Abstract
Myelin associated glycoprotein (Siglec-4) is a myelin adhesion receptor that is well established for its role as an inhibitor of axonal outgrowth in nerve injury, mediated by binding to sialic acid containing ligands on the axonal membrane. Because disruption of myelin-ligand interactions promotes axon outgrowth, we have sought to develop potent ligand based inhibitors using natural ligands as scaffolds. Although natural ligands of MAG are glycolipids terminating in the sequence NeuAcα2–3Galβ1–3(±NeuAcα2–6)GalNAcβ-R, we previously established that synthetic O-linked glycoprotein glycans with the same sequence α-linked to Thr exhibited ~1000 fold increased affinity (~1 µM). Attempts to increase potency by introducing a benzoylamide substituent at C-9 of the α2–3 sialic acid afforded only a 2-fold increase, instead of increases of >100 fold observed for other sialoside ligands of MAG. Surprisingly, however, introduction of a 9-N-fluoro-benzoyl substituent on the α2–6 sialic acid increased affinity 80 fold, resulting in a potent inhibitor with a Kd of 15 nM. Docking this ligand to a model of MAG based on known crystal structures of other siglecs suggests that the Thr positions the glycan such that aryl substitution of the α2–3 sialic acid produces a steric clash with the GalNAc, while attaching an aryl substituent to the other sialic acid positions the substituent near a hydrophobic pocket that accounts to the increase in affinity.
Keywords: Myelin associated glycoprotein, MAG, siglec, Sialic acid, lectin
Myelin associated glycoprotein (MAG, Siglec-4) is a member of the sialic acid immunoglobulin lectin (siglec) family, which bind sialic acid containing glycans as ligands. 8 While most of the 15 human siglecs described to date are expressed on various white blood cells of the immune system, 8 MAG is unique in that it is exclusively expression by glial cells that produce the myelin wrapped around the axons of neuronal cells. It’s function is well established as a cell adhesion protein that is important in maintaining the integrity of the myelin-axon organization, mediated by the interaction of MAG expressed on the innermost leaflet on the myelin membrane, interacting with sialic acid containing gangliosides as ligands on the axon. 9 Gene knockout mice that are missing either MAG or its ganglioside ligands exhibit increased demyelination and axon degeneration in the central and peripheral nervous system, with resulting neuronal deficiencies. 9
In addition to its role in stabilizing axon-myelin interactions, MAG is one of several glial receptors that contribute to inhibition of axon growth following nerve injury. 9–11 Others include neurite outgrowth inhibitor (Nogo) and oligodenrocyte myelin glycoprotein MOgp. These receptors mediate inhibition of the outgrowth of axons in injured nerve by interacting with their respective ligands on the axon. Although MAG is unique in its recognition of axonal gangliosides as ligands, Nogo, MAG and MOgp all interact with the Nogo receptors, NgR1 and NgR2, as ligands. Although there is not consensus on the mode of MAG interaction with NgRs, 9, 12 several reports suggest that the interaction is sialic acid dependent. 13–15
