but only some of the modified proteins will be functionally affected. Experimentally, it is easier to identify 4-HNE adducts, e.g., by high-throughput mass spectrometry, than to determine their functional consequences, if any. The latter needs to be done individually for each candidate protein.
Although a distinction is often made between age-associated diseases and “normal” aging, it is obvious that many degenerative diseases shorten life span. 4-HNE is not only elevated in the brain of Alzheimer's disease patients, but modifies, among others, the amyloid β (Aβ) peptide and facilitates its misfolding and aggregation [194,205,206]. Thus, 4-HNE is likely to contribute to the progression of Alzheimer's disease by targeting specific protein(s).
Whereas functional modifications by 4-HNE of any given target, for example the Aβ peptide discussed above, usually has rather narrow and specific biological consequences, a much broader range of outcomes can be expected if 4-HNE modifies a regulatory protein. The modulation by 4-HNE of the activity of transcription factors provides a good illustration. In an unbiased, global approach, cells were treated with 4-HNE, and the total set of 4-HNE-modified proteins was determined by mass spectrometry, while shifts in gene transcription were documented at the same time using microarrays [207]. A subsequent bioinformatic analysis revealed the possible regulatory links between the two sets of data. Among these, transcription factors were prominent [207]. In particular, several heat shock proteins (Hsps) were found to have formed adducts with 4-HNE [208] and heat shock-regulated transcripts were significantly elevated [209] in response to 4-HNE treatment. These results were interpreted in terms of a release, by the 4-HNE-adducted Hsps, of the heat shock factor 1 (HSP1) which, under basal conditions, remains in an inactive cytoplasmic complex with Hsps. Following release, HSP1 is now able to translocate to the nucleus and to induce the expression of a large set of protective genes, including but not restricted to those that encode Hsps [207]. In the context of aging, this represents a compensatory response in which 4-HNE evokes mechanisms that protect the cell against 4-HNE, other electrophiles, and other destabilizing factors. The effect on life span can be complex: the protective response may be weak, in which case 4-HNE could accelerate aging, or very strong, perhaps leading to overcompensation, masking of any underlying detrimental effects of 4-HNE, and – overall – an extended life span, as observed by us in the mGsta4 null mouse in the C57BL genetic background [183]. Between the two extremes, there will be a situation without any measurable change of life span. As seen in this example, a conclusion from any one negative result that 4-HNE is irrelevant to aging would be premature and overly simplistic.
A high-throughput approach, such as that summarized in [207], provides a global view and indicates which candidate processes are important and which may be marginal, but it suffers from several drawbacks: the results are correlative and do not demonstrate causality, proteins or transcripts of low abundance may escape detection even if biologically significant, the conclusions depend on existing gene ontology classifications and would thus miss unexpected connections, and, in the case being discussed [207], post-transcriptional outcomes are ignored in the analysis which is restricted to transcriptional shifts. Thus, as most global approaches, the unbiased analysis of 4-HNE effects [207] should be viewed not as the final word but as an extremely useful tool to suggest candidate proteins for subsequent molecular and functional analysis. Such individual analyses have been carried out previously and have uncovered several important transcription factors that are modulated by 4-HNE. In the context of aging, the already discussed Nrf2 (SKN-1 in C. elegans) is of particular importance as it activates anti-electrophile and antioxidant defenses [106–108,139] in response to an electrophilic or oxidative stimulus [210,211]. Thus, activation of Nrf2 is another example of a compensatory longevity-assurance mechanism evoked by 4-HNE. A specific example of Nrf2 action is the biosynthesis of glutathione. Cells contain very high (millimolar) concentrations of glutathione, a compound with strong nucleophilic properties attributable to its thiol group [212]. Even though further accelerated by GSTs, the spontaneous reaction of glutathione with most electrophiles is rapid. Thus, glutathione has, among many others, an anti-electrophile protective function, both non-enzymatic and GST-dependent. The rate-limiting step in the de novo synthesis of glutathione is catalyzed by glutamate cysteine ligase, a heterodimeric enzyme that consists of a catalytic and modulatory subunit. The expression of genes encoding both subunits is induced by active Nrf2 in conjunction with active JNK signaling [213,214]. Interestingly, the catalytic subunit of glutamate cysteine ligase can be directly modified by 4-HNE, resulting in an increase of enzymatic activity even in the absence of the modulatory subunit. This is a rare example of a gain of function following adduct formation with 4-HNE. Overall, glutamate cysteine ligase activity is upregulated in response to 4-HNE by both a transcriptional and a posttranslational mechanism.
A number of other signaling pathways, such as stress response, NFκB-mediated processes, apoptosis, cell cycling and inflammation are modulated by 4-HNE. It is, however, not clear how these pathways affect aging and longevity assurance. Many appear to have a dual role; for example, apoptosis is essential for maintaining homeostasis but becomes destabilizing if excessive or occurring in the wrong place at the wrong time. Therefore, the possible mechanisms by which 4-HNE affects these pathways will not be further discussed here. Because of the prominence of IIS in orchestrating longevity assurance, it would be important to know whether IIS is modulated by 4-HNE. However, no direct data are available on this topic.
An intriguing mechanism by which 4-HNE could contribute to longevity is the partial uncoupling of mitochondria. When respiratory chain substrates are available but ATP is not synthesized, the proton-motive force is high, as is the local oxygen partial pressure. Under such conditions, the generation of ROS by mitochondria is high [31]. A partial uncoupling will lower the proton-motive force and the oxygen concentration; ROS production will decrease at the cost of dissipating some of the available free energy as heat. It has been proposed that 4-HNE activates mitochondrial uncoupling proteins to limit ROS production [215–217], even though this conclusion has been disputed [218]. Given the doubts surrounding the oxidative stress theory of aging, it remains uncertain whether a partial uncoupling of mitochondria affects longevity [219].
As has been already discussed, evidence has been accruing that loss of homeostasis of the proteome plays a prominent role in aging. In addition to exerting, at high concentrations, a generalized destabilizing effect, 4-HNE specifically modulates processes that are directly involved in the maintenance of the proteome. 4-HNE and/or related α,β-unsaturated carbonyl compounds not only modify [208] but also inhibit the function of chaperones of the Hsp70 and Hsp90 classes [220,221]. The effect of this inhibition on the release of HSP1 and induction of protective genes has been already discussed. However, a deficiency in Hsp function will have a destabilizing effect on the global proteome. Similarly, 4-HNE partially inhibited the activity of protein disulfide isomerase [222], a chaperone that facilitates the correct formation of disulfide bonds in newly synthesized endoplasmic reticulum proteins. Thus, excess 4-HNE can trigger ER stress, a factor that contributes to aging [87,223].
There are two major degradation pathways for damaged proteins: autophagy and degradation by the proteasome. Because autophagy targets mainly long-lived proteins whereas the proteasome is primarily responsible for the removal of proteins with shorter half-lives [224–226], autophagy may be more important in preventing the accumulation of persistent damage. Nevertheless, both degradative processes are modulated by 4-HNE, albeit in a complex way. 4-HNE may inhibit the proteasome directly [227]. Proteins that are only moderately modified by 4-HNE are preferred substrates for the proteasome, while heavily modified proteins inhibit the proteasome and are not cleared efficiently [228,229]. The biphasic (non-monotonic) response of proteasomal function to 4-HNE levels may be very relevant to aging. Proteins that carry 4-HNE adducts and initially escape degradation could be further modified to become more effective proteasomal inhibitors. In such case, the protein would not only permanently escape future proteolysis but would also prevent the degradation of other proteins, thus initiating a positive-feedback cycle leading to accelerating and detrimental accumulation of damaged material.
The modulation of autophagy by 4-HNE is even more complex and has not been extensively studied; most of the available information is from studies of ischemia/reperfusion (I/R) injury to the heart muscle. TOR signaling is of central importance for the process (Fig. 8; the mammalian nomenclature is used in this Figure). Autophagy is negatively regulated by mTOR which is inhibited by AMPK which, in turn, is activated by the upstream kinase LKB1. The latter kinase is modified and inhibited by 4-HNE [230,231]. Thus, high levels of 4-HNE will lead to low autophagy (branch 1 in the scheme of Fig. 8), as has been experimentally verified for the ischemia phase of I/R [232]. If the acute situation of I/R can be used as a model of chronic processes relevant to aging, elevated 4-HNE will limit autophagy and, therefore, promote aging.
Fig. 8.
Modulation by 4-HNE of selected signaling pathways that affect autophagy. Branches of the pathways, labeled 1 through 4 in the scheme, are referred to in the text.
A shift in signaling has been proposed for the reperfusion phase of heart I/R [232]. In this situation, the insulin signaling pathway (branch 2 in Fig. 8), which also converges on mTOR, was considered to prevail over AMPK-mediated regulation [232]. Insulin signaling, or IIS, is antagonized by the protein phosphatase PTEN which is a substrate for the LKB1 kinase [233]. As phosphorylation of PTEN by LKB1 inhibits the function of PTEN [232,234], and as 4-HNE inhibits LKB1 [230,231], high 4-HNE would result in high autophagy (branch 3 in Fig. 8). The opposite effects of 4-HNE on autophagy in the ischemia and reperfusion phases of I/R (mediated by branches 1 and 3, respectively, in the scheme of Fig. 8) were proposed to act together to adjust autophagy to optimal levels for cardiomyocyte survival during I/R [232]. However, 4-HNE can directly modify and inhibit PTEN, thus activating IIS [235] (branch 4 in Fig. 8). In this scenario, high 4-HNE would lead to low autophagy, in agreement with the outcome of AMPK-mediated regulation (branch 1). Thus, perhaps in tissues other than the heart muscle or under chronic conditions, as opposed to the acute I/R injury, elevated 4-HNE can be reasonably predicted to limit autophagy (branches 1 and 4 of the scheme) and to promote aging.
