Abstract
Background
The reduced immunogenicity of the H5 hemagglutinin (HA), compared to seasonal HA serotypes, has stimulated searches for effective adjuvants to improve H5 vaccine efficacy. This study examined the immunogenicity and protective efficacy in ferrets immunized with a split-virion H5N1 vaccine combined with Advax™, a novel delta inulin-based polysaccharide adjuvant technology that has previously demonstrated ability to augment humoral and cellular immunity to co-administered antigens.
Methods
Ferrets were vaccinated twice 21 days apart with 7.5 µg or 22.5 µg of a split-virion preparation of A/Vietnam/1203/2004 with or without adjuvant. An additional group received just one immunization with 22.5 µg HA plus adjuvant. Serum antibodies were measured by hemagglutination inhibition and microneutralization assays. Vaccinated animals were challenged intranasally 21 days after the last immunization with 106 EID50 of the homologous strain. Morbidity was assessed by observed behavior, weight loss, temperature, cytopenias, histopathology, and viral load.
Results
No serum neutralization antibody was detected after two immunizations with unadjuvanted vaccine. Two immunizations with high or low dose adjuvanted vaccine stimulated high neutralizing antibody titers. Survival was 100% in all groups receiving adjuvanted-vaccine including the single dose group, compared to 67% survival with unadjuvanted vaccine, and 0% survival in saline or adjuvant-alone controls. Minimal morbidity was seen in all animals receiving adjuvanted vaccine, and was limited to rhinorrhea and mild thrombocytopenia, without fever, weight loss, or reduced activity. H5N1 virus was cleared from the nasal wash by day 4 post-challenge only in animals receiving adjuvanted vaccine which also prevented viral invasion of the brain in most animals.
Conclusions
In this initial study, Advax™ adjuvant formulations improved the protective efficacy of a split-virion H5N1vaccine as measured by significantly enhanced immunogenicity, survival, and reduced morbidity.
1. Introduction
Highly pathogenic avian influenza of the H5N1 serotype spread rapidly in Asian wild bird populations in 2003, then spread to Europe and Africa, and has sporadically appeared in domestic bird populations in various countries [1]. In spite of only 500 reported human infections, the high mortality rate of ~ 60% raises concern for pandemic potential if avian H5N1 strains were to acquire efficient human-to-human transmission through mutation or recombination.
Vaccination is the most effective strategy to control the spread of a pandemic influenza virus. Developing a pre-pandemic vaccine for H5N1 strains presents a number of challenges [2,3,4,5,6]. First, the world population has little prior exposure to H5 hemagglutinin (HA), reducing potential cross-reactive immunity. Second, the evolution of 10 clades of H5N1 strains has been relatively rapid during the last decade, necessitating a level of heterotypic protection from stockpiled vaccines that is not usually necessary for seasonal vaccines. Third, the logistics of rapidly immunizing a large population requires optimal dose-sparing, ideally requiring just a single dose for protection. To achieve these objectives, the immunogenicity of pandemic vaccines must be optimized to ensure they are sufficiently robust to prevent both disease and transmission [7].
Inactivated whole virus H5N1 vaccines have been shown to be protective against H5N1 challenge in immunized ferrets and to be immunogenic in humans [8,9] but induce high reactogenicity. Split-virion vaccines are preferred for their lower reactogenicity [4] but are less immunogenic than whole virus H5N1 vaccines. In ferrets, two 7.5–15 µg doses of split-viron H5N1 A/Vietnam/1194/04 vaccine failed to induce neutralizing antibody or reduce fever and viral shedding, provided at best 50% protection against homologous lethal challenge and failed to protect against heterologous challenge [10] [11]. A similar lack of protection was observed in macaques [12]. In human trials, two 90µg doses of split-virion H5N1 vaccine induced hemagglutination inhibition (HI) titers of ≥ 40 in only half of subjects [13,14]. Improvement in H5N1 vaccine immunogenicity has been attained by additional booster doses [15], higher initial priming doses [13], or addition of adjuvant. Squalene oil-in-water adjuvants [5] were superior to aluminum salts, which have provided equivocal results in human influenza trials [14, 16] and have concerns about long-term toxicity [17].
Particulate adjuvants derived from inulin polymers have broad adjuvanticity [18,19,20] and have been shown to improve the immunogenicity of seasonal influenza vaccines in mice [21]. Advax™ is the latest generation of adjuvants made from delta inulin, a newly-described highly stable inulin isoform with potent immunological activity [22]. Advax™ adjuvants have been used to enhance the immunogenicity of vaccines against Japanese encephalitis virus [23], human immunodeficiency virus [24] amongst others. We used a validated ferret challenge model to test whether the protective efficacy of a split-virion H5N1 Clade 1 vaccine could be enhanced by formulation with delta inulin adjuvant. The Advax™ adjuvant formulations tested were safe and well tolerated in the ferret model and significantly improved the protective efficacy of the H5N1 vaccine by enhanced immunogenicity and prevention of mortality and most signs of morbidity.
2. Methods and Materials
2.1. Ferrets
All procedures were conducted under protocols approved by the Institutional Animal Care and Use Committee (IACUC) at Lovelace Respiratory Research Institute, and all facilities were accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC). Castrated ferrets (Mustela putorious furo, Triple F Farms, Sanger, PA) aged 11– 14 weeks weighing 0.7 to 1.9 kg were held for fourteen days for acclimation and quarantine. Ferrets were seronegative for currently circulating influenza A H1 and H3, influenza B viruses, and to H5 antigen. Animals were pair-housed in plastic bottom rabbit/ferret cages (Allentown Inc., Allentown, NJ) during quarantine and vaccination and in a bioBubble® (bioBubble Inc, Fort Collins, CO) inside an ABSL3+ containment area for virus challenge.
