Abstract
Toll-like receptor agonists are promising adjuvants for immune therapy of cancer, but their potential efficacy as single or combinatorial agents has yet to be fully evaluated. Here we report that among all TLR agonists tested, dendritic cells (DCs) stimulated with the TLR3 agonist polyI:C displayed the strongest activity in stimulating pro-inflammatory responses and the production of melanoma antigen-specific CD8+ T cells. Simultaneous treatment with TLR7/8 agonists further improved these responses, but the inclusion of bacterial LPS, a TLR4 agonist, suppressed pro-inflammatory cytokine production. This inhibition was contingent upon rapid induction of the suppressive cytokine IL-10 by LPS, leading to dysregulated immune responses and it could be reversed by STAT3 knockdown, p38 blockade or antibodies to IL-10 and its receptor. Our findings show how certain TLR agonist combinations can enhance or limit dendritic cell responses associated with anti-tumor immunity, through their relative ability to induce IL-10 pathways that are immune suppressive.
Introduction
Toll-like receptor (TLR) agonists are molecular components associated with microbial pathogens, and natural mediators of inflammation. Through ligation of specific receptors on antigen presenting cells (APCs), they initiate innate immune responses and facilitate the induction of adaptive immunity. Dendritic cells (DCs) are the most potent antigen presenting cells playing a crucial role at the crosstalk of innate and adaptive immune systems. In the last decade the unique features of TLR agonists and DCs have been tested in immunotherapy trials for cancer patients (1, 2). Exploiting TLR agonists in vivo as adjuvants to tumor antigen delivery has succeeded in inducing potent immune responses and in some cases evidence of tumor regression or delayed time to recurrence. The first adjuvant approved for use in human cancer was live, attenuated Mycobacterium bovis (stimulant of TLR2 and TLR4) for the treatment of bladder carcinoma in situ and superficial bladder cancers. In the clinic it performed better than standard chemotherapy (3). Another promising adjuvant is detoxified LPS, Monophosphoryl A (MPL) (targeting TLR4) which is a component of many immune therapeutic strategies. It is currently being co-administered with MAGE A3 (a cancer testis antigen) in patients with advanced melanoma and non-small cell lung cancer (NSCLC) (4). So far, the results in Phase I/II trials indicate very low toxicity and suggest clinical benefit with prolonged disease-free survival for patients with resected stage I and II NSCLC (5). Agonists of TLR7 and TLR7/8, Imiquimod and Resiquimod, respectively, succeeded in augmenting immunologic responses in melanoma patients treated with either peptide or protein vaccines (6, 7). Combination of TLR7/8 agonists with TRL4 agonist resulted in increased CD8+ T cells retaining CD28 (8). Since the new formulation of the TLR3 agonist, Poly-ICLC, also shown to act through the intracellular receptor MDA5, has an improved half-life in vivo, it has been reexamined with reported benefits in the induction of the adaptive CD4+ T cell immune responses(9).
In this study we sought to examine the potential of single and combined TLR agonists for their ability to induce potent anti-tumor antigen responses, while limiting the potential for activating inhibitory pathways or skewing T cell responses towards Th2 like profile. We show that certain TLR combinations are synergistic while others are limiting in their ability to induce potent immune responses, and that IL-10 secretion is a key homeostatic control mechanism that governs this outcome. Upon specific blockade of IL-10, the potency of tested adjuvants is greatly augmented as shown by an improved induction of both innate and adaptive immune responses targeted against the model melanoma antigen MelanA/MART-1.
METHODS
Peptides
For the human studies, Flu MP58–66 (GILGFVFTL), MelanA/Mart-126–35 (ELA modified) ELAGIGILTV, and HIV Gag77–85 SLYNTVATL peptides were synthesized by Genemed Synthesis Inc.
Human monocyte-derived DCs
Buffy coats were purchased from the New York Blood Center and leukaphereses from BRT Laboratories. These served as sources for peripheral blood mononuclear cells (PBMCs). Human monocyte-derived DC (mo-DCs) were differentiated from monocyte fractions of PBMCs as described previously (10). Briefly, monocytes that attached to the tissue culture plates following plating of peripheral blood mononuclear cells were cultured at 37ºC on 10cm2 plates in 10ml of culture media containing 300IU/ ml recombinant human (rHu) IL-4 (Immunex) and 116IU/ ml rHu GM-CSF (R&D). On days 2 and 4 of culture, additional IL-4 and GM-CSF was added. Immature DCs were harvested on day 5 for use in experiments.
Tetramers
Mart26–35-specific, HIV Gag77–85 specific tetramers were generated by the Vaccine and Cell Therapy facility at New York University School of Medicine.
Human Antibodies
Unless otherwise specified all antibodies ( CD3, CD4, CD45RO, CD62L, CD8, CD80, CD86, PDL-1) for staining human cells were purchased from BD Pharmingen.
Measurement Of Cytokine And Chemokine Secretion
Human mo-DCs cultured with various stimuli:LPS at 100ng/ml ( Sigma), PolyI:C at 2–5 ug/ml from Amersham and InVivogen (used both in vitro and in vivo), or Oncovir’s Hiltonol® ( just in vitro, in Figures 2, 3 and Supplemental Figures 3 and 4) ( all 3 sources gave similar in vitro results when compared side by side), R848 at 1uM – 10uM (3M) were incubated for 18–24 hours (unless otherwise stated in the figure) at 37 ºC before their culture supernatants were collected and tested for the presence of the following cytokines: IL-12p70, TNF-α, IL-10, IL-6, IL-1β and IL-8 by flow cytometry using a cytometric bead array (BD Biosciences). Alternatively IL-12p70, TNF-α (BD Pharmingen), and IFNα (PBL, Piscataway, NJ); were measure by enzyme-linked immunosorbent assay (ELISA). Also, a panel of 22 cytokines was measured using the Luminex platform, and a kit from Millipore. Blocking antibodies to IL-10 and IL-10R and recombinant human IL-10 (used at 300ng/ml) were purchased from Biolegend as was control IgG2A/IgG1, all blocking antibodies were used at 5ug/ml.
