Abstract
Mitochondria participate in apoptosis through a range of mechanisms that vary between vertebrates and invertebrates. In vertebrates, they release intermembrane space proteins, such as cytochrome c, to promote caspase activation in the cytosol. This process is the result of the loss of integrity of the outer mitochondrial membrane caused by proapoptotic members of the Bcl-2 family. This event is always accompanied by a fissioning of the organelle. Fission of mitochondria has also been reported to participate in apoptosis in Drosophila and Caenorhabditis elegans. However, in these organisms, mitochondrial membrane permeabilization does not occur and the mechanism by which mitochondrial dynamics participates in cell death remains elusive.
Introduction
Mitochondria are essential organelles because they supply the cell with metabolic energy in the form of ATP generated by oxidative phosphorylation. In addition they perform a number of other key metabolic reactions. Their shape, from spherical to elongated, is continually remodeled by fusion and fission events that link all the organelles within a cell into a continuum over time. Although why mitochondria are so dynamic is not known, the process is essential for mitochondrial maintenance in yeast (Hoppins et al., 2007) and mammals (Chen et al., 2003). Recently, mitochondrial fission has been linked to the cellular death program of apoptosis. Mitochondria are involved in the so-called intrinsic pathway of apoptosis where they release soluble proteins, including cytochrome c, from the intermembrane space to initiate caspase activation in the cytosol (Kroemer et al., 2007; Vaux, 2011). The release of these proteins is a consequence of the integrity of the mitochondrial outer membrane (OMM) being compromised, a process called mitochondrial outer membrane permeabilization (MOMP). So far, this process has only been validated in vertebrates; it does not seem to be required in C. elegans and is debated in Drosophila. As reviewed here, in these invertebrates, mitochondria may be involved in apoptosis through distinct mechanisms. In vertebrates, MOMP is under the control of the proapoptotic Bcl-2 family members. Here, we will focus on the mechanisms by which Bcl-2 family members elicit MOMP during apoptosis. We will also review the link that connects some Bcl-2 family proteins with fission and fusion of the organelle in apoptosis and in healthy cells.
Bcl-2 family members
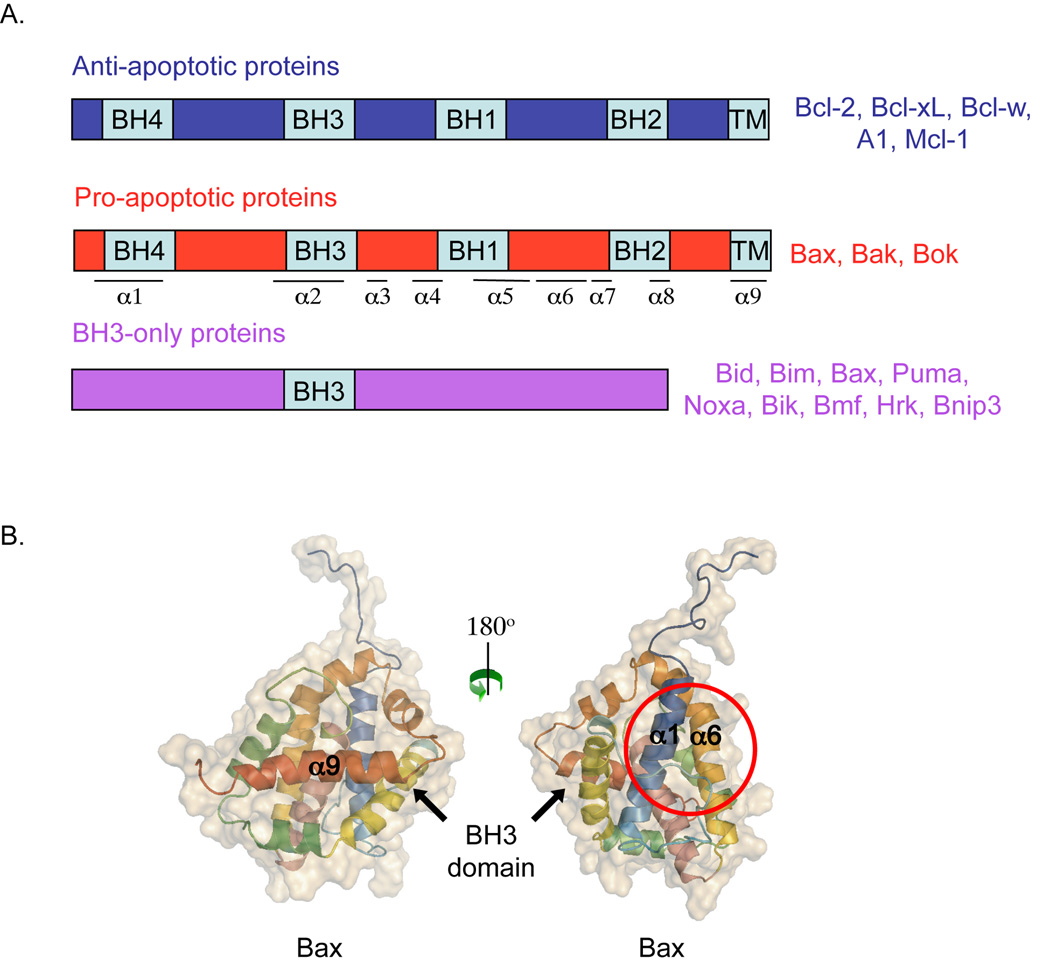