Conditions and cell type appear to be important because it has been reported that in vascular smooth muscle cells, exposure to relatively high (50 μM) 4-HNE caused modification and/or crosslinking of multiple proteins and activation of autophagy [236], while in the retina, 4-HNE-modified proteins resisted lysosomal (autophagic) degradation and inhibited the degradation of other proteins [237]. Moreover, autophagy may not always correlate with a life span extension in C. elegans [238]. Clearly, the relationship between lipid peroxidation, 4-HNE, autophagy, and longevity has been only partially defined, in spite of suggestive data, summarized above, that the pro-aging effect of 4-HNE [110,174,175] is at least in part mediated by inhibition of autophagy.
Conclusions and future directions
The effects of 4-HNE and other endogenous electrophiles on longevity are best described by a non-monotonic function. Just like low levels of ROS, low 4-HNE concentrations are probably required for homeostasis: lipid peroxidation and 4-HNE signal the state of the cell and trigger an appropriate metabolic adaptation. At moderately elevated 4-HNE levels, the adjustments are protective; they also enable the cell to survive future electrophilic stresses (hormetic response). Many of the responses aim at lowering the 4-HNE level. Therefore, in some situations the relevant parameter may not be the steady-state 4-HNE concentration but the flux through the pathways, i.e., the rate of 4-HNE generation and the rate of its disposal. If these rates are high, a metabolic and regulatory cost is exacted from the cell, even if the resulting steady-state 4-HNE level is suppressed. If the adaptive responses are overwhelmed by still higher 4-HNE supply, apoptosis will be triggered. Superimposed on these adaptive processes are the destabilizing consequences of chemical 4-HNE reactivity with cellular nucleophiles. Overall, the biological responses to 4-HNE are complex and nonlinear.
In the context of aging, the low (physiological) range of 4-HNE concentrations is most relevant. Thus, the emphasis of future research should be on signaling, rather than on toxic effects of 4-HNE that manifest themselves at high concentrations. Unlike many other biological processes, aging is primarily affected by factors that are chronic and cumulative. This also applies to 4-HNE exposure, and presents unique experimental challenges. It is a well-known temptation for the experimentalist to increase the dose of an agent until a readily measurable response is obtained. In the case of 4-HNE, the non-monotonic nature of the response precludes such an approach, or at best, must be acknowledged in the interpretation of results. Another experimental difficulty derives from the already mentioned dual nature of a reactive compound such as 4-HNE: it is destabilizing but, at the same time, it induces defense mechanisms. As these opposing responses may occur concomitantly, their sum can have a negative, positive, or no effect on longevity, depending on the level of exposure to the electrophile, time, target tissues, and other conditions. It is therefore important to devise experimental approaches that would permit a separate characterization of the various molecular effects of 4-HNE and/or other electrophiles. Such dissection of a multifaceted response is difficult but necessary to understand the mechanisms by which electrophiles affect aging.
In summary, there is ample experimental evidence that electrophiles have the ability to modulate life span at different levels, probably by both destabilizing biological systems and evoking protective responses. This dual nature of biological effects of electrophiles poses significant conceptual and experimental challenges. Moreover, the relative contribution of electrophiles versus other factors that affect aging and longevity assurance remains to be established. In spite of these difficulties, progress has been made. Advances include the understanding that electrophilic stress is chemically distinct from oxidative stress and has different biological outcomes. Experimentally, interventions designed to alter directly the level of electrophilic stress made it possible to establish causal relationships between electrophiles and longevity. Having developed the necessary basic tools, the field is in the position to gain a fuller understanding of the role of electrophiles in aging.
Acknowledgments
The work carried out in the author's laboratory was supported by NIH/NIA grants R01 AG028088 and R01 AG032643. The author acknowledges infrastructure support and a Research Career Scientist Award from the Department of Veterans Affairs. Helpful comments and suggestions of Drs. Robert J. Shmookler Reis, Andrzej Zimniak, Ludwika Zimniak, and two anonymous reviewers of the manuscript are appreciated.
List of Abbreviations
- 4-HNE
4-hydroxynon-2-enal
- GST
glutathione transferase
- Hsp
heat shock protein
- I/R
ischemia/reperfusion
- IIS
insulin/insulin-like signaling
- MUFA
monounsaturated fatty acid
- PI3K
phosphatidylinositol 3-kinase
- PUFA
polyunsaturated fatty acid
- RNAi
RNA interference
- ROS
reactive oxygen species
- TOR
target of rapamycin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Pinkston JM, Garigan D, Hansen M, Kenyon C. Mutations that increase the life span of C. elegans inhibit tumor growth. Science. 2006;313:971–975. doi: 10.1126/science.1121908. [DOI] [PubMed] [Google Scholar]
- [2].Pinkston-Gosse J, Kenyon C. DAF-16/FOXO targets genes that regulate tumor growth in Caenorhabditis elegans. Nat. Genet. 2007;39:1403. doi: 10.1038/ng.2007.1. [DOI] [PubMed] [Google Scholar]
- [3].March J. Reactions, mechanisms, and structure. Wiley; New York: 1985. Advanced organic chemistry. [Google Scholar]
- [4].Mayr H, Ofial AR. Kinetics of electrophile-nucleophile combinations: A general approach to polar organic reactivity. Pure Appl. Chem. 2005;77:1807–1821. [Google Scholar]
- [5].Pearson RG. Hard and soft acids and bases - the evolution of a chemical concept. Coord. Chem. Rev. 1990;100:403–425. [Google Scholar]
- [6].Schultz TW, Carlson RE, Cronin MT, Hermens JL, Johnson R, O'Brien PJ, Roberts DW, Siraki A, Wallace KB, Veith GD. A conceptual framework for predicting the toxicity of reactive chemicals: modeling soft electrophilicity. SAR QSAR Environ. Res. 2006;17:413–428. doi: 10.1080/10629360600884371. [DOI] [PubMed] [Google Scholar]
- [7].Metzler DE, Metzler CM. Biochemistry: the chemical reactions of living cells. Harcourt/Academic Press; San Diego, CA: 2001. [Google Scholar]
- [8].Williams DA. Drug metabolism. In: Lemke TL, Williams DA, Roche VF, Zito SW, editors. Foye's principles of medicinal chemistry. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 253–326. [Google Scholar]
- [9].Jorquera R, Tanguay RM. The mutagenicity of the tyrosine metabolite, fumarylacetoacetate, is enhanced by glutathione depletion. Biochem. Biophys. Res. Commun. 1997;232:42–48. doi: 10.1006/bbrc.1997.6220. [DOI] [PubMed] [Google Scholar]
- [10].Bergeron A, Jorquera R, Orejuela D, Tanguay RM. Involvement of endoplasmic reticulum stress in hereditary tyrosinemia type I. J. Biol. Chem. 2006;281:5329–5334. doi: 10.1074/jbc.M506804200. [DOI] [PubMed] [Google Scholar]
- [11].Held PK. Disorders of tyrosine catabolism. Mol. Genet. Metab. 2006;88:103–106. doi: 10.1016/j.ymgme.2006.04.002. [DOI] [PubMed] [Google Scholar]
- [12].van Dyk E, Steenkamp A, Koekemoer G, Pretorius PJ. Hereditary tyrosinemia type 1 metabolites impair DNA excision repair pathways. Biochem. Biophys. Res. Commun. 2010;401:32–36. doi: 10.1016/j.bbrc.2010.09.002. [DOI] [PubMed] [Google Scholar]
- [13].Fisher AL, Page KE, Lithgow GJ, Nash L. The C. elegans K10C2.4 gene encodes a member of the fumarylacetoacetate hydrolase family. A C. elegans model of type I tyrosinemia. J. Biol. Chem. 2008;283:9127–9135. doi: 10.1074/jbc.M708341200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee SS, Kennedy S, Tolonen AC, Ruvkun G. DAF-16 target genes that control C-elegans life-span and metabolism. Science. 2003;300:644–647. doi: 10.1126/science.1083614. [DOI] [PubMed] [Google Scholar]
- [15].Ovadi J, Srere PA. Macromolecular compartmentation and channeling. Int. Rev. Cytol. 2000;192:255–280. doi: 10.1016/s0074-7696(08)60529-x. [DOI] [PubMed] [Google Scholar]
