Abstract
Little is known on the role of neuronal structures for spatial navigation. Our goal was to examine how Parkinson's disease (PD) and cerebellar ataxia, as human lesion models of the basal ganglia and cerebellum, affect spatial navigation round a circular walking path, blindfolded. Twelve subjects with idiopathic PD (ON and OFF medication), 8 subjects with cerebellar ataxia and a control group of 20 age-matched healthy subjects participated. All groups performed well when walking around the circle with eyes open. In the eyes-closed condition, control subjects overshot the outlined trajectory but returned to their initial position, thus walking a further distance with eyes closed than with eyes open. When OFF medication, PD subjects navigated a larger radius than controls with eyes closed. When ON levodopa, PD subjects walked a similar distance as controls but with even larger errors in endpoint. Surprisingly, cerebellar patients navigated the circular walking task in the eyes closed condition with even more accuracy (i.e. following the outlined circle) than control and PD subjects. We conclude that blindfolded navigation around a previously seen circle requires intact basal ganglia, but not cerebellar input.
Keywords: Levodopa, Kinematics, Task Performance and Analysis, Gait, Navigation
1. Introduction
Navigation is a complex process requiring integration of both environmental (external) and self-movement (internal) cues. In blind navigation, environmental cues (e.g., visual, auditory, olfactory) are generally limited to a remembered target and/or path (Loomis et al., 1993, Wallace et al., 2010). In that case, self-motion cues (e.g., proprioception and vestibular) are the basis for navigating through the environment and are used to update an online representation of direction and distance travelled (Berthoz et al., 1995). Path integration (or dead reckoning) is a parallel process that operates on self-movement cues and results in an estimate of the direction and distance from the position where movement was initiated (Wallace et al., 2010). A number of studies (Loomis et al., 1993, Takei et al., 1996, Takei et al., 1997) have looked at the ability of humans to walk blindfolded around different path shapes (straight line, circular, triangular, etc.; (Pham and Hicheur, 2009). When healthy subjects walk blindfolded around a circular path, they consistently overshoot the ideal radius, undershoot the total angle and overshoot the total path length, independent of the size of the circle (Takei et al., 1997).
Currently, little is known about the role of neuronal structures for navigation. Systematic biases in processing of incoming somatosensory/sensory information may contribute to potential abnormalities in spatial navigation in subjects with Parkinson's disease (PD) and cerebellar ataxia (Bowen et al., 1972, Rondi-Reig and Burguiere, 2005, Crenna et al., 2007). However, the contribution of basal ganglia and cerebellum in non-visual locomotor navigation is currently unknown. PD is a movement disorder in which visuospatial and kinesthetic awareness is affected in addition to the classic motor deficits of bradykinesia, rigidity, tremor and balance disorders (Amick et al., 2006). Difficulty with somatosensory kinesthesia has been proposed to be responsible for undershooting of reaching targets in patients with PD (Demirci et al., 1997, Konczak et al., 2007, Wright et al., 2007a). It has been hypothesized that these kinesthesic deficits may also be responsible for undershooting walking distance and particular difficulties with making turns while walking (Crenna et al., 2007, Wright et al., 2010). Damage to the cerebellum not only results in ataxia (hypermetric stepping and lateral postural sway while walking), it may also affect the structural network involved in spatial navigation such as the spatial representation of the environment and adapting locomotion to a specific context (Petrosini et al., 1998, Rondi-Reig and Burguiere, 2005).
Thus, the aim of this study was to compare distance and rotational error when walking around a remembered circular path without visual feedback in PD, cerebellar and control subjects. The results from this study will enable us to better understand the contribution of basal ganglia and cerebellum for path integration.
2. Experimental Procedures
2.1 Subjects
Twelve subjects with a clinical diagnosis of “idiopathic” PD, treated with levodopa, eight subjects with cerebellar ataxia and two respective control groups participated in the study. The subjects in the control groups had no prior history of neurological diseases. All subjects were screened with a health history evaluation to ensure that they were free of musculoskeletal and any other neurological impairments that could contribute to postural instability or movement dysfunction. The control subjects were matched for age, weight and height (see Table 1 for subject characteristics). All subjects were ambulatory and able to stand without an assisting device for the experiment. The PD subjects had no history suggesting “atypical” PD symptoms, as defined by Hughes et al. (Hughes et al., 1992) or other existing neuromuscular disorders, including severely flexed posture. PD subjects included in the study had Hoehn &Yahr scores of 2 or 3. Severity of cerebellar ataxia was assessed with the Scale for the Assessment and Rating of Ataxia (SARA) and scores are presented in Table 1. Three of the cerebellar subjects were diagnosed with idiopathic cerebellar ataxia, three subjects as spinocerebellar ataxia type 6 (SCA-6), one subjects as SCA-15 and one subject as olivopontocerebellar atrophy. All subjects provided informed consent in accordance to the Oregon Health & Science University Internal Review Board regulations for human subjects' studies and the Helsinki Declaration.