Bcl-2 family proteins are subdivided into three groups on the basis of their pro- or antiapoptotic action and the Bcl-2 Homology (BH) domains they possess (Figure 1A)(Schinzel et al., 2004; Youle and Strasser, 2008). Antiapoptotic Bcl-2-like proteins (e.g. Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and A1/Bfl-1) and proapoptotic Bax-like proteins (e.g. Bax, Bak and Bok/Mtd) display 4 BH domains (Kvansakul et al., 2008). The proapoptotic BH3-only proteins (e.g. Bid, Bim/Bod, Bad, Bmf, Bik/Nbk, Blk, Noxa, Puma/Bbc3 and Hrk/DP5), on the other hand, possess only a short motif called the BH3 domain as their name indicates. BH3 proteins integrate and transmit death signals that emanate from defective cellular processes to other Bcl-2 family members. Through their BH3 domain, these proteins either interact with antiapoptotic proteins to inhibit their function and/or to interact directly with multidomain proteins such as Bax or Bak to stimulate their activity. The former are often referred to as ‘sensitizers’, whereas the later are classified as ‘activators’ (reviewed in Giam et al., 2008). The multidomain proapoptotic proteins, Bax and Bak, and perhaps Bok in some tissues, are responsible for MOMP and are the master effectors of apoptosis as cells lacking Bax and Bak fail to undergo MOMP and apoptosis in response to many death stimuli (Wei et al., 2001).
Figure 1. Structure of Bcl-2 family proteins.
A) The Bcl-2 family is divided into three groups based on their Bcl-2 Homology domains (BH). Pro- and antiapoptotic proteins contain 4 BH domains, while BH3-only proteins, as their name indicates, contain only the BH3 domain. B) Structure of Bax showing the alpha-helix 9 (transmembrane domain) embedded in the hydrophobic groove (left structure). A 180° rotation (structure on the right) shows the alpha-helices 1 and 6. The red circle represents the binding site for the Bim BH3 domain.
Mechanisms of activation of proapoptotic Bcl-2 family members
Activation of the proapoptotic Bcl-2 family protein Bax results from a highly regulated multistep process involving its translocation from the cytosol to the OMM where it inserts and oligomerizes. In contrast to Bax, Bak is constitutively inserted in the OMM by a C-terminal transmembrane domain. Its insertion can be facilitated by the voltage-dependent anion channel isoform 2 with which it was found to interact (Cheng et al., 2003; Lazarou et al. 2010; Roy et al., 2009). In this section, we will describe what maintains Bax in the cytosol in healthy cells and how BH3-only proteins, together with the lipid bilayer, cooperate to promote oligomerization of Bax and Bak in the OMM during apoptosis.