2.2. Vaccine and adjuvant
The H5N1 A/Vietnam/1203/2004 Monovalent Influenza Subvirion Vaccine: Fisher Repository stock number – CLAG-1170 (lot# U007827) was obtained from the NIAID repository and was stored at 2 to 8°C. Each dose of vaccine was diluted with the appropriate Advax™ adjuvant formulation in physiological saline. The vaccine was administered by intramuscular (IM) thigh injection in a volume of 0.5 mL and the other thigh for the second vaccination. Control animals received either adjuvant alone or an equal volume of buffered saline. Two formulations of Advax™ adjuvant;, Lot# VAX-SPL-0910-03 (Ad 1) and Lot# VAX-SPL-0910-04 (Ad 2) were supplied by Vaxine Pty Ltd, Adelaide Australia. Ad1 represented standard delta inulin, whereas Ad2 comprised standard delta inulin plus a synthetic oligonucleotide (699188) at 300ug/mL. Adjuvant was stored at 2–8°C, as directed by the manufacturer and combined with vaccine immediately before use.
2.3. Virus
Influenza virus A/Vietnam/1203/2004 (H5N1) (VN/1203) was obtained from the Centers for Disease Control and Prevention (CDC). This stock had been passaged twice in embryonated eggs at the CDC and once in 10-day embryonated chicken eggs at LRRI to generate the master stock and once again in eggs to generate the virus for all subsequent challenges (Lot 03142007EP2.2) [26]. Aliquots of 0.5 mL to 1.0 mL were stored at −80 °C. After storage, the virus was determined in three separate assays to have a concentration of 1.4×108 plaque forming units (PFU)/mL, 5.8×108 50% tissue culture infectious dose (TCID50)/mL, and 1×108 50% egg infectious dose (EID50)/mL. As a Risk Group 3 pathogen all manipulations were carried out in a BSL3/ABSL3+ containment facility.
2.4. Experimental Design
Animals were assigned to groups using a stratified (body weight) randomization procedure by a computerized data acquisition system (e.g., Path-Tox; Xybion, Cedar Knolls, NJ). A total of 49 ferrets were assigned to one of ten groups;. Four groups of 7 ferrets each received adjuvanted vaccine twice 21 days apart: 7.5 µg vaccine + Ad1; 7.5 µg vaccine + Ad2; 22.5 µg + Ad1; 22.5 µg vaccine + Ad2. −Two groups of 3 ferrets each received vaccine twice without adjuvant: 22.5 µg + No Ad; 7.5 µg + No Ad. Three Control groups of three ferrets each received twice: saline + Ad1; saline + Ad2; saline + Saline. One additional group of 6 ferrets received 22.5 µg vaccine + Ad2 administered only once at the time of priming of other groups. Ferrets were infected three weeks after the vaccine booster dose, or six weeks after the priming dose in the group vaccinated only once. Following anesthesia with intramuscular ketamine (20mg/kg) and xylazine (4 mg/kg), 106 EID50 of VN/1203 was instilled in 500 µL into each nare, and the challenge dilution was cultured to ensure consistent infections. Nasal washes were collected by instilling into each nare 1.0 mL of saline containing 1% bovine serum albumin, 100 units penicillin/mL, 100 µg/mL streptomycin, and 0.25 µg amphotericin B/mL. Whole blood for complete blood count was obtained by superior vena cava puncture on day 4 after challenge.
2.5. Observations
Twice daily observations recorded ocular discharge, nasal discharge, sneezing, coughing, stool characteristics, and activity score. During morning observations and, as directed, weights and temperatures from shoulder and hip implanted microchips (IPTT-300 Implantable Programmable Temperature and Identification Transponder; Bio Medic Data Systems, Inc, (BMDS) Seaford, DE) were recorded. An activity score (0 = alert and playful, 1 = alert but playful only when stimulated, 2 = alert but not playful when stimulated, and 3 = neither alert nor playful when stimulated) was obtained each time the ferrets were observed. Moribund animals were designated for humane euthanasia by any one of the following criteria: a temperature of less than 33.3 °C, weight loss ≥ 25%, unresponsiveness to touch, self-mutilation, paralysis, movement disorder, or respiratory distress. Vaccine injection sites were palpated for nodular masses but none were identified.
2.6. Viral Load
In upper respiratory tract samples obtained during life, nasal washes were obtained 2 and 4 days after viral challenge, and throat swabs were obtained 1, 2, 3, 4, and 6, days after challenge. In tissues harvested at necropsy, influenza virus was cultured from lavage of a caudal lung lobe and from four 250 mg fragments of homogenized (TissueLyser, QIAGEN, Valencia, CA) lung, brain, spleen, tracheobronchial lymph nodes, and two tracheal rings. The 200 µL aliquots of homogenate were incubated on Madin-Darby Canine Kidney (MDCK) cells under agarose overlay for 2 days by modified standard techniques [25].
2.7. Serology
Serum was collected by vena cava puncture on the day of first vaccination and 14, 21, and 28 days after first vaccination; day 14 post vaccination corresponds to day −28 before challenge, and day 28 post vaccination corresponds to day −7 before challenge. Serological assays were modified from previously described procedures [26,27]. Serum samples were inactivated by receptor-destroying enzyme (Denka-Seiken, Tokyo, Japan) at 37°C for 16–20 hours followed by heat inactivation at 56 °C for 30 minutes. Hemagglutination inhibition (HI) was performed using horse red blood cells [28]. Titers of neutralizing antibodies were measured by the microneutralization assay (MN). One hundred tissue culture infectious dose 50% (100 TCID50) of VN/1203 virus was mixed with an equal volume of serial dilutions of serum in quadruplicate, incubated for 1 hour at 37°C and 100 µL of the mixture was added to a prewashed monolayer of MDCK cells in 96 well plates. The plates were incubated for 3 days and the cytopathic effect (CPE) was enumerated using an inverted microscope. The highest serum dilution protecting more than half of the wells was recorded as geometric mean titers and a negative titer was denoted as 10.