Numerous studies suggest that disruption of the interaction of MAG, Nogo and MOgp with their ligands can release the inhibition of axonal outgrowth and promote nerve regeneration following nerve injury. 9–11 This has been elegantly demonstrated for MAG in in vivo experiments that show improvement of nerve regeneration by administration of sialidase to destroy sialic acid dependent ligands. 16 Demonstration that sialoside ligands can reverse MAG dependent inhibition of axon outgrowth in vitro 17 has suggested the possibility that small molecules of sufficient potency could also promote nerve regeneration in vivo. This has stimulated our interest in the development of inhibitors of MAG based on its natural sialoside ligands. 1–4, 6, 7, 18–23
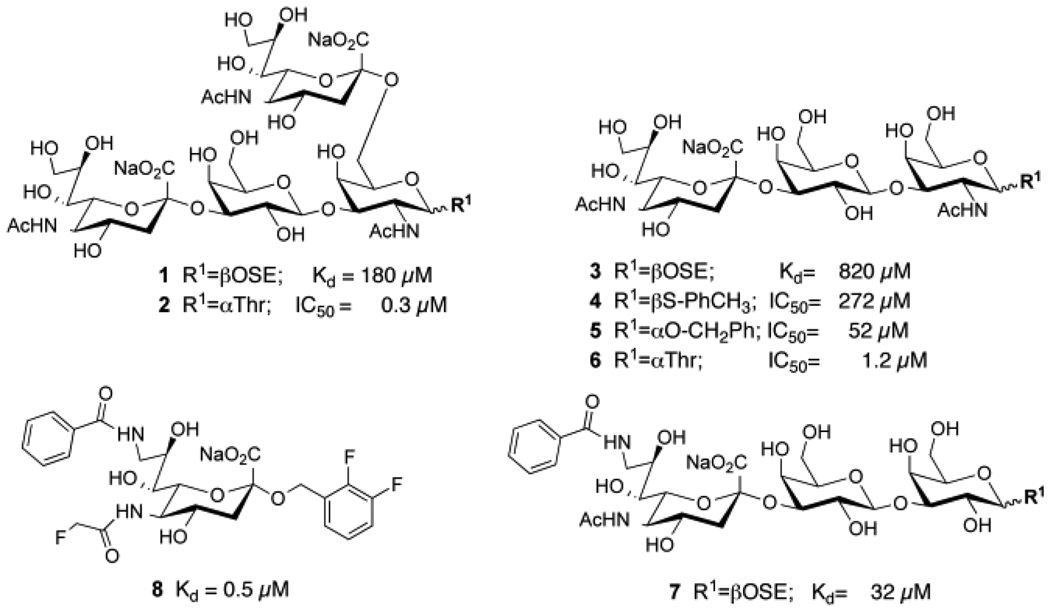
Early investigations into the natural axonal ligands of MAG documented that it binds preferentially to gangliosides that contain the terminal sequence NeuAcα2–3Galβ1–3GalNAcβ-R found in GD1a and GT1b, and the di-sialylated sequence NeuAcα2–3Galβ1–3(NeuAcα2–6)GalNAcβ-R found in rare gangliosides such as GQ1bα. 9, 24 These same sequences are found as O-linked glycans of glycoproteins in α-linkage to threonine. These structures β-linked to synthetic aglycons (1 and 3) exhibited Kd values for MAG of 820 µM and 180 µM, respectively, 2 affinities that are typical of glycan binding proteins that mediate cell-to-cell contact through multivalent binding of receptors on one cell and glycan ligands on the other. 25–28 However, in contrast to the β-glycosides, the same sialoside sequences α-linked to threonine exhibit potencies over 100 fold higher (Figure 1).
Figure 1.
Relative potencies of representative sialoside ligands of MAG. Natural sialoside sequences (1–6) β-linked (1, 3, 4) 1, 2 or α-linked (2, 5, 6) 1 to synthetic aglycons correspond to MAG ligands found in some gangliosides (e.g. GQ1bα) and O-linked glycans of glycoproteins, respectively. Modification of sialic acid with aryl substituents at C-9 and/or C-2 afford significant gains in affinity (7,8) 3–7, 19
Modifications of sialic acids with substituents at the C-9, C-5 and C-2 positions have also been documented to afford substantial increases in affinity (Figure 1). Addition of an aromatic substituent at C-9 typically results in affinity increases of 60–250 fold, even for simple sialosides such as α-methyl-NeuAc, and other linear sialosides such as NeuAcα2–3Galβ1–4GlcNAc. 2–4, 6, 7, 18–23, 29–31 Systematic analysis of the additive gains in affinity with substituents added at the C-9, C-5 and C-2 positions of sialic acid have yielded sialic acid analogs with Kd values of 150–500 nM (e.g. 8), representing affinity gains of over 1000 fold over most natural sialosides (e.g. 1, 3), 1, 6, 7, 18, 19, 31 with the exception of the synthetic O-linked sialosides with an α-Thr aglycon, which already exhibited affinities in this range (2, 6). 1
Since the sialosides α-linked to Thr already exhibited low/sub µM potencies (2 and 6), we sought to achieve significant increased affinity by addition of an aromatic substituent at C-9 of the sialic acid linked α2–3 to Gal. To our surprise, only a modest 2-fold increase in affinity was observed. Thus, the affinity increases afforded by the aromatic substituent at C-9 (7) and the α-Thr aglycon (6) were not additive, a seemingly counter intuitive finding since the substituents were at the non-reducing and reducing ends of the sialoside, respectively.
