Abstract
Neuroinflammation mediated by microglia is a pathological hallmark of many CNS disorders. Cell lines derived from inbred C57Bl/6 and outbred ICR/CD1 mice (BV2 and N9 respectively), are often used to study microglial inflammatory activities. Although many studies demonstrate different responses of these cell lines to the same stimulus, no comparisons have been done in vivo. Because inbreeding reduces resistance to pathogens and parasites, we hypothesized that microglia from outbred ICR/CD1 mice would have a stronger response to centrally administered LPS than microglia from inbred C57Bl/6 mice. The evaluation of gene expression in freshly-isolated CD11b+ cells from brain revealed that microglia from ICR/CD1 mice were more pro-inflammatory than those from C57Bl/6 mice, although these differences did not appear to result from alterations in the expression levels of the LPS receptors TLR4 or CD14. Notably, the timing of inflammatory gene expression did not correlate with CD11b+ cell proliferation/infiltration. The highest expression of TNFα, IL-6 and iNOS occurred 3 hours after LPS injection when the number of CD11b+ cells was not changed. Whereas the expression of these pro-inflammatory genes had returned to basal by 48 hours when the highest number of CD11b+ cells in the brain was found, the expression of the anti-inflammatory cytokine IL-10 was still significantly up-regulated. This is important because the increased presence of CD11b+ cells in the CNS is often used as an indicator of neuroinflammation. While LPS did not affect the expression of the growth factors VEGF or BDNF, we observed that mechanical injury (caused by intraparenchymal injection) induced distinct patterns of microglial activation characterized by increased expression of VEGF and down-regulation of BDNF. It remains to be determined which type of microglia is more beneficial/detrimental to the CNS, but our data suggest that genetic traits determining microglial properties may have profound effect on many CNS pathologies.
Keywords: LPS, microglia, inflammation, growth factors, strain differences
In recent years, the active role of the immune system, and particularly microglia, has been increasingly recognized in many CNS disorders. Neuroinflammation is a hallmark feature of chronic neurodegenerative diseases, stroke, ischemia, traumatic injury, chronic pain and epilepsy. Microglia, CNS resident innate immune cells of myeloid origin, are the primary mediators of inflammation here. Microglial inflammatory responses include migration, proliferation and release of soluble factors (Kreutzberg 1996) including cytokines, chemokines and growth factors that not only propagate immune responses, but are also involved in repair functions (Graeber 2010; Kreutzberg 1996). However, the specific role of these cells in pathology is controversial since their activities can be both neuroprotective and injurious. Studies employing techniques to alter or ablate microglial activities revealed that absent or inadequate microglial responses often worsen damage after an acute neuronal insult or during neurodegenerative pathology (Skripuletz et al. 2008; Turrin and Rivest 2006). On the other hand, disregulation or overproduction of pro-inflammatory cytokines may create a chronic inflammatory environment and promote neuronal injury and death, exacerbating existing brain pathology.
Many studies suggest that genes regulating inflammatory responses of immune cells may profoundly affect CNS pathologies. For example, the susceptibility to multiple sclerosis (MS) or experimental allergic encephalomyelitis, an animal model of MS, is significantly affected by HLA and MHC II genes expressed on monocytes in humans and rodents, respectively (Kalman and Lublin 1999; Muhallab et al. 2005; Storch et al. 2006). Strain-dependent differences in central immune responses have been reported for MPTP-induced neurotoxicity (Hamre et al. 1999), autoimmune encephalomyelitis (Maron et al. 1999), focal cerebral ischemia (Lambertsen et al. 2002) and others.
Studies have also shown strain-dependent differences in microglial inflammatory properties in vitro either in cell lines or primary microglial cells (McLaughlin et al. 2006; Nickles et al. 2008; Wei and Lin 2009). A comparison of the vast literature on BV-2 and N9 cells, the most commonly used microglial cell lines, suggests dissimilarities in their immune responsiveness (Baker et al. 2004; Fleisher-Berkovich et al. 2010; Horigome et al. 1994; Susaki et al. 1996; Zhang et al. 2003). One can argue that differential responses of BV-2 and N9 microglia may be due to different immortalization techniques used to derive these cell lines (Blasi et al. 1990; Righi et al. 1989). BV2 and N9 cells were immortalized using different retroviruses, and the microglia used for immortalization were from primary cultures prepared from different brain regions at different stages of CNS development. The BV-2 cell line was generated by immortalization of primary microglial cells derived from newborn brains of C57Bl/6 mice by infection with the J2 retrovirus carrying the v-raf and v-myc oncogenes (Blasi et al. 1990), whereas N9 cells were generated by immortalization of embryonic (E12–13) primary cultures derived from the ventral mesencephalon and cerebral cortex of ICR mice with a retrovirus carrying the v-myc and the v-mil oncogenes of the avian retrovirus MH2 (Righi et al. 1989). However, also important, is that these two microglial cell lines were derived from mice of different genetic backgrounds: inbred C57Bl/6 and outbred ICR/CD1. Inbred mice are often used in research for their homogeneity, the generation of transgenic animals and susceptibility to many disorders. However, inbreeding is associated with profound effects on the immune system reducing immunocompetence and resistance to pathogens and parasites (Hofer et al. 2010; Ilmonen et al. 2008).
Despite differences observed between N9 and BV-2 microglial cell lines, no comparisons have been made in microglial responsiveness in vivo in C57Bl/6 and ICR/CD1 mice, or between primary microglial cultures derived from these animals. We hypothesized that microglia from outbred ICR/CD1 mice will mount a stronger immune response to centrally administered LPS than microglia from inbred C57Bl/6 mice. To address this hypothesis, we investigated in vivo microglial activities of these two mouse strains in response to centrally administered LPS. Moreover, we have compared LPS-induced production of pro-inflammatory mediators in BV-2 and N9 cells with primary microglial cultures derived from their respective mouse strains. We found that microglia from ICR/CD1 mice mounted a stronger pro-inflammatory response to LPS than microglia from C57Bl/6 mice regardless of whether the studies were done in vivo or in vitro. These results support the idea that the genetic predisposition of microglia may have a profound effect on many CNS disorders, and may contribute to individual variations/susceptibilities in the population. However, more studies are needed to identify genes that regulate microglial inflammatory responses which could be eventually targeted to enhance beneficial microglial activities and suppress those that are detrimental to the CNS.
MATERIALS AND METHODS
Animals
ICR/CD1 and C57Bl/6 male 12-week old mice or pregnant females were purchased from Charles River (Wilmington, MA, USA) and housed under standard conditions. All experiments were conducted in ALAAAC accredited facilities under protocols approved by the University of Wisconsin Institutional Animal Care and Use Committee.
Primary microglial cells
Primary microglial cells were prepared as we described previously (Nikodemova et al. 2007). Briefly, brains from 3–5 day old mice were dissected and dissociated with 0.25% trypsin supplemented with EDTA followed by trituration with a Pasteur pipette until a single cell suspension was obtained. Cells were resuspended in DMEM supplemented with 10% FBS and 100 units/ml penicillin/streptomycin, and plated in 80 mm2 cell culture flasks. After 10–12 days, flasks were gently shaken for one hour, medium was harvested and centrifuged for 10 min to collect microglia. Cells were plated on a 96-well plate at the density of 75,000 cells/well. Microglia were treated the following day with LPS (1 μg/ml) for 24 hours. Media were collected and assayed for TNFα, IL-10 and nitrite presence.