- [16].Marnett LJ, Riggins JN, West JD. Endogenous generation of reactive oxidants and electrophiles and their reactions with DNA and protein. J. Clin. Invest. 2003;111:583–593. doi: 10.1172/JCI18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- [18].Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog. Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- [19].Schneider C, Porter NA, Brash AR. Routes to 4-hydroxynonenal: Fundamental issues in the mechanisms of lipid peroxidation. J. Biol. Chem. 2008;283:15539–15543. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Poli G, Schaur RJ, Siems WG, Leonarduzzi G. 4-Hydroxynonenal: a membrane lipid oxidation product of medicinal interest. Med. Res. Rev. 2008;28:569–631. doi: 10.1002/med.20117. [DOI] [PubMed] [Google Scholar]
- [21].Schneider C. An update on products and mechanisms of lipid peroxidation. Mol. Nutr. Food Res. 2009;53:315–321. doi: 10.1002/mnfr.200800131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Catala A. A synopsis of the process of lipid peroxidation since the discovery of the essential fatty acids. Biochem. Biophys. Res. Commun. 2010;399:318–323. doi: 10.1016/j.bbrc.2010.07.087. [DOI] [PubMed] [Google Scholar]
- [23].Long EK, Picklo MJ., Sr. Trans-4-hydroxy-2-hexenal, a product of n-3 fatty acid peroxidation: Make some room HNE…. Free Radic. Biol. Med. 2010;49:1–8. doi: 10.1016/j.freeradbiomed.2010.03.015. [DOI] [PubMed] [Google Scholar]
- [24].Lee SH, Blair IA. Characterization of 4-oxo-2-nonenal as a novel product of lipid peroxidation. Chem. Res. Toxicol. 2000;13:698–702. doi: 10.1021/tx000101a. [DOI] [PubMed] [Google Scholar]
- [25].Doorn JA, Petersen DR. Covalent adduction of nucleophilic amino acids by 4-hydroxynonenal and 4-oxononenal. Chem. Biol. Interact. 2003;143:93–100. doi: 10.1016/s0009-2797(02)00178-3. [DOI] [PubMed] [Google Scholar]
- [26].LoPachin RM, Gavin T, Petersen DR, Barber DS. Molecular mechanisms of 4-hydroxy-2-nonenal and acrolein toxicity: nucleophilic targets and adduct formation. Chem. Res. Toxicol. 2009;22:1499–1508. doi: 10.1021/tx900147g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wood PM. The potential diagram for oxygen at pH 7. Biochem. J. 1988;253:287–289. doi: 10.1042/bj2530287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Koppenol WH, Stanbury DM, Bounds PL. Electrode potentials of partially reduced oxygen species, from dioxygen to water. Free Radic. Biol. Med. 2010;49:317–322. doi: 10.1016/j.freeradbiomed.2010.04.011. [DOI] [PubMed] [Google Scholar]
- [29].Halliwell B, Gutteridge JMC. Free radicals in biology and medicine. Oxford University Press; Oxford: 1999. [Google Scholar]
- [30].Lane N. Oxygen: the molecule that made the world. Oxford University Press; New York, NY: 2002. [Google Scholar]
- [31].Murphy MP. How mitochondria produce reactive oxygen species. Biochem. J. 2009;417:1–13. doi: 10.1042/BJ20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brown GC, Borutaite V. There is no evidence that mitochondria are the main source of reactive oxygen species in mammalian cells. Mitochondrion. 2011 doi: 10.1016/j.mito.2011.02.001. in press. [DOI] [PubMed] [Google Scholar]
- [33].Tretter L, Adam-Vizi V. Generation of reactive oxygen species in the reaction catalyzed by alpha-ketoglutarate dehydrogenase. J. Neurosci. 2004;24:7771–7778. doi: 10.1523/JNEUROSCI.1842-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Starkov AA, Fiskum G, Chinopoulos C, Lorenzo BJ, Browne SE, Patel MS, Beal MF. Mitochondrial alpha-ketoglutarate dehydrogenase complex generates reactive oxygen species. J. Neurosci. 2004;24:7779–7788. doi: 10.1523/JNEUROSCI.1899-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ushio-Fukai M. Compartmentalization of redox signaling through NADPH oxidase-derived ROS. Antioxid. Redox Signal. 2009;11:1289–1299. doi: 10.1089/ars.2008.2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat. Rev. Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- [37].Xia Y, Zweier JL. Superoxide and peroxynitrite generation from inducible nitric oxide synthase in macrophages. Proceedings of the National Academy of Sciences. 1997;94:6954–6958. doi: 10.1073/pnas.94.13.6954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Halliwell B. Free radicals and antioxidants - quo vadis? Trends Pharmacol. Sci. 2011;32:125–130. doi: 10.1016/j.tips.2010.12.002. [DOI] [PubMed] [Google Scholar]
- [39].Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem. Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Salganik RI. The benefits and hazards of antioxidants: controlling apoptosis and other protective mechanisms in cancer patients and the human population. J. Am. Coll. Nutr. 2001;20:464S–472S. doi: 10.1080/07315724.2001.10719185. [DOI] [PubMed] [Google Scholar]
- [41].Halliwell B. Phagocyte-derived reactive species: salvation or suicide? Trends Biochem. Sci. 2006;31:509–515. doi: 10.1016/j.tibs.2006.07.005. [DOI] [PubMed] [Google Scholar]
- [42].Tlili A, Dupré-Crochet S, Erard M, Nüße O. Kinetic analysis of phagosomal production of reactive oxygen species. Free Radic. Biol. Med. 2011;50:438–447. doi: 10.1016/j.freeradbiomed.2010.11.024. [DOI] [PubMed] [Google Scholar]
- [43].Forman HJ. Reactive oxygen species and α,β-unsaturated aldehydes as second messengers in signal transduction. Ann. N.Y. Acad. Sci. 2010;1203:35–44. doi: 10.1111/j.1749-6632.2010.05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Chaudhary P, Sharma R, Sharma A, Vatsyayan R, Yadav S, Singhal SS, Rauniyar N, Prokai L, Awasthi S, Awasthi YC. Mechanisms of 4-hydroxy-2-nonenal induced pro- and anti-apoptotic signaling. Biochemistry. 2010;39:6263–6275. doi: 10.1021/bi100517x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Gueraud F, Atalay M, Bresgen N, Cipak A, Eckl PM, Huc L, Jouanin I, Siems W, Uchida K. Chemistry and biochemistry of lipid peroxidation products. Free Radic. Res. 2010;44:1098–1124. doi: 10.3109/10715762.2010.498477. [DOI] [PubMed] [Google Scholar]
- [46].Porter NA, Caldwell SE, Mills KA. Mechanisms of free radical oxidation of unsaturated lipids. Lipids. 1995;30:277–290. doi: 10.1007/BF02536034. [DOI] [PubMed] [Google Scholar]
- [47].Doktorov AB, Lukzen NN, Pedersen JB. Analysis of lipid peroxidation kinetics. 1. Role of recombination of alkyl and peroxyl radicals. J. Phys. Chem. B. 2008;112:11854–11861. doi: 10.1021/jp709921m. [DOI] [PubMed] [Google Scholar]
- [48].Bochkov VN, Oskolkova OV, Birukov KG, Levonen AL, Binder CJ, Stockl J. Generation and biological activities of oxidized phospholipids. Antioxid. Redox Signal. 2010;12:1009–1059. doi: 10.1089/ars.2009.2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Negre-Salvayre A, Auge N, Ayala V, Basaga H, Boada J, Brenke R, Chapple S, Cohen G, Feher J, Grune T, Lengyel G, Mann GE, Pamplona R, Poli G, Portero-Otin M, Riahi Y, Salvayre R, Sasson S, Serrano J, Shamni O, Siems W, Siow RC, Wiswedel I, Zarkovic K, Zarkovic N. Pathological aspects of lipid peroxidation. Free Radic. Res. 2010;44:1125–1171. doi: 10.3109/10715762.2010.498478. [DOI] [PubMed] [Google Scholar]
- [50].Halliwell B. Oxidative stress and neurodegeneration: where are we now? J. Neurochem. 2006;97:1634–1658. doi: 10.1111/j.1471-4159.2006.03907.x. [DOI] [PubMed] [Google Scholar]
- [51].Pearl R. The rate of living. University of London Press; London: 1928. [Google Scholar]
- [52].Harman D. Aging: a theory based on free radical and radiation chemistry. J. Gerontol. 1956;11:298–300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- [53].Medawar PB. An unsolved problem of biology. H. K. Lewis; London: 1952. [Google Scholar]
- [54].Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. [Google Scholar]
- [55].Beckman KB, Ames BN. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- [56].Kenyon CJ. The genetics of ageing. Nature. 2010;464:504–512. doi: 10.1038/nature08980. [DOI] [PubMed] [Google Scholar]
- [57].Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle. 2010;9:3151–3156. doi: 10.4161/cc.9.16.13120. [DOI] [PubMed] [Google Scholar]
- [58].Longo VD, Mitteldorf J, Skulachev VP. Programmed and altruistic ageing. Nat. Rev. Genet. 2005;6:866–872. doi: 10.1038/nrg1706. [DOI] [PubMed] [Google Scholar]
- [59].Goldsmith TC. Aging, evolvability, and the individual benefit requirement; medical implications of aging theory controversies. J. Theor. Biol. 2008;252:764–768. doi: 10.1016/j.jtbi.2008.02.035. [DOI] [PubMed] [Google Scholar]
- [60].Lithgow GJ. Why aging isn't regulated: A lamentation on the use of language in aging literature. Exp. Gerontol. 2006;41:890–893. doi: 10.1016/j.exger.2006.06.051. [DOI] [PubMed] [Google Scholar]