Table 1.
Characteristics of the Parkinson's Disease, Cerebellar and control subjects.
| Parkinson's Disease | Cerebellar | |||
|---|---|---|---|---|
| Patients (n=12) | Controls (n=12) | Patients (n=8) | Controls (n=8) | |
| Age (yrs) | 64 ± 9 (46-81) | 64 ± 9 (43-81) | 58 ±7 (48-68) | 57 ± 6 (47-64) |
| Gender | 12 M | 12 M | 2 M, 6 F | 2 M, 6 F |
| Height (cm) | 176 ± 6 (165-185) | 174 ± 6 (165-183) | 169 ± 11 (154-185) | 170 ± 11 (150-183) |
| Weight (kg) | 82 ± 8 (68-94) | 80 ± 11 (62-99) | 75 ± 13 (64-100) | 75 ± 13 (64-99) |
| Duration (yrs) | 6 ± 4 (2-12) | - | 5 ± 3 (2-9)* | - |
| Motor UPDRS-ON | 23 ± 8 (14-41) | - | - | - |
| Motor UPDRS-OFF | 32 ± 10 (18-47) | - | - | - |
| Hoehn & Yahr ON | 2.0 ± 0.1 (2.0-2.5) | - | - | - |
| Hoehn & Yahr OFF | 2.3 ± 0.4 (2.0-3.0) | - | - | - |
| SARA | - | - | 14 ± 3 (9-18) | - |
Values are mean ± SD (range). UPDRS=Unified Parkinson Disease Rating Scale, SARA= Scale for the Assessment and Rating of Ataxia.
= excluding one subject with SCA-15 suspected, not confirmed genetically, affected for >20 yrs.
2.2 Protocol
The subjects walked one revolution around a 1.2m-radius circle marked on the floor. Walking direction was alternated between each trial to avoid vestibular decay that might affect gait. The subjects were asked to maintain their head erect and not fixate the floor in order to standardize the body position across subjects and avoid leaning over to stare at the circle. To standardize upper body position and avoid arm movements that would hide body markers from the cameras, subjects walked with their arms crossed. The subjects started by executing 10 revolutions around the circle (5 in each direction) with eyes opened, immediately followed by 10 additional revolutions around the circle with eyes closed (and with a blindfold). The instructions to the subjects were to walk one full turn around the circle as they had performed in the eyes opened condition and to stop once they thought they were back to their starting position. Only after the subjects had stopped were they allowed to open their eyes and lift up the blindfold to look at their current position and return to the initial position to start another trial in the opposite direction. Hence, subjects received feedback about their final position but not on how far they deviated from the circle. The only instance where subjects received feedback concerning deviation from the circle was when they were stopped because they were about to hit a wall or an obstacle (chair or desk). When stopped, the subjects were asked to return to their start position and start the next trial. Table 2 describes the number of subjects who deviated away from the circle enough to be stopped during the trial. The subjects wore a safety harness equipped with a handle that could be quickly held by an assistant who was ready to catch the subjects in case of a fall. No such incident occurred for any of the subjects.
Table 2.
Number of subjects who were stopped during eyes closed walking around the circle
| Group | No of trials stopped | No. of different subjects |
|---|---|---|
| Controls | 6* | 4** |
| PD OFF meds | 12 | 6 |
| PD ON meds | 3 | 3 |
| Ataxia | 3 | 2 |
1 subject was stopped when matching speed
1 subject was stopped in both comfortable and matched speeds
2.3 Protocol for Subjects with PD and Cerebellar Ataxia
PD subjects were tested off medication (OFF) the morning after abstaining from levodopa overnight (washout period ≥12 h). After completing the full protocol in the OFF condition, the PD subjects were given their usual morning dose of medication, followed by a rest period of one hour. After the rest period, once the subjects reported that they felt “ON”, the protocol was repeated with PD subjects on medication (ON). The motor part III of the UPDRS (Fahn et al., 1987) was used to characterize the state of disease OFF (before starting the protocol) and ON (after the rest period) medication (Table 1).
The PD in OFF and ON condition and cerebellar subjects walked at their comfortable/preferred speed around the circle 10 times in each direction with eyes open, followed by walking around the circle 10 times in each direction with eyes closed.