Bax travels back and forth from the cytosol to the OMM in healthy cells
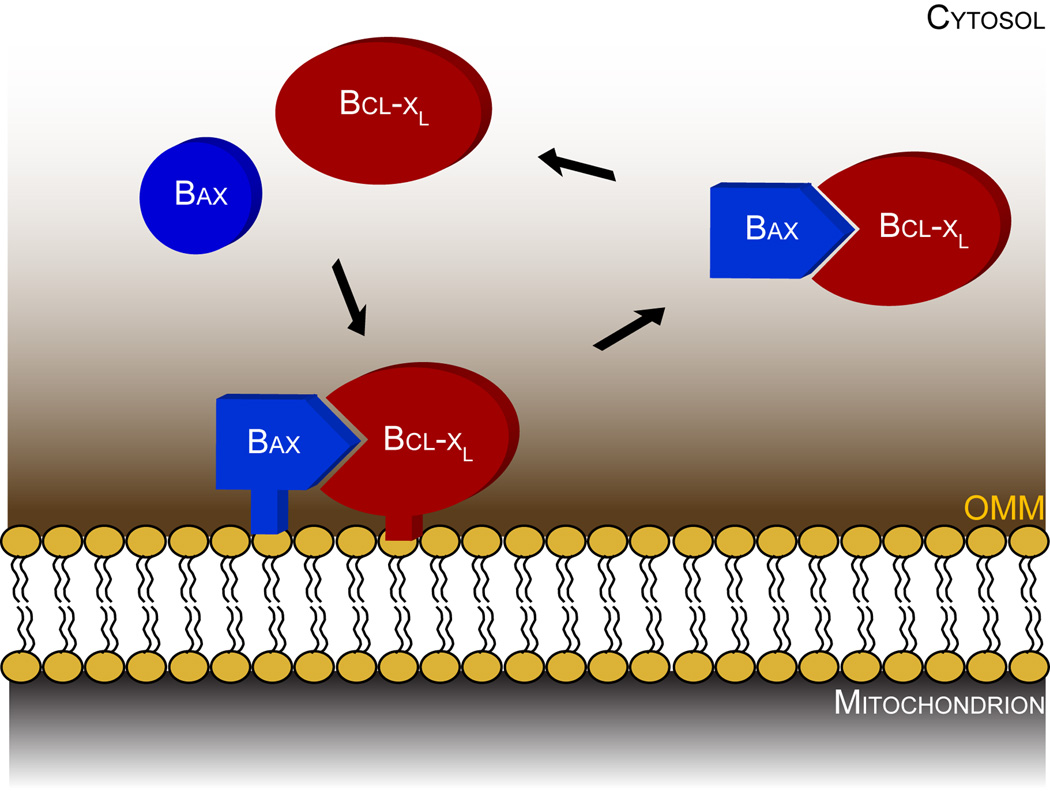
In healthy cells, Bax is mainly cytosolic with a minor fraction loosely attached to mitochondria. In contrast to what is observed in cultured cells, the amount of membrane-bound Bax is negligible in tissues such as liver or brain. This suggests that the mitochondrial sub-population of Bax at the mitochondria could be a measure of the stress experienced by the cells, which increases with in vitro culture. Using fluorescence loss in photobleaching, Edlich et al. (2011) observed that Bax constantly travels back and forth from the cytoplasm to mitochondria (Figure 2). Once attached to the OMM, it can be retrotranslocated to the cytosol by Bcl-xL by a mechanism that is not yet fully understood. Bax retrotranslocation by Bcl-xL and possibly by other prosurvival Bcl-2 proteins, would ensure that Bax does not chronically accumulate at the OMM to reach a critical level that could promote its autoactivation. It is still unclear what allows Bax to attach to the OMM specifically in healthy cells. The most likely explanation is that Bax undergoes discrete and reversible conformational changes that would expose not only its BH3 domain but also its N- and C-terminal domains. Consistently, it was previously observed that during detachment of cells from the extracellular matrix, the N-terminus of Bax undergoes a conformational change that can be reversed upon re-attachment of cells to substrate (Gilmore et al., 2000). Understanding what triggers and controls changes in Bax conformation in healthy cells requires further investigations. A reversible conformational change in Bax could be triggered by a simple contact of the protein with the lipid bilayer of the OMM, as suggested by experiments performed with liposomes (Yethon et al., 2003). Other triggers, including BH3-only proteins, Prostaglandins (Lalier et al.), p53 (Chipuk et al., 2004), pH variations (Khaled et al., 1999), or post-translational modifications (Kutuk and Letai, 2008), all could also be responsible for sending Bax to the OMM. But what would prevent a complete conformational change in Bax in healthy cells? The structure of the OMM may counteract Bax activation. Its cholesterol content has been shown to prevent Bax insertion or oligomerization in the OMM (Christenson et al., 2008; Lucken-Ardjomande et al., 2008). Moreover, previous data have shown that stress-induced mitochondrial hyperfusion, which leads to highly elongated organelles, delays Bax activation upon exposure of cells to apoptotic stimuli (Tondera et al., 2009). Thus, the lipid content and the shape of the OMM may be refractory to complete Bax activation in healthy cells.