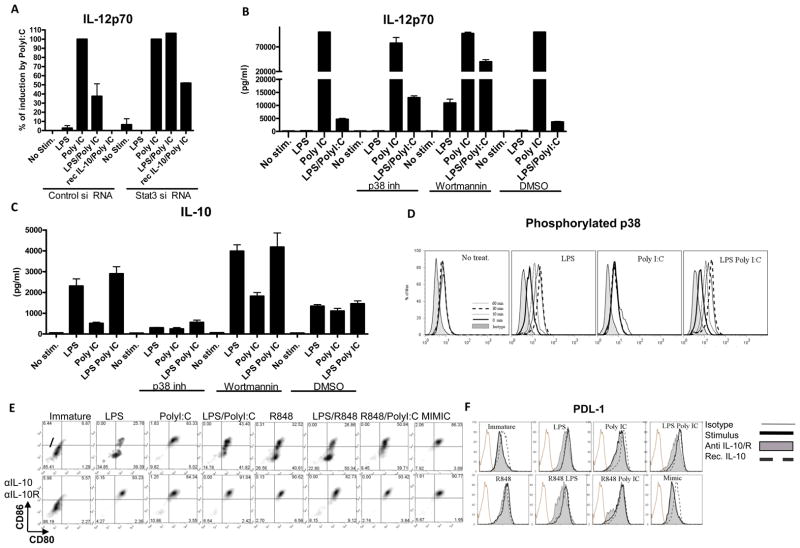
Figure 2. Inhibition of PolyI:C induced responses by LPS is mediated by IL-10, Stat3 and partially by P38.
MoDCs were electroporated with STAT3 siRNA and the amount of total protein was measure by western blot (A) and quantified using densitometry (B). These cells were treated with LPS, PolyI:C or their combination, and IL-12p70 was measured (A). MoDCs prior to exposure to LPS, PolyI:C or their combination were pretreated with a P38 inhibitor 10ng/ml or wortmannin 100nM/ml, and IL-12p70 and IL-10 was quantified (B and C respectively). Phosphorylation of P38 was monitored over time upon ligation of TLR4, TLR3 or their combination (D). MoDCs were exposed to 100ng/ml of LPS, 5ug/ml of PolyI:C, 10uM of R848 and/or their combination as well as Mimic cytokine cocktail or left untreated with or without blocking antibodies to IL-10 and IL-10R with or without recombinant IL-10. Cells were then stained with anti CD80 and anti-CD86 (E) anti- PDL-1 antibody (F) or with the isotype control.
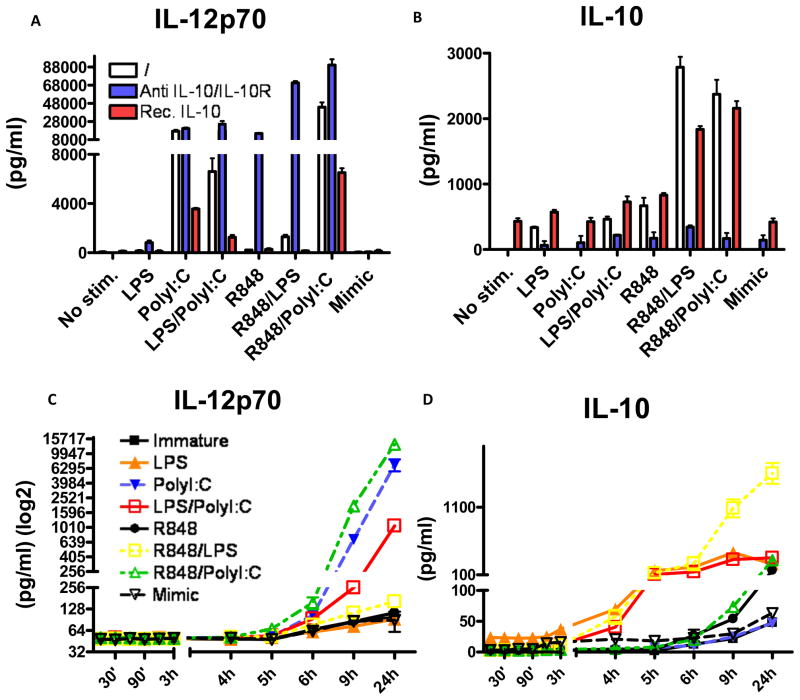
Figure 3. Kinetics of IL-10 secretion explains inflammatory capacity of TLR agonists.
MoDCs were monitored for the secretion of IL-12p70 and IL-10 when either untreated or stimulated with LPS, PolyI:C, LPS/PolyI:C, R848, R848/LPS, R848/PolyI:C and Mimic. IL-12p70 and IL-10 was also monitored using the same conditions with or without blocking IL-10 and IL-10R, and with or without pretreatment with recombinant IL-10 (A, B respectively). The same experiment was repeated by monitoring IL-12p70 and IL-10 in supernatants collected over time (C, D respectively). Each experiment was performed in triplicates and repeated at least with 3 different donors.
In mouse in vivo experiments, animals were injected intra-peritoneal with 100ug of PolyI:C or 100ul of PBS and 4 hours later blood was collected and IL-6 and TNF-α were measured in sera using cytometric bead array (BD Biosciences).