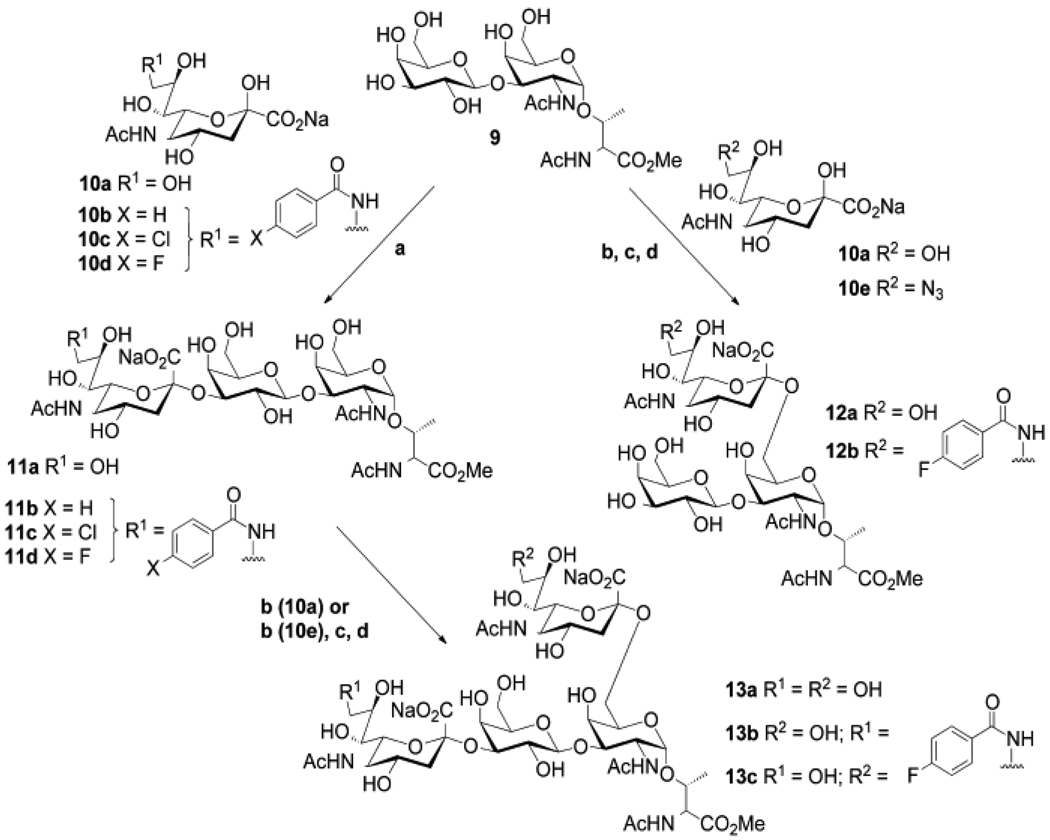
To explore this finding further, we evaluated the effect of adding single ring aromatic substituents selected from our previous studies 3, 6, 19, 22 to each sialic acid of the disialyl-T-antigen (2). As shown in Scheme 1, T-antigen (9) was prepared chemically as previously described, 32 and was enzymatically sialylated with either NeuAc or C-9 substituted analogs of NeuAc using porcine ST3Gal I or chicken/human ST6GalNAc I. For C-9 substituted analogs of the NeuAcα2–3Gal sequence, 9-N substituted NeuAc analogs (10b–d) were activated to the corresponding CMP-NeuAc donor substrate and transferred directly to the acceptor glycan using the porcine ST3Gal I. For C-9 substituted analogs of the NeuAcα2–6GalNAc sequence, 9-azido-NeuAc was activated to CMP-9-azido-NeuAc and transferred to T-antigen (9) or sialyl-T-antigen (11a) using ST6GalNAc I. Once transferred, the 9-azide was reduced to the 9-amine, which was then acylated with the corresponding benzoyl chloride. The strategy of introducing the 9-azido-NeuAc followed by reduction and acylation was used because the chicken ST6GalNAc I did not efficiently transfer the 9-amino or 9-N-substituted sialic acids.
Scheme 1.