2.4 Protocol for Healthy Control Subject
Healthy controls performed the circular walk task twice. The first time they walked without any instructions regarding their walking velocity, thereby walking at their comfortable/preferred speed. Once they completed their 10 trials with eyes open and 10 trials with eyes closed, the subjects were instructed to adjust their walking speed to that of the subjects they were matched with (matched speed), reducing their speed. To ensure that the healthy control subjects' speed matched that of the patient, the number of steps to walk around the circle and time to walk around the circle was monitored. The control subjects were not told about the number of steps to be taken or duration of the turn in order to avoid their counting the number of steps. Instead, they were instructed to walk slower or faster or with smaller or longer steps until they had reached values similar to those of the patients.
2.5 Measurements
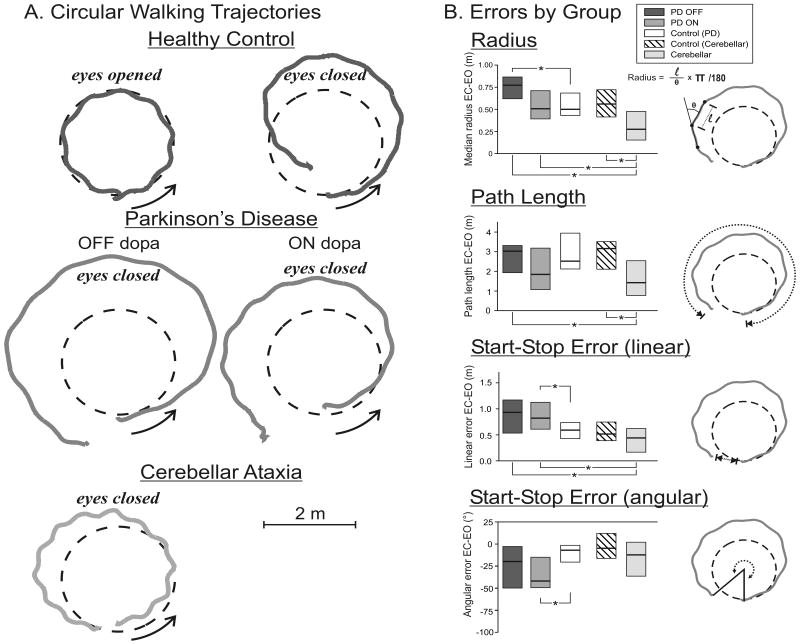
All subjects were instrumented with reflective markers positioned over their bilateral anterior superior iliac spines (ASIS) and bilateral greater trochanters. The position of the markers were recorded at 60 Hz in 3 dimensions with an 8-camera video system (Hi-Res, Motion Analysis System, Santa Rosa, CA). As can be observed in the Figures, many subjects deviated from their trajectory and walked toward the limits of the calibrated volume. For this specific reason, we selected the ASIS marker because it was the most visible in all subjects and reflects well the overall walking trajectory of the subjects. The inside ASIS marker was used to quantify the subject's trajectory (i.e., right ASIS for turns in the clockwise walking direction and left ASIS for counter-clockwise walking). When the ASIS marker was invisible for more than 120 frames (2 seconds of recording), the ipsilateral trochanter marker was used instead (< 10% of data). The linear and angular components of the trajectories were calculated as follows: The linear component corresponds to the tangential component of the trajectory computed with the Euclidean metric between each sampled point. The angular component corresponds to the rotational component between each pair of sampled points (see Figure 1, top trajectory).
Figure 1.
A. Representative walking trajectories from one healthy control (top panels), one subject with Parkinson's Disease (PD; middle panel) and a subject with cerebellar ataxia (bottom panel). The black dashed circles represent the outlined circle on the floor and gray traces, the subjects' trajectories. Arrows indicate the direction in which subjects walked (counterclockwise direction presented). The eyes open condition in the healthy control subject is representative of the same condition in all subject groups. B. Group medians for all trajectory metrics are presented has horizontal lines and boxes represent the interquartile range of the data (median+IQR). Each metric corresponds to the dotted lines illustrated in the icons on the right side of each bar graph. * denotes p <0.05
The outcomes used for analysis were calculated for each individual trial as (1) the median radius outputted, calculated as the linear/angular ratio, (2) the total distance travelled (or path length) obtained by calculating the cumulative sum of the tangential components (walking velocity was obtained by computing the first derivative of the linear path), (3) the linear end position error and (4) the angular end position error, computed as the difference between the start and end position. When comparing turning direction (CW vs. CCW), there were no differences within groups on any of the outcome measures. Thus, scores for both CW and CCW walking directions were averaged across all 20 trials for the group comparisons. To control for inter-individual variability in task performance, we used the mean change in performance from the eyes-closed to eyes-opened condition for each metric computed from the circular walking path.