BV2 and N9 cells
BV-2 and N9 cells were cultured in the same media as used for primary cultures. Cells were plated at the density of 120,000 cells/well on a 12-well plate. Cells were treated the following day in the same manner as primary microglial cultures with LPS (1 μg/ml) for 24 hours. Media were collected and assayed for TNFα and nitrite presence.
LPS injections and microglia isolation
For this part of the study 12-week old male mice were used. Bilateral, intraparenchymal injections with lipopolysaccharide (2 μg LPS/side) were performed at final stereotaxic coordinates of 0.5 mm rostral of bregma, 1 mm right/left of midline and 3.0 mm ventral. To minimize injury from injection we used a 26s Hamilton syringe with a sharp needle (Hamilton, Nevada). Control animals received the same volume of vehicle (PBS). Mice were euthanized 3, 18 or 48 hours after injection followed by perfusion with ice-cold PBS. Brains were dissected out and immediately used for microglial isolation.
CD11b+ cells were isolated from the CNS as we have described previously (Crain et al. 2009). Briefly: after perfusion with PBS, whole brains were removed, weighed and dissociated into a single cell suspension using the Neural Tissue Dissociation Kit according to the manufacturer’s protocol (Miltenyi Biotec, Germany). Cells were resuspended in 0.9 M sucrose followed by centrifugation at 850g for 10 min to remove myelin. After washing in HBSS, cells were resuspended in IMAG buffer (PBS supplemented with 0.5% BSA and 2 mM EDTA) and immunostained with PE-conjugated anti-CD11b antibodies followed by incubation with anti-PE secondary antibodies conjugated to magnetic beads. CD11b+ cells were separated using Miltenyi MS columns placed in a magnetic field according to the manufacturer’s protocol. This method yields a CD11b+ cell population with a purity of >95% as determined by flow cytometry (Crain et al. 2009). Cells were counted and used immediately for total RNA isolation using TriReagent (Sigma-Aldrich, St. Louis, MO, USA) according to the manufacturer’s protocol.
Quantitative RT-PCR (qRT-PCR)
cDNA was synthesized using 0.5 μg total RNA and MMLV reverse transcriptase (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocol, in a total volume of 20 μl. The reaction product was diluted to 80 μl with nuclease-free water. Quantitative PCR was done using 3 μl of cDNA reaction and Power SYBR Green (Applied Biosystems, Warrington, UK). All samples were amplified in duplicates and gene expression was normalized to 18S. Primer sequences for genes evaluated in this study are presented in Table 1.
Table 1.
| Forward primer | Reverse primer | |
|---|---|---|
| 18S | 5′-CGG GTG CTC TTA GCT GAG TGT CCC G-3′ | 5′-CTC GGG CCT GCT TTG AAC AC-3′ |
| BDNF | 5′- CAC TGA GCA AAG CCG AAC TTC -3′ | 5′- AAT GTG GCT TTG CTG TCC TGG -3′ |
| CD14 | 5′- GCC AAA TTG GTC GAA CAA GC-3′ | 5′- CCA TGG TCG GTA GAT TCT GAA AGT |
| IL-6 | 5′- ACT TCC ATC CAG TTG CCT TC -3′ | 5′- GTC TCC TCT CCG GAC TTG TG -3′ |
| IL-10 | 5′- CCT GGG TGA GAA GCT GAA GA-3′ | 5′- TTT TCA CAG GGG AGA AAT CG-3′ |
| iNOS | 5′- TGA CGC TCG GAA CTG TAG CAC | 5′- TGA TGG CCG ACC TGA TGT T-3′ |
| TNFα | 5′- TGT AGC CCA VGT VGT AGC AA-3′ | 5′- AGG TAC AAC CCA TCG GCT GG-3′ |
| TLR4 | 5′- CGA GGC TTT TCC ATC CAA TA-3′ | 5′- AGG CAG CAG GTG GAA TTG TAT-3′ |
| VEGF | 5′- TTG AGA CCC TGG TGG ACA TCT-3′ | 5′- CAC ACA GGA CGG CTT GAA GA-3′ |
TNFα ELISA
Protein levels of TNFα released by microglial cells into the culture media were assayed by ELISA (TNFα DuoSet Elisa, R&D Systems) according to the manufacturer’s protocols. TNFα standards were prepared in range 60–2000 pg/ml. Cytokines were assayed in 100 μl of culture media harvested 24 hours after LPS treatment.