Figure 2. Bax moves back and forth from the cytosol to the mitochondrial outer membrane.
In healthy cells, Bax (blue) and Bclx-L (red) are translocated from the cytosol to the outer mitochondrial membrane (OMM) where they attach loosely. The triangle in Bax represents its BH3 domain, which needs to be exposed in order for Bcl-xL-mediated retrotranslocation to occur. OMM: outer mitochondrial membrane.
The role of BH3-only proteins and of the lipid bilayer
The protein tBid has been originally described as a direct activator of Bax, able to induce a conformational change of the Bax N-terminus, as well as modulate Bax insertion and oligomerization in the OMM (Desagher et al., 1999; Eskes et al., 2000). Many groups have confirmed and extended these observations to other BH3-only proteins, including Bim and Puma (Gallenne et al., 2009; Kim et al., 2009; Kuwana et al., 2005; Merino et al., 2009; Ren et al. 2010). Importantly, these studies have also provided details about the mechanisms by which BH3-only proteins trigger conformational changes in Bax, leading to its insertion and oligomerization in the OMM. Most BH3-only proteins, except Bid, appear to be intrinsically unstructured proteins. However, their BH3 domain is known to form an alpha-helix upon binding to the hydrophobic groove that is on the surface of antiapoptotic proteins. In Bax, this hypdrophobic groove is occupied by alpha-helix 9 (the transmembrane domain) (Figure 1B). Until recently, it was unclear where BH3 activators impact Bax to induce the structural changes required for its insertion and assembly in the OMM. To answer this question, Walenski and colleagues used peptides whose alpha-helicity was stabilized by a chemical modification termed ‘hydrocarbon stapling’. They derived staple peptides from the BH3 domain of Bid or Bim and reported the first evidence of a direct interaction between the BH3 domain of Bim and Bid with Bax (Gavathiotis et al., 2008; Walensky et al., 2006). Moreover, consistent with previous data (Cartron et al., 2004), their studies localized the binding site of the BH3 domain of Bim to the junction of alpha-helices 1 and 6 on the N-terminal side of Bax, i.e. on the opposite side of the hydrophobic groove (Gavathiotis et al., 2008) (Figure 1B). Thus, BH3-only proteins appear to interact with different sites in Bax and in prosurvival Bcl-2 proteins in which the hydrophobic groove is the receiving domain. According to a model derived from recent NMR studies, upon interaction with Bim, Bax structural metamorphosis begins with the displacement of its alpha-helices 1 and 2 from a closed to an open position, which leads to the release of the alpha-helix 9 from the hydrophobic groove and exposure of the BH3 domain (Gavathiotis et al. 2010). Whether the results obtained with peptides in solution in these studies faithfully reflect what occurs in the cell, at the OMM, will require further investigation (discussed in Czabotar et al., 2009). Moreover, it will be important to clarify where BH3-only proteins impact Bak, as a previous report defined the hydrophobic groove as the receiving domain of the tBid BH3 domain (Moldoveanu et al., 2006).
In contrast to the stapled Bim peptide, tBid is unable to interact with full length Bax or Bcl-xL in solution (Lovell et al., 2008) begging the question of where in the cell do interactions between BH3-only proteins and Bax or Bak occur. Following its cleavage by caspase 8, the N-terminus of Bid (N-Bid) remains attached to the C-terminal portion of the protein (C-Bid), possibly masking the BH3 domain in C-Bid. However, in the presence of a lipid bilayer with a phospholipid composition mimicking that of the OMM, it was shown that only C-Bid inserts while N-Bid remains in solution (Lovell et al., 2008). Thus, upon interaction with the membrane, tBid is fully operational to recruit Bax to the OMM as shown by fluorescence resonance energy transfer (Lovell et al., 2008). These results emphasize the importance of the lipid environment for activation of these Bcl-2 proteins and prompted Andrews and colleagues to propose the ‘membrane-embedded together’ model. According to this model, tBid first inserts in the membrane, then recruits Bax that in turn inserts and oligomerizes. According to these authors, Bcl-xL and other antiapoptotic proteins such as Bcl-w that are in part cytosolic, are also recruited and activated at the membrane by both the activator and sensitizer BH3-only proteins (Shamas-Din et al. 2011). This part of the model confers on BH3-only proteins an ambiguous role since, in theory, they could recruit both pro- or antiapoptotic proteins. If this is the case, and if the role of antiapoptotic Bcl-2 proteins in the OMM is to prevent Bax or Bak oligomerization, then BH3-only proteins should be classified as antiapoptotic proteins, which is not consistent with their known function. Therefore, it is unlikely that the BH3-only proteins recruit antiapoptotic Bcl-2 family members into the OMM to inhibit Bax or Bak function. Rather, BH3-only proteins may recruit antiapoptotic proteins and remain tightly bound to them to inhibit their function. Soluble and membrane bound antiapoptotic Bcl-2 proteins could therefore be inhibited either in the cytosol or at the membrane level by soluble or membrane-associated BH3-only proteins, respectively. Moreover, antiapoptotic Bcl-2 proteins, whether constitutively present in membranes (such as Bcl-2), or cytosolic (such as Bcl-xL and Bcl-w), have the possibility to directly interact with the BH3-only domain of Bax or Bak during the process of their oligomerization. This implies that the affinity of antiapoptotic proteins for the BH3 domain of Bax or Bak must be very high to compete with other Bax or Bak molecules and prevent their homo-oligomerization.