In Vitro T Cell Priming
Human mo-DCs derived from healthy donors were matured in the presence of the agonist of choice and 1uM Mart26–35 peptide over night at 1×106/ml. The day after, cells were washed 2x with RPMI and resuspended at 1×105 cells/ml. Naïve CD8+ T cells were isolated by depletion of CD45RO positive cells. Bead isolated CD8+T cells from autologous donors were resuspended at 1×106/ml. DCs were co-cultured with CD8+ T cells in 24 well plates at a ratio of 1:10 in 10% PHS in RPMI. After 4 days of culture 5ng/ml of IL-7 (R&D) and 5 ng/ml IL-15 (R&D) were added to cultures. Priming cultures were restimulated with autologous irradiated Mart26–35 peptide pulsed PBMCs at day 10. IL-7 and IL-15 were replenished every 2 days. On day 21 the T cells from each well were tested for antigen-specificity by staining with Mart-1/MelanA specific tetramers.
Antigen Specific Cell Capture and Expansion
On day 22 of the priming experiments, the cells were stained with Mart-1 tetramer PE for 45 min at 4ºC, washed 2x with PBS, and then enriched with anti-PE beads (Miltenyi) for 15 min at 4ºC. Cells were placed in 96 well plates containing 1×105 allogeneic irradiated PBMCs per well in CTL media supplemented with 150 U/ml of IL-2 and 1ug/ml of phytohemaglutinin (PHA-L) (Sigma). Cells were then cultured for 2+ weeks, with media change as needed before they were functionally evaluated.
DC migration assay
Immature human monocyte derived DCs were treated described above and then placed in the top chamber of Transwell plates that contained CCR7-specific ligand CCL21(300U/ml) in the medium in the bottom chamber. Three hours later, the cells that migrated towards the lower chamber were counted. Fold change over the cells that migrated spontaneously in the absence of CCL21 is reported. Assays were performed in triplicate and shown is a representative experiment.
Allogeneic T Cell Reaction
Naïve CD8+ T cells were isolated from PBMCs using CD45RO and CD8 magnetic beads from Myltenyi Biotech. MoDCs were pepared as described above. After 24h stimulation with the agonist of choice, MoDCs were washed 3 times, and then incubated with allogeneic naïve CD8+ t cells in 96 well round bottom tissue culture plates at different ratios 1:10–1:90, and left in the incubator at 37C for 6days in the presence of vehicle (−), rhIL-12 (1ng/ml) from R&D, anti-IL12 (2ug/ml), or control IgG (2ug/ml) from Biolegend before being stimulated with CD3/CD28 dynabeads (Invitrogen) according to the manufacturers protocol. Day after supernatants were collected and levels of IFNγ was assessed.
Flow Cytometry and Cell Sorting
Flow cytometry was performed using either the FACS Calibur (BD Biosciences) or the LSR II (BD Biosciences) flow cytometers. Analysis was done using FlowJo software (Tree Star).
Statistical Analysis
All statistical analysis was performed using student’s t test. We have performed 2 way ANOVA test on the results of kinetics of IL-10 at 5h and 6h.
Results
TLR4 engagement inhibits TLR3-induced proinflammatory cytokine production in dendritic cells
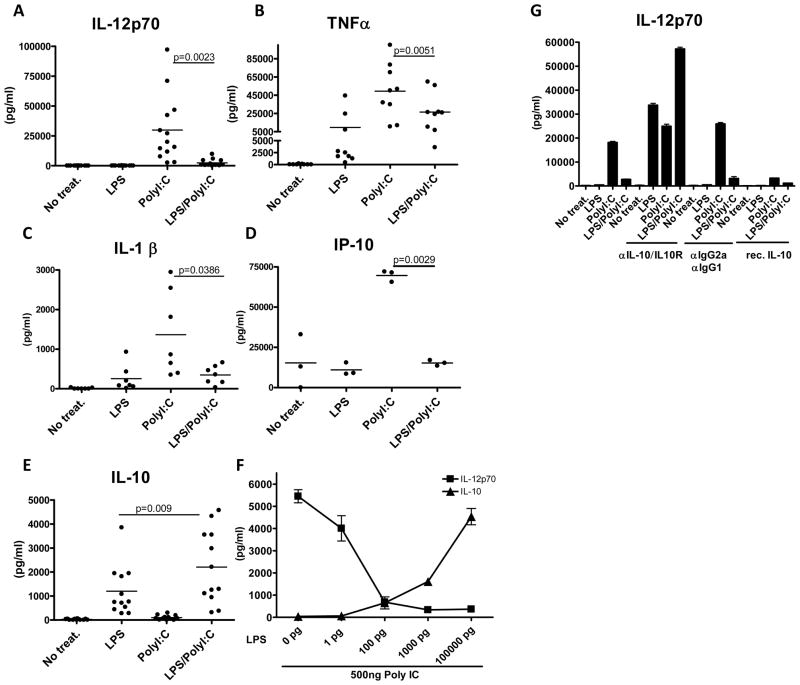
Initially we compared the effects of the TLR4 and TLR3 agonists, LPS and PolyI:C, respectively, and their combination to induce cytokine secretion in immature monocyte-derived DCs (MoDCs.) We chose to begin with LPS as it is known to mature DCs that induce potent anti-tumor immunity in mice, and its derivative MPL has shown promise in human immunization protocols (11). PolyI:C has been less extensively studied than LPS but in vitro studies generated impressive results (9). For most studies we used Poly-ICLC, which is PolyI:C stabilized with carboxymethyl cellulose and poly-L-lysine to confer resistance to ribonuclease. In our study PolyI:C induced high levels of the proinflammatory cytokines IL-12p70, TNF-α, IL-1β, and IP-10 (Figure 1A, 1B, 1C and 1D respectively), in addition to Type I IFNs and IL-6 (Supplementary Figure 1A and 1B). LPS was a poor inducer of bioactive IL-12, but a potent stimulator of IL-10. Interestingly, when TLR4 and TLR3 were engaged simultaneously, the pro-inflammatory cytokine response, in particular IL-12, was dramatically reduced (p=0.0023 for IL12p70 and p=0.0051 for TNF-α) in comparison to TLR3 ligation alone, and resembled the pattern induced by LPS (Figure 1A and 1B respectively). In contrast, IL-10 levels were significantly increased when TLR4 and TLR3 were co-ligated compared to PolyI:C or LPS alone (Figure 1E).