2.6 Statistical analysis
Statistical analysis was done using STATISTICA software (Statsoft, version 9, Tulsa, OK). To investigate differences in the circular walking outcomes between the groups the Mann-Whitney U test was used for each dependent variable. For repeated measurement comparisons to test for the effect of medication in the PD group (ON vs. OFF), effect of vision (eyes open vs. eyes closed), effect of walking speed (comfortable vs. matched), and the effect of asymmetries in walking direction (CW vs. CCW as well as affected vs. non-affected side), the Sign test was used. A p value less than or equal to 0.05 was considered statistically significant. All data reported in text corresponds to median ± interquartile range (IQR).
3. Results
3.1 Walking velocity during circular walking
The comfortable walking velocity of both PD and cerebellar subjects was significantly slower than velocity of the healthy control subjects [PD: 0.453 ± 0.065m/s vs. Control PD: 0.531 ± 0.084m/s, U(12)=29.00, Z=-2.45, p=0.012 and Cerebellar: 0.388 ± 0.048m/s vs. Control Cerebellar: 0.563 ± 0.082m/s, U(8)=0.00, Z=-3.31, p=0.000]. Therefore, control subjects were asked to match the walking velocity of the subjects and successful matching was obtained [Matched PD controls: 0.425 ± 0.072m/s, U(12)=42.00, Z=1.70, p=0.088 and Matched Cerebellar controls:0.411 ± 0.058 m/s, U(8)=29.00, Z=-0.26, p=0.793].
To ensure that the control subjects' ability to walk around the circle was not affected by the imposed walking speed, we tested the effect of comfortable walking vs. matched walking in control subjects on all our performance outcomes and found no difference in outcomes between walking speeds (p>0.386). All ensuing analyses are performed with the control groups walking at matched speed.
3.2 Circular walking with eyes open and blindfolded
As expected, walking around the circle with eyes open was navigated very accurately by all subjects. There was no difference in path length, end position or radius of walking between PD (either ON or OFF) and control subjects when walking around the circle with eyes open (p>0.05). Subjects with cerebellar ataxia also walked around the circle with similar accuracy as control subjects with eyes open, except in the linear end position error, for which cerebellar subjects stopped short (∼20 cm) of their target compared to control subjects [U(8)=7.00, Z=2.57, p=0.010]. Figure 1A shows a typical example of a healthy subject's walking trajectory with eyes open (values are presented in Table 3).
Table 3.
Median (IQR) of navigation parameters for each group.
| EO | EC | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PD ON |
PD OFF |
PD Ctrl |
CA | CA Ctrl |
PD ON |
PD OFF |
PD Ctrl |
CA | CA Ctrl |
|
| Radius (m) | 1.05 (0.03) |
1.05 (0.03) |
1.09 (0.05) |
1.09 (0.13) |
1.09 (0.02) |
1.54 (0.32) |
1.79 (0.26) |
1.57 (0.29) |
1.42 (0.35) |
1.64 (0.38) |
| Path length (m) | 6.80 (0.32) |
6.72 (0.23) |
6.83 (0.27) |
6.84 (1.06) |
6.83 (0.24) |
8.58 (1.75) |
9.59 (1.12) |
9.32 (1.85) |
8.68 (1.60) |
9.80 (0.96) |
| Linear End Point Error (m) | 0.16 (0.16) |
0.13 (0.11) |
0.10 (0.05) |
0.30 (0.24) |
0.11 (0.04) |
0.91 (0.59) |
1.14 (0.75) |
0.68 (0.31) |
0.74 (0.28) |
0.65 (0.37) |
| Angular End Point Error (deg) | 363 (10) |
362 (8) |
359 (5) |
350 (13) |
356 (2) |
323 (33) |
340 (47) |
352 (21) |
331 (28) |
353 (26) |
CA=cerebellar ataxia; ctrl=control
PD ctrl and CA ctrl are at matched speed
With eyes closed, performance decreased for all groups. As seen in Figure 1B and Table 3, executing the same task with eyes closed resulted in a longer path length (40% larger with eyes closed, main effect: p=0.000) and larger radius (52%, main effect: p=0.000) for all groups, including healthy control subjects. In a few trials (∼2%), subjects overshot their trajectory in such a way that they were heading toward the limits of the room and were stopped to avoid collision (see Table 2).