Is the lipid composition of the membrane crucial for tBid-induced Bax oligomerization?
Using liposomes containing phosphatidylcholine and phosphatidylglycerol, it was previously found that tBid can recruit Bax to the membrane and drive formation of small pores that could allow passage of small molecules (such as carboxyfluorescein) but were impermeable to cytochrome c (Roucou et al., 2002). Analysis of the quaternary structure of Bax revealed that the protein was monomeric under this condition. At the same time, Kuwana et al. (2002) and later others (Lucken-Ardjomande et al., 2008; Terrones et al., 2004), reported that cardiolipin is required for the formation of tBid-induced Bax oligomers and of giant pores. Thus the presence of cardiolipin in the membrane appears to promote tBid-induced Bax oligomerization and formation of cytochrome c permeable pores. Cardiolipin is a negatively charged phospholipid, exclusively found in mitochondria, mainly in the IMM where it is important for the activity of several complexes of the respiratory chain and of some transporters (Hasler, 2011; Schlame et al., 2000; Schug and Gottlieb, 2009). Subfractionation of the mitochondrial membranes, together with the accessibility of cardiolipin to enzymatic degradation by Phospholipase 2 added to isolated mitochondria, indicate that small amounts of this phospholipid are also present in the OMM, probably enriched at contact sites between the OMM and the IMM (Ardail et al., 1990; Daum, 1985; Epand et al., 2007; Gebert et al., 2009; Hovius et al., 1990). tBid was found to bind cardiolipin through central alpha-helices, which would explain its recruitment to mitochondria, principally at contact sites between the IMM and the OMM (Kim et al., 2004; Lutter et al., 2000). In addition to promoting membrane insertion of tBid, cardiolipin could be involved in the Bax oligomerization process itself as suggested by the experiment described above (Roucou et al., 2002). Besides cardiolipin, Gross and colleagues recently identified MTCH2/MIMP, a protein similar to mitochondrial carriers of the adenine nucleotide translocator family, as a receptor for tBid (Zaltsman et al. 2010). In the absence of this protein, tBid is recruited less efficiently to the OMM and apoptosis is delayed when cells are exposed to tBid-dependent death stimuli. Thus MTCH2/MIMP seems to accelerate the recruitment of tBid to the OMM, and may cooperate with cardiolipin to provide the OMM with an optimal concentration of tBid. Further experiments, in particular using proteoliposomes prepared with recombinant MTCH2/MIMP, are required to determine how tBid binds this carrier protein. Moreover, the requirement of cardiolipin for Bax activation and MOMP in the cell requires further investigation as several reports have shown that this phospholipid can be dispensable for Bax activation (Gonzalvez et al., 2008; Iverson et al., 2003; Polcic et al., 2005). Importantly, in most studies using liposomes, the process of Bax oligomerization induced by tBid does not appear to be optimal as only a small fraction of Bax oligomerizes, even when high concentrations of tBid are used and despite the presence of cardiolipin in membranes (Kuwana et al., 2002; Schafer et al., 2009). This suggests that, in vitro, either important components that facilitate Bax insertion and/or oligomerization are missing or that the lipid bilayer structure in these experimental se-ups is not optimal to allow efficient Bax oligomerization.