2.8. Histopathology
Lung tissue and brain with olfactory bulbs were collected at necropsy from ferrets moribund on days 6 to 8 post-challenge and from surviving ferrets free of symptoms at day 14 post-challenge. After fixation in buffered formalin, standardized locations of tissue were trimmed for histopathology from the left cranial, right middle and right caudal lung lobes. Standardized sampling of brains included olfactory bulbs and coronal sections through the entire brain at the frontal region, parietal region and cerebellum with brainstem. Paraffin embedded 4 µm sections were stained with hematoxylin & eosin, and read by a board certified veterinary pathologist (APG) blinded to the vaccination history. Standard, subjective grading of lesions was based on the severity of the change within affected areas and the extent of tissue affected by the change: 0 (none) = essentially no tissue affected; 1 (minimal) = ~1 to 5% affected; 2 (mild) = ~6 to 25% affected; 3 (moderate) = ~26 to 50% affected; 4 (marked) = ~50 to ~100% affected with a severe change.
2.9. Statistics
Statistical analyses were performed using GraphPad Prism (version 5.03, GraphPad Software, Inc. La Jolla, CA). Serum antibody response was analyzed by analysis of variance (ANOVA) using the Bonferroni post-test correction. Survival proportions were tested using the Log-Rank test. Morbidity by increasing activity score was examined by Fisher’s exact test. Viral load was analyzed by the repeated measure ANOVA for each tissue and fluid. Hematological measurements were analyzed by Student's t-test.
3. Results
3.1. Serum Antibody Response
Antibody titers pre- and post-immunization and post-challenge were determined by both HI and MN assays. Neither controls nor ferrets immunized with unadjuvanted vaccine had detectable neutralizing antibody prior to challenge (Figure 1). After challenge survivors in unadjuvanted vaccine groups did develop antibody with titers ranging from 160 – 3840 at day 14 post-challenge (data not shown). In contrast, ferrets receiving two doses of adjuvanted vaccine all demonstrated neutralizing antibody pre-challenge, regardless of antigen dose or adjuvant formulation (Figure 1 B+D). In ferrets receiving Advax™-adjuvanted vaccine, peak GMT neutralizing antibody one week after booster immunization was > 512 in all animals, with a non-significant decrease during the 3 week interval prior to challenge. At 21 days after the priming dose, Ad2-adjuvanted vaccine recipients had significantly higher serum HI antibody than the Ad1 groups (p < 0.03, Log Rank-sum test). Thus delta inulin adjuvants significantly enhanced the immunogenicity of the H5N1 vaccine as indicated by high levels of neutralizing antibody.
Figure 1. HI and MN titers measured at the time of the booster dose (21 days prior to challenge) and 14 days after the booster dose (7 days prior to challenge).
Plots for each group denote the mean (+) and the 95% confidence intervals (whiskers) with dots representing individual data points. The samples that were below the limit of detection (BLD, 10 units) are drawn at the bottom of each column.
3.2. Survival
All ferrets that received high or low dose vaccine with either Advax™-adjuvant formulation survived (Figure 2). By contrast no saline or adjuvant alone controls survived, with a mean time to death of 5.75 dpi. In non-adjuvanted vaccine groups two out of three (67%) survived in both dose groups, with a mean time to death of 7.3 dpi, (reduced survival significantly lower than the adjuvanted groups at p = 0.05, Log Rank-sum test). Partial protection by non-adjuvanted vaccine is consistent with the results with the unadjuvanted vaccine obtained in previous studies by the investigators [29]. Thus delta inulin adjuvanted vaccine resulted enhanced protection from lethal challenge, with complete protection even after a single dose of Ad2-adjuvanted vaccine.
Figure 2. Survival Proportions Demonstrate Adjuvant Advantage.
Each of the 10 groups is denoted by survival percent: vaccine dose (or saline) + adjuvant identity (or saline). The survival of each the 5 adjuvanted-vaccine groups are significantly different from the two unadjuvanted vaccine groups (Log-Rank test, p = 0.05) and from the three control groups (Log-Rank test, p<0.001).
3.3. Morbidity
Recipients of two doses of Ad1-adjuvanted vaccine lost only 5% of body weight then recovered, while animals receiving two doses of Ad2-adjuvanted vaccine did not lose weight (Figure 3A). The recipients of just a single dose of Ad2-adjuvanted vaccine lost 5–15% of body weight by day 2, but then all recovered. Recipients of non-adjuvanted vaccine lost greater than 15% of body weight by day 5 pi and the four survivors failed to recover lost weight. All control ferrets lost 15–20% of body weight by day 5 pi prior to death. All ferrets in the control and non-adjuvanted vaccine groups demonstrated fever (temperature increase greater than 2°C above baseline) on multiple days after challenge (Figure 3B). Just 4 ferrets in the Ad1-adjuvanted vaccine groups demonstrated fever lasting for only one day and no ferrets in the Ad2-adjuvanted group demonstrated fever.
Figure 3. Group Mean Weight Change (A) and Body Temperature Change (B).
Change is calculated from pre-challenge baseline value of each animal in each group for each day following challenge (DPI). Control ferrets receiving only adjuvant or saline are combined in one graph. As all moribund ferrets were euthanized by day 8 dpi, subsequent days are denoted by ‘0’ change.
No vaccinated ferrets showed signs of coughing or sneezing and only two ferrets in the control groups exhibited respiratory distress when moribund. Neurological signs including paralysis of limbs and seizures were not observed in adjuvanted-vaccine groups but occurred in 2/6 (33%) non-adjuvanted vaccine recipients and 4/9 (44%) controls, appearing after day 5 pi and persisting until death. Ocular discharge was observed between day 3–6 pi in 7/34 (20.5%) adjuvanted-vaccine recipients, 6/6 (100%) non-adjuvanted vaccine recipients, and 9/9 (100%) controls. Nasal discharge was a common sign in all groups, occurring as early as day 1 pi, resolving in vaccinated animals by day 10–11 pi, but persisting in controls until death. Diarrhea occurred in only 18% of adjuvanted-vaccine recipients whereas all unvaccinated controls experienced diarrhea until death.