3.3 Group-specific differences in circular walking with eyes closed
As shown in Figure 1B, when walking with eyes closed, PD OFF showed a larger radius than controls [U(12)=34.00, Z=2.17, p=0.030] but not a longer path length than control subjects [U(12)=72.00, Z=0.00, p=1.000]. PD OFF did not stop further away from their initial position [distance: U(12)=44.00, Z=1.59, p=0.112 and angle: U(12)=54.00, Z=-1.01, p=0.312].
After taking levodopa medication, the PD ON subjects navigated with a similar radius [U(12)=72.00, Z=0.00, p=1.000] and path length [U(12)=72.00, Z=0.00, p=1.000] as the control subjects but had a significantly increased angular end point error compared to control subjects [U(12)=28.00, Z=-2.51, p=0.010].
In contrast to PD subjects, cerebellar subjects were remarkably accurate in how well they followed the circle outlined on the floor with their eyes closed. In fact, compared to control subjects who increased their walking radius by 52% (p=0.013) when they closed their eyes, cerebellar subjects increased their radius by only 27% (p=0.013; Figure 1B). Cerebellar subjects were as accurate as control subjects in returning to their initial linear position [U(8)=23.00, Z=-0.89, p=0.372] and angular position [U(8)=28.00, Z=-0.37, p=0.713], consequently walking significantly smaller distance [path length: U(8)=12.00, Z=-2.05, p=0.041].
3.4 Asymmetries in circular walking
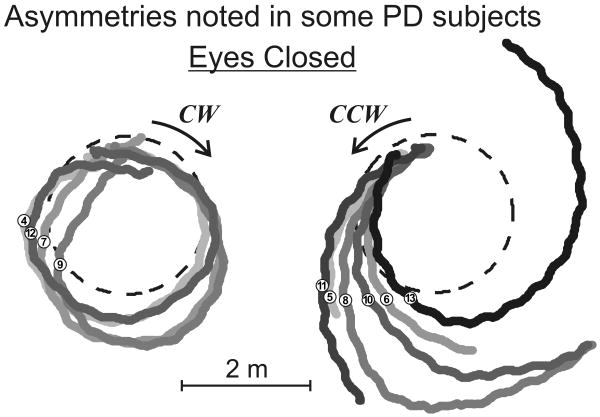
We observed rather large asymmetries in trajectories in half of the PD subjects (4/12 with large asymmetries as shown in Figure 2). No such asymmetries were observed in cerebellar and healthy control subjects. As shown by group medians in Table 4, PD ON subjects were noticeably more asymmetric between walking directions although this difference was not statistically significant. Forty-two percent of PD ON subjects had a path length asymmetry >1m vs. controls where only 21% of subjects exhibited such an asymmetry between turn direction. Similarly, when comparing the linear start-stop error, 58% of PD ON subjects showed an asymmetry >0.4m vs. 29% in healthy controls. However, we found no significant or consistent asymmetries of walking performance related to the affected side, walking direction, disease severity or recorded asymmetry in the UPDRS Motor score (p>0.05).
Figure 2.
Example of a PD subject presenting with marked asymmetry in walking direction around the circle (dashed line) with eyes closed. Numbers indicate the order in which trials were performed. 4/12 PD subjects had similar asymmetry, 2/12 showed less discrepancies between walking directions and 6/12 were similar to cerebellar and control subjects. Trials 1 to 3 were excluded as they were excluded from the analysis to avoid learning effects from the task.
Table 4.
Difference between clockwise and counterclockwise gait (absolute differences)
| PD OFF | PD ON | CONTROLS | ||
|---|---|---|---|---|
| Path length (m) | ||||
| Range | 2.55 | 1.55 | 1.57 | |
| Median | 0.43 | 0.65 | 0.57 | |
| IQR | 0.81 | 1.13 | 0.56 | |
| Start-stop error, linear (m) | ||||
| Range | 0.97 | 0.89 | 0.83 | |
| Median | 0.23 | 0.42 | 0.23 | |
| IQR | 0.43 | 0.50 | 0.33 | |
No significant differences between groups (p>0.22).
4. Discussion
This study is the first to compare circular navigation abilities in control, PD and cerebellar subjects. We showed that, despite characteristic pathological gaits, all groups navigated equally well when walking around the circle with eyes opened, apart from cerebellar subjects stopping short of a full revolution around the circle. Cerebellar subjects showed larger variability in trajectory than control subjects, which is likely explained by their ataxia. Furthermore, the low stamina of cerebellar subjects may also have caused them to stop earlier than control subjects. With eyes closed and when OFF levodopa, PD subjects navigated a larger walking radius, especially in one direction. When ON medication, PD subjects navigated a similar walking radius as control subjects but stopped before reaching their starting position. Surprisingly, cerebellar subjects with eyes closed performed the circular walking task with more accuracy (i.e. followed the outlined circle) than either controls or PD subjects.