The role of mitochondrial fission
Mitochondria are dynamic organelles that fuse and divide continuously. The core components of the fusion and fission machineries have been identified as the dynamin-related proteins Drp1, mitofusins (Mfn) 1 and 2 and Opa1 (Box 1) (reviewed in Westermann, 2010). Fission of mitochondria is a constant in apoptosis (Figure 3)(Martinou and Youle, 2006). The mechanism underlying this event appears to involve Drp1, the most downstream component of the fission machinery (Frank et al., 2001). The precise mechanism by which Drp1 is recruited to the mitochondria during apoptosis remains vague. Under normal conditions, Drp1 moves back and forth from the cytosol to the OMM. However, following Bax activation, Drp1 stably associates with the OMM due to Bax/Bak-dependent SUMO modification (Wasiak et al., 2007). However, the mechanistic link between activation of Bax or Bak and Drp1 SUMOylation is still missing. Importantly, it should be mentioned that inhibition of Drp1, delays, but does not completely prevent, mitochondrial fission (Ishihara et al., 2009). This suggests that a Drp1-independent mechanism also seems to participate in the fission of the organelle during apoptosis. A link between mitochondrial fission and Bax activation was suggested by the detection of active Bax in discrete foci at mitochondrial fission sites (Karbowski et al., 2002). Bak, which initially surrounds the organelle, also coalesces into foci at mitochondrial fission sites during apoptosis. In most cases, (although not always), these foci appear to be located in proximity to Drp1 and Mfn2 and form independently of Drp1 activity (Frank et al., 2001; Ishihara et al., 2009; Montessuit et al. 2010). By inhibiting Drp1 and delaying mitochondrial fission, Karbowski et al. (2002) could observe these Bax spots at the surface of elongated mitochondria. Interestingly, some of them were located at constriction sites that the authors described as ‘aberrant mitochondrial scission attempts’. This observation further supports the existence of a Drp1-independent mechanism responsible for constricting, and possibly fissioning, the organelle during apoptosis. Such constriction sites have also been observed in healthy Drp1-deficient cells, not only on mitochondria (Ishihara et al., 2009), but also on peroxisomes, which rely on Drp1 for division (Koch et al., 2004). These sites may provide the appropriate membrane curvature, or lipid composition, for the assembly of Drp1 rings and the formation of Bax foci during apoptosis.
Box 1: Dynamin- related proteins involved in the fusion and fission mitochondrial machineries.
Drp1 is a large GTPase of the dynamin family that is required for mitochondrial fission. The protein is cytosolic and translocates to the mitochondria where it binds to receptors such as Mff and Fis1. It assembles into rings around mitochondria and constricts and breaks down their membrane by a mechanism that is not completely understood.
Mitofusins: two mitofusins (Mfn), Mfn1 and Mfn2, are required in the fusion of the outer mitochondrial membrane. These proteins are integrated in the OMM, facing the cytosol, and mediate the docking of mitochondria to one another during fusion by engaging in trans-homo-oligomeric and hetero-oligomeric complexes.
Opa1 (Optic atrophy 1): As its name implies this protein was found to be mutated in the disease optic atrophy, which leads to degeneration of the optic nerve. Opa1 is required for fusion of the inner mitochondrial membrane. Different isoforms of this protein that form a complex integrated in the IMM, facing the intermembrane space of mitochondria, have been identified.
Figure 3. Model for Bax activation and mitochondrial outer membrane permeabilization: the role of membrane hemifission and hemifusion intermediates.
A) During apoptosis, Drp1 and Bax are recruited to mitochondrial foci. As a result MOMP occurs concomitantly with mitochondrial fission. The release of cytochrome c promotes caspase activation in the cytosol. A magnification of what occurs at the level of mitochondrial fission sites is shown in (B). According to the ‘embeded together model’, tBid first inserts in the outer mitochondrial membrane (OMM) to expose its BH3 domain. Upon interaction with the Bid BH3 domain, Bax is in turn recruited to the OMM where it inserts and oligomerizes. This process does not occur randomly at the OMM surface. Bax mainly oligomerizes at mitochondrial fission sites and at contact sites between the OMM and the IMM. C) (Left) A detailed structure of the hemifission intermediate that forms before fission of the organelle. The membrane forms a non bilayer structure that represents a privileged site for the formation of a membrane hole. Upon its oligomerization in the OMM, Bax forms a lipidic pore or triggers the formation of a hole at the edge of the hemifission intermediate. (Right) A detailed structure of the membrane at OMM and inner mitochondrial membrane (IMM) contact sites. The model postulates that Bax forms a pore in the membrane at the edges of membrane hemifusion intermediates that may form between the OMM and the IMM.