Activity was diminished to a ‘1’ score in only 3/34 (8.8%) ferrets in the adjuvantedvaccine groups, whereas 100% of the non-adjuvanted vaccine and control ferrets attained an activity score of 2 or more, including the 4 survivors in the non-adjuvanted vaccine groups (Figure 4). Thus vaccination with delta inulin adjuvant almost completely prevented infection-associated morbidity after lethal challenge.
Figure 4. Group Mean Activity Scores for each day after challenge.
Adjuvanted vaccine recipients are graphed according to dose (22.5 µg-inulin;blue, and 7.5 µg-inulin:green), combining the results of the Ad1 and Ad2 groups, denoted as ‘inulin’. The two doses of unadjuvanted vaccine (VXX-saline) are combined, and graphed according to survival (orange) or non-survival (maroon). Control recipients of adjuvant alone (saline-inulin:pink) are separated from control recipients of saline alone (olive).
3.4. Hematology
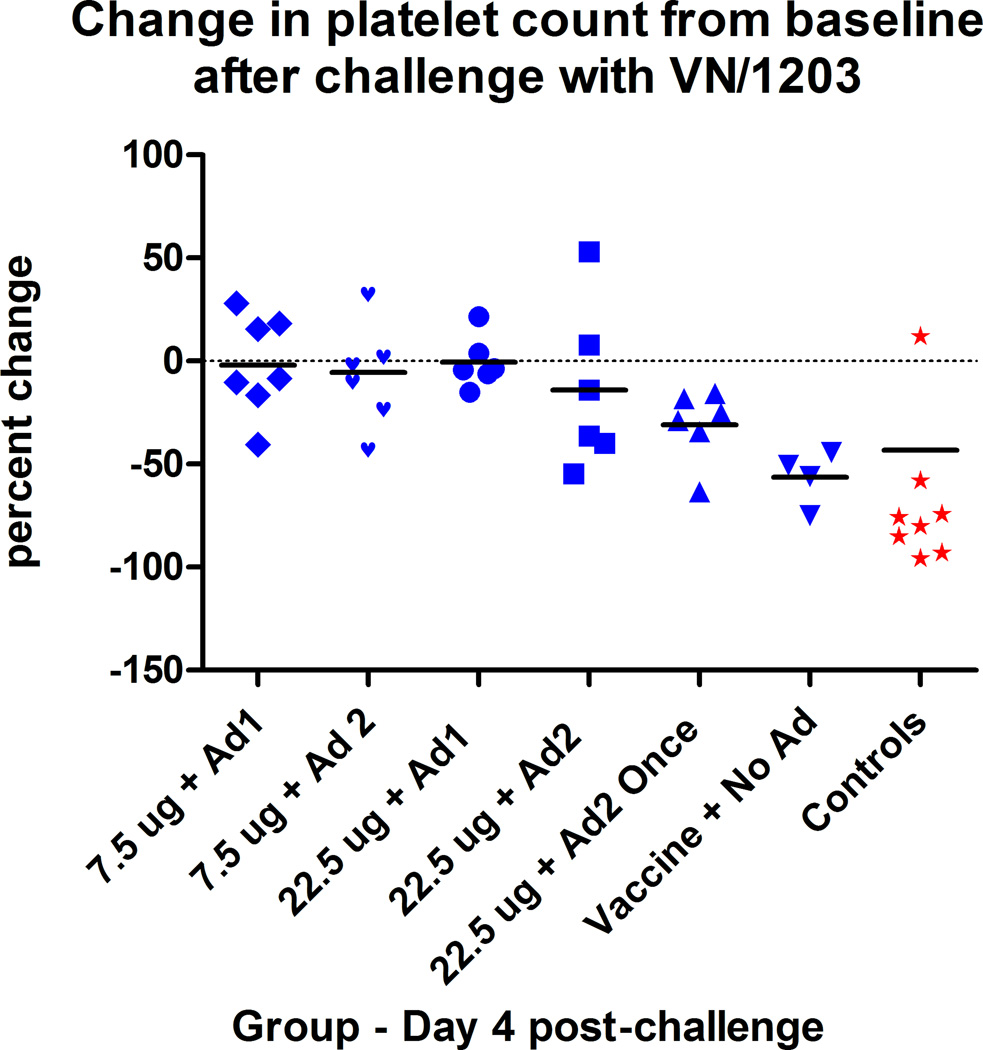
Based on prior experience, complete blood counts were obtained only on day 4 pi as this day identifies the nadir of lymphocyte and platelet counts in ferrets challenged with lethal doses of VN/1203. Group means of lymphocyte counts on day 4 in adjuvanted-vaccine and non-adjuvanted vaccine recipients were not different from baseline means, but control animals showed reduced lymphocyte counts as expected (data not shown). Platelet counts tended to be reduced only in the once-immunized Ad2-adjuvanted vaccine recipients (range −10% to −60% reduction, p < 0.01) but not in the twice-immunized adjuvanted-vaccine groups (p > 0.5) (Figure 5). Non-adjuvanted vaccine recipients had marked thrombocytopenia but platelet counts in all of these groups had recovered to baseline values by day 14 pi. Controls and ultimately moribund non-adjuvanted vaccine recipients had significantly reduced platelet counts on day 4 pi compared to all other groups (paired t test, p<0.001).
Figure 5. Post-challenge thrombocytopenia on day 4 post-challenge.
Thrombocytopenia is expressed as the percentage change from pre-challenge collection value. Values from ultimate survivors (blue) are compared with ultimately moribund ferrets (red).