Figure 1. LPS inhibits PolyI:C induced proinflammatory cytokines in human dendritic cells.
MoDCs were exposed to 100ng/ml of LPS, 5ug/ml of PolyI:C, or their combination. IL-12p70 was measured (A, n=13) as was TNFα (B, n=9), IL-1β (C, n=7), IP-10 (D, n=3) and IL-10 (E, n=13) by cytokine bead array (CBA) and Luminex (range from 3–13 healthy donors). The combination of LPS and PolyI:C induced significantly lower amounts of IL-12p70, TNFα, IL-1β and IP-10 than PolyI:C alone, while inducing significantly higher amounts of IL-10 than LPS alone. MoDCs were exposed to a suboptimal dose of 500ng/ml of PolyI:C and given increasing doses of LPS while monitoring levels of IL-12p70 and IL-10 (F). IL-12p70 levels were measured in MoDCs that were either left untreated, exposed to blocking antibodies to IL-10 and IL-10R, IgG2/IgG1 control antibody or they were pre exposed to recombinant IL-10 (G, performed in triplicates, in 3 donors).
Suppression of PolyI:C induced DC cytokine responses by LPS is mediated by IL-10
To determine whether IL-10 was responsible for the suppression of IL-12, TNF-α and other cytokines tested we added increasing doses of LPS to immature MoDC while keeping the dose of PolyI:C constant at a suboptimal level. The addition of as little as 1pg/ml of LPS noticeably reduced IL-12p70 levels (Figure 1F), and maximal inhibition was observed at the dose of 100ng/ml of LPS, which we use routinely. As IL-12p70 levels fell with augmented doses of LPS, there was a corresponding increase of IL-10 secreted (Figure 1F). This finding may explain discrepancies in the literature as they pertain to relative IL-12p70 induction by LPS and PolyI:C. For example, LPS is often considered to be an effective inducer of IL-12 in DCs but levels of only a few hundred pg/ml are reported, similar to our own results. As minute amounts of LPS (far below Limulus assay detection limits) are able to suppress PolyI:C induced IL-12, it is possible that contamination with LPS may obscure the real effects of this and other agonists. To see if the inhibitory potential of LPS could be reversed, we added blocking antibodies to IL-10 and the IL-10 receptor (IL-10R). The inhibition of IL-12p70 in the LPS/PolyI:C condition was not only overturned, but IL-12p70 levels increased substantially. Furthermore, LPS treated DCs now also produced high levels of IL-12p70, indicating that IL-10 is the source of self-induced inhibition (Figure 1G). Isotype matched antibodies and recombinant human (rh) IL-10 were used as controls (Figure 1G). The latter inhibited IL-12 (Figure 1G), TNFα, IP-10 and IL-1β (data not shown) consistent with a direct role for IL-10 in blocking inflammation. Therefore the inhibitory profile observed during co-ligation of TLR4 and TLR3 receptors, is mediated by TLR4 induced IL-10.
Inhibition of Poly IC induced responses by LPS is mediated by Stat3 and partially by p38
IL-10 signals through IL-10R, JAK1 and ultimately STAT3 to suppress pro-inflammatory signaling (12). Therefore, we silenced STAT3 using specific siRNA, achieving about 50% reduction in total STAT3 levels (Supplemantary Figure 1C and 1D). That level of down regulation was sufficient to completely rescue the LPS-induced inhibition of IL-12 (Figure 2A). Knockdown of STAT3 also partially reversed rhIL-10 inhibition of PolyI:C-induced IL-12 (Figure 2A). As phosphoinositide 3-kinase (PI3K) has been hypothesized to block IL-12 by blocking p38, JNK, ERK and NF-kappaB pathways in the early phase of TLR signaling (13) we blocked PI3K activity with wortmannin at 100nM, a concentration that does not inhibit endocytosis (14). Inhibition of PI3K allowed induction of IL-12p70 by LPS alone, as well as reversal of the inhibitory profile of LPS on PolyI:C (Figure 2B ). However it also increased levels of IL-10 (Figure 2C) suggesting that PI3K plays an important role in overall suppression of TLR signaling, and indicating that its effects are IL-10 independent. Inhibition of p38 suppressed induction of IL-10 and partially reversed the inhibition of IL-12p70 when MoDC were co-ligated with TLR4 and TLR3 agonists (Figure 2B and 2C, respectively), consistent with the fact that p38 activation participates in LPS-induced IL-10 transcription (15) and that the levels of IL-10 were reduced. In line with these observations, we noted phosphorylation of p38 as early as 10 min within moDC when both LPS or LPS/PolyI:C were used (but not PolyI:C), reaching peak levels between 30 and 60 minutes (Figure 2D). Altogether, these results indicate that LPS induced suppression is mediated at least in part by p38 that is required for IL-10 induction, and which inhibits IL-12 secretion in a STAT3-dependent manner.