Synthesis of the sialosides a) pST3Gal I, buffer, yields 83–94%; b) ChST6GalNAc I, 92%; c) PMe3, CH3OH/THF/H2O, 87%, d), RCOCl, DMF, Et3N, 78–89%
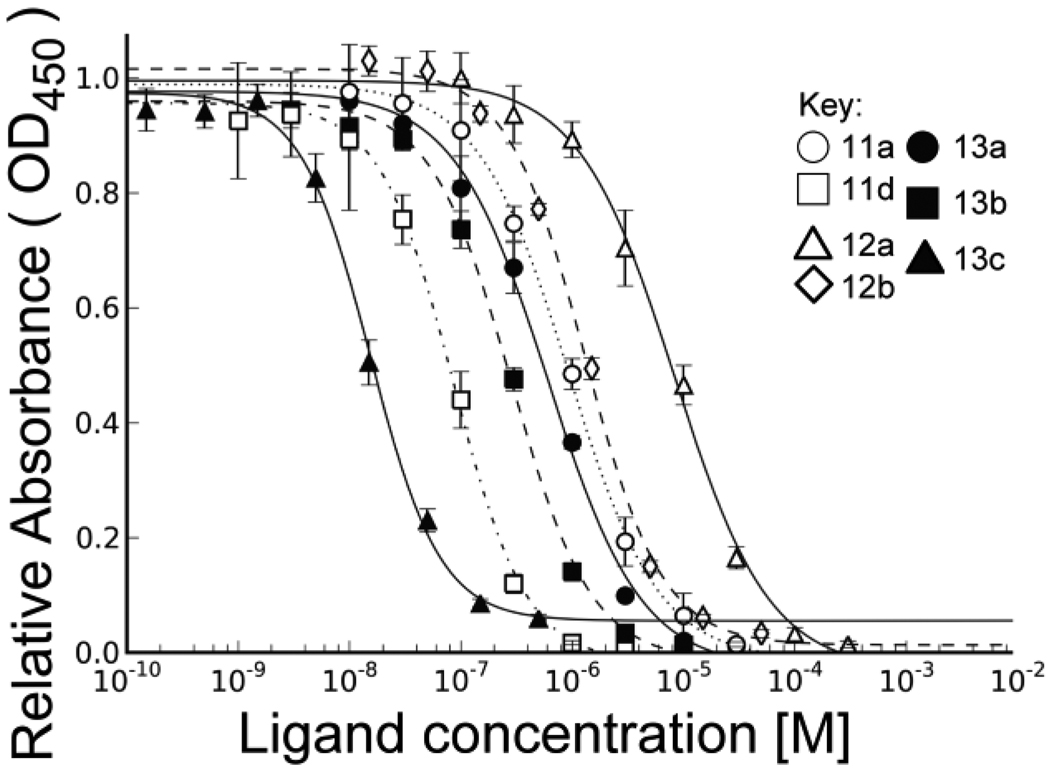
Each of the sialylated products was assessed for their ability to compete the binding of a MAG-Fc chimera to NeuAcα2–3Galβ1–4GlcNAc-R coupled to biotinylated polyacrylamide, which is adsorbed to streptavidin coated 96 well plates (Figure 2). To ensure that day-to-day variation in the assay was not a factor in for comparing their potency, each compound was analyzed three times on separate days, with at least one other compound to ensure that there was no significant ‘drift’ in the assay. The IC50 values in Table 1 represent the mean and standard deviation of the values obtained for the three separate assays. The standard error ranged from 5–15%, demonstrating the reproducibility of the results over course of the analysis of all compounds.
Figure 2.
Analysis of the inhibitory potency of sialoside analogs in MAG binding assay. Each compound is analyzed in triplicate for inhibition of the binding of MAG-Fc chimera to polymeric ligand adsorbed to the wells of 96 well plates as described in Supporting Materials.
Table 1.
Inhibitory potencies of C-9 substituted analogs of sialylated Core 1 O-linked glycans for MAG
| Compounda | R1 (NeuAca2–3) |
R2 (NeuAca2–6) |
IC50b (nM) |
rIPc | Apparent Kdd (µM) |
|---|---|---|---|---|---|
| 11a | HO- | -- | 1200 ± 110 | 1.0 | 1.9 |
| 11b |
|
-- | 650 ± 73 | 1.8 | 1.0 |
| 11c | -- | 610 ± 61 | 1.9 | 1.0 | |
| 11d | -- | 79 ± 12 | 14 | 0.13 | |
| 12a | -- | HO- | 7900 ± 420 | 0.15 | 13 |
| 12b | -- | 640 ± 54 | 1.9 | 1.0 | |
| 12c | -- | 1100 ± 230 | 1.1 | 1.760 | |
| 13a | HO- | HO- | 750 ± 170 | 1.6 | 1.2 |
| 13b | HO- | 300 ± 53 | 4 | 0.48 | |
| 13c | HO- | 9 ± 1 | 129 | 0.015 |
See structures in Scheme 1
IC50 values were determined by competition assays as described in Methods
Relative inhibitory potency (rIP) is based on the IC50 values relative to 11a.
Apparant Kd values are calculated from the IC50 values with reference to the Kd determined for 13a by isothermal titration (Figure 2).