3.5. Viral load
In the adjuvanted vaccine groups, nasal washes and throat swab cultures were negative on day 1 pi, commonly positive on 2 day pi, less frequently positive on day 4 pi, and always negative on day 6 pi and at necropsy on day 14 pi (Figure 6). Throat swab titers in Ad2 vaccine recipients on days 2, 3, and 4 pi were significantly lower (Mann-Whitney, p=0.0018), a trend to lower loads in Ad1-vaccine recipients (Mann-Whitney, p=0.06), but no reduced loads in single-dose Ad2-vaccine recipients, compared to unadjuvanted vaccine recipients. Upper airway samples from the control groups were positive for virus from day 1 pi until necropsy and all pulmonary and extrapulmonary tissues from all moribund non-survivors contained moderate to high levels of virus. All pulmonary and extrapulmonary tissues from survivors necropsied on day 14 post-challenge were negative by culture. Thus delta inulin adjuvant enhanced the ability of the H5N1 vaccine to mediate early clearance of upper respiratory tract infectious virus after challenge.
Figure 6. Viral load in respiratory tract samples collected during life.
Throat swabs were collected on days 1, 2, 3, 4 and 6 post-challenge, and at necropsy (Nx). Nasal wash (NW) samples were obtained on day 2, 4, and at necropsy. Viral cultures <10 were below limit of detection (LOD). All tissue samples from moribund ferrets obtained at necropsy (lung lavage, four sections of lung, tracheal rings, turbinates, and brain) were positive by culture (data not shown).
3.6. Histopathology
Lungs of non-survivor animals in the control and non-adjuvanted vaccine groups revealed severe pneumonia characterized by extensive necrosis of parenchyma, fibrin exudation, neutrophil and macrophage influx and centri-acinar hemorrhage with intervening regions of normal appearing lung. All survivors in the immunized groups had evidence of resolving pneumonia (data not shown). The brain tissue of non-survivors was diffusely involved with multifocal lesions of lympho-histiocytic meningoencephalitis typical of H5N1 influenza, characterized by necrosis with abundant pyknosis and karyorrhexis of cells and increased cellularity with lymphocytes, histiocytes and neutrophils. Lesions were often more severe in olfactory bulbs and in the more rostral and ventral brain regions with decreasing severity in caudal sections. Resolving brain inflammation in survivors was indicated by either recent major brain necrosis with malacia and cavities due to the loss of neural tissue with clearance of cellular debris, or with minor brain involvement with perivascular lymphocytic cuffing. There was a complete absence of lesions of resolving brain inflammation in 15/28 (54%) of recipients of 2 doses of adjuvanted-vaccine, while 4/4 (100%) survivors in the non-adjuvanted vaccine group had evidence of major brain involvement (Table 1). This suggests that formulation with delta inulin adjuvant enhanced the ability of the H5N1 vaccine to prevent viral replication in the brain.
TABLE 1.
Central Nervous System Pathology in Vaccinated Survivors1
| Vaccinated Twice (no adjuvant)3 |
Vaccinated once (22.5 µg + adjuvant) |
Vaccinate d twice (7.5 µg + adjuvant)4 |
Vaccinate d twice (22.5 µg + adjuvant)5 |
|
|---|---|---|---|---|
| Tissues examined2 | [n=8] | [n=12] | [n=28] | [n=28] |
| Evidence of major CNS involvement Malacia with cavitation (perivascular cuffing may be present) | 88%6 (3.0) |
42% (2.4) |
11% (2.7) |
39% (2.2) |
| Evidence of minor CNS reaction Perivascular cuffing (ONLY) | 0% (-) |
33% (1.5) |
18% (1.5) |
25% (1.4) |
Cells in body of table contain incidence as a percent (number affected divided by number of examined CNS tissues—olfactory bulb and brain proper—among survivors in that group. All survivors were euthanized 14 days after challenge and tissues obtained from necropsy. Number within parentheses denotes the average lesion severity (from 1 – 4) among animals demonstrating that lesion
Combines all CNS tissues – olfactory lobe and brain as brainstem, cerebral cortex and cerebellum.
Vaccinated without adjuvant received either 22.5 µg or 7.5 µg vaccine alone.
Vaccinated twice with 7.5 µg vaccine and either adjuvant.
Vaccinated twice with 22.0 µg vaccine and either adjuvant.
Vaccination without adjuvant significantly associated with higher incidence of major CNS involvement compared to vaccination with adjuvant by z test of proportions with Yates corrections: control (vaccine no adjuvant) vs 7.5 µg group, z=3.83, p<0.001; vs 22.5 µg group, z=2.004, p=0.045; vs 22.5 µg vaccinated once not significant.
4. Discussion
We show here that the addition of Advax™ adjuvant formulations to a split-virion H5N1 vaccine enhanced protection against lethal challenge and reduced infection-associated morbidity while providing evidence for antigen-sparing. By contrast, the non-adjuvanted H5N1 vaccine failed to fully protect against homologous challenge even at the higher 22.5µg dose, confirming the results of other studies with non-adjuvanted vaccines [2, 10, 11, 29]. The ferret model of H5N1 infection mimics human disease with respect to kinetics, histopathology and antibody response and is considered an appropriate challenge model [30, 31, 32]. Challenge with clade 1.0 strain VN/1203 is fatal in ferrets at intranasal doses ranging from 101 to 107 EID50 [33]. The ferret data indicating poor immunogenicity of non-adjuvanted H5N1 vaccine is mirrored by human immunogenicity data showing only 22% of adults responded to two 15 µg VN/1203 doses and only 54% responded to two 90µg doses [13]. Trials of other human split-virion H5N1 vaccines have also shown poor immunogenicity [34, 35].
Adjuvants have been used to improve the immunogenicity of split-virion influenza vaccines [36, 37]. Aluminum adjuvants enhanced the immunogenicity of H5N1 vaccine in ferrets [11,29] but failed to enhance vaccine immunogenicity in humans [38, 39, 40, 41]. MF59, a squalene oil emulsion adjuvant, was shown in ferrets to enhance multi-clade protection against H5N1 strains [42], and in humans improve immunogenicity of H5N1 antigens and H1N1 2009 pandemic vaccines [43, 44]. Nevertheless, MF59 has not been approved for use by the FDA, leaving a major need to identify suitable safe and effective adjuvants for use in pandemic influenza vaccines. Advax™ is a novel polysaccharide adjuvant derived from delta inulin that acts at least in part through activation of the alternative complement pathway, a TLR-independent means of enhancing adaptive immune responses [19]. Delta inulin stimulates both humoral and CD4 and CD8 memory responses [23, 24], and as seen in this study, is well tolerated and safe. This study is the first to show formulations of H5N1 vaccine with either Advax™ formulations provided 100% protection. The fact that the Ad2 formulation induced slightly higher HI titers, less morbidity and earlier viral clearance than Ad1 suggests that there is room for further adjuvant optimization in this model to increase H5N1 vaccine effectiveness even further.