Kinetics of IL-10 secretion account for the differential co-stimulatory and pro-inflammatory capacities of TLR agonists
To determine whether the results we observed with PolyI:C and LPS applied to other TLR agonists, we tested R848, a TLR7/8 agonist. R848 alone induced very low levels of IL-12p70 as compared to PolyI:C (Supplementary Table 1). In every condition inducing appreciable amounts of IL-10, blocking this cytokine during the maturation of MoDCs subsequently resulted in highly increased CD80 and CD86 co-stimulatory molecule up-regulation (Figure 2E). Negative co-stimulation, as measured by the expression levels of PDL-1 was further increased with the addition of rhIL-10, while blocking IL-10 reversed this up-regulation in the conditions where IL-10 was secreted (Figure 2F). Similarly, when IL10 and IL10R were blocked with specific antibodies, IL-12p70 and TNF-α levels were dramatically increased (p<0.0001, Figure 3A and data not shown). The combination of LPS and R848 gave a synergistic profile, as previously described (16), inducing higher amounts of IL-12p70 compared to either agonist alone, despite high levels of IL-10 (Figure 3B) but still fairly small levels of IL12p70 in comparison to PolyI:C alone (Figure 3A). However, blocking IL-10 upon stimulation in this dual agonist combination resulted in dramatically increased amounts of pro-inflammatory cytokines, as exemplified by IL-12p70, suggesting that IL-10 production blocked the synergistic effects of the two agonists on IL-12 production (Figure 3A and Supplementary Table 1). While the combination of TLR3 and TLR7/8 ligation induced high amounts of IL-12p70, these were similar to levels induced by PolyI:C alone (p=0.47, Supplementary Figure 1E). In contrast, the levels of TNFα were significantly higher if PolyI:C and R848 were combined (p=0.02, Supplementary Figure 1F) compared to TLR3 or TLR7/8 ligation alone. Interestingly, although the ability of R848 to produce IL-12 was dampened by the simultaneous production of IL-10 (Figure 3A) it did not appear to inhibit the endogenous IL-12 production by PolyI:C when the two agonists were combined. However, blockade of IL-10 did further augment the IL-12 levels induced by these two agonists (p=0.0048).
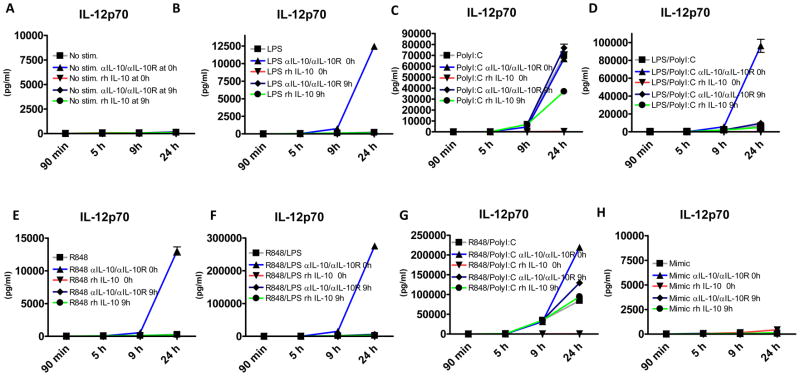
We next measured levels of IL-10 produced under these various stimulation conditions. Significant IL-10 secretion was observed upon LPS stimulation, and synergistic induction of IL-10 upon R848/ LPS and R848/PolyI:C combined stimulation (Figure 3B). Exogenously added rhIL-10 was used as a control. Strikingly, addition of only 300pg/ml of recombinant IL-10 before stimulation induced far greater suppression of IL-12 production than that induced by LPS or R848-induced IL-10 (Figures 3A and 3B) in combination with PolyI:C. This suggested that the timing of IL-10 secretion, with respect to that of pro-inflammatory cytokine secretion, is important for its suppressive effect. We therefore measured IL-12p70 and IL-10 secretion kinetics in response to R848, PolyI:C and LPS. When secreted, IL-12p70 was measurable in supernatants at 5–6 hours in all conditions (Figure 3C). The timing of IL-10 secretion, however, was dependent on the agonist used. Namely, if LPS was used alone or in combination with other agonists, IL-10 was measurable between 3 and 4 hours, before any IL-12 is significantly secreted. In contrast, R848 used alone or in a combination with any agonist other than LPS, induced IL-10 between 6 and 9 hours (Figure 3D). We performed a statistical test on the results of kinetics of IL-10 production at 5h and 6h, and found a significant difference (p<0.001) in LPS vs. R848/PolyI:C or any other condition, provided it didn’t contain LPS. When LPS was compared to another condition that also included LPS, the significance at these early time points of 5h and 6h was lost. This difference in timing explains how the addition of 5–10 fold lower amounts of recombinant IL-10 prior to TLR agonist stimulation (Figure 2A) caused superior suppression of IL-12p70 compared to combinations of LPS/Poly I:C and LPS/R848, which induce higher amounts of IL-10, but later. It also explains why TLR7/8 and TLR3 co-ligation resulted in significant inflammatory cytokine production, given the relatively delayed IL-10 secretion. In order to confirm these findings and to explain why R848 despite inducing later secretion of IL-10, does not produce much IL-12p70, but does so if blocking antibodies to IL-10/IL10R are introduced, we performed a similar kinetic experiment but this time also adding rhIL-10 or αIL10/IL10R antibodies at 9h after stimulation (Figure 4A–H). Early addition of rhIL-10 resulted in complete abrogation of IL12 production in all conditions tested. We noted that if IL-10 was blocked at 0h when MoDCs were stimulated with R848 (Figure 4E), the majority of production of IL-12 did not occur until after 9h, well after IL-10 is normally induced by this agonist without blocking IL-10 (Figure 2D). R848 thus induces IL-12 relatively late allowing for also late induction of IL-10 to suppress further secretion of IL12. These data explain the paucity of IL-12 in the supernatant of MoDCs stimulated with R848. In addition we show that addition of rhIL-10 at 9h in PolyI:C stimulated DCs only had a partial effect with suppressive capacity being much lower than any condition where there was IL-10 early (Figure 4C). Addition of rhIL-10 at 9h to R848/PolyI:C stimulated DCs had little effect as there was enough IL-10 by that time point (Figure 4G). We observed similar temporal regulation of TNF-α by IL-10 in the same experimental setting (Supplementary Figure 1G). In addition to cytokine control, we tested whether blocking IL-10/IL-10R, would have an impact on the migratory capacity of DCs towards CCL21. In addition no observed differences in CCR7 expression (data not shown) blocking of IL10/IL10R did not enhance any of the conditions tested (Supplementary Figure 1H) suggesting that migration capacity is independent of IL-10.