Although the earlier results of ours and others that aryl substituents at C-9 of sialic acid afford ~100 fold increased affinity, 9-N-benzoyl (11b) or 9-N-p-chloro-benzoyl (11c) substituents in the NeuAcα2–3 linked sialic acid of the 3’-sialyl-T antigen revealed affinity increases of only 2 fold. The one exception in this series was 11d with the p-fluoro-benzoyl substituent, which gave a 14 fold increase in affinity with an IC50 of 80 nM. However, with the same substituent on the NeuAcα2–3 linked sialic acid of the disialyl-T-antigen (13b) increased affinity by only 2 fold. In contrast, the p-fluoro-benzoyl substituent on the NeuAcα2–6 sialic acid (13c) resulted in an 80-fold increase in potency, with an IC50 of 9 nM. This represents a potency at least 10 fold higher than any other synthetic ligand of MAG reported to date.
In order to more directly compare the affinities of these synthetic ligands with others reported by us and other groups using various related assays, we determined the Kd of the disialyl-T antigen (13a) by isothermal titration calorimetry. This gave a Kd value of 1.2 µM, in good agreement with the IC50 of 0.75 ± 0.17 µM (see Supplementary material). Using this as a reference, we have calculated the Kd apparent for each of the compounds assuming that the IC50 values are proportional (Table 1).
The apparent Kd of the most potent compound 13c is 15 nM, representing at least 10,000 fold higher than the native structure 1. This is remarkable since 13c is identical except for αThr as the aglycon, and the 9-N-p-fluoro-benzoyl substituent on the sialic acid linked α2–6 to GalNAc. To better understand the basis for these dramatic affinity increases, we investigated the docking of this ligand to a homology model of MAG that is based on existing crystal structures of several siglecs.
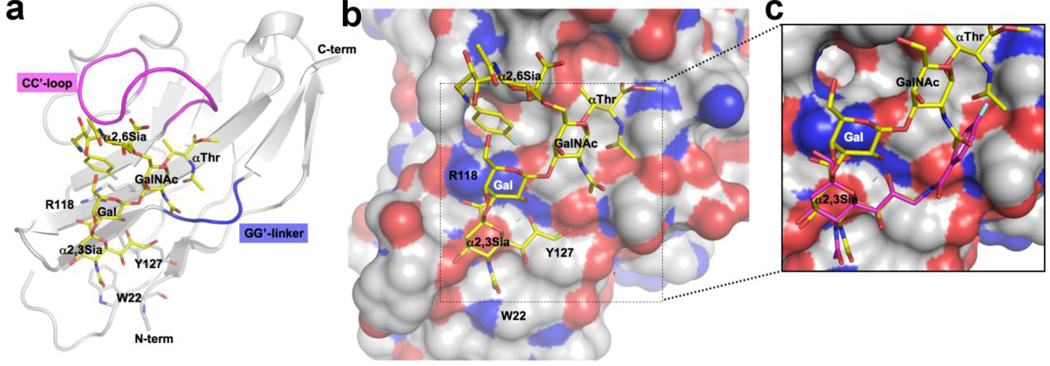
A homology model of the carbohydrate binding domain of MAG was constructed based on the available crystal structures determined for sialoadhesin in complex with a 9-N-biphenyl-carbonyl-NeuAcαCH3, siglec-5 in complex with α2,3-sialylated lactose and siglec-7 in complex with an α2,8 linked ganglioside (See Figure S3). Distinct structural features were picked based on careful analysis of the X-ray data in combination with structural sequence alignment and homology modeling tools to enable the interpretation of the binding data. A modular approach was used, considering the unique features of MAG relative to the other siglecs. Although the backbone structure surrounding the conserved sialoside binding site is assumed to be the same for MAG as for the available template structures, we considered the CC’-loop and the GG’-linker as critical variable elements of the homology model. 33 Accordingly, we used the sialoadhesion structure as the basis for the backbone and essential parts of the sialic acid recognition site. This included the position of the essential arginine for the salt bridge with the carboxyl of the Neu5Ac, the 5’ site for the N-acetyl group, Trp29 stabilizing the glycerol side chain and the positioning of the C-9 aryl substituents. Sialoadhesin was also used as a template for the GG’-linker, a crucial determinant of the hydrophobic pocket at the C-9 position. This represents a conservative assumption since 9-aryl analogs of α–methyl-NeuAc are documented to dramatically increase affinity for both sialoadhesin and MAG. 29 The second determinant of the carbohydrate recognition is the CC’-loop, which is known to vary in siglecs, and influence receptor specificity. 33, 34 Siglec-5 and -7 have the same number of amino acids in the CC’-loop as MAG and were therefore served as templates.