T cell-mediated immunity plays an incompletely defined role in influenza protection [45]. In this study some ferrets vaccinated with one dose of adjuvanted-vaccine or two doses of non-adjuvanted vaccine had no detectable pre-challenge serum neutralizing antibody yet survived lethal challenge, replicating the observations of other studies [10,46]. A possible explanation for this finding is that this vaccine-induced protection was T cell-mediated. Unfortunately, ferret T-cell reagents and assays are currently lacking to construct a more complete picture of the protective immune repertoire in this model [2, 31]. Measures of morbidity following challenge may provide insight into the mechanisms of protection. All surviving recipients of non-adjuvanted vaccine experienced severe weight loss, high fever, anorexia, decreased activity and thrombocytopenia for up to 6 – 10 days after challenge and had histopathologic evidence of marked brain invasion. Thus, any protective effect of the unadjuvanted vaccine appeared to be mediated relatively late after day 5 post-infection, timing that is consistent with the onset of T-cell immunity [47]. In contrast, ferrets receiving adjuvanted vaccine did not experience any major morbidity, And most of the ferrets had no evidence of brain invasion, including the olfactory bulbs [48 and unpublished data]. Prevention of virus spread to the brain had been similarly noted in a study of alum-adjuvanted whole virion H5N1 vaccine [49], consistent with the idea that higher levels of neutralizing antibody induced by adjuvanted vaccine reduced brain invasion through olfactory neurons early in infection [50].
In this initial study, the addition of delta-inulin adjuvant formulations to the split-virion H5N1 vaccine resulted in a marked improvement in ferret mortality and morbidity. Further studies of this novel adjuvant technology are warranted with particular attention to mapping the correlates of vaccine protection at the humoral, cellular and mucosal level and identifying the optimal dose and formulation for influenza protection. Given the limited immunogenicity in humans of the current licensed H5N1 vaccine, new formulations including suitable adjuvants are a high priority for evaluation and addition to the pandemic vaccine stockpile.
Acknowledgements
The project was funded by NIAID contract HHSN 266-200-400-095I, Task Order C-22. The authors would like to thank the program officers, Drs. Martin Crumrine and Rachelle Solomon, of DMID/NIAID/NIH for the discussions and guidance during the preparation of the study designs. We thank the Animal Care and the ABSL3+ staffs, led by Lara Coleman, Sara Lemoine, and Krystle Pacheco. We would also like to thank Thomas Rowe and Dr. David Kelvin for their assistance and direction with the serological assays. The development of Advax™ adjuvant was supported in part by contracts U01AI061142 and HHSN272200800039C from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, Department of Health and Human Services. We would like to thank Farukh Khambaty, Linda Lambert and David Cho, NIAID, for their suggestions and assistance during the development of Advax adjuvant. This paper's contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the National Institute of Allergy and Infectious Diseases.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain
REFERENCES
- 1.Lebarbenchon C, Feare CJ, Renaud F, Thomas F, Gauthier-Clerc M. Persistence of highly pathogenic avian influenza viruses in natural ecosystems. Emerg Infect Dis. 2010;16:1057–1062. doi: 10.3201/eid1607.090389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hampson AW. Ferrets and the challenges of H5N1 vaccine formulation. J Infect Dis. 2006;194:143–145. doi: 10.1086/505229. [DOI] [PubMed] [Google Scholar]
- 3.Stephenson I, Gust I, Kieny MP, Pervikov Y. Development and evaluation of influenza pandemic vaccines. Lancet Infect Dis. 2006;6:71–72. doi: 10.1016/S1473-3099(06)70364-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subbarao K, Joseph T. Scientific barriers to developing vaccines against avian influenza viruses. Nat Rev Immunol. 2007;7:267–278. doi: 10.1038/nri2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saville M, Marsh G, Hoffenbach A. Improving seasonal and pandemic influenza vaccines. Influenza Other Respi Viruses. 2008;2:229–235. doi: 10.1111/j.1750-2659.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palache B. New vaccine approaches for seasonal and pandemic influenza. Vaccine. 2008;26:6232–6236. doi: 10.1016/j.vaccine.2008.07.022. [DOI] [PubMed] [Google Scholar]