4.1 Basal ganglia disease affects walking around a circle blindfolded
Walking around a circle blindfolded requires accurate space estimation and somatosensory-vestibular integration of body motion (Takei et al., 1997). When going around a circle blindfolded, subjects need to form an internal representation of the intended path, create an accurate motor plan and use sensory feedback of the movements they generate while walking. It is not clear which of these processes are affected by PD.
Nevertheless, subjects with PD went around the circle blindfolded with significantly less precision than control subjects. Recent studies show that, in addition to bradykinesia, tremor and postural instability, individuals with PD present deficits that impairs joint position sense (Zia et al., 2000, Zia et al., 2002, Wright et al., 2007a, Wright et al., 2007b, Wright et al., 2010), distal (Endo et al., 2009, Marusiak et al., 2010) and axial (Wright et al., 2007b, Franzen et al., 2009) tone as well as spatial perception (Wright et al., 2007a, Wright et al., 2010). It has been suggested that these impairments may affect gating or integration of sensory information that affect motor control (Abbruzzese and Berardelli, 2003, Konczak et al., 2009). The basal ganglia play an especially important role in non-visual walking/locomotion since kinesthetic cues predominate in this task (Takei et al., 1997).
Our previous study showed that that axial rigidity in PD subjects also affects turning ability, probably because the usual motor drive needed to rotate body segments might not be sufficient to overcome the increase in tone (Wright et al., 2007b, Franzen et al., 2009). Thus, axial rigidity may have contributed to the larger path radius in PD subjects OFF, but not ON, when levodopa reduces rigidity. In addition, the reduced proprioception observed in PDs (Zia et al., 2000, Zia et al., 2002, Wright et al., 2007a, Wright et al., 2007b, Wright et al., 2010) likely affects proprioceptive feedback from movement execution, resulting in erroneous corollary discharge such that PD subjects may not accurately detect their movement errors (Demirci et al., 1997). These turning difficulties during curvilinear walking in people with PD could also be due to a disrupted intersegmental coordination due to problems automating complex movements since turning requires sequential coordination of multiple segments as well as coordination between posture and locomotion (Bowen et al., 1972, Vaugoyeau et al., 2006, Crenna et al., 2007). It is unlikely that errors in circular navigation are due to PD motor deficits alone, since they were able to complete the task normally in the eyes opened condition.
In contrast to recent studies showing perceptomotor asymmetries related to the first affected side (Bowen et al., 1972, Wright et al., 2007a, Wright et al., 2010), we found no consistent asymmetries in walking trajectories across PD subjects walking around a CW versus CCW circle with or without vision. However, a few PD subjects were noticeably asymmetric in their performance (Figure 2 and Table 4) and it might be related to most affected side differences although this study was not able to detect these due to a lack of power.
4.2 Levodopa improves the rotational component in circular walking with eyes closed
When subjects were ON levodopa, they significantly improved their turning ability around the circle by navigating a walking radius more similar to that of control subjects. Yet, when subjects were ON levodopa, they did not improve their ability to return to their starting position. In fact, when OFF levodopa, more PD subjects were stopped while going around the circle (6/12 subjects, as shown in Table 2) because of their excessively large trajectory.
It seems that levodopa shortens the radius of a circular navigation path as subjects in our study turned more. In a previous study, we assessed the effect of levodopa on an objective measure of postural axial tone in these same PD subjects (Franzen et al., 2009). Levodopa did not decrease postural tone about the hips or trunk when subjects were ON or OFF levodopa. Thus, it is unlikely that changes in rigidity are responsible for the improvement in turning. Barnett-Cowan et al (2010), have suggested that when ON medication, subjects with PD might rely more on their vestibular cues and rely less on their internal representation, which might explain the differences we observed. Wright et al (2010) also found reduced axial kinesthetic sensitivity in PD subject when ON levodopa. More studies are needed to investigate the role of levodopa in non-visual locomotion in PD.
4.3 Cerebellar deficits do not deteriorate navigation round a circle
A primary feature of our results is the difference in performance between cerebellar and PD patients. Thus, whereas PD pathology substantially worsened eyes closed performance compared to controls, cerebellar deficiency did not. A strength of the present study is that we controlled for possible differences in gait that could be related to gait speed. We also controlled for motor control deficits associated with PD and cerebellar disorders by normalizing navigation with eyes closed for each subject's navigation with eyes open. Thus, the differences between performance in PD and cerebellar subjects presumably arise from the different pathologies, rather than differences in velocity control or in motor control deficits.