Homology models using these criteria were further subjected to an automated docking of known 9’substituted trisaccharide inhibitors of MAG (see Supplementary material). When bound ligands violated the conserved features of sialic acid recognition at the C-5 N-acetyl, C7–C9 polyhydroxy side chain or C-1 carboxylic acid sites, the model was excluded from further consideration. The final model comprises all features of siglec/sialic acid recognition conserved in available structures of sialoadhesion, siglec-5 and -7 (Figure 3).
Figure 3.
Proposed binding of sialyl-T antigen analogs to MAG. a) Homology model of MAG constructed from the solved structures siglecs-1, -5 and -7 depicting the binding of an analog of disialyl-T antigen (13c). b) Model highlighting the spatial relationship of the α2–3 sialic acid bound to the conserved arginine (R118), the αThr and the 9-N-fluoro-benzolamide on the α2–6 sialic acid (see Supplementary material for analysis of glycan torsion angles; Figure S1). c) Inset showing the steric clash of the 9-N-fluoro-benzolamide on the α2–3 sialic acid with the N-acetyl groups of the GalNAc and αThr.
The model accommodated sialosides with α2,3 or α2,6 linked sialic acids (e.g. 3’- or 6’- sialyllactose), consistent with the binding of both linkage types to MAG. 1, 6 Di-sialylated ligands such as disialyl-T-antigen (2) invoked the question whether the α2,3 or α2,6 linked sialic acid occupied the conserved site. Taking into account the significant gain in affinity when substituting the reducing end with αThr, we assumed there would be an interaction of this group with the protein. Accordingly, we performed docking of the corresponding monosialylated derivatives 11a and 12a to investigate the position of the αThr with the sialic acid bound to the conserved site. With the α2,6-linked Neu5Ac of 12a fixed to the conserved site, there was no interaction of αThr with the protein. In contrast, positioning the α2,3-linked sialic acid of 11a in the conserved site allows the αThr to interact favorably with the protein. In the model, the αThr is harbored in a shallow grove formed by the CC’-loop and the G’-strand. This pose anchors the reducing end GalNAc with the N-acetyl group pointing towards the GG’-linker (Figure 3). This directs the N-acetyl methyl group into the strongly favored hydrophobic site accessed by C-9 substituents, a site well described for other siglecs. 30 The placement of the N-acetyl group in this hydrophobic site then imposes steric restrictions for the substitution of the α2–3 sialic acid glycerol chain in the C-9 position which would occupy the same site (Figure 3c). This model is entirely consistent with the observation that introducing an aryl substituent at C-9 enhances affinity of α-methyl-NeuAc, but does not enhance the affinity of the sialyl-T antigens (11a and 13a), which are anchored by the αThr. The 14-fold increase in affinity observed for the 9-N-para-flurobenzyl substituent of the α2,3-linked Neu5Ac (11d) can be accommodated by the successful competition with the GalNAc and αThr N-acetyl groups pointing into the same space.
With the glycan anchored by the α2–3 linked sialic acid at one end, and the αThr at the other, the α2–6 linked sialic acid has a minimal interaction with the protein. Although alternative positioning of the CC’ loop might provide such interactions, in the absence of evidence that such interactions exist, they were not further explored. However, the 9-N-p-fluorobenzyl substituent that affords 80-fold increase in affinity (13c) is in position to engage in protein contacts. In this model, the gain of affinity is attributed to a stacking interaction of the para-fluorobenzyl moiety with the conserved arginine (R118) (Figure 3a,b; and Figure SZ). This is a known mode of interaction of fluoro-aryl inhibitors to proteins as described for the binding of type 2 statins to HMG CoA reductase (see also Figure S2). 35 As an alternative, the fluorobenzyl moiety could interact with a hydrophobic pocket seen in Figure 3 below the phenyl ring, previously suggested to account for the increased affinity of aryl substituents attached at C-6 of the GalNAc in place of sialic acid. 4 However, we believe that stacking interaction of the fluorobenzyl with R118 better accounts for the high affinity observed.