Measurement of NO production
Nitric oxide production was assessed indirectly by measurement of nitrite, a stable by-product of NO generation, in culture media. Nitrite levels were assayed in 100 μl culture media using the Greiss reagent as we described previously (Nikodemova et al. 2006; Watters et al. 2002).
Statistical analysis
Data are expressed as mean ± SEM. All experiments were repeated at least 3 times with multiple n in each experiment. Data obtained by qRT-PCR are expressed as fold change over vehicle at 3h (set to 1) for each mouse strain. Data were analyzed using SigmaStat software. Student’s t-test and one-way ANOVA were used for two group or multiple group comparisons, respectively. Differences were considered statistically significant at p<0.05.
RESULTS
Strain differences in inflammatory responses in primary microglia and cell lines
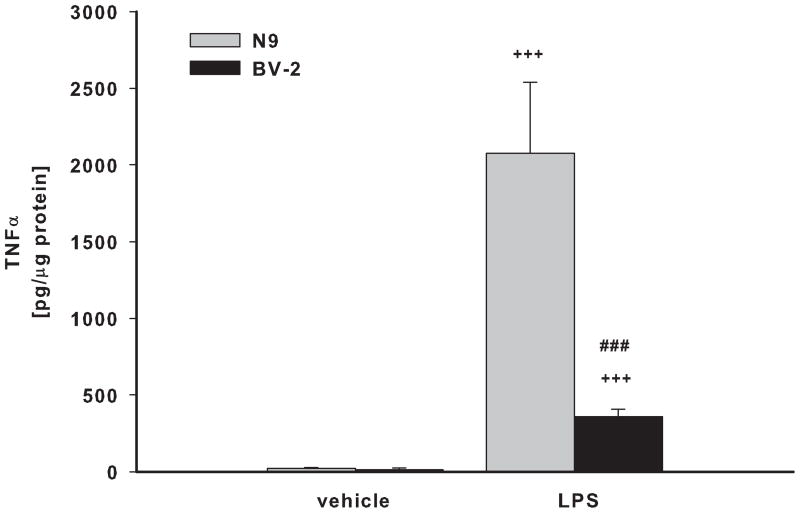
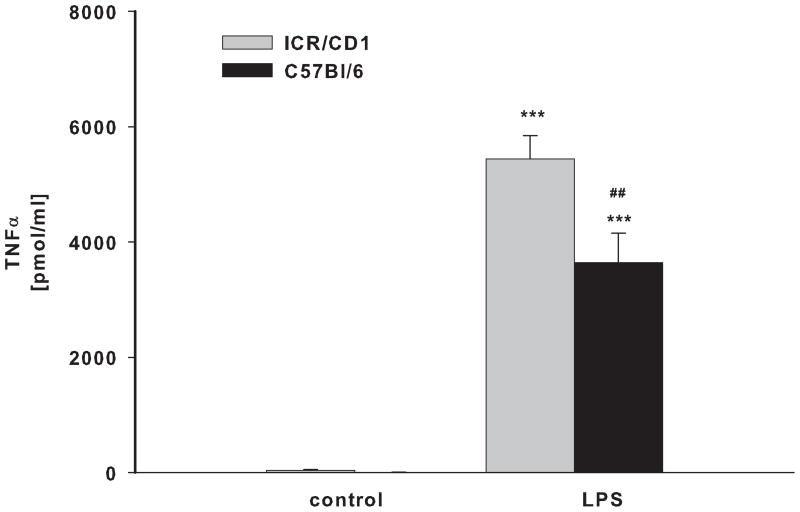
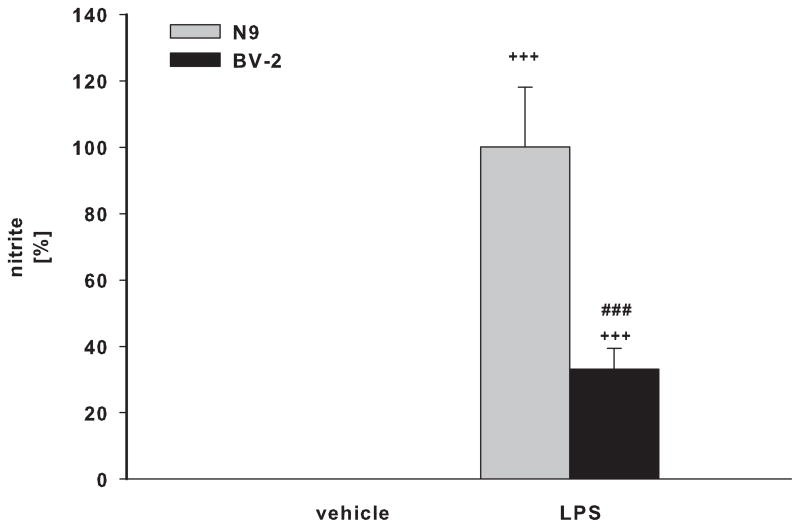
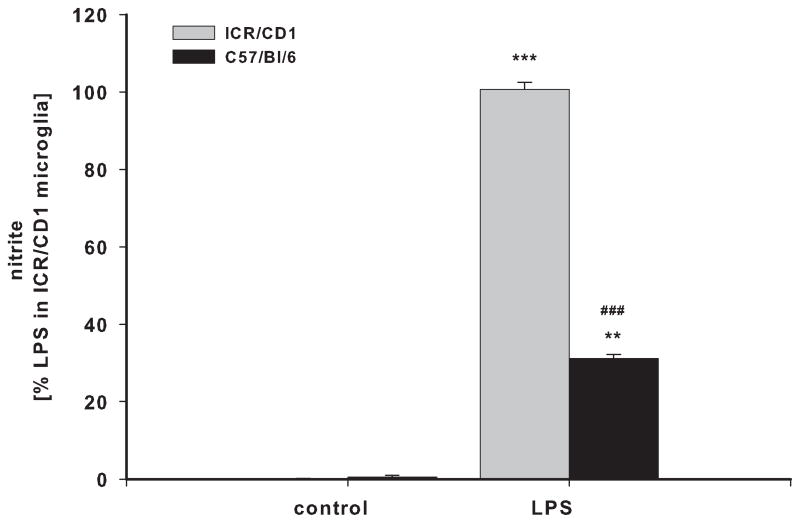
BV-2 and N9 cells, and primary microglia from ICR/CD1 and C57Bl/6 mice were exposed to LPS for 24 hours. Media was collected and assayed for the presence of TNFα (Fig 1A, B) and nitric oxide (Fig. 1C, D). N9 cells produced 5.8 and 2.9 times more TNFα and NO than BV-2 cells, respectively (Fig 1A, C). Primary microglia from ICR/CD1 mice released 1.7 and 3.2 times more TNFα (Fig 1B) and NO (Fig 1D) than microglia from C57Bl/6 mice, respectively. Our data show that ICR/CD1 primary microglia and N9 cells derived from ICR/CD1 mice mount a stronger pro-inflammatory response to LPS than C57Bl/6 microglia and BV-2 cells. These data demonstrate that: a) genetic factors may play an important role in the magnitude of microglial responses, and b) the responsiveness to LPS of immortalized and primary microglial cells derived from the same mouse strain are similar.
Fig 1. TNFα and nitric oxide production in microglial cultures.
BV2 and N9 cell lines (A, C) and primary microglia (B, D) derived from C57Bl/6 and ICR/CD1 brains were stimulated with LPS (1 μg/ml) for 24 hours. Culture media were collected and assayed for the presence of TNFα (A, B) and nitric oxide (C, D). n=4–9; **p<0.01 vs vehicle, ***p<0.001 vs vehicle; ##p<0.01 between strains, ###p<0.001 between strains
The number of CD11b+ cells increases in ICR/CD1 brains after LPS
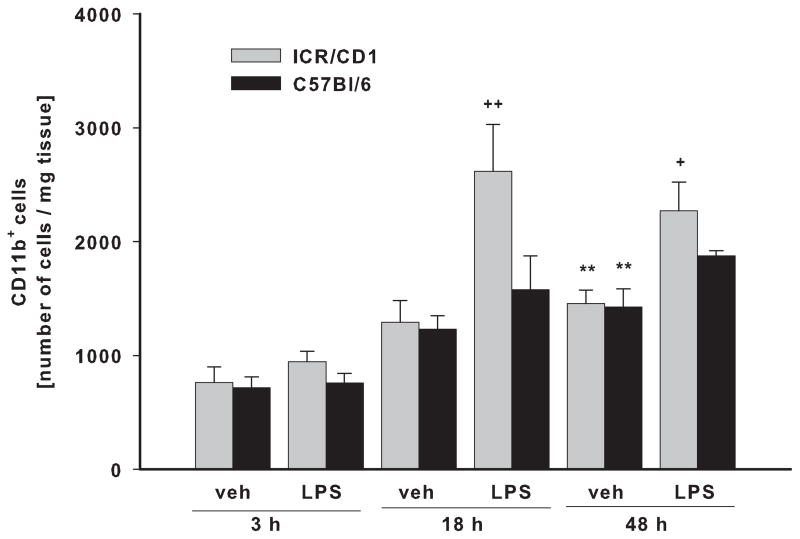
To determine if differences in microglial responses from each mouse strain were also evident in vivo, we injected LPS into the parenchyma of ICR/CD1 and C57Bl/6 mice. Because an increased number of CD11b+ cells in the CNS (usually detected by immunohistochemistry) is often regarded as an indicator of microglial activation and proliferation, we compared the number of freshly-isolated CD11b+ cells from brains of ICR/CD1 and C57Bl/6 mice 3, 18 and 48 hours after LPS and PBS (control) injections. There was no difference between the strains in the yield of microglia obtained from naïve mouse brains (not shown) or at 3 or 18 hours after PBS injection into the brain parenchyma (Fig. 2). However, 48 hours post-vehicle injection, the number of microglia significantly increased approximately 2 times in both mouse strains suggesting a microglial response to injection injury. In ICR/CD1 mice, LPS induced a further 2 and 1.5 fold increase in CD11b+ cells at 18 and 48 hours after LPS injection, respectively. Interestingly, LPS had no effect on the number of microglial cells obtained from C57Bl/6 mice in which the increased number of CD11b+ cells was attributable to injection injury, indicating a strain difference in microglial responsiveness to LPS.