Figure 4. Delayed addition of rhIL-10 and blocking antibodies to IL-10/IL-10R further elucidates the mechanism of TLR agonists.
Monocyte derived dendritic cells were exposed to 100ng of LPS, 5ug of PolyI:C, 10uM of R848 and/or their combination as well as Mimic cytokine cocktail or left untreated +/− rhIL-10 (300ng/ml) at 0h and 9h and +/− anti IL-10/IL-10R (2ug/ml) at 0 or 9h, IL-12p70 was monitored over time in triplicate wells (n=2) by CBA (A–H).
IL-10 has a well-established anti-inflammatory role in TLR signaling, however we show for the first time that the kinetics of its secretion by MoDCs ultimately affects the induction of inflammatory cytokines by TLR3, TLR4 and TLR7/8 agonists or their combinations.
Increased levels of inflammation by MoDCs translate into higher and stronger specific CD8 T cell responses
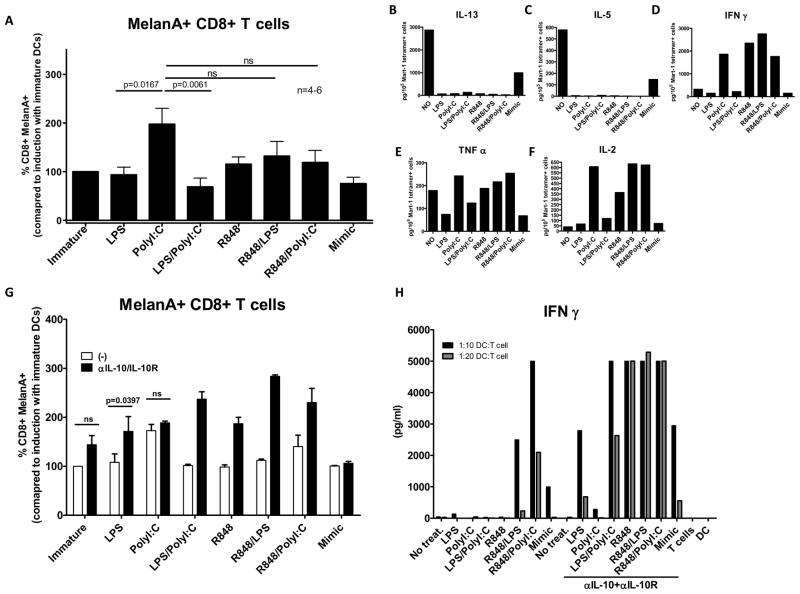
To test whether these differences in cytokine secretion actually translate into functionally different T cell responses, we analyzed CD8+ T cell priming against the melanoma-associated antigen Melan-A/Mart-1 using its HLA-A2 restricted Melan-A/Mart-1 (ELAGIGILTV) epitope as an immunogen. PolyI:C-matured MoDCs consistently induced higher frequencies of specific T cell responses in comparison to LPS or LPS/PolyI:C matured MoDCs (Figure 5A and Supplementary Figure 2). The combinations of R848/LPS and R848/PolyI:C resulted in levels of primed CD8+ T cells similar to PolyI:C – matured DC alone, although the trend was higher in the latter (Figure 5A and Supplementary Figure 2A). When the quality of these tetramer positive polyclonal populations was evaluated, immature and mimic matured MoDCs were found to induce CD8+ T cells that secreted IL-13 and IL-5 (Figure 5B and 5C respectively), typical of Tc2-like CD8+ T cells. On the other hand, TLR agonist ligation of MoDCs induced Tc1 CD8+ cells, regardless of the agonist, but with varying quality. PolyI:C, R848 and the combination of LPS/R848 and R848/PolyI:C were able to induce Melan-A/MART-1 CD8+ T cells capable of secreting higher amounts of IFN-γ, TNF-α and IL-2 upon restimulation (Figure 5D, 5E and 5F respectively). Thus, secretion of inflammatory cytokines by DC upon TLR triggering correlates with their T cell stimulation capacity, inducing functionally differential anti-tumor responses.
Figure 5. Increased levels of inflammation in MoDCs translate into higher numbers of specific CD8 T cell responses.
Healthy donor naïve A2.1+ CD8+ T cells were primed with peptide encoding for the HLA A2.1 restricted Mart-1/MelanA (ELAGIGILTV) epitope and the frequency of tetramer positive cells was monitored (n=6) (B). Tetramer positive cells from all conditions from a donor were expanded and examined functionally for secretion of IL-13, IL-5, IFNγ, TNFα and IL-2 (B, C, D, E and F, respectively). Using the same priming experimental setup 2 additional donors were used with a condition of αIL-10/IL10R Abs for each treatment, showing a representative donor (triplicate wells) (G). Differentially matured MoDCs and allogeneic naïve CD8+T cells were co-cultured for 7 days in a MLR reaction. Proliferated cells were stimulated over night with αCD3/CD28 beads and evaluated for the production of IFNγ (H).
We next formally assessed the contribution of IL-12 in the priming of naïve CD8+ T cells by TLR agonist-matured DC, by repleting the cytokine through addition of rhIL-12, or blocking endogenously produced IL-12. After 6 days of co-culture, the ability of T cells to secrete IFNγ was measured (Supplementary Figure 2B). We saw substantial increases in IFN-γ production with the addition of rhIL-12 in all conditions tested, and we also observed complete abrogation of IFN-γ secretion by T cells after IL-12 was blocked suggesting a crucial role for IL-12 in our system.