We previously reported that the disialyl-T antigen (11a) exhibited activity for reversing MAG inhibition of neurite outgrowth in an in vitro cell culture model. 17 However, it is not optimal for in vivo studies due to its relatively low (µM) potency, and the rapid clearance of small oligosaccharides from the blood. Since 13c exhibits significantly higher potency (Kd=15 nM), and has increased hydrophobicity due to the 9-aryl substituent, it will be of interest to determine if it has suitable phamacokinetic properties to evaluate its ability to promote axonal outgrowth in animal models of nerve injury. 16 This would provide an important proof of concept for the use of small molecule inhibitors of MAG for treatment of nerve injury. Longer term, however, we believe that the approach of minimizing the structural complexity of such inhibitors is ultimately the best route to obtaining pharmaceutically acceptable inhibitors. The detailed understanding of the basis for the potent inhibitory potency of 13c may aid in the rational design of such sialoside mimic inhibitors.
Supplementary Material
Acknowledgments
The authors thank Ola Blixt, Tasneem Islam, and Karin Norgard Sumnicht for discussions and preliminary experiments on the nature of O-linked glycans as inhibitors of MAG, and Anna Crie with help in preparation of the manuscript and Figures. These studies were supported by NIH grant GM60928 (JCP), EMBO fellowship (CR) and Swiss National Science Foundation (project 200020-120628 (BE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Supplementary Material may be found online.
References and notes
- 1.Blixt O, Collins BE, van den Nieuwenhof IM, Crocker PR, Paulson JC. J Biol Chem. 2003;278:31007. doi: 10.1074/jbc.M304331200. [DOI] [PubMed] [Google Scholar]
- 2.Shin SY, Gathje H, Schwardt O, Gao GP, Ernst B, Kelm S, Meyer B. Chembiochem. 2008;9:2946. doi: 10.1002/cbic.200800485. [DOI] [PubMed] [Google Scholar]
- 3.Mesch S, Lemme K, Koliwer-Brandl H, Strasser DS, Schwardt O, Kelm S, Ernst B. Carbohydr Res. 2010;345:1348. doi: 10.1016/j.carres.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 4.Schwardt O, Gathje H, Vedani A, Mesch S, Gao GP, Spreafico M, von Orelli J, Kelm S, Ernst B. J Med Chem. 2009;52:989. doi: 10.1021/jm801058n. [DOI] [PubMed] [Google Scholar]
- 5.Kelm S, Brossmer R, Isecke R, Gross HJ, Strenge K, Schauer R. Eur J Biochem. 1998;255:663. doi: 10.1046/j.1432-1327.1998.2550663.x. [DOI] [PubMed] [Google Scholar]
- 6.Blixt O, Han S, Liao L, Zeng Y, Hoffmann J, Futakawa S, Paulson JC. J Am Chem Soc. 2008;130:6680. doi: 10.1021/ja801052g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwardt O, Koliwer-Brandl H, Zimmerli R, Mesch S, Rossato G, Spreafico M, Vedani A, Kelm S, Ernst B. Bioorg Med Chem. 2010;18:7239. doi: 10.1016/j.bmc.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 8.Crocker PR, Paulson JC, Varki A. Nat Rev Immunol. 2007;7:255. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 9.Schnaar RL, Lopez PH. J Neurosci Res. 2009;87:3267. doi: 10.1002/jnr.21992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zorner B, Schwab ME. Ann N Y Acad Sci. 2010;1198 Suppl 1:E22. doi: 10.1111/j.1749-6632.2010.05566.x. [DOI] [PubMed] [Google Scholar]
- 11.Cao Z, Gao Y, Deng K, Williams G, Doherty P, Walsh FS. Mol Cell Neurosci. 2010;43:1. doi: 10.1016/j.mcn.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 12.Worter V, Schweigreiter R, Kinzel B, Mueller M, Barske C, Bock G, Frentzel S, Bandtlow CE. PLoS One. 2009;4:e5218. doi: 10.1371/journal.pone.0005218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robak LA, Venkatesh K, Lee H, Raiker SJ, Duan Y, Lee-Osbourne J, Hofer T, Mage RG, Rader C, Giger RJ. J Neurosci. 2009;29:5768. doi: 10.1523/JNEUROSCI.4935-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Venkatesh K, Chivatakarn O, Lee H, Joshi PS, Kantor DB, Newman BA, Mage R, Rader C, Giger RJ. J Neurosci. 2005;25:808. doi: 10.1523/JNEUROSCI.4464-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strenge K, Schauer R, Kelm S. FEBS Lett. 1999;444:59. doi: 10.1016/s0014-5793(99)00029-0. [DOI] [PubMed] [Google Scholar]