Fig 2. Total CD11b+ cell number isolated from brain.
ICR/CD1 and C57Bl/6 mice were bilaterally injected with LPS (2μg/side) or PBS (control) into the brain parenchyma. Three, 18 or 48 hours later, mice were perfused followed by immunomagnetic isolation of CD11b+ cells. Cell numbers were normalized per mg of tissue. In ICR/CD1 mice, LPS induced a significant increase in the number of CD11b+ cells 18 and 48 hours after injection. Mechanical injury caused by injection resulted in a significant increase in CD11b+ cells in both mouse strains. n = 4–6, **p<0.01 (vs. vehicle at 3h), +p<0.05, ++p<0.01 (vs. vehicle at same time point).
Pro-inflammatory gene expression is higher in ICR/CD1 microglia in response to LPS
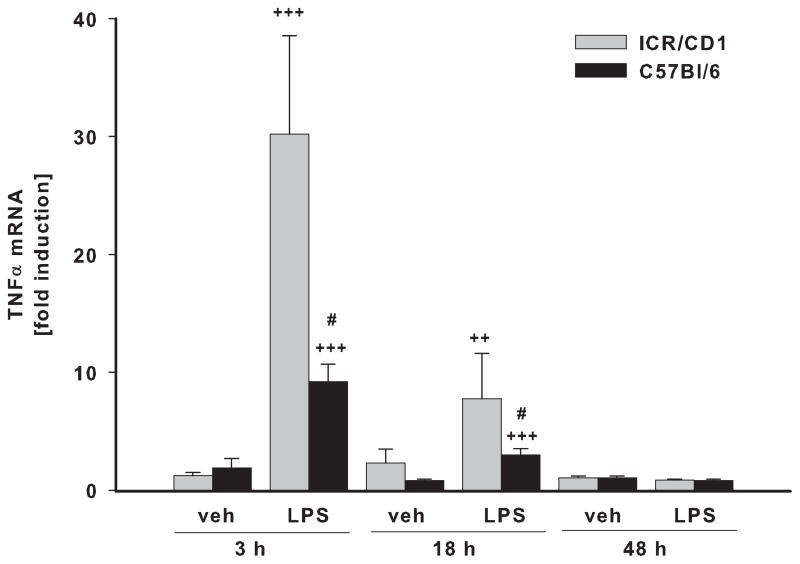
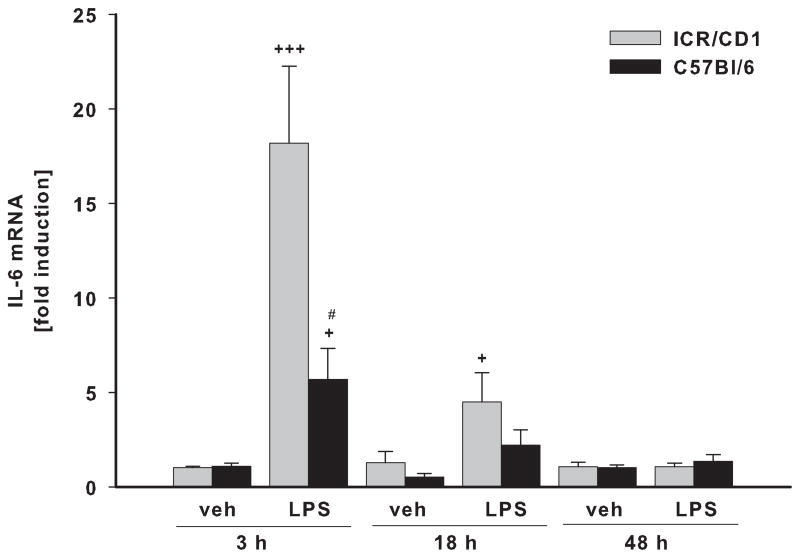
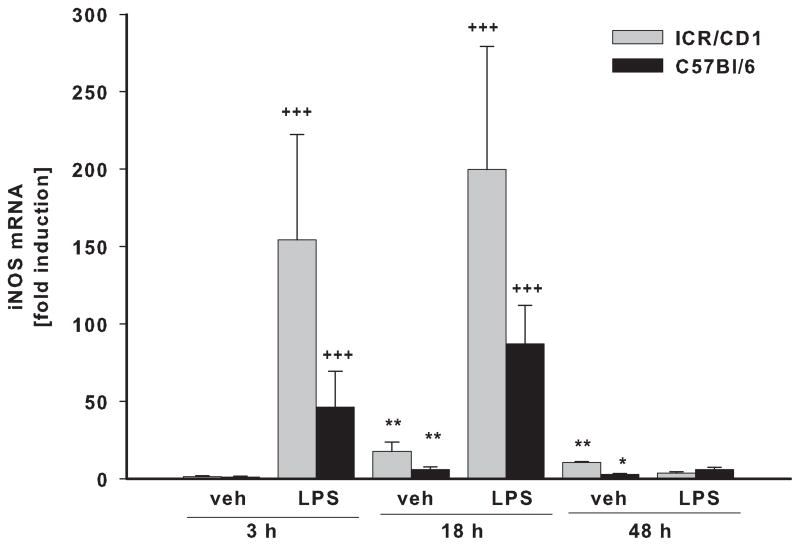
In freshly-isolated CD11b+ cells, we evaluated the expression of three pro-inflammatory genes: TNFα (Fig. 3A), IL-6 (Fig. 3B) and iNOS (Fig. 3C) in response to LPS. Vehicle (PBS) injection did not affect the expression of TNFα or IL-6 at any time point, although it did induce a small but significant increase in iNOS expression at 18 and 48 hours post-injection in both strains. The highest expression of TNFα, IL-6 and iNOS in response to LPS was observed at 3 and 18 hours after injection with expression returning to basal levels by 48 hours. Although we observed a similar time course of inflammatory gene expression in both strains, the magnitude of the response was considerably different. The up-regulation of the TNFα and IL-6 genes were 3 times higher in ICR/CD1 microglia compared to C57Bl/6 microglia at 3 hours after LPS, and was still significantly higher in ICR/CD1 microglia 18 hours after LPS. The expression of iNOS in response to LPS was 2.5 times higher in ICR/CD1 microglia compared to C57Bl/6 microglia, although this difference did not reach statistical significance. Overall, our data show a stronger pro-inflammatory response in ICR/CD1 microglia suggesting more severe neuroinflammation in response to centrally administered LPS in this mouse strain.
Fig 3. Pro-inflammatory cytokine expression in CD11b+ cells in response to LPS.
The levels of TNFα (A), IL-6 (B) and iNOS (C) mRNA were evaluated in freshly-isolated CD11b+ cells 3, 18 and 48 h after LPS by qRT-PCR. ICR/CD1 microglia display higher levels of pro-inflammatory gene expression than C57Bl/6 microglia, suggesting more severe neuroinflammation in ICR/CD1 mice. In both strains, the highest expression occurred 3 hours post-LPS injection and was downregulated to basal levels by 48 hours. PBS did not affect TNFα or IL-6, but induced a small increase in iNOS mRNA 18 and 48 h after injection. n = 5–10, * p<0.05, **p<0.01 (vs. vehicle at 3h); +p<0.05, ++p<0.01, +++p<0.001 (vs. vehicle at same time point); # p<0.05 (vs. other strain at same time point).