- 7.Riley S, Wu JT, Leung GM. Optimizing the Dose of Pre-Pandemic Influenza Vaccines to Reduce the Infection Attack Rate. PLoS Med. 2007;4:e218. doi: 10.1371/journal.pmed.0040218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerhard W. The role of the antibody response in influenza virus infection. Curr Top Microbiol Immunol. 2001;260:171–190. doi: 10.1007/978-3-662-05783-4_9. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann E, Lipatov AS, Webby RJ, Govorkova EA, Webster RG. Role of specific hemagglutinin amino acids in the immunogenicity and protection of H5N1 influenza virus vaccines. Proc Natl Acad Sci U S A. 2005;102:12915–12920. doi: 10.1073/pnas.0506416102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baras Bt, Stittelaar KJ, Simon JH, Thoolen RJMM, Mossman SP, et al. Cross-Protection against Lethal H5N1 Challenge in Ferrets with an Adjuvanted Pandemic Influenza Vaccine. PLoS ONE. 2008;3:e1401. doi: 10.1371/journal.pone.0001401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Middleton D, Rockman S, Pearse M, Barr I, Lowther S, et al. Evaluation of vaccines for H5N1 influenza virus in ferrets reveals the potential for protective single-shot immunization. J Virol. 2009;83:7770–7778. doi: 10.1128/JVI.00241-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ruat C, Caillet C, Bidaut A, Simon J, Osterhaus AD. Vaccination of macaques with adjuvanted formalin-inactivated influenza A virus (H5N1) vaccines: protection against H5N1 challenge without disease enhancement. J Virol. 2008;82:2565–2569. doi: 10.1128/JVI.01928-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treanor JJ, Campbell JD, Zangwill KM, Rowe T, Wolff M. Safety and immunogenicity of an inactivated subvirion influenza A (H5N1) vaccine. N Engl J Med. 2006;354:1343–1351. doi: 10.1056/NEJMoa055778. [DOI] [PubMed] [Google Scholar]
- 14.Chotpitayasunondh T, Thisyakorn U, Pancharoen C, Pepin S, Nougarede N. Safety, humoral and cell mediated immune responses to two formulations of an inactivated, split-virion influenza A/H5N1 vaccine in children. PLoS One. 2008;3:e4028. doi: 10.1371/journal.pone.0004028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zangwill KM, Treanor JJ, Campbell JD, Noah DL, Ryea J. Evaluation of the safety and immunogenicity of a booster (third) dose of inactivated subvirion H5N1 influenza vaccine in humans. J Infect Dis. 2008;197:580–583. doi: 10.1086/526537. [DOI] [PubMed] [Google Scholar]
- 16.Nolan T, Richmond PC, Formica NT, Hoschler K, Skeljo MV, et al. Safety and immunogenicity of a prototype adjuvanted inactivated split-virus influenza A (H5N1) vaccine in infants and children. Vaccine. 2008;26:6383–6391. doi: 10.1016/j.vaccine.2008.08.046. [DOI] [PubMed] [Google Scholar]
- 17.Krewski D, Yokel RA, Nierboer E, Borchelt D, Cohen J, et al. Human health risk assessment for aluminum, aluminum oxide, and aluminum hydroxide. J Toxicol Environ Health B Crit Rev. 2007;10:1–269. doi: 10.1080/10937400701597766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Silva DG, Cooper PD, Petrovsky N. Inulin-derived adjuvants promote both Th1 and Th2 immune responses. Immunol Cell Biol. 2004;82:611–616. doi: 10.1111/j.1440-1711.2004.01290.x. [DOI] [PubMed] [Google Scholar]
- 19.Petrovsky N. Novel human polysaccharide adjuvants with dual Th1 and th2 potentiating activity. Vaccine. 2006;24S2:S2/26–S22/29. doi: 10.1016/j.vaccine.2005.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrovsky N. Freeing vaccine adjuvants from dangerous immunological dogma. Exper Rev Vaccines. 2008;7:7–10. doi: 10.1586/14760584.7.1.7. [DOI] [PubMed] [Google Scholar]
- 21.Cooper PD, Steele EJ. The adjuvanticity of gamma inulin. Immunol Cell Biol. 1988;66:345–352. doi: 10.1038/icb.1988.45. [DOI] [PubMed] [Google Scholar]
- 22.Cooper PD, Petrovsky N. Delta inulin: a novel, immunologically-active, stable packing structure comprising β-D-[2→1]poly(fructo-furanosyl) α-D-glucose polymers. Glycobiology. 2010 doi: 10.1093/glycob/cwq201. pub online 7 Dec 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lobigs M, Pavy M, Hall RA, Lobigs P, Cooper P, Komiya T, Toriniwa H, Petrovsky N. An inactivated Vero cell-grown Japanese encephalitis vaccine formulated with Advax, a novel inulin-based adjuvant, induces protective neutralizing antibody against homologous and heterologous flaviviruses. J Gen Virol. 2010;91:1407–1417. doi: 10.1099/vir.0.019190-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cristillo AD, Grazia Ferrari M, Hudacik L, Lewis B, Galmin L, Bowen B, et al. Induction of mucos al and systemic antibody and T-cell responses following prime-boost immunization with novel adjuvanted human immunodeficiency virus-1-vaccine formulations. J Gen Virol. 2011;92:128–140. doi: 10.1099/vir.0.023242-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Szretter KJ, Balish AL, Katz JM. Influenza: propagation, quantification, and storage Chapter 15. Curr Protoc Microbiol. 2006:11. doi: 10.1002/0471729256.mc15g01s3. Unit 15G. [DOI] [PubMed] [Google Scholar]
- 26.Harmon MW, Rota PA, Walls HH, Kendal AP. Antibody response in humans to influenza virus type B host-cell-derived variants after vaccination with standard (egg-derived) vaccine or natural infection. J Clin Microbiol. 1988;26:333–337. doi: 10.1128/jcm.26.2.333-337.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rowe T, Abernathy RA, Hu-Primmer J, Thompson WW, Lu X, et al. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kayali G, Setterquist SF, Capuano AW, Myers KP, Gill JS, et al. Testing human sera for antibodies against avian influenza viruses: horse RBC hemagglutination inhibition vs. microneutralization assays. J Clin Virol. 2008;43:73–78. doi: 10.1016/j.jcv.2008.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Layton RC, Gigliotti A, Armijo P, Myers L, Knight J, Donart N, et al. Enhanced Immunogenicity, Mortality Protection, and Reduced Viral Brain Invasion by Alum Adjuvant with an H5N1 Split-virion Vaccine in the Ferret. PLoS ONE. 2011;8 doi: 10.1371/journal.pone.0020641. e20641. http://dx.plos.org/10.1371/journal.pone.0020641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maher JA, DeStefano J. The ferret: an animal model to study influenza virus. Lab Anim (NY) 2004;33:50–53. doi: 10.1038/laban1004-50. [DOI] [PubMed] [Google Scholar]