Blocking IL-10 during MoDC maturation increases the quantity and quality of CD8 T cell responses
Given that IL-10 plays an important role in controlling inflammation induced by MoDCs during TLR ligation, we tested if blocking it translated into differential magnitude and quality of CD8 T cell priming. As shown in Figure 5G, inhibition of IL-10 during DC activation dramatically enhanced Melan-A-specific T cell generation, except when IL-10 is not secreted. i.e. upon PolyI:C stimulation possibly through the combined effects of enhanced co-stimulation and dampened negative co-stimulation (Figure 2E and 2F). We also tested whether neutralization of IL-10 improved stimulation of allogeneic T cell responses by TLR agonist matured DCs. After 7 days the ability of T cells to secrete IFNγ was measured. Blockade of IL-10 significantly increased IFNγ secretion by primed T cells (Figure 5H). Lack of IL-10 secretion by DCs upon ligation with PolyI:C explains why this adjuvant as a single agent is superior to others tested. The priming with combinations of LPS/R848 and R848/PolyI:C can be further improved by blocking IL-10. Apart from PolyI:C, all tested TLR agonists or their combinations, use IL-10 as a mechanism to control the priming of T cells. In immuno-compromised melanoma patients blocking of IL-10 or usage of adjuvants which induce low levels of IL-10, like PolyI:C may be beneficial in boosting their anti-melanoma immunity.
Discussion
Using TLR agonists as adjuvants to mature DCs either ex vivo or in situ is an attractive method for initiating or boosting anti-tumor responses. Here we show that certain combinations of TLR agonists are synergistic in their ability to mature DCs and induce T cell responses, whereas others are immunosuppressive. Synergism was observed with LPS and R848 as previously described for cytokine production (16). We show for the first time that this also translates into more efficient CD8+ T cell priming. Inhibition of both inflammatory cytokine production and T cell priming was observed when LPS and PolyI:C were combined. Similar inhibition occurred when zymosan was added to PolyI:C (Supplementary Figure 3), which was recently confirmed in a study showing that TLR2 ligation controls inflammation induced by PolyI:C (17). Altogether these findings suggest that general co-ligation of the TLR2/TLR4 with the TLR3 pathways will lead to suppressed levels of pro-inflammatory cytokines.
Our study shows that the antagonism of LPS on TLR ligation occurs through an IL-10 dependent mechanism. The involvement of IL-10 was demonstrated by using blocking antibodies to IL-10 and IL-10R, which completely rescued the inhibitory profile elicited by LPS. Phosphorylation of p38 MAP kinase was required for maximal IL-10 secretion as selective inhibition of p38 MAP kinase phosphorylation reduced IL-10 secretion. We postulate that the consistent partial rescue is due to the fact that p38 MAP kinase is also required for 12p70 production (18). IL-10 activity required STAT3 as verified by using siRNA specific for STAT3. Altogether these results indicate that LPS activates p38 MAP kinase, leading to IL-10 (as well as IL-12) production. IL-10 subsequently mediates its inhibitory effects via IL-10R and STAT3, which exert the immunosuppressive activity. IL-10 has recently been shown to lead to ubiquitination of MyD88 associated signaling molecules (19), suggesting a possible additional mechanism of action in attenuating TLR dependent responses.
We also verified the previous findings (13) that PI3K is a negative regulator of TLR signaling (18). PI3K has been implicated in the negative regulation of the p38 and NFkappa B pathways that are crucial for full TLR signaling cascades (13). Although our data suggest an IL-10 independent activity of PI3K on TLR signaling, as PI3K blockade resulted in higher amounts of IL-10, it remains possible that PI3K regulates IL-10 uptake or its downstream effects.
Interestingly, in human DCs other TLR agonist combinations such as R848/PolyI:C or R848/LPS showed at least additive or synergistic effects in their ability to induce high levels of inflammatory cytokines in vitro despite high levels of IL-10. We explained these phenomena by showing that the kinetics of IL-10 secretion is different among the TLR agonists used. Namely, LPS is capable of inducing IL-10 very early, at about 3h, while R848 does so at about 6–9 h. This delay allows for synergism of R848/PolyI:C to occur, that can be further augmented if blocking antibodies to IL-10 and IL-10R are present. The R848/LPS combination also displayed a weak synergistic profile especially in the induction of IL-12p70, despite early secretion of IL-10. Here we postulate that the combined signals from TLR4 and TLR8 were sufficiently potent to allow for small amounts of IL-12 to be secreted, but which is still dependent on IL-10, as its blockade released several fold higher levels of all cytokines measured (e.g. IL-12p70, TNFα, IL-6, etc.). These findings indicate that the timing of IL-10 secretion influences synergy between TLR agonists.
The levels of inflammation also translated into differential priming capability of DCs. IL-10 suppressed expression of CD80 and CD86, while it up regulated PDL-1. If MoDCs were matured with PolyI:C while pulsed with MelanA/Mart-1 (ELAGIGILTV) peptide, they yielded a higher frequency of tetramer positive cells compared to the LPS or LPS/PolyI:C stimuli tested. Furthermore, CD8+ MelanA/Mart-1+ cells generated from conditions inducing strong inflammatory profile also secreted higher levels of IFNγ, TNFα, and IL-2. Altogether, blocking IL-10 augmented the frequency of CD8+ MelanA/Mart-1+ cells induced, in all conditions where IL-10 was initially secreted, most apparently through higher expression of IL-12. The higher levels of CD80 and CD86 and lower expression of PDL-1 also contribute to the amplification in priming. No significant change/ enhancement was seen with PolyI:C which induces little to no IL-10.