IL-10 and growth factor expression displayed no strain differences
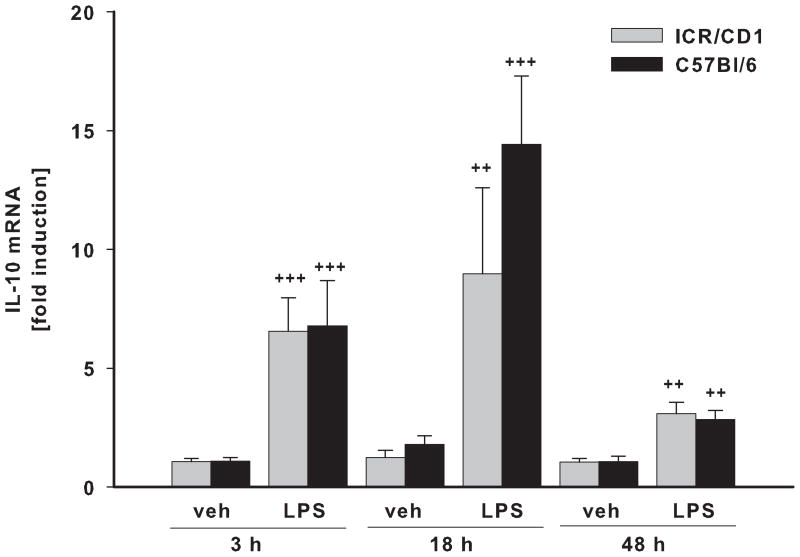
IL-10 is an important anti-inflammatory cytokine that plays a key role in modulation of immune responses. We found that the time course of IL-10 gene induction after LPS treatment was different from that of pro-inflammatory genes (Fig. 4A). IL-10 mRNA levels were significantly elevated 3 hours after LPS injection, and they remained elevated for at least 48 hours. Vehicle injection had no effect on IL-10 expression at any time point. We did not observe any differences in the magnitude of IL-10 up-regulation between strains.
Fig 4. IL-10 and trophic factor expression in CD11b+ cells following LPS.
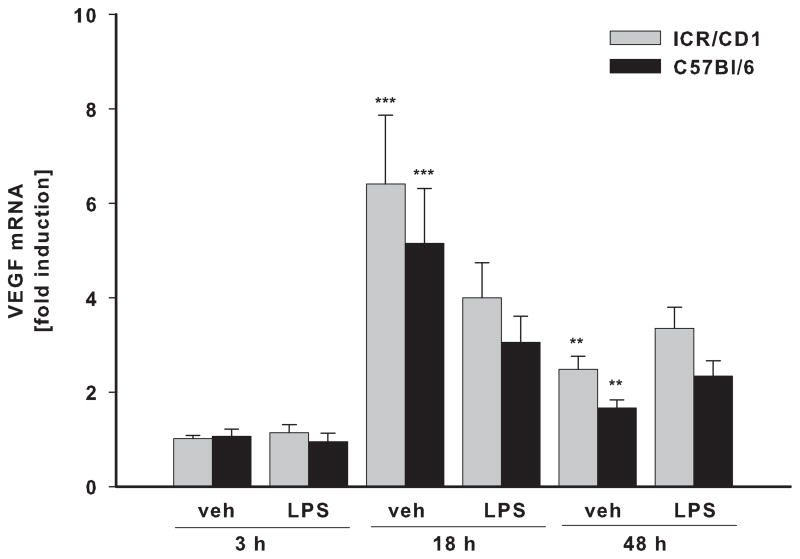
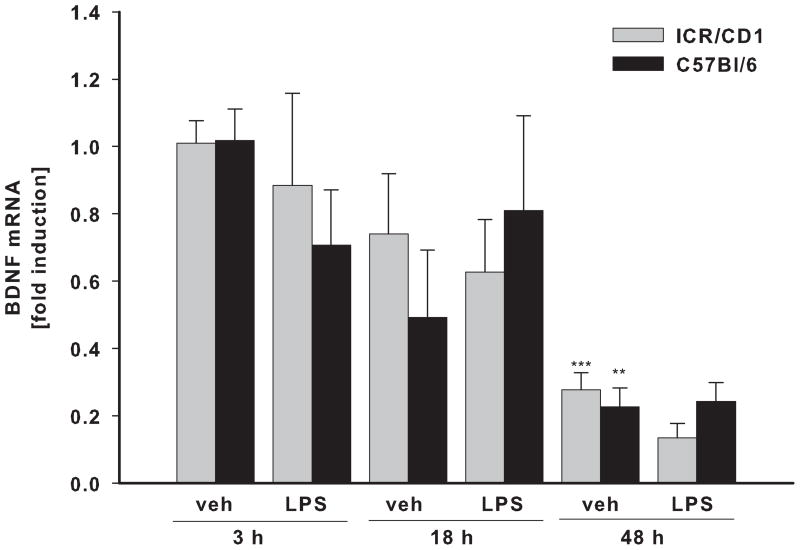
The levels of IL-10 (A), VEGF (B) and BDNF (C) mRNA were evaluated in freshly-isolated CD11b+ cells 3, 18 and 48 h after LPS by qRT-PCR. There was no difference in IL-10, VEGF and BDNF expression in microglia between the strains. Contrary to pro-inflammatory genes, LPS-induced expression of IL-10 remained highly elevated throughout the time course. LPS did not change VEGF or BDNF expression, however, these genes were affected by injection injury (veh). VEGF was upregulated at 18 and 48 hours whereas BDNF expression was inhibited 48 hours after injury. n = 4–11. *p<0.05, ** p<0.01, ***p<0.001 (vs. vehicle at 3h); ++p<0.01, +++p<0.001 (vs. vehicle at same time point).
Besides inflammatory cytokines, activated microglia also produce a number of growth factors such as VEGF and BDNF that can promote neuronal survival and plasticity. Interestingly, we found that LPS had no effect on the expression of VEGF (Fig. 4B) or BDNF (Fig. 4C) in either mouse strain. However, gene expression of these trophic factors changed in response to injection injury itself. BDNF mRNA levels were significantly down-regulated 48 hours post-injection in both strains. On the contrary, VEGF expression was significantly up-regulated 18 and 48 hours after injection; we observed no strain differences in the expression of these two genes.