In conclusion, basal ganglia and cerebellar impairments result in a different effect on the ability to walk blindfolded around a circular path. The current evidence suggests that PD subjects overshoot the circle radius and stop short of their initial position, whereas cerebellar subjects navigate the circle with eyes closed even more accurately than control subjects, despite their ataxia. Understanding non-visual, curvilinear navigation is important for the care and rehabilitation of individuals with PD and cerebellar ataxia to improve safe mobility and reduce falls.
Research Highlights.
Blindfolded navigation is differently affected by Basal ganglia & cerebellar diseases
Cerebellar subjects navigate with eyes closed more accurately than controls
OFF levodopa, PD subjects output a larger radius than controls
ON levodopa, PD subjects improve radius and distance but have larger endpoint errors
Acknowledgments
The authors thank Triana Nagel for help in the patient recruitment and data collection. This research was supported by the NIH R37-AG006457, NIH R01-DC004082, the Center for Health Care Sciences at Karolinska Institutet, Sweden.
Abbreviations
- ANOVA
Analysis of Variance
- ASIS
Anterior Superior Iliac Spines
- CA
Cerebellar Ataxia
- CCW
Counter-clockwise
- ctrl
Control
- CW
Clockwise
- EC
Eyes Closed
- EO
Eyes Open
- IQR
Interquartile range
- OFF
Off levodopa medication
- ON
On levodopa medication
- PD
Parkinson's Disease
- SARA
Scale for the Assessment and Rating of Ataxia
- SCA
Spinocerebellar Ataxia
- SD
Standard Deviation
- UPDRS
Unified Parkinson's Disease Rating Scale
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Erika Franzén, Email: erika.franzen@ki.se.
Geoffrey Melvill Jones, Email: gmelvill@ucalgary.ca.
Fay B Horak, Email: horakf@ohsu.edu.
References
- Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18:231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- Amick MM, Schendan HE, Ganis G, Cronin-Golomb A. Frontostriatal circuits are necessary for visuomotor transformation: mental rotation in Parkinson's disease. Neuropsychologia. 2006;44:339–349. doi: 10.1016/j.neuropsychologia.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Barnett-Cowan M, Dyde RT, Fox SH, Moro E, Hutchison WD, Harris LR. Multisensory determinants of orientation perception in Parkinson's disease. Neuroscience. 2010;167:1138–1150. doi: 10.1016/j.neuroscience.2010.02.065. [DOI] [PubMed] [Google Scholar]
- Berthoz A, Israel I, Georges-Francois P, Grasso R, Tsuzuku T. Spatial memory of body linear displacement: what is being stored? Science. 1995;269:95–98. doi: 10.1126/science.7604286. [DOI] [PubMed] [Google Scholar]
- Bowen FP, Hoehn MM, Yahr MD. Parkinsonism: alterations in spatial orientation as determined by a route-walking test. Neuropsychologia. 1972;10:355–361. doi: 10.1016/0028-3932(72)90027-9. [DOI] [PubMed] [Google Scholar]
- Crenna P, Carpinella I, Rabuffetti M, Calabrese E, Mazzoleni P, Nemni R, Ferrarin M. The association between impaired turning and normal straight walking in Parkinson's disease. Gait Posture. 2007;26:172–178. doi: 10.1016/j.gaitpost.2007.04.010. [DOI] [PubMed] [Google Scholar]
- Demirci M, Grill S, McShane L, Hallett M. A mismatch between kinesthetic and visual perception in Parkinson's disease. Ann Neurol. 1997;41:781–788. doi: 10.1002/ana.410410614. [DOI] [PubMed] [Google Scholar]
- Endo T, Okuno R, Yokoe M, Akazawa K, Sakoda S. A novel method for systematic analysis of rigidity in Parkinson's disease. Mov Disord. 2009;24:2218–2224. doi: 10.1002/mds.22752. [DOI] [PubMed] [Google Scholar]
- Franzen E, Paquette C, Gurfinkel VS, Cordo PJ, Nutt JG, Horak FB. Reduced performance in balance, walking and turning tasks is associated with increased neck tone in Parkinson's disease. Exp Neurol. 2009;219:430–438. doi: 10.1016/j.expneurol.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees AJ. What features improve the accuracy of clinical diagnosis in Parkinson's disease: a clinicopathologic study. Neurology. 1992;42:1142–1146. doi: 10.1212/wnl.42.6.1142. [DOI] [PubMed] [Google Scholar]