- [61].Zimniak P. Detoxification reactions: relevance to aging. Ageing Res. Rev. 2008;7:281–300. doi: 10.1016/j.arr.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Mitteldorf J. Aging is not a process of wear and tear. Rejuvenation Res. 2010;13:322–326. doi: 10.1089/rej.2009.0967. [DOI] [PubMed] [Google Scholar]
- [63].Taverna DM, Goldstein RA. Why are proteins marginally stable? Proteins. 2002;46:105–109. doi: 10.1002/prot.10016. [DOI] [PubMed] [Google Scholar]
- [64].Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J. Gerontol. Ser. A: Biol. Sci. & Med. Sci. 2009;64:167–170. doi: 10.1093/gerona/gln071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].David DC, Ollikainen N, Trinidad JC, Cary MP, Burlingame AL, Kenyon C. Widespread protein aggregation as an inherent part of aging in C. elegans. PLoS Biol. 2010;8:e1000450. doi: 10.1371/journal.pbio.1000450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Dillin A, Cohen E. Ageing and protein aggregation-mediated disorders: from invertebrates to mammals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:94–98. doi: 10.1098/rstb.2010.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu. Rev. Bioch. 2006;75:333–366. doi: 10.1146/annurev.biochem.75.101304.123901. [DOI] [PubMed] [Google Scholar]
- [68].Luheshi LM, Dobson CM. Bridging the gap: from protein misfolding to protein misfolding diseases. FEBS Lett. 2009;583:2581–2586. doi: 10.1016/j.febslet.2009.06.030. [DOI] [PubMed] [Google Scholar]
- [69].Harman D. Free radical theory of aging: an update: increasing the functional life span. Ann. N.Y. Acad. Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- [70].Gems D, Doonan R. Antioxidant defense and aging in C. elegans: Is the oxidative damage theory of aging wrong? Cell Cycle. 2009;8:1077–1083. doi: 10.4161/cc.8.11.8595. [DOI] [PubMed] [Google Scholar]
- [71].Perez VI, Bokov A, Van Remmen H, Mele J, Ran Q, Ikeno Y, Richardson A. Is the oxidative stress theory of aging dead? Biochim. Biophys. Acta. 2009;1790:1005–1014. doi: 10.1016/j.bbagen.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Lapointe J, Hekimi S. When a theory of aging ages badly. Cell. Mol. Life Sci. 2010;67:1–8. doi: 10.1007/s00018-009-0138-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Speakman JR, Selman C. The free-radical damage theory: Accumulating evidence against a simple link of oxidative stress to ageing and lifespan. Bioessays. 2011;33:255–259. doi: 10.1002/bies.201000132. [DOI] [PubMed] [Google Scholar]
- [74].Cocheme HM, Murphy MP. Can antioxidants be effective therapeutics? Curr. Opin. Investig. Drugs. 2010;11:426–431. [PubMed] [Google Scholar]
- [75].Pun PBL, Gruber J, Tang SY, Schaffer S, Ong RLS, Fong S, Ng LF, Cheah I, Halliwell B. Ageing in nematodes: do antioxidants extend lifespan in Caenorhabditis elegans? Biogerontology. 2009;11:17–30. doi: 10.1007/s10522-009-9223-5. [DOI] [PubMed] [Google Scholar]
- [76].Lee C-YJ, Seet RCS, Huang SH, Long LH, Halliwell B. Different patterns of oxidized lipid products in plasma and urine of dengue fever, stroke, and Parkinson's disease patients: cautions in the use of biomarkers of oxidative stress. Antioxid. Redox Signal. 2009;11:407–420. doi: 10.1089/ars.2008.2179. [DOI] [PubMed] [Google Scholar]
- [77].Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Yang W, Hekimi S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010;8:e1000556. doi: 10.1371/journal.pbio.1000556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Woo HA, Yim SH, Shin DH, Kang D, Yu D-Y, Rhee SG. Inactivation of peroxiredoxin I by phosphorylation allows localized H2O2 accumulation for cell signaling. Cell. 2010;140:517–528. doi: 10.1016/j.cell.2010.01.009. [DOI] [PubMed] [Google Scholar]
- [80].Gems D, Partridge L. Stress-response hormesis and aging: “That which does not kill us makes us stronger”. Cell Metabol. 2008;7:200–203. doi: 10.1016/j.cmet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- [81].Bourg EL. Hormesis, aging and longevity. Biochim. Biophys. Acta. 2009;1790:1030–1039. doi: 10.1016/j.bbagen.2009.01.004. [DOI] [PubMed] [Google Scholar]
- [82].Rudiger HA, Graf R, Clavien PA. Sub-lethal oxidative stress triggers the protective effects of ischemic preconditioning in the mouse liver. J. Hepatol. 2003;39:972–977. doi: 10.1016/s0168-8278(03)00415-x. [DOI] [PubMed] [Google Scholar]
- [83].Ristow M, Zarse K. How increased oxidative stress promotes longevity and metabolic health: the concept of mitochondrial hormesis (mitohormesis) Exp. Gerontol. 2010;45:410–418. doi: 10.1016/j.exger.2010.03.014. [DOI] [PubMed] [Google Scholar]
- [84].McElwee JJ, Schuster E, Blanc E, Thomas JH, Gems D. Shared transcriptional signature in Caenorhabditis elegans dauer larvae and long-lived daf-2 mutants implicates detoxification system in longevity assurance. J. Biol. Chem. 2004;279:44533–44543. doi: 10.1074/jbc.M406207200. [DOI] [PubMed] [Google Scholar]
- [85].Gems D, McElwee JJ. Broad spectrum detoxification: the major longevity assurance process regulated by insulin/IGF-1 signaling? Mech. Ageing Dev. 2005;126:381–387. doi: 10.1016/j.mad.2004.09.001. [DOI] [PubMed] [Google Scholar]
- [86].McElwee J, Schuster E, Blanc E, Piper M, Thomas J, Patel D, Selman C, Withers D, Thornton J, Partridge L, Gems D. Evolutionary conservation of regulated longevity assurance mechanisms. Genome Biol. 2007;8:R132. doi: 10.1186/gb-2007-8-7-r132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat. Rev. Mol. Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].McCormick MA, Tsai SY, Kennedy BK. TOR and ageing: a complex pathway for a complex process. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:17–27. doi: 10.1098/rstb.2010.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Kapahi P, Chen D, Rogers AN, Katewa SD, Li PW, Thomas EL, Kockel L. With TOR, less is more: a key role for the conserved nutrient-sensing TOR pathway in aging. Cell Metabol. 2010;11:453–465. doi: 10.1016/j.cmet.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- [91].Foster KG, Fingar DC. Mammalian target of rapamycin (mTOR): conducting the cellular signaling symphony. J. Biol. Chem. 2010;285:14071–14077. doi: 10.1074/jbc.R109.094003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Blagosklonny MV. Aging and immortality: quasi-programmed senescence and its pharmacologic inhibition. Cell Cycle. 2006;5:2087–2102. doi: 10.4161/cc.5.18.3288. [DOI] [PubMed] [Google Scholar]
- [93].Blagosklonny MV. Rapamycin and quasi-programmed aging: Four years later. Cell Cycle. 2010;9:1859–1862. doi: 10.4161/cc.9.10.11872. [DOI] [PubMed] [Google Scholar]
- [94].Goto M. Inflammaging (inflammation + aging): A driving force for human aging based on an evolutionarily antagonistic pleiotropy theory? Biosci. Trends. 2008;2:218–230. [PubMed] [Google Scholar]
- [95].Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- [96].Evans DS, Kapahi P, Hsueh WC, Kockel L. TOR signaling never gets old: Aging, longevity and TORC1 activity. Ageing Res. Rev. 2011;10:225–237. doi: 10.1016/j.arr.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- [98].Kirkwood TB. A systematic look at an old problem. Nature. 2008;451:644–647. doi: 10.1038/451644a. [DOI] [PubMed] [Google Scholar]
- [99].Kirkwood TB. Systems biology of ageing and longevity. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011;366:64–70. doi: 10.1098/rstb.2010.0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Tatar M. The plate half-full: status of research on the mechanisms of dietary restriction in Drosophila melanogaster. Exp. Gerontol. 2011;46:363–368. doi: 10.1016/j.exger.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Truscott RJW. Macromolecular deterioration as the ultimate constraint on human lifespan. Ageing Res. Rev. 2011 doi: 10.1016/j.arr.2010.12.001. in press. [DOI] [PubMed] [Google Scholar]