The expression of LPS receptors display small strain differences
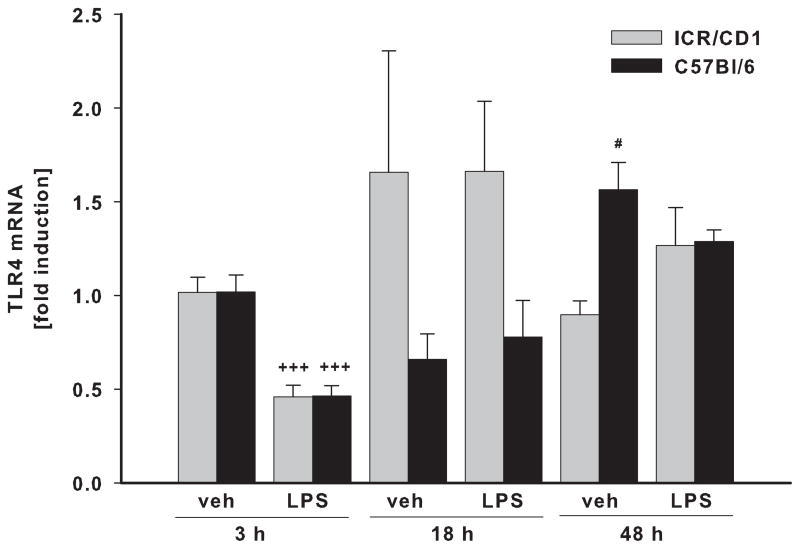
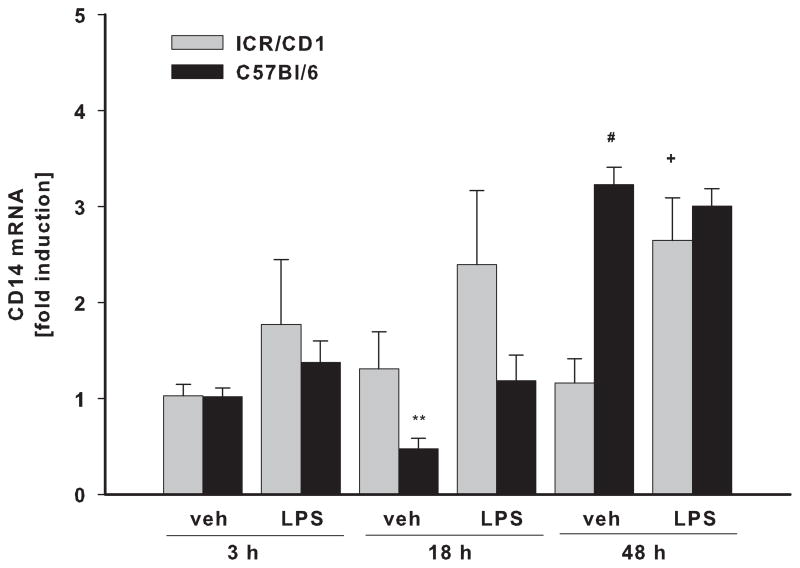
To assess whether the differences in LPS responsiveness between ICR/CD1 and C57Bl/6 microglia are due to differences in the expression of LPS receptors, we examined mRNA levels for TLR4 and CD14 in freshly-isolated CD11b+ cells. We did not observe any strain differences in basal levels of TLR4 or CD14. In response to LPS, TLR4 receptor expression was significantly down-regulated by 50% at 3 hours post-injection in both strains, but was restored to basal levels by 18 hours after injection (Fig. 5A). CD14 expression was not changed 3 or 18 hours after injection, but at 48 hours, CD14 mRNA levels were elevated in both strains (Fig. 5B). However, while CD14 mRNA in ICR/CD1 microglia was increased in response to LPS, in C57Bl/6 microglia, CD14 expression was increased in response to injection injury and was not further affected by LPS treatment, suggesting differential regulation of CD14 in both mouse strains.
Fig 5. TLR4 and CD14 expression in CD11b+ cells following LPS.
TLR4 (A) and CD14 (B) mRNA levels were evaluated by qRT-PCR in freshly isolated CD11b+ cells 3, 18 and 48 h after LPS injection. LPS treatment resulted in rapid but transient downregulation of TLR4 three hours after injection. CD14 receptor expression was mainly unaffected but we observed a small increase in its expression 48 hours after injection. n = 3–6, *p<0.05 (vs. vehicle at 3h); +p<0.05, +++p<0.001 (vs. vehicle at same time point); # p<0.05 (vs. other strain at same time point).
DISCUSSION
The data in this study show significant strain differences in the pro-inflammatory activities of microglial cells in outbred ICR/CD1 and inbred C57Bl/6 mice. Our in vitro experiments show that N9 cells and ICR/CD1 primary microglia produced significantly more TNFα and NO in response to LPS than BV-2 and C57Bl/6 primary microglia. These results are in line with other evidence indicating differential responses in these commonly used microglial cell lines. Importantly, we found that BV-2 and N9 microglia (derived from C57Bl/6 and ICR/CD1 mice respectively), respond similarly to LPS as do primary microglial cells derived from the same mouse strains. Although we have examined the production of only a limited number of inflammatory mediators here, and cannot exclude the possibility of differences in other microglial activities, the observed similarities between these microglial cell lines and their respective primary microglial cultures is reassuring. BV-2 and N9 cells are often used for simplicity and ease of manipulation; our results indicate that they can provide valuable and accurate information on microglial properties. However, differential responses between mouse strains should be considered when extrapolating or generalizing findings of microglial immune activities in a particular mouse model.
Because of differences in the magnitude of pro-inflammatory response in microglia in vitro, we were interested in whether microglial activities also differed in vivo in ICR/CD1 and C57Bl/6 mice. Strain-dependent differences in the macrophage immune responses to bacterial, parasitic or fungal infections most likely reflecting differences in genetic predisposition of these cells have been reported before (Lichtenstein et al. 2010; Sun et al. 2011; Vecchio et al. 2010). Since microglia share many similarities with macrophages, it is reasonable to expect that microglial properties would also be strain-dependent. In this study we investigated the immune activities of microglia in vivo in response to LPS, a stimulus that activates microglia through TLR4/CD14 receptors. Since LPS does not cross the blood-brain barrier (Qin et al. 2007; Singh and Jiang 2004), any microglial responses to peripheral LPS would be indirect and likely mediated via activities of the peripheral immune system. C57Bl/6 mice often display Th1 polarized immune responses (Anderson et al. 2005; Brown et al. 1996; Muller et al. 2002), however, this polarization is stimulus-dependent as a recent study showed that C57Bl/6 mice can also display Th2-skewed immune activities, for example to Cryptococus neoformans (Chen et al. 2008). Hence, microglial responses to peripheral LPS would reflect potential strain differences in the genetic predisposition of the peripheral immune system that would be difficult to distinguish from strain differences in central microglial immune responses. For these reasons, we chose to activate microglial cells directly by injecting LPS into the brain parenchyma.
LPS induced a rapid microglial inflammatory response as judged by the up-regulation of pro-inflammatory genes in both mouse strains. This response was significantly stronger in ICR/CD1 mice and is in good agreement with our in vitro observations in microglial cells. The pro-inflammatory response was resolved within 48 hours after LPS injection in both strains. The up-regulated expression of the anti-inflammatory cytokine IL-10 overlapped with the expression of pro-inflammatory mediators at 3 and 18 hours. However, contrary to pro-inflammatory genes, IL-10 mRNA expression was elevated for at least 48 hours after LPS injection, thus likely preventing sustained expression of pro-inflammatory genes. We did not find any strain differences in IL-10 mRNA expression suggesting that microglia from both strains are equally competent in suppressing inflammatory activities.
Another important observation from this study is that the increased number of CD11b+ cells in the CNS does not correlate with the up-regulation of inflammatory gene expression. The highest pro-inflammatory gene mRNA levels were observed 3 hr after LPS, before any change in CD11b+ cell number. On the contrary, the highest number of CD11b+ cells was observed 48 hours after injections when IL-10 was still significantly elevated but pro-inflammatory gene expression had already returned to basal levels. Increased CD11b+ immunoreactivity in the CNS, which may result from either microglial proliferation, increased infiltration of peripheral macrophages (or other CD11b+ cells) or both, is often regarded as a marker of “microglial activation” and neuroinflammation. Indeed, increased CD11b immunoreactivity is found in many acute and chronic CNS disorders. However, considering the limitations of immunostaining for cytokines, only a few studies have been able to directly link the expression of pro-inflammatory cytokines to morphological changes or increased number of CD11b+ cells. Our data show that increased CD11b expression may not be the most accurate indicator of microglial “activation” and neuroinflammation because the peak of the inflammatory response preceded CD11b+ cells proliferation/infiltration. Therefore, a mere increase in microglial number should not be assumed to be indicative of their pro-inflammatory activities.