It was recently found that the formation of membrane hemifusion intermediates is a process able to promote tBid-induced Bax oligomerization (Montessuit et al. 2010). This membrane remodeling can be promoted by Drp1 in a cell free system and may recapitulate the membrane remodeling that occurs at mitochondrial fission sites in cells undergoing apoptosis (Figure 3). When membranes divide or fuse, contacting leaflets fuse to form a stalk. The stalk then expands to form a metastable non-bilayer membrane intermediate also called hemifission or hemifusion intermediate for membrane fission and fusion respectively (Chernomordik and Kozlov, 2008; Kozlovsky and Kozlov, 2003). For fusion, this transient state would evolve towards the formation of a fusion pore, while for membrane fission, it would decay spontaneously into two separate membranes, thereby completing the fission process. The mechanism by which these membrane intermediates promote Bax oligomerization is still unclear. Hemifusion or hemifission intermediates are expected to occur during fusion or fission of the organelle, respectively. These structures may also possibly form at contact sites between the OMM and the IMM, which are enriched in cardiolipin and phosphatidylethanolamine, two phospholipids that have a propensity to form non-bilayer structures (Figure 3). The limited number of such membrane structures at the surface of mitochondria may explain why Bax or Bax oligomers are not randomly distributed in the OMM in apoptotic cells.
How does Bax or Bak permeabilize the outer mitochondrial membrane?
The mechanism by which Bax, or Bak, permeabilizes membranes to allow efflux of large proteins is still unclear, although several models having been proposed and challenged. These models predict that Bax, alone or combined with other proteins, forms channels large enough to allow passage of cytochrome c and other proteins. Alternatively, Bax or Bak could modulate the opening of existing channels such as the so-called permeability transition pore, to induce MOMP. However, opening of the permeability transition pore has been questioned by a number of genetic studies that have excluded the requirement of some of its major components (reviewed in Tait and Green 2010; Westphal et al. 2011). It is therefore unlikely that the permeability transition pore plays a role in Bax and Bak-induced MOMP.
Proteinaceous or lipidic pores
The model of Bax or Bak pore formation finds its roots in the determination of the 3D structure of Bcl-xL (Muchmore et al., 1996). This protein exhibits a structural similarity to bacterial pore-forming toxins - certain colicins and the translocation domain of diphtheria toxin-. Other members of the Bcl-2 family were later determined to have a similar structure. Consistent with this structural resemblance, Bax and Bcl-2 were found to form pores in planar lipid bilayers (Antonsson et al., 1997; Schlesinger et al., 1997). The ability of Bax and Bak to induce MOMP seems to reside at least in part in the nature of their central alpha-helices 5 and 6. Thus, replacing the alpha-helix 5 of Bcl-xL by the equivalent in Bax is sufficient to turn Bcl-xL into a ‘killer’ protein (George et al., 2007). In addition, differences in the ability of pro- and anti-survival proteins to oligomerize could explain their capacity to induce MOMP. Biochemical data support a model in which Bak and Bax oligomers are formed by two interfaces involving the BH3:groove and alpha-helix 6: alpha-helix 6 interfaces, while alpha-helices 5, 6 and 9 are embedded in the membrane (Dewson et al., 2008; Zhang et al. 2010). Although a model for Bax or Bak oligomerization is beginning to emerge, the atomic details of membrane-embedded Bax and Bak oligomer are still missing. In particular the number of units present in the Bax or Bak oligomers remains elusive and estimates vary considerably depending on the approaches used. Assessing the minimal size of the Bax or Bak oligomer that is responsible for MOMP is critical, but difficult. Indeed, once oligomerization has been initiated, self-oligomerization proceeds which may lead to the recruitment of hundreds of Bax molecules, that then protrude out of the OMM (Nechushtan et al., 2001b). Whether all these molecules are required for pore-formation in the membrane is questionable. Such heterogeneity of oligomers may hamper resolution of the 3D structure of these proteins within a membrane. Moreover the nature of the pore formed by Bax -- whether proteinaceous or lipidic (Box 2) -- is still under debate. Although some reports favor formation of a proteinaceous channel (Dejean et al., 2005; Epand et al., 2002), other results are consistent with formation of a lipidic pore (Basanez et al., 1999; Garcia-Saez et al., 2007; Garcia-Saez et al., 2006). In favor of the latter model, alpha-helix 5 of Bax, which is sufficient to perforate membranes, was found to form lipidic pores in a synthetic membrane by X-ray diffraction (Qian et al., 2008). However, one must be cautious with overall structural predictions of Bax based on data obtained with only minimal domains of the protein. Nevertheless, there are other reasons to favor the lipidic pore model for Bax and Bak. Recently, analysis of Bax-permeabilized liposomes by cryo-electron microscopy revealed large openings of the membrane, from 25 to 100 nm, compatible with the release of megadalton dextrans. Importantly, the edges of these pores were devoid of protein (Bleicken et al. 2010; Schafer et al., 2009). Whether pores of this type are formed in the OMM of mitochondria undergoing MOMP remains now to be shown.