- [102].Tullet JMA, Hertweck M, An JH, Baker J, Hwang JY, Liu S, Oliveira RP, Baumeister R, Blackwell TK. Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans. Cell. 2008;132:1025–1038. doi: 10.1016/j.cell.2008.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Langston W, Circu ML, Aw TY. Insulin stimulation of γ-glutamylcysteine ligase catalytic subunit expression increases endothelial GSH during oxidative stress: influence of low glucose. Free Radic. Biol. Med. 2008;45:1591–1599. doi: 10.1016/j.freeradbiomed.2008.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–184. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- [105].Sykiotis GP, Habeos IG, Samuelson AV, Bohmann D. The role of the antioxidant and longevity-promoting Nrf2 pathway in metabolic regulation. Curr. Opin. Clin. Nutr. Metabol. Care. 2011;14:41–48. doi: 10.1097/MCO.0b013e32834136f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Zhang H, Forman HJ. Signaling pathways involved in phase II gene induction by α,β-unsaturated aldehydes. Toxicol. Ind. Health. 2009;25:269–278. doi: 10.1177/0748233709102209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Klaassen CD, Reisman SA. Nrf2 the rescue: effects of the antioxidative/electrophilic response on the liver. Toxicol. Appl. Pharmacol. 2010;224:57–65. doi: 10.1016/j.taap.2010.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Lewis KN, Mele J, Hayes JD, Buffenstein R. Nrf2, a guardian of healthspan and gatekeeper of species longevity. Integr. Comp. Biol. 2010;50:829–843. doi: 10.1093/icb/icq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Wang J, Robida-Stubbs S, Tullet JM, Rual JF, Vidal M, Blackwell TK. RNAi screening implicates a SKN-1-dependent transcriptional response in stress resistance and longevity deriving from translation inhibition. PLoS Genet. 2010;6:e1001048. doi: 10.1371/journal.pgen.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ayyadevara S, Dandapat A, Singh SP, Beneš H, Zimniak L, Shmookler Reis RJ, Zimniak P. Lifespan extension in hypomorphic daf-2 mutants of Caenorhabditis elegans is partially mediated by glutathione transferase CeGSTP2-2. Aging Cell. 2005;4:299–307. doi: 10.1111/j.1474-9726.2005.00172.x. [DOI] [PubMed] [Google Scholar]
- [111].Oh SW, Mukhopadhyay A, Dixit BL, Raha T, Green MR, Tissenbaum HA. Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat. Genet. 2006;38:251–257. doi: 10.1038/ng1723. [DOI] [PubMed] [Google Scholar]
- [112].Schuster E, McElwee JJ, Tullet JM, Doonan R, Matthijssens F, Reece-Hoyes JS, Hope IA, Vanfleteren JR, Thornton JM, Gems D. DamID in C. elegans reveals longevity-associated targets of DAF-16/FoxO. Mol. Syst. Biol. 2010;6:399. doi: 10.1038/msb.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Manjithaya R, Subramani S. Autophagy: a broad role in unconventional protein secretion? Trends Cell Biol. 2011;21:67–73. doi: 10.1016/j.tcb.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Madeo F, Tavernarakis N, Kroemer G. Can autophagy promote longevity? Nat. Cell Biol. 2010;12:842–846. doi: 10.1038/ncb0910-842. [DOI] [PubMed] [Google Scholar]
- [115].Morimoto RI, Driessen AJM, Hegde RS, Langer T. The life of proteins: the good, the mostly good and the ugly. Nat. Struct. Mol. Biol. 2011;18:1–4. doi: 10.1038/nsmb0111-1. [DOI] [PubMed] [Google Scholar]
- [116].Kikis EA, Gidalevitz T, Morimoto RI. Protein homeostasis in models of aging and age-related conformational disease. Adv. Exp. Med. Biol. 2010;694:138–159. doi: 10.1007/978-1-4419-7002-2_11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Douglas PM, Dillin A. Protein homeostasis and aging in neurodegeneration. J. Cell Biol. 2010;190:719–729. doi: 10.1083/jcb.201005144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ben-Zvi A, Miller EA, Morimoto RI. Collapse of proteostasis represents an early molecular event in Caenorhabditis elegans aging. Proc. Natl. Acad. Sci. USA. 2009;106:14914. doi: 10.1073/pnas.0902882106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Perez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, Ward W, Richardson A, Chaudhuri A. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc. Natl. Acad. Sci. USA. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Salmon AB, Leonard S, Masamsetti V, Pierce A, Podlutsky AJ, Podlutskaya N, Richardson A, Austad SN, Chaudhuri AR. The long lifespan of two bat species is correlated with resistance to protein oxidation and enhanced protein homeostasis. FASEB J. 2009;23:2317–2326. doi: 10.1096/fj.08-122523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Tower J. Hsps and aging. Trends Endocrin. Metabol. 2009;20:216–222. doi: 10.1016/j.tem.2008.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Hansen M, Taubert S, Crawford D, Libina N, Lee S-J, Kenyon C. Lifespan extension by conditions that inhibit translation in Caenorhabditis elegans. Aging Cell. 2007;6:95–110. doi: 10.1111/j.1474-9726.2006.00267.x. [DOI] [PubMed] [Google Scholar]
- [123].Bonawitz ND, Chatenay-Lapointe M, Pan Y, Shadel GS. Reduced TOR signaling extends chronological life span via increased respiration and upregulation of mitochondrial gene expression. Cell Metabol. 2007;5:265–277. doi: 10.1016/j.cmet.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Zid BM, Rogers AN, Katewa SD, Vargas MA, Kolipinski MC, Lu TA, Benzer S, Kapahi P. 4E-BP extends lifespan upon dietary restriction by enhancing mitochondrial activity in Drosophila. Cell. 2009;139:149–160. doi: 10.1016/j.cell.2009.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Papa S, Skulachev VP. Reactive oxygen species, mitochondria, apoptosis and aging. Mol. Cell. Biochem. 1997;174:305–319. [PubMed] [Google Scholar]
- [126].Stefanatos R, Sanz A. Mitochondrial complex I: A central regulator of the aging process. Cell Cycle. 2011;10:1528–1532. doi: 10.4161/cc.10.10.15496. [DOI] [PubMed] [Google Scholar]
- [127].Pamplona R, Portero-Otín M, Requena JR, Thorpe SR, Herrero A, Barja G. A low degree of fatty acid unsaturation leads to lower lipid peroxidation and lipoxidation-derived protein modification in heart mitochondria of the longevous pigeon than in the short-lived rat. Mech. Ageing Dev. 1999;106:283–296. doi: 10.1016/s0047-6374(98)00121-3. [DOI] [PubMed] [Google Scholar]
- [128].Pamplona R, Portero-Otin M, Riba D, Ledo F, Gredilla R, Herrero A, Barja G. Heart fatty acid unsaturation and lipid peroxidation, and aging rate, are lower in the canary and the parakeet than in the mouse. Aging (Milan, Italy) 1999;11:44–49. [PubMed] [Google Scholar]
- [129].Pamplona R, Portero-Otin M, Ruiz C, Gredilla R, Herrero A, Barja G. Double bond content of phospholipids and lipid peroxidation negatively correlate with maximum longevity in the heart of mammals. Mech. Ageing Dev. 2000;112:169–183. doi: 10.1016/s0047-6374(99)00045-7. [DOI] [PubMed] [Google Scholar]
- [130].Hulbert AJ, Rana T, Couture P. The acyl composition of mammalian phospholipids: an allometric analysis. Comp. Biochem. Physiol. Part B. 2002;132:515–527. doi: 10.1016/s1096-4959(02)00066-0. [DOI] [PubMed] [Google Scholar]
- [131].Buttemer WA, Battam H, Hulbert AJ. Fowl play and the price of petrel: long-living Procellariiformes have peroxidation-resistant membrane composition compared with short-living Galliformes. Biol. Lett. 2008;4:351–354. doi: 10.1098/rsbl.2008.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Buffenstein R. Negligible senescence in the longest living rodent, the naked mole-rat: insights from a successfully aging species. J. Comp. Physiol. B. 2008;178:439–445. doi: 10.1007/s00360-007-0237-5. [DOI] [PubMed] [Google Scholar]
- [133].Hulbert AJ, Faulks SC, Buffenstein R. Oxidation-resistant membrane phospholipids can explain longevity differences among the longest-living rodents and similarly-sized mice. J. Gerontol. Ser. A: Biol. Sci. & Med. Sci. 2006;61:1009–1018. doi: 10.1093/gerona/61.10.1009. [DOI] [PubMed] [Google Scholar]
- [134].Mitchell TW, Buffenstein R, Hulbert AJ. Membrane phospholipid composition may contribute to exceptional longevity of the naked mole-rat (Heterocephalus glaber): A comparative study using shotgun lipidomics. Exp. Gerontol. 2007;42:1053–1062. doi: 10.1016/j.exger.2007.09.004. [DOI] [PubMed] [Google Scholar]
- [135].Andziak B, O'Connor TP, Buffenstein R. Antioxidants do not explain the disparate longevity between mice and the longest-living rodent, the naked mole-rat. Mech. Ageing Dev. 2005;126:1206–1212. doi: 10.1016/j.mad.2005.06.009. [DOI] [PubMed] [Google Scholar]
- [136].Andziak B, O'Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H, Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- [137].Andziak B, Buffenstein R. Disparate patterns of age-related changes in lipid peroxidation in long-lived naked mole-rats and shorter-lived mice. Aging Cell. 2006;5:525–532. doi: 10.1111/j.1474-9726.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- [138].Buffenstein R, Edrey YH, Yang T, Mele J. The oxidative stress theory of aging: embattled or invincible? Insights from non-traditional model organisms. Age (Dordr.) 2008;30:99–109. doi: 10.1007/s11357-008-9058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Dinkova-Kostova AT, Holtzclaw WD, Kensler TW. The role of Keap1 in cellular protective responses. Chem. Res. Toxicol. 2005;18:779–1791. doi: 10.1021/tx050217c. [DOI] [PubMed] [Google Scholar]