- 16.Mountney A, Zahner MR, Lorenzini I, Oudega M, Schramm LP, Schnaar RL. Proc Natl Acad Sci U S A. 2010;107:11561. doi: 10.1073/pnas.1006683107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyas AA, Blixt O, Paulson JC, Schnaar RL. J Biol Chem. 2005;280:16305. doi: 10.1074/jbc.M500250200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shelke SV, Cutting B, Jiang X, Koliwer-Brandl H, Strasser DS, Schwardt O, Kelm S, Ernst B. Angew Chem Int Ed Engl. 2010;49:5721. doi: 10.1002/anie.200907254. [DOI] [PubMed] [Google Scholar]
- 19.Mesch S, Moser D, Strasser DS, Kelm A, Cutting B, Rossato G, Vedani A, Koliwer-Brandl H, Wittwer M, Rabbani S, Schwardt O, Kelm S, Ernst B. J Med Chem. 2010;53:1597. doi: 10.1021/jm901517k. [DOI] [PubMed] [Google Scholar]
- 20.Bhunia A, Schwardt O, Gathje H, Gao GP, Kelm S, Benie AJ, Hricovini M, Peters T, Ernst B. Chembiochem. 2008;9:2941. doi: 10.1002/cbic.200800458. [DOI] [PubMed] [Google Scholar]
- 21.Shelke SV, Gao GP, Mesch S, Gathje H, Kelm S, Schwardt O, Ernst B. Bioorg Med Chem. 2007;15:4951. doi: 10.1016/j.bmc.2007.04.038. [DOI] [PubMed] [Google Scholar]
- 22.Gao G, Smiesko M, Schwardt O, Gathje H, Kelm S, Vedani A, Ernst B. Bioorg Med Chem. 2007;15:7459. doi: 10.1016/j.bmc.2007.07.033. [DOI] [PubMed] [Google Scholar]
- 23.Schwizer D, Gathje H, Kelm S, Porro M, Schwardt O, Ernst B. Bioorg Med Chem. 2006;14:4944. doi: 10.1016/j.bmc.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 24.Collins BE, Yang LJ, Mukhopadhyay G, Filbin MT, Kiso M, Hasegawa A, Schnaar RL. J Biol Chem. 1997;272:1248. doi: 10.1074/jbc.272.2.1248. [DOI] [PubMed] [Google Scholar]
- 25.Cairo CW, Gestwicki JE, Kanai M, Kiessling LL. J Am Chem Soc. 2002;124:1615. doi: 10.1021/ja016727k. [DOI] [PubMed] [Google Scholar]
- 26.Gestwicki JE, Cairo CW, Strong LE, Oetjen KA, Kiessling LL. J Am Chem Soc. 2002;124:14922. doi: 10.1021/ja027184x. [DOI] [PubMed] [Google Scholar]
- 27.Collins BE, Paulson JC. Curr Opin Chem Biol. 2004;8:617. doi: 10.1016/j.cbpa.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Lee RT, Ichikawa Y, Kawasaki T, Drickamer K, Lee YC. Arch Biochem Biophys. 1992;299:129. doi: 10.1016/0003-9861(92)90254-t. [DOI] [PubMed] [Google Scholar]
- 29.Zaccai NR, Maenaka K, Maenaka T, Crocker PR, Brossmer R, Kelm S, Jones EY. Structure. 2003;11:557. doi: 10.1016/s0969-2126(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 30.Zaccai NR, May AP, Robinson RC, Burtnick LD, Crocker PR, Brossmer R, Kelm S, Jones EY. J Mol Biol. 2007;365:1469. doi: 10.1016/j.jmb.2006.10.084. [DOI] [PubMed] [Google Scholar]
- 31.Abdu-Allah HH, Watanabe K, Completo GC, Sadagopan M, Hayashizaki K, Takaku C, Tamanaka T, Takematsu H, Kozutsumi Y, Paulson JC, Tsubata T, Ando H, Ishida H, Kiso M. Bioorg Med Chem. 2011;19:1966. doi: 10.1016/j.bmc.2011.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blixt O, Allin K, Pereira L, Datta A, Paulson JC. J Am Chem Soc. 2002;124:5739. doi: 10.1021/ja017881+. [DOI] [PubMed] [Google Scholar]
- 33.Zhuravleva MA, Trandem K, Sun PD. J Mol Biol. 2008;375:437. doi: 10.1016/j.jmb.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yamaji T, Teranishi T, Alphey MS, Crocker PR, Hashimoto Y. J Biol Chem. 2002;277:6324. doi: 10.1074/jbc.M110146200. [DOI] [PubMed] [Google Scholar]
- 35.Istvan ES, Deisenhofer J. Science. 2001;292:1160. doi: 10.1126/science.1059344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.