- Konczak J, Corcos D, Horak F, Poizner H, Shapiro M, Tuite P, Volkmann J, Maschke M. Proprioception and Motor Control in Parkinson's Disease. Journal of Motor Behavior. 2009;41:543–552. doi: 10.3200/35-09-002. [DOI] [PubMed] [Google Scholar]
- Konczak J, Krawczewski K, Tuite P, Maschke M. The perception of passive motion in Parkinson's disease. Journal of Neurology. 2007;254:655–663. doi: 10.1007/s00415-006-0426-2. [DOI] [PubMed] [Google Scholar]
- Loomis JM, Klatzky RL, Golledge RG, Cicinelli JG, Pellegrino JW, Fry PA. Nonvisual navigation by blind and sighted: assessment of path integration ability. J Exp Psychol Gen. 1993;122:73–91. doi: 10.1037//0096-3445.122.1.73. [DOI] [PubMed] [Google Scholar]
- Marusiak J, Kisiel-Sajewicz K, Jaskolska A, Jaskolski A. Higher muscle passive stiffness in Parkinson's disease patients than in controls measured by myotonometry. Arch Phys Med Rehabil. 2010;91:800–802. doi: 10.1016/j.apmr.2010.01.012. [DOI] [PubMed] [Google Scholar]
- Petrosini L, Leggio MG, Molinari M. The cerebellum in the spatial problem solving: a co-star or a guest star? Prog Neurobiol. 1998;56:191–210. doi: 10.1016/s0301-0082(98)00036-7. [DOI] [PubMed] [Google Scholar]
- Pham QC, Hicheur H. On the open-loop and feedback processes that underlie the formation of trajectories during visual and nonvisual locomotion in humans. J Neurophysiol. 2009;102:2800–2815. doi: 10.1152/jn.00284.2009. [DOI] [PubMed] [Google Scholar]
- Rondi-Reig L, Burguiere E. Is the cerebellum ready for navigation? Prog Brain Res. 2005;148:199–212. doi: 10.1016/S0079-6123(04)48017-0. [DOI] [PubMed] [Google Scholar]
- Takei Y, Grasso R, Amorim MA, Berthoz A. Circular trajectory formation during blind locomotion: a test for path integration and motor memory. Exp Brain Res. 1997;115:361–368. doi: 10.1007/pl00005705. [DOI] [PubMed] [Google Scholar]
- Takei Y, Grasso R, Berthoz A. Quantitative analysis of human walking trajectory on a circular path in darkness. Brain Res Bull. 1996;40:491–495. doi: 10.1016/0361-9230(96)00147-5. discussion 495-496. [DOI] [PubMed] [Google Scholar]
- Vaugoyeau M, Viallet F, Aurenty R, Assaiante C, Mesure S, Massion J. Axial rotation in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2006;77:815–821. doi: 10.1136/jnnp.2004.050666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DG, Koppen JR, Jones JL, Winter SS, Wagner SJ. Navigating with fingers and feet: Analysis of human (homo sapiens) and rat (rattus norvegicus) movement organization during nonvisual spatial tasks. J Comp Psychol. 2010;124:381–394. doi: 10.1037/a0020546. [DOI] [PubMed] [Google Scholar]
- Wright WG, Gurfinkel V, King L, Horak F. Parkinson's disease shows perceptuomotor asymmetry unrelated to motor symptoms. Neurosci Lett. 2007a;417:10–15. doi: 10.1016/j.neulet.2007.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WG, Gurfinkel VS, King LA, Nutt JG, Cordo PJ, Horak FB. Axial kinesthesia is impaired in Parkinson's disease: Effects of levodopa. Exp Neurol. 2010;225:202–209. doi: 10.1016/j.expneurol.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WG, Gurfinkel VS, Nutt J, Horak FB, Cordo PJ. Axial hypertonicity in Parkinson's disease: direct measurements of trunk and hip torque. Exp Neurol. 2007b;208:38–46. doi: 10.1016/j.expneurol.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zia S, Cody F, O'Boyle D. Joint position sense is impaired by Parkinson's disease. Ann Neurol. 2000;47:218–228. [PubMed] [Google Scholar]
- Zia S, Cody FW, O'Boyle DJ. Identification of unilateral elbow-joint position is impaired by Parkinson's disease. Clin Anat. 2002;15:23–31. doi: 10.1002/ca.1087. [DOI] [PubMed] [Google Scholar]