Microglia react to any disturbances in homeostasis (Kreutzberg 1996), therefore it is not surprising that they responded in injection injury itself. The number of CD11b+ cells was doubled by 48 hours after injury. And although we found a small induction of iNOS expression, there was no up-regulation of the pro-inflammatory cytokines TNFα and IL-6. Interestingly, while LPS did not change the expression of BDNF and VEGF, injection injury affected the expression of these trophic factors profoundly. VEGF expression was up-regulated (6.3 and 5.1 times in ICR/CD1 and C57Bl/6, respectively) 18 hours after injury and BDNF expression was gradually down-regulated to 20% of basal levels within 48 hours. However, it will be important to determine the effects of LPS and/or injection injury on protein levels of these trophic factors as their translation and secretion are important points in their regulation. Overall, our data suggest that microglial responses are tailored to specific stimuli. LPS that mimics bacterial infection induced a rapid and strong up-regulation of pro-inflammatory cytokines and iNOS, whereas mechanical injury (i.e. the injection process itself) caused delayed up-regulation of VEGF and down-regulation of BDNF accompanied by increasing number of CD11b+ cells.
To determine whether strain differences in microglial responsiveness to LPS are the result of different expression levels of LPS receptors, we examined mRNA levels of TLR4 and CD14 on freshly isolated CD11b+ cells. We did not find any differences in basal mRNA levels for these receptors, suggesting that other factors may be responsible for observed differences in microglial inflammatory activities. Because we found similar strain-dependent differences in the expression of multiple pro-inflammatory genes, it is likely that they result from alterations in common upstream elements rather than from genetic variability in pro-inflammatory genes individually. Therefore, signaling pathways downstream of TLR4/CD14 and common transcription factors regulating these pro-inflammatory genes are also likely involved and should be considered. Because we have observed similar strain differences in microglial activities in vivo and in in vitro cultures, our data suggest that differences in pro-inflammatory responses between strains in vivo reflects differences in microglial properties themselves, rather than indirect effects from other CNS cell types. A recent study by Hofer et al (2010) showed 2 times lower levels of serum G-CSF, a cytokine that stimulates proliferation and differentiation of bone marrow stem cells into granulocytes, in ICR mice than in C57Bl/6 mice. This enabled outbred ICR mice to have a stronger response to a granulopoiesis-enhancing stimulus resulting in the higher production of granulocyte-macrophage progenitor cells in the event of infection compared to inbred mouse strains (Hofer et al. 2010). Therefore, it is possible that the decreased pro-inflammatory responses of C57Bl/6 microglia is a result of inbreeding depression which has been shown to have profound effects on many elements of the immune system across many vertebrate species including fish, birds, mammals and humans (Hofer et al. 2010; Ilmonen et al. 2008).
Although we found that microglial activities are strain-dependent, it remains to be determined which type of microglia are more beneficial to the CNS. We should not assume that microglia producing higher levels of pro-inflammatory cytokines will be more neurotoxic, or vice versa. For example, a recent study by Turrin et al. showed that TNFα is necessary for mediating microglial reactivity to acute neurotoxicity that helps to eliminate cell debris, limit subsequent damage and restore homeostasis (Turrin and Rivest 2006). Many cytokines have a wide range of biological functions and their concentration, the timing of their production and the receptors expressed by recipient cells, will determine if they act in a neuroprotective or neurotoxic manner. We hypothesize that the overall effect of microglial activities will depend on ongoing pathology or injury, and microglia that mount a stronger or weaker pro-inflammatory response may be either beneficial or detrimental depending on the circumstance. However, more studies are needed to delineate what types of microglial activities are harmful or beneficial in any given pathology.
HIGHLIGHTS.
Neuroinflammation is a hallmark of many CNS disorders
Severity of neuroinflammation mediated by microglia is strain-dependent
Inflammatory gene expression does not correlate with CD11b proliferation/infiltration
Mechanical injury and LPS induce different patterns of microglial activation
Acknowledgments
We would like to thank Ms. Lisa Rantala for excellent technical assistance. This work was supported by NIH T32HL007654, K12AG019247 (MN) and R01 NS049033 (JJW).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson CF, Mendez S, Sacks DL. Nonhealing infection despite Th1 polarization produced by a strain of Leishmania major in C57BL/6 mice. J Immunol. 2005;174(5):2934–2941. doi: 10.4049/jimmunol.174.5.2934. [DOI] [PubMed] [Google Scholar]
- Baker AE, Brautigam VM, Watters JJ. Estrogen modulates microglial inflammatory mediator production via interactions with estrogen receptor beta. Endocrinology. 2004;145(11):5021–5032. doi: 10.1210/en.2004-0619. [DOI] [PubMed] [Google Scholar]
- Blasi E, Barluzzi R, Bocchini V, Mazzolla R, Bistoni F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J Neuroimmunol. 1990;27(2–3):229–237. doi: 10.1016/0165-5728(90)90073-v. [DOI] [PubMed] [Google Scholar]
- Brown JA, Titus RG, Nabavi N, Glimcher LH. Blockade of CD86 ameliorates Leishmania major infection by down-regulating the Th2 response. J Infect Dis. 1996;174(6):1303–1308. doi: 10.1093/infdis/174.6.1303. [DOI] [PubMed] [Google Scholar]
- Chen GH, McNamara DA, Hernandez Y, Huffnagle GB, Toews GB, Olszewski MA. Inheritance of immune polarization patterns is linked to resistance versus susceptibility to Cryptococcus neoformans in a mouse model. Infect Immun. 2008;76(6):2379–2391. doi: 10.1128/IAI.01143-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain JM, Nikodemova M, Watters JJ. Expression of P2 nucleotide receptors varies with age and sex in murine brain microglia. J Neuroinflammation. 2009;6:24. doi: 10.1186/1742-2094-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher-Berkovich S, Filipovich-Rimon T, Ben-Shmuel S, Hulsmann C, Kummer MP, Heneka MT. Distinct modulation of microglial amyloid beta phagocytosis and migration by neuropeptides (i) J Neuroinflammation. 2010;7:61. doi: 10.1186/1742-2094-7-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graeber MB. Changing face of microglia. Science. 2010;330(6005):783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- Hamre K, Tharp R, Poon K, Xiong X, Smeyne RJ. Differential strain susceptibility following 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) administration acts in an autosomal dominant fashion: quantitative analysis in seven strains of Mus musculus. Brain Res. 1999;828(1–2):91–103. doi: 10.1016/s0006-8993(99)01273-1. [DOI] [PubMed] [Google Scholar]