- [140].Hulbert AJ, Beard LA, Grigg GC. The exceptional longevity of an egg-laying mammal, the short-beaked echidna (Tachyglossus aculeatus) is associated with peroxidation-resistant membrane composition. Exp. Gerontol. 2008;43:729–733. doi: 10.1016/j.exger.2008.05.015. [DOI] [PubMed] [Google Scholar]
- [141].Haddad LS, Kelbert L, Hulbert AJ. Extended longevity of queen honey bees compared to workers is associated with peroxidation-resistant membranes. Exp. Gerontol. 2007;42:601–609. doi: 10.1016/j.exger.2007.02.008. [DOI] [PubMed] [Google Scholar]
- [142].Hulbert AJ, Faulks SC, Harper JM, Miller RA, Buffenstein R. Extended longevity of wild-derived mice is associated with peroxidation-resistant membranes. Mech. Ageing Dev. 2006;127:653–657. doi: 10.1016/j.mad.2006.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [143].Puca AA, Andrew P, Novelli V, Anselmi CV, Somalvico F, Cirillo NA, Chatgilialoglu C, Ferreri C. Fatty acid profile of erythrocyte membranes as possible biomarker of longevity. Rejuvenation Res. 2008;11:63–72. doi: 10.1089/rej.2007.0566. [DOI] [PubMed] [Google Scholar]
- [144].Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- [145].Hulbert AJ. On the importance of fatty acid composition of membranes for aging. J. Theor. Biol. 2005;234:277–288. doi: 10.1016/j.jtbi.2004.11.024. [DOI] [PubMed] [Google Scholar]
- [146].Hulbert AJ. Metabolism and longevity: Is there a role for membrane fatty acids? Integr. Comp. Biol. 2010;50:808–817. doi: 10.1093/icb/icq007. [DOI] [PubMed] [Google Scholar]
- [147].Geiser F. Influence of polyunsaturated and saturated dietary lipids on adipose tissue, brain and mitochondrial membrane fatty acid composition of a mammalian hibernator. Biochim. Biophys. Acta. 1990;1046:159–166. doi: 10.1016/0005-2760(90)90183-x. [DOI] [PubMed] [Google Scholar]
- [148].Abbott SK, Else PL, Hulbert AJ. Membrane fatty acid composition of rat skeletal muscle is most responsive to the balance of dietary n-3 and n-6 PUFA. Br. J. Nutr. 2010;103:522–529. doi: 10.1017/S0007114509992133. [DOI] [PubMed] [Google Scholar]
- [149].Pamplona R, Barja G, Portero-Otin M. Membrane fatty acid unsaturation, protection against oxidative stress, and maximum life span: a homeoviscous-longevity adaptation? Ann. N.Y. Acad. Sci. 2002;959:475–490. doi: 10.1111/j.1749-6632.2002.tb02118.x. [DOI] [PubMed] [Google Scholar]
- [150].Laganiere S, Yu BP. Anti-lipoperoxidation action of food restriction. Biochem. Biophys. Res. Commun. 1987;145:1185–1191. doi: 10.1016/0006-291x(87)91562-2. [DOI] [PubMed] [Google Scholar]
- [151].Laganiere S, Yu BP. Modulation of membrane phospholipid fatty acid composition by age and food restriction. Gerontology. 1993;39:7–18. doi: 10.1159/000213509. [DOI] [PubMed] [Google Scholar]
- [152].Faulks SC, Turner N, Else PL, Hulbert AJ. Calorie restriction in mice: effects on body composition, daily activity, metabolic rate, mitochondrial reactive oxygen species production, and membrane fatty acid composition. J. Gerontol. Ser. A: Biol. Sci. & Med. Sci. 2006;61:781–794. doi: 10.1093/gerona/61.8.781. [DOI] [PubMed] [Google Scholar]
- [153].Ramsey JJ, Hagopian K, Kenny TM, Koomson EK, Bevilacqua L, Weindruch R, Harper M-E. Proton leak and hydrogen peroxide production in liver mitochondria from energy-restricted rats. Am. J. Physiol. 2004;286:E31–E40. doi: 10.1152/ajpendo.00283.2003. [DOI] [PubMed] [Google Scholar]
- [154].Solfrizzi V, D'Introno A, Colacicco AM, Capurso C, Palasciano R, Capurso S, Torres F, Capurso A, Panza F. Unsaturated fatty acids intake and all-causes mortality: a 8.5-year follow-up of the Italian Longitudinal Study on Aging. Exp. Gerontol. 2005;40:335–343. doi: 10.1016/j.exger.2005.01.003. [DOI] [PubMed] [Google Scholar]
- [155].Panza F, Solfrizzi V, Colacicco AM, D'Introno A, Capurso C, Torres F, Del Parigi A, Capurso S, Capurso A. Mediterranean diet and cognitive decline. Public Health Nutr. 2004;7:959–963. doi: 10.1079/phn2004561. [DOI] [PubMed] [Google Scholar]
- [156].Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am. J. Clin. Nutr. 2006;83:1505S–1519S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- [157].Lombardo YB, Chicco AG. Effects of dietary polyunsaturated n-3 fatty acids on dyslipidemia and insulin resistance in rodents and humans. J. Nutr. Biochem. 2006;17:1–13. doi: 10.1016/j.jnutbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- [158].Schmitz G, Ecker J. The opposing effects of n-3 and n-6 fatty acids. Prog. Lipid Res. 2008;47:147–155. doi: 10.1016/j.plipres.2007.12.004. [DOI] [PubMed] [Google Scholar]
- [159].Hibbeln JR, Nieminen LRG, Blasbalg TL, Riggs JA, Lands WEM. Healthy intakes of n-3 and n-6 fatty acids: estimations considering worldwide diversity. Am. J. Clin. Nutr. 2006;83:1483S–1493S. doi: 10.1093/ajcn/83.6.1483S. [DOI] [PubMed] [Google Scholar]
- [160].Cordain L, Eaton SB, Sebastian A, Mann N, Lindeberg S, Watkins BA, O'Keefe JH, Brand-Miller J. Origins and evolution of the Western diet: health implications for the 21st century. Am. J. Clin. Nutr. 2005;81:341–354. doi: 10.1093/ajcn.81.2.341. [DOI] [PubMed] [Google Scholar]
- [161].Shmookler Reis RJ, Xu L, Lee H, Chae M, Thaden JJ, Bharill P, Tazearslan C, Siegel E, Alla R, Zimniak P, Ayyadevara S. Modulation of lipid biosynthesis contributes to stress resistance and longevity of C. elegans mutants. Aging. 2011;3:125–147. doi: 10.18632/aging.100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Hanoune J, Defer N. Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharm. Toxicol. 2001;41:145–174. doi: 10.1146/annurev.pharmtox.41.1.145. [DOI] [PubMed] [Google Scholar]
- [163].Yan L, Vatner DE, O'Connor JP, Ivessa A, Ge H, Chen W, Hirotani S, Ishikawa Y, Sadoshima J, Vatner SF. Type 5 adenylyl cyclase disruption increases longevity and protects against stress. Cell. 2007;130:247–258. doi: 10.1016/j.cell.2007.05.038. [DOI] [PubMed] [Google Scholar]
- [164].Sanchez-Roman I, Gomez J, Naudi A, Ayala V, Portero-Otin M, Lopez-Torres M, Pamplona R, Barja G. The β-blocker atenolol lowers the longevity-related degree of fatty acid unsaturation, decreases protein oxidative damage, and increases extracellular signal-regulated kinase signaling in the heart of C57BL/6 mice. Rejuvenation Res. 2010;13:683–693. doi: 10.1089/rej.2010.1062. [DOI] [PubMed] [Google Scholar]
- [165].Ayyadevara S, Alla R, Thaden JJ, Shmookler Reis RJ. Remarkable longevity and stress resistance of nematode PI3K-null mutants. Aging Cell. 2008;7:13–22. doi: 10.1111/j.1474-9726.2007.00348.x. [DOI] [PubMed] [Google Scholar]
- [166].Tazearslan C, Ayyadevara S, Bharill P, Shmookler Reis RJ. Positive feedback between transcriptional and kinase suppression in nematodes with extraordinary longevity and stress resistance. PLoS Genet. 2009;5:e1000452. doi: 10.1371/journal.pgen.1000452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Pamplona R. Advanced lipoxidation end-products. Chem. Biol. Interact. 2011 doi: 10.1016/j.cbi.2011.01.007. in press. [DOI] [PubMed] [Google Scholar]
- [168].Siems W, Grune T. Intracellular metabolism of 4-hydroxynonenal. Mol. Aspects Med. 2003;24:167–175. doi: 10.1016/s0098-2997(03)00011-6. [DOI] [PubMed] [Google Scholar]
- [169].Riahi Y, Cohen G, Shamni O, Sasson S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am. J. Physiol. 2010;299:E879–E886. doi: 10.1152/ajpendo.00508.2010. [DOI] [PubMed] [Google Scholar]
- [170].Sun M, Salomon RG. Oxidative fragmentation of hydroxy octadecadienoates generates biologically active γ-hydroxyalkenals. J. Am. Chem. Soc. 2004;126:5699–5708. doi: 10.1021/ja038756w. [DOI] [PubMed] [Google Scholar]
- [171].Petersen DR, Doorn JA. Reactions of 4-hydroxynonenal with proteins and cellular targets. Free Radic. Biol. Med. 2004;37:937–945. doi: 10.1016/j.freeradbiomed.2004.06.012. [DOI] [PubMed] [Google Scholar]
- [172].Forman HJ, Dickinson DA, Iles KE. HNE - signaling pathways leading to its elimination. Mol. Aspects Med. 2003;24:189–194. doi: 10.1016/s0098-2997(03)00013-x. [DOI] [PubMed] [Google Scholar]