- 31.Tripp RA, Tompkins SM. Animal models for evaluation of influenza vaccines. Curr Top Microbiol Immunol. 2009;333:397–412. doi: 10.1007/978-3-540-92165-3_19. [DOI] [PubMed] [Google Scholar]
- 32.Belser JA, Szretter KJ, Katz JM, Tumpey TM. Use of animal models to understand the pandemic potential of highly pathogenic avian influenza viruses. Adv Virus Res. 2009;73:55–97. doi: 10.1016/S0065-3527(09)73002-7. [DOI] [PubMed] [Google Scholar]
- 33.Maines TR, Chen LM, Matsuoka Y, Chen H, Rowe T, et al. Lack of transmission of H5N1 avian-human reassortant influenza viruses in a ferret model. Proc Natl Acad Sci U S A. 2006;103:12121–12126. doi: 10.1073/pnas.0605134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson KG, Colegate AE, Podda A, Stephenson I, Wood J, et al. Safety and antigenicity of non-adjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a randomised trial of two potential vaccines against H5N1 influenza. Lancet. 2001;357:1937–1943. doi: 10.1016/S0140-6736(00)05066-2. [DOI] [PubMed] [Google Scholar]
- 35.Leroux-Roels I, Borkowski A, Vanwolleghem T, Drame M, Clement F, et al. Antigen sparing and cross-reactive immunity with an adjuvanted rH5N1 prototype pandemic influenza vaccine: a randomised controlled trial. Lancet. 2007;370:580–589. doi: 10.1016/S0140-6736(07)61297-5. [DOI] [PubMed] [Google Scholar]
- 36.Tripp RA, Tompkins SM. Recombinant vaccines for influenza virus. Curr Opin Investig Drugs. 2008;9:836–845. [PubMed] [Google Scholar]
- 37.Atmar RL, Keitel WA. Adjuvants for pandemic influenza vaccines. Curr Top Microbiol Immunol. 2009;333:323–344. doi: 10.1007/978-3-540-92165-3_16. [DOI] [PubMed] [Google Scholar]
- 38.Bresson JL, Perronne C, Launay O, Gerdil C, Saville M, et al. Safety and immunogenicity of an inactivated split-virion influenza A/Vietnam/1194/2004 (H5N1) vaccine: phase I randomised trial. Lancet. 2006;367:1657–1664. doi: 10.1016/S0140-6736(06)68656-X. [DOI] [PubMed] [Google Scholar]
- 39.Keitel WA, Campbell JD, Treanor JJ, Walter EB, Patel SM, et al. Safety and immunogenicity of an inactivated influenza A/H5N1 vaccine given with or without aluminum hydroxide to healthy adults: results of a phase I-II randomized clinical trial. J Infect Dis. 2008;198:1309–1316. doi: 10.1086/592172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brady RC, Treanor JJ, Atmar RL, Keitel WA, Edelman R, et al. Safety and immunogenicity of a subvirion inactivated influenza A/H5N1 vaccine with or without aluminum hydroxide among healthy elderly adults. Vaccine. 2009;27:5091–5095. doi: 10.1016/j.vaccine.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bernstein DI, Edwards KM, Dekker CL, Belshe R, Talbot HK, et al. Effects of adjuvants on the safety and immunogenicity of an avian influenza H5N1 vaccine in adults. J Infect Dis. 2008;197:667–675. doi: 10.1086/527489. [DOI] [PubMed] [Google Scholar]
- 42.Forrest HL, Khalenkov AM, Govorkova EA, Kim J-K, Del Giudice G, Webster RG. Single- and multiple-clade influenza A H5N1 vaccines induce cross protection in ferrets. Vaccine. 2009;27:4187–4195. doi: 10.1016/j.vaccine.2009.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Keitel W, Groth N, Lattanzi M, Praus M, Hilbert AK, et al. Dose ranging of adjuvant and antigen in a cell culture H5N1 influenza vaccine: safety and immunogenicity of a phase 1/2 clinical trial. Vaccine. 2010;28:840–848. doi: 10.1016/j.vaccine.2009.10.019. [DOI] [PubMed] [Google Scholar]
- 44.Clark TW, Pareek M, Hoschler K, Dillon H, Nicholson KG, et al. Trial of 2009 influenza A (H1N1) monovalent MF59-adjuvanted vaccine. New Engl J Med. 2009;361:2424–2435. doi: 10.1056/NEJMoa0907650. [DOI] [PubMed] [Google Scholar]
- 45.Webby RJ, Andreansky S, Stambas J, Rehg JE, Webster RG, et al. Protection and compensation in the influenza virus-specific CD8+ T cell response. Proc Natl Acad Sci U S A. 2003;100:7235–7240. doi: 10.1073/pnas.1232449100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lipatov AS, Hoffmann E, Salomon R, Yen HL, Webster RG. Cross-Protectiveness and Immunogenicity of Influenza A/Duck/Singapore/3/97(H5) Vaccines against Infection with A/Vietnam/1203/04(H5N1) Virus in Ferrets. J Infect Dis. 2006;194:1040–1043. doi: 10.1086/507709. [DOI] [PubMed] [Google Scholar]
- 47.Miao H, Hollenbaugh J, Zand M, Holden-Wiltse J, Mosmann TR, et al. Quantifying the early immune response and adaptive immune response kinetics in mice infected with influenza A virus. J Virol. 2010;84:6687–6698. doi: 10.1128/JVI.00266-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Layton RC, Armijo P, Myers L, Knight J, Donart N, et al. Ferret model of avian influenza demonstrates dose and strain dependant pathology and viral load in brain. Procedia in Vaccinology. 2009;1:30–34. [Google Scholar]
- 49.Govorkova EA, Webby RJ, Humberd J, Seiler JP, Webster RG. Immunization with Reverse Genetics Produced H5N1 Influenza Vaccine Protects Ferrets against Homologous and Heterologous Challenge. J Infect Dis. 2006;194:159–167. doi: 10.1086/505225. [DOI] [PubMed] [Google Scholar]
- 50.Shinya K, Makino A, Hatta M, Watanabe S, Kim JH, et al. Subclinical brain injury caused by H5N1 influenza virus infection. J Virol. 2011;85:5202–5207. doi: 10.1128/JVI.00239-11. [DOI] [PMC free article] [PubMed] [Google Scholar]