- Hofer M, Pospisil M, Dusek L, Hola J, Hoferova Z, Weiterova L. Basal and induced granulopoiesis in outbred, F1 hybrid and inbred mice: can inbreeding depression influence the experimental practice? Exp Biol Med (Maywood) 2010;235(8):928–931. doi: 10.1258/ebm.2010.010032. [DOI] [PubMed] [Google Scholar]
- Horigome K, Bullock ED, Johnson EM., Jr Effects of nerve growth factor on rat peritoneal mast cells. Survival promotion and immediate-early gene induction. J Biol Chem. 1994;269(4):2695–2702. [PubMed] [Google Scholar]
- Ilmonen P, Penn DJ, Damjanovich K, Clarke J, Lamborn D, Morrison L, Ghotbi L, Potts WK. Experimental infection magnifies inbreeding depression in house mice. J Evol Biol. 2008;21(3):834–841. doi: 10.1111/j.1420-9101.2008.01510.x. [DOI] [PubMed] [Google Scholar]
- Kalman B, Lublin FD. The genetics of multiple sclerosis. A review. Biomed Pharmacother. 1999;53(8):358–370. doi: 10.1016/s0753-3322(99)80107-3. [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19(8):312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- Lambertsen KL, Gregersen R, Finsen B. Microglial-macrophage synthesis of tumor necrosis factor after focal cerebral ischemia in mice is strain dependent. J Cereb Blood Flow Metab. 2002;22(7):785–797. doi: 10.1097/00004647-200207000-00004. [DOI] [PubMed] [Google Scholar]
- Lichtenstein JH, Molina RM, Donaghey TC, Amuzie CJ, Pestka JJ, Coull BA, Brain JD. Pulmonary responses to Stachybotrys chartarum and its toxins: mouse strain affects clearance and macrophage cytotoxicity. Toxicol Sci. 2010;116(1):113–121. doi: 10.1093/toxsci/kfq104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maron R, Hancock WW, Slavin A, Hattori M, Kuchroo V, Weiner HL. Genetic susceptibility or resistance to autoimmune encephalomyelitis in MHC congenic mice is associated with differential production of pro- and anti-inflammatory cytokines. Int Immunol. 1999;11(9):1573–1580. doi: 10.1093/intimm/11.9.1573. [DOI] [PubMed] [Google Scholar]
- McLaughlin P, Zhou Y, Ma T, Liu J, Zhang W, Hong JS, Kovacs M, Zhang J. Proteomic analysis of microglial contribution to mouse strain-dependent dopaminergic neurotoxicity. Glia. 2006;53(6):567–582. doi: 10.1002/glia.20294. [DOI] [PubMed] [Google Scholar]
- Muhallab S, Dahlman I, Wallstrom E. Disparate MHC class II haplotypes in myelin oligodendrocyte glycoprotein- and myelin basic protein-induced experimental autoimmune encephalomyelitis. J Neuroimmunol. 2005;161(1–2):155–161. doi: 10.1016/j.jneuroim.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Muller A, Schott-Ohly P, Dohle C, Gleichmann H. Differential regulation of Th1-type and Th2-type cytokine profiles in pancreatic islets of C57BL/6 and BALB/c mice by multiple low doses of streptozotocin. Immunobiology. 2002;205(1):35–50. doi: 10.1078/0171-2985-00109. [DOI] [PubMed] [Google Scholar]
- Nickles D, Abschuetz A, Zimmer H, Kees T, Geibig R, Spiess E, Regnier-Vigouroux A. End-stage dying glioma cells are engulfed by mouse microglia with a strain-dependent efficacy. J Neuroimmunol. 2008;197(1):10–20. doi: 10.1016/j.jneuroim.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Duncan ID, Watters JJ. Minocycline exerts inhibitory effects on multiple mitogen-activated protein kinases and IkappaBalpha degradation in a stimulus-specific manner in microglia. J Neurochem. 2006;96(2):314–323. doi: 10.1111/j.1471-4159.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- Nikodemova M, Watters JJ, Jackson SJ, Yang SK, Duncan ID. Minocycline down-regulates MHC II expression in microglia and macrophages through inhibition of IRF-1 and protein kinase C (PKC)alpha/betaII. J Biol Chem. 2007;282(20):15208–15216. doi: 10.1074/jbc.M611907200. [DOI] [PubMed] [Google Scholar]
- Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55(5):453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righi M, Mori L, De Libero G, Sironi M, Biondi A, Mantovani A, Donini SD, Ricciardi-Castagnoli P. Monokine production by microglial cell clones. Eur J Immunol. 1989;19(8):1443–1448. doi: 10.1002/eji.1830190815. [DOI] [PubMed] [Google Scholar]
- Singh AK, Jiang Y. How does peripheral lipopolysaccharide induce gene expression in the brain of rats? Toxicology. 2004;201(1–3):197–207. doi: 10.1016/j.tox.2004.04.015. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Lindner M, Kotsiari A, Garde N, Fokuhl J, Linsmeier F, Trebst C, Stangel M. Cortical demyelination is prominent in the murine cuprizone model and is strain-dependent. Am J Pathol. 2008;172(4):1053–1061. doi: 10.2353/ajpath.2008.070850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch MK, Bauer J, Linington C, Olsson T, Weissert R, Lassmann H. Cortical demyelination can be modeled in specific rat models of autoimmune encephalomyelitis and is major histocompatibility complex (MHC) haplotype-related. J Neuropathol Exp Neurol. 2006;65(12):1137–1142. doi: 10.1097/01.jnen.0000248547.13176.9d. [DOI] [PubMed] [Google Scholar]
- Sun K, Gan Y, Metzger DW. Analysis of murine genetic predisposition to pneumococcal infection reveals a critical role of alveolar macrophages in maintaining the sterility of the lower respiratory tract. Infect Immun. 2011;79(5):1842–1847. doi: 10.1128/IAI.01143-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susaki Y, Shimizu S, Katakura K, Watanabe N, Kawamoto K, Matsumoto M, Tsudzuki M, Furusaka T, Kitamura Y, Matsuda H. Functional properties of murine macrophages promoted by nerve growth factor. Blood. 1996;88(12):4630–4637. [PubMed] [Google Scholar]
- Turrin NP, Rivest S. Tumor necrosis factor alpha but not interleukin 1 beta mediates neuroprotection in response to acute nitric oxide excitotoxicity. J Neurosci. 2006;26(1):143–151. doi: 10.1523/JNEUROSCI.4032-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vecchio D, Arezzini B, Pecorelli A, Valacchi G, Martorana PA, Gardi C. Reactivity of Mouse Alveolar Macrophages to Cigarette Smoke is Strain Dependent. Am J Physiol Lung Cell Mol Physiol. 2010 doi: 10.1152/ajplung.00013.2009. [DOI] [PubMed] [Google Scholar]
- Watters JJ, Sommer JA, Pfeiffer ZA, Prabhu U, Guerra AN, Bertics PJ. A differential role for the mitogen-activated protein kinases in lipopolysaccharide signaling: the MEK/ERK pathway is not essential for nitric oxide and interleukin 1beta production. J Biol Chem. 2002;277(11):9077–9087. doi: 10.1074/jbc.M104385200. [DOI] [PubMed] [Google Scholar]
- Wei R, Lin CM. Strain-dependent inflammatory responsiveness of rat microglial cells. J Neuroimmunol. 2009;211(1–2):23–38. doi: 10.1016/j.jneuroim.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Zhang J, Geula C, Lu C, Koziel H, Hatcher LM, Roisen FJ. Neurotrophins regulate proliferation and survival of two microglial cell lines in vitro. Exp Neurol. 2003;183(2):469–481. doi: 10.1016/s0014-4886(03)00222-x. [DOI] [PubMed] [Google Scholar]