- [173].Amunom I, Stephens LJ, Tamasi V, Cai J, Pierce WM, Jr., Conklin DJ, Bhatnagar A, Srivastava S, Martin MV, Guengerich FP, Prough RA. Cytochromes P450 catalyze oxidation of αβ-unsaturated aldehydes. Arch. Biochem. Biophys. 2007;464:187–196. doi: 10.1016/j.abb.2007.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Ayyadevara S, Dandapat A, Singh SP, Siegel ER, Shmookler Reis RJ, Zimniak L, Zimniak P. Life span and stress resistance of Caenorhabditis elegans are differentially affected by glutathione transferases metabolizing 4-hydroxynon-2-enal. Mech. Ageing Dev. 2007;128:196–205. doi: 10.1016/j.mad.2006.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Ayyadevara S, Engle MR, Singh SP, Dandapat A, Lichti CF, Beneš H, Shmookler Reis RJ, Liebau E, Zimniak P. Lifespan and stress resistance of Caenorhabditis elegans are increased by expression of glutathione transferases capable of metabolizing the lipid peroxidation product 4-hydroxynonenal. Aging Cell. 2005;4:257–271. doi: 10.1111/j.1474-9726.2005.00168.x. [DOI] [PubMed] [Google Scholar]
- [176].Burcham PC, Kaminskas LM, Fontaine FR, Petersen DR, Pyke SM. Aldehyde-sequestering drugs: tools for studying protein damage by lipid peroxidation products. Toxicology. 2002;181–182:229–236. doi: 10.1016/s0300-483x(02)00287-1. [DOI] [PubMed] [Google Scholar]
- [177].Guiotto A, Calderan A, Ruzza P, Osler A, Rubini C, Jo DG, Mattson MP, Borin G. Synthesis and evaluation of neuroprotective α,β-unsaturated aldehyde scavenger histidyl-containing analogues of carnosine. J. Medicinal Chem. 2005;48:6156–6161. doi: 10.1021/jm050507q. [DOI] [PubMed] [Google Scholar]
- [178].Burcham PC, Pyke SM. Hydralazine inhibits rapid acrolein-induced protein oligomerization: role of aldehyde scavenging and adduct trapping in cross-link blocking and cytoprotection. Mol. Pharmacol. 2006;69:1056–1065. doi: 10.1124/mol.105.018168. [DOI] [PubMed] [Google Scholar]
- [179].Tang SC, Arumugam TV, Cutler RG, Jo DG, Magnus T, Chan SL, Mughal MR, Telljohann RS, Nassar M, Ouyang X, Calderan A, Ruzza P, Guiotto A, Mattson MP. Neuroprotective actions of a histidine analogue in models of ischemic stroke. J. Neurochem. 2007;101:729–736. doi: 10.1111/j.1471-4159.2006.04412.x. [DOI] [PubMed] [Google Scholar]
- [180].Aldini G, Dalle-Donne I, Colombo R, Maffei Facino R, Milzani A, Carini M. Lipoxidation-derived reactive carbonyl species as potential drug targets in preventing protein carbonylation and related cellular dysfunction. ChemMedChem. 2006;1:1045–1058. doi: 10.1002/cmdc.200600075. [DOI] [PubMed] [Google Scholar]
- [181].Galvani S, Coatrieux C, Elbaz M, Grazide MH, Jean Claude T, Parini A, Uchida K, Kamar N, Rostaing L, Baltas M, Salvayre R, Negre-Salvayre A. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free Radic. Biol. Med. 2008;45:1457–1467. doi: 10.1016/j.freeradbiomed.2008.08.026. [DOI] [PubMed] [Google Scholar]
- [182].Bouguerne B, Belkheiri N, Bedos-Belval F, Vindis C, Uchida K, Duran H, Grazide M-H, Baltas M, Salvayre R, Nègre-Salvayre A. Antiatherogenic effect of bisvanillyl-hydralazone, a new hydralazine derivative with antioxidant, carbonyl scavenger, and antiapoptotic properties. Antioxid. Redox Signal. 2011;14:2093–2106. doi: 10.1089/ars.2010.3321. [DOI] [PubMed] [Google Scholar]
- [183].Singh SP, Niemczyk M, Saini D, Sadovov V, Zimniak L, Zimniak P. Disruption of the mGsta4 gene increases life span of C57BL mice. J. Gerontol. Ser. A: Biol. Sci. & Med. Sci. 2010;65A:14–23. doi: 10.1093/gerona/glp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Zimniak P, Singhal SS, Srivastava SK, Awasthi S, Sharma R, Hayden JB, Awasthi YC. Estimation of genomic complexity, heterologous expression, and enzymatic characterization of mouse glutathione S-transferase mGSTA4-4 (GST 5.7) J. Biol. Chem. 1994;269:992–1000. [PubMed] [Google Scholar]
- [185].Xiao B, Singh SP, Nanduri B, Awasthi YC, Zimniak P, Ji X. Crystal structure of a murine glutathione S-transferase in complex with a glutathione conjugate of 4-hydroxynon-2-enal in one subunit and glutathione in the other: evidence of signaling across the dimer interface. Biochemistry. 1999;38:11887–11894. doi: 10.1021/bi990468i. [DOI] [PubMed] [Google Scholar]
- [186].Engle MR, Singh SP, Czernik PJ, Gaddy D, Montague DC, Ceci JD, Yang Y, Awasthi S, Awasthi YC, Zimniak P. Physiological role of mGSTA4-4, a glutathione S-transferase metabolizing 4-hydroxynonenal: generation and analysis of mGsta4 null mouse. Toxicol. Appl. Pharmacol. 2004;194:296–308. doi: 10.1016/j.taap.2003.10.001. [DOI] [PubMed] [Google Scholar]
- [187].Singh SP, Niemczyk M, Saini D, Awasthi YC, Zimniak L, Zimniak P. Role of the electrophilic lipid peroxidation product 4-hydroxynonenal in the development and maintenance of obesity in mice. Biochemistry. 2008;47:3900–3911. doi: 10.1021/bi702124u. [DOI] [PubMed] [Google Scholar]
- [188].Halliwell B. The wanderings of a free radical. Free Radic. Biol. Med. 2009;46:531–542. doi: 10.1016/j.freeradbiomed.2008.11.008. [DOI] [PubMed] [Google Scholar]
- [189].Schauenstein E, Esterbauer H, Jaag G, Taufer M. Über die Wirkungen von Aldehyden auf gesunde und maligne Zellen, 1. Mitt.: Hydroxy-octenal, ein neuer Fettaldehyd. Monatsh. Chemie. 1964;95:180–183. [Google Scholar]
- [190].Benedetti A, Comporti M, Esterbauer H. Identification of 4-hydroxynonenal as a cytotoxic product originating from the peroxidation of liver microsomal lipids. Biochim. Biophys. Acta. 1980;620:281–296. doi: 10.1016/0005-2760(80)90209-x. [DOI] [PubMed] [Google Scholar]
- [191].Schaur RJ. Basic aspects of the biochemical reactivity of 4-hydroxynonenal. Mol. Aspects Med. 2003;24:149–159. doi: 10.1016/s0098-2997(03)00009-8. [DOI] [PubMed] [Google Scholar]
- [192].Stopforth A, Burger BV, Crouch AM, Sandra P. Urinalysis of 4-hydroxynonenal, a marker of oxidative stress, using stir bar sorptive extraction-thermal desorption-gas chromatography/mass spectrometry. J. Chromatogr. B. 2006;834:134–140. doi: 10.1016/j.jchromb.2006.02.038. [DOI] [PubMed] [Google Scholar]
- [193].Niknahad H, Siraki AG, Shuhendler A, Khan S, Teng S, Galati G, Easson E, Poon R, O'Brien PJ. Modulating carbonyl cytotoxicity in intact rat hepatocytes by inhibiting carbonyl-metabolizing enzymes. I. Aliphatic alkenals. Chem. Biol. Interact. 2003;143–144:107–117. doi: 10.1016/s0009-2797(02)00185-0. [DOI] [PubMed] [Google Scholar]
- [194].Liu L, Komatsu H, Murray IV, Axelsen PH. Promotion of amyloid beta protein misfolding and fibrillogenesis by a lipid oxidation product. J. Mol. Biol. 2008;377:1236–1250. doi: 10.1016/j.jmb.2008.01.057. [DOI] [PubMed] [Google Scholar]
- [195].Koster JF, Slee RG, Montfoort A, Lang J, Esterbauer H. Comparison of the inactivation of microsomal glucose-6-phosphatase by in situ lipid peroxidation-derived 4-hydroxynonenal and exogenous 4-hydroxynonenal. Free Radic. Res. Commun. 1986;1:273–287. doi: 10.3109/10715768609051637. [DOI] [PubMed] [Google Scholar]
- [196].Dick RA, Kwak MK, Sutter TR, Kensler TW. Antioxidative function and substrate specificity of NAD(P)H-dependent alkenal/one oxidoreductase. A new role for leukotriene B4 12-hydroxydehydrogenase/15-oxoprostaglandin 13-reductase. J. Biol. Chem. 2001;276:40803–40810. doi: 10.1074/jbc.M105487200. [DOI] [PubMed] [Google Scholar]
- [197].Pillon NJ, Soulere L, Vella RE, Croze M, Care B, Soula HA, Doutheau A, Lagarde M, Soulage CO. Quantitative structure-activity relationship for 4-hydroxy-2-alkenal induced cytotoxicity in L6 muscle cells. Chem. Biol. Interact. 2010;188:171–180. doi: 10.1016/j.cbi.2010.06.015. [DOI] [PubMed] [Google Scholar]
- [198].Nathan C. Specificity of a third kind: reactive oxygen and nitrogen intermediates in cell signaling. J. Clin. Invest. 2003;111:769–778. doi: 10.1172/JCI18174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Forman HJ, Dickinson DA. Introduction to serial reviews on 4-hydroxy-2-nonenal as a signaling molecule. Free Radic. Biol. Med. 2004;37:594–596. doi: 10.1016/j.freeradbiomed.2004.06.009. [DOI] [PubMed] [Google Scholar]
- [200].Yang Y, Yang Y, Xu Y, Lick SD, Awasthi YC, Boor PJ. Endothelial glutathione-S-transferase A4-4 protects against oxidative stress and modulates iNOS expression through NF-κB translocation. Toxicol. Appl. Pharmacol. 2008;230:187–196. doi: 10.1016/j.taap.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Awasthi YC, Sharma R, Sharma A, Yadav S, Singhal SS, Chaudary P, Awasthi S. Self-regulatory role of 4-hydroxynonenal in signaling for stress-induced programmed cell death. Free Radic. Biol. Med. 2008;45:111–118. doi: 10.1016/j.freeradbiomed.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Dwivedi S, Sharma A, Patrick B, Sharma R, Awasthi YC. Role of 4-hydroxynonenal and its metabolites in signaling. Redox Rep. 2007;12:4–10. doi: 10.1179/135100007X162211. [DOI] [PubMed] [Google Scholar]