The implications of these findings are many. PolyI:C appears to be the most potent TLR adjuvant tested due to its induction of pro-inflammatory cytokines in the absence of IL-10, and maintenance of high levels of CD80 and CD86. PolyI:C is already approved for clinical testing in humans, and can be used to mature DC ex vivo prior to their administration in vivo or by in situ administration. The use of PolyI:C in animal models has thus far yielded impressive results in terms of proinflammatory cytokines secretion by bone marrow derived DCs and priming of polyvalent CD4+ responses (9). Our results point to ways to enhance vaccines that engender immunity.
In summary, the ability of LPS to mediate IL-10 dependent suppression on the PolyI:C induced inflammation suggests complex interactions of TLR agonists with each other. It also unveils an important role temporal secretion of IL-10 has in the biologic system, while possibly allowing us to exploit it in vaccine design.
Supplementary Material
Acknowledgments
We would like to thank Philip Greenberg and Jeff Pufnock for providing us with the protocol for CD8+ T cell priming and for helpful discussions. This work was supported by the Bill and Melinda Gates Foundation, Melanoma Research Alliance, Cancer Research Institute, Alliance for Lupus Research, NIH R01 AI071078, NIH R01 AI044628 and the Emerald Foundation.
References
- 1.Bhardwaj N. Harnessing the immune system to treat cancer. J Clin Invest. 2007;117:1130–6. doi: 10.1172/JCI32136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–203. doi: 10.1172/JCI31205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lamm DL, Blumenstein BA, Crawford ED, et al. A randomized trial of intravesical doxorubicin and immunotherapy with bacille Calmette-Guerin for transitional-cell carcinoma of the bladder. N Engl J Med. 1991;325:1205–9. doi: 10.1056/NEJM199110243251703. [DOI] [PubMed] [Google Scholar]
- 4.Brichard VG, Lejeune D. GSK’s antigen-specific cancer immunotherapy programme: pilot results leading to Phase III clinical development. Vaccine. 2007;25 (Suppl 2):B61–71. doi: 10.1016/j.vaccine.2007.06.038. [DOI] [PubMed] [Google Scholar]
- 5.Vansteenkiste J. Final results of a multi-center, double-blind, randomized, placebo-controlled phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC) Proc Am Soc Clin Oncol. 2007 [Google Scholar]
- 6.Shackleton M, Davis ID, Hopkins W, et al. The impact of imiquimod, a Toll-like receptor-7 ligand (TLR7L), on the immunogenicity of melanoma peptide vaccination with adjuvant Flt3 ligand. Cancer Immun. 2004;4:9. [PubMed] [Google Scholar]
- 7.Adams S, O’Neill DW, Nonaka D, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–84. doi: 10.4049/jimmunol.181.1.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pufnock JS, Cigal M, Rolczynski LS, et al. Priming CD8+ T cells with dendritic cells matured using TLR4 and TLR7/8 ligands together enhances generation of CD8+ T cells retaining CD28. Blood. doi: 10.1182/blood-2010-11-317966. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Longhi MP, Trumpfheller C, Idoyaga J, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. J Exp Med. 2009;206:1589–602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.O’Neill DW, Bhardwaj N. Differentiation of peripheral blood monocytes into dendritic cells. Curr Protoc Immunol. 2005;Chapter 22(Unit 22F):4. doi: 10.1002/0471142735.im22f04s67. [DOI] [PubMed] [Google Scholar]
- 11.Vandepapeliere P, Horsmans Y, Moris P, et al. Vaccine adjuvant systems containing monophosphoryl lipid A and QS21 induce strong and persistent humoral and T cell responses against hepatitis B surface antigen in healthy adult volunteers. Vaccine. 2008;26:1375–86. doi: 10.1016/j.vaccine.2007.12.038. [DOI] [PubMed] [Google Scholar]
- 12.O’Garra A, Murphy KM. From IL-10 to IL-12: how pathogens and their products stimulate APCs to induce T(H)1 development. Nat Immunol. 2009;10:929–32. doi: 10.1038/ni0909-929. [DOI] [PubMed] [Google Scholar]
- 13.Fukao T, Koyasu S. PI3K and negative regulation of TLR signaling. Trends Immunol. 2003;24:358–63. doi: 10.1016/s1471-4906(03)00139-x. [DOI] [PubMed] [Google Scholar]
- 14.Lee HK, Lund JM, Ramanathan B, Mizushima N, Iwasaki A. Autophagy-dependent viral recognition by plasmacytoid dendritic cells. Science. 2007;315:1398–401. doi: 10.1126/science.1136880. [DOI] [PubMed] [Google Scholar]
- 15.Ma W, Lim W, Gee K, et al. The p38 mitogen-activated kinase pathway regulates the human interleukin-10 promoter via the activation of Sp1 transcription factor in lipopolysaccharide-stimulated human macrophages. J Biol Chem. 2001;276:13664–74. doi: 10.1074/jbc.M011157200. [DOI] [PubMed] [Google Scholar]
- 16.Napolitani G, Rinaldi A, Bertoni F, Sallusto F, Lanzavecchia A. Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat Immunol. 2005;6:769–76. doi: 10.1038/ni1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai Y, Di Nardo A, Nakatsuji T, et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat Med. 2009;15:1377–82. doi: 10.1038/nm.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fukao T, Tanabe M, Terauchi Y, et al. PI3K-mediated negative feedback regulation of IL-12 production in DCs. Nat Immunol. 2002;3:875–81. doi: 10.1038/ni825. [DOI] [PubMed] [Google Scholar]
- 19.Chang J, Kunkel SL, Chang CH. Negative regulation of MyD88-dependent signaling by IL-10 in dendritic cells. Proc Natl Acad Sci U S A. 2009;106:18327–32. doi: 10.1073/pnas.0905815106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.