- [203].Grimsrud PA, Xie H, Griffin TJ, Bernlohr DA. Oxidative stress and covalent modification of protein with bioactive aldehydes. J. Biol. Chem. 2008;283:21837–21841. doi: 10.1074/jbc.R700019200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Isom AL, Barnes S, Wilson L, Kirk M, Coward L, Darley-Usmar V. Modification of cytochrome c by 4-hydroxy- 2-nonenal: evidence for histidine, lysine, and arginine-aldehyde adducts. J. Am. Soc. Mass Spectrom. 2004;15:1136–1147. doi: 10.1016/j.jasms.2004.03.013. [DOI] [PubMed] [Google Scholar]
- [205].Bieschke J, Zhang Q, Bosco DA, Lerner RA, Powers ET, Wentworth P, Jr., Kelly JW. Small molecule oxidation products trigger disease-associated protein misfolding. Acc. Chem. Res. 2006;39:611–619. doi: 10.1021/ar0500766. [DOI] [PubMed] [Google Scholar]
- [206].Butterfield DA, Bader Lange ML, Sultana R. Involvements of the lipid peroxidation product, HNE, in the pathogenesis and progression of Alzheimer's disease. Biochim. Biophys. Acta. 2010;1801:924–929. doi: 10.1016/j.bbalip.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].Jacobs AT, Marnett LJ. Systems analysis of protein modification and cellular responses induced by electrophile stress. Acc. Chem. Res. 2010;43:673–683. doi: 10.1021/ar900286y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Vila A, Tallman KA, Jacobs AT, Liebler DC, Porter NA, Marnett LJ. Identification of protein targets of 4-hydroxynonenal using click chemistry for ex vivo biotinylation of azido and alkynyl derivatives. Chem. Res. Toxicol. 2008;21:432–444. doi: 10.1021/tx700347w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].West JD, Marnett LJ. Alterations in gene expression induced by the lipid peroxidation product, 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2005;18:1642–1653. doi: 10.1021/tx050211n. [DOI] [PubMed] [Google Scholar]
- [210].Li W, Kong AN. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009;48:91–104. doi: 10.1002/mc.20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Kaspar JW, Niture SK, Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol. Aspects Med. 2009;30:1–12. doi: 10.1016/j.mam.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Dickinson DA, Iles KE, Watanabe N, Iwamoto T, Zhang HQ, Krzywanski DM, Forman HJ. 4-hydroxynonenal induces glutamate cysteine ligase through JNK in HBE1 cells. Free Radic. Biol. Med. 2002;33:974–987. doi: 10.1016/s0891-5849(02)00991-7. [DOI] [PubMed] [Google Scholar]
- [214].Zhang H, Court N, Forman HJ. Submicromolar concentrations of 4-hydroxynonenal induce glutamate cysteine ligase expression in HBE1 cells. Redox Rep. 2007;12:101–106. doi: 10.1179/135100007X162266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Talbot DA, Brand MD. Uncoupling protein 3 protects aconitase against inactivation in isolated skeletal muscle mitochondria. Biochim. Biophys. Acta. 2005;1709:150–156. doi: 10.1016/j.bbabio.2005.07.001. [DOI] [PubMed] [Google Scholar]
- [216].Esteves TC, Parker N, Brand MD. Synergy of fatty acid and reactive alkenal activation of proton conductance through uncoupling protein 1 in mitochondria. Biochem. J. 2006;395:619–628. doi: 10.1042/BJ20052004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Toime LJ, Brand MD. Uncoupling protein-3 lowers reactive oxygen species production in isolated mitochondria. Free Radic. Biol. Med. 2010;49:606–611. doi: 10.1016/j.freeradbiomed.2010.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Cannon B, Shabalina IG, Kramarova TV, Petrovic N, Nedergaard J. Uncoupling proteins: a role in protection against reactive oxygen species-or not? Biochim. Biophys. Acta. 2006;1757:449–458. doi: 10.1016/j.bbabio.2006.05.016. [DOI] [PubMed] [Google Scholar]
- [219].Mookerjee SA, Divakaruni AS, Jastroch M, Brand MD. Mitochondrial uncoupling and lifespan. Mech. Ageing Dev. 2010;131:463–472. doi: 10.1016/j.mad.2010.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [220].Carbone DL, Doorn JA, Kiebler Z, Sampey BP, Petersen DR. Inhibition of Hsp72-mediated protein refolding by 4-hydroxy-2-nonenal. Chem. Res. Toxicol. 2004;17:1459–1467. doi: 10.1021/tx049838g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [221].Carbone DL, Doorn JA, Kiebler Z, Ickes BR, Petersen DR. Modification of heat shock protein 90 by 4-hydroxynonenal in a rat model of chronic alcoholic liver disease. J. Pharmacol. Exp. Ther. 2005;315:8–15. doi: 10.1124/jpet.105.088088. [DOI] [PubMed] [Google Scholar]
- [222].Carbone DL, Doorn JA, Kiebler Z, Petersen DR. Cysteine modification by lipid peroxidation products inhibits protein disulfide isomerase. Chem. Res. Toxicol. 2005;18:1324–1331. doi: 10.1021/tx050078z. [DOI] [PubMed] [Google Scholar]
- [223].Viswanathan M, Kim SK, Berdichevsky A, Guarente L. A role for SIR-2.1 regulation of ER stress response genes in determining C. elegans life span. Dev. Cell. 2005;9:605–615. doi: 10.1016/j.devcel.2005.09.017. [DOI] [PubMed] [Google Scholar]
- [224].Paludan C, Schmid D, Landthaler M, Vockerodt M, Kube D, Tuschl T, Münz C. Endogenous MHC Class II processing of a viral nuclear antigen after autophagy. Science. 2005;307:593–596. doi: 10.1126/science.1104904. [DOI] [PubMed] [Google Scholar]
- [225].Stromhaug PE, Klionsky DJ. Approaching the molecular mechanism of autophagy. Traffic. 2001;2:524–531. doi: 10.1034/j.1600-0854.2001.20802.x. [DOI] [PubMed] [Google Scholar]
- [226].Princiotta MF, Finzi D, Qian S-B, Gibbs J, Schuchmann S, Buttgereit F, Bennink JR, Yewdell JW. Quantitating protein synthesis, degradation, and endogenous antigen processing. Immunity. 2003;18:343–354. doi: 10.1016/s1074-7613(03)00051-7. [DOI] [PubMed] [Google Scholar]
- [227].Ferrington DA, Kapphahn RJ. Catalytic site-specific inhibition of the 20S proteasome by 4-hydroxynonenal. FEBS Lett. 2004;578:217–223. doi: 10.1016/j.febslet.2004.11.003. [DOI] [PubMed] [Google Scholar]
- [228].Friguet B, Szweda LI. Inhibition of the multicatalytic proteinase (proteasome) by 4-hydroxy-2-nonenal cross-linked protein. FEBS Lett. 1997;405:21–25. doi: 10.1016/s0014-5793(97)00148-8. [DOI] [PubMed] [Google Scholar]
- [229].Grune T, Davies KJA. The proteasomal system and HNE-modified proteins. Mol. Aspects Med. 2003;24:195–204. doi: 10.1016/s0098-2997(03)00014-1. [DOI] [PubMed] [Google Scholar]
- [230].Wagner TM, Mullally JE, Fitzpatrick FA. Reactive lipid species from cyclooxygenase-2 inactivate tumor suppressor LKB1/STK11: cyclopentenone prostaglandins and 4-hydroxy-2-nonenal covalently modify and inhibit the AMP-kinase kinase that modulates cellular energy homeostasis and protein translation. J. Biol. Chem. 2006;281:2598–2604. doi: 10.1074/jbc.M509723200. [DOI] [PubMed] [Google Scholar]
- [231].Dolinsky VW, Chan AY, Frayne IR, Light PE, Des Rosiers C, Dyck JR. Resveratrol prevents the prohypertrophic effects of oxidative stress on LKB1. Circulation. 2009;119:1643–1652. doi: 10.1161/CIRCULATIONAHA.108.787440. [DOI] [PubMed] [Google Scholar]
- [232].Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur. Heart J. 2011;32:1025–1038. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [233].Mehenni H, Lin-Marq N, Buchet-Poyau K, Reymond A, Collart MA, Picard D, Antonarakis SE. LKB1 interacts with and phosphorylates PTEN: a functional link between two proteins involved in cancer predisposing syndromes. Hum. Mol. Genet. 2005;14:2209–2219. doi: 10.1093/hmg/ddi225. [DOI] [PubMed] [Google Scholar]
- [234].Mocanu MM, Yellon DM. PTEN, the Achilles' heel of myocardial ischaemia/reperfusion injury? Br. J. Pharmacol. 2007;150:833–838. doi: 10.1038/sj.bjp.0707155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [235].Covey TM, Edes K, Coombs GS, Virshup DM, Fitzpatrick FA. Alkylation of the tumor suppressor PTEN activates Akt and β-catenin signaling: a mechanism linking inflammation and oxidative stress with cancer. PLoS ONE. 2010;5:e13545. doi: 10.1371/journal.pone.0013545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [236].Hill BG, Haberzettl P, Ahmed Y, Srivastava S, Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth muscle cell. Biochem. J. 2008;410:525–534. doi: 10.1042/BJ20071063. [DOI] [PubMed] [Google Scholar]
- [237].Kaemmerer E, Schutt F, Krohne TU, Holz FG, Kopitz J. Effects of lipid peroxidation-related protein modifications on RPE lysosomal functions and POS phagocytosis. Invest. Ophthalmol. Vis. Sci. 2007;48:1342–1347. doi: 10.1167/iovs.06-0549. [DOI] [PubMed] [Google Scholar]
- [238].Hashimoto Y, Ookuma S, Nishida E. Lifespan extension by suppression of autophagy genes in Caenorhabditis elegans. Genes Cells. 2009;14:717–726. doi: 10.1111/j.1365-2443.2009.01306.x. [DOI] [PubMed] [Google Scholar]