Box 2: Proteinaceous vs lipidic pores.
Many toxins are known to spontaneously induce transmembrane pores in lipid bilayers under certain conditions. Depending on their structure, they can either form proteinaceous or lipidic pores (Yang et al., 2001).
Proteinaceous pores (also called barrel-stave pores): toxins form a barrel-like pore that spans the membrane. The pore lumen is lined by peptides that are perpendicularly inserted in the membrane
Lipidic pores (also called toroidal pores): insertion of the peptides in the membrane triggers lipid monolayer bending such that the outer and inner leaflets of the membrane are continuous. The pore is lined by both the peptides and the lipid head-groups.
Mechanistic link between mitochondrial dynamics and MOMP: the role of membrane hemifusion/hemifission intermediates
The notion that MOMP and mitochondrial fission are mechanistically linked remains controversial. In some reports, inhibition of mitochondrial fission or stimulation of fusion, significantly delayed apoptosis (Cassidy-Stone et al., 2008; Frank et al., 2001; Germain et al., 2005; Merrill et al. 2011). Puzzlingly, cells from the neural crest failed to die in Drp1-knockout mouse embryos (Wakabayashi et al., 2009), whereas mouse embryonic fibroblast cell lines generated from these Drp1-deficient mice displayed little (Wakabayashi et al., 2009) or partial (Ishihara et al., 2009) resistance to apoptosis. In other studies, inhibiting mitochondrial fission in HeLa cells had no or only a minor impact on the kinetics of MOMP and cell death (Estaquier and Arnoult, 2007; Ishihara et al., 2009; Parone et al., 2006; Sheridan et al., 2008). Moreover, some studies showed that it was possible to dissociate mitochondrial fission from MOMP. James et al. (2003) reported that Bcl-xL could inhibit MOMP and apoptosis due to overexpression of the putative Drp1 receptor hFis1, without preventing Fis-1-induced fission. On the other hand, Sheridan et al. (2008) observed that Bcl-xL, as well as other members of the apoptosis-inhibitory subset of the Bcl-2 family, antagonized Bax and/or Bak-induced cytochrome c release but failed to block mitochondrial fragmentation associated with Bax/Bak activation. Several studies also reported that inhibition of mitochondrial fission did not prevent the efflux of Smac/DIABLO, a protein present in the intermembrane space of mitochondria, although it could delay cytochrome c release (Estaquier and Arnoult, 2007; Parone et al., 2006). These data favor a model in which mitochondrial fission inhibition could retard cytochrome c release by preventing the remodeling of mitochondria cristae, a process that is thought to be required for efficient cytochrome c mobilization (Cipolat et al., 2006; Yamaguchi et al., 2008). Re-evaluation of these conflicting data, in particular in light of the new results by Montessuit et al. (2010) on the role of mitochondrial membrane hemifusion/hemifission intermediates in Bax oligomerization, provides at least two explanations for why inhibiting Drp1 can only delay cytochrome c release and cell death. First, this is because the inhibition of known components of the mitochondrial fission machinery cannot completely prevent mitochondrial fission as previously mentioned. Second this is because it appears that rather the fission of mitochondria per se, it is the formation of membrane hemifission intermediates that may play a central role in Bax oligomerization and MOMP. According to theoretical studies, a place where the cost in energy to form a membrane hole would be minimal is at the edge of a membrane hemifusion structure (Katsov et al., 2004). The observation that Bax is confined at discrete foci at the surface of mitochondria, some of which coincide with mitochondrial fission sites, together with the new role of membrane hemifusion or hemifission intermediates in the oligomerization of Bax during apoptosis, raise the possibility that Bax may opportunistically target those sites that are optimally designed for it to oligomerize and to perforate the membrane (Figure 3).
The role of mitochondria in Drosophila cell apoptosis
Steps of apoptosis upstream of caspases are regulated differently in flies and mammals (Salvesen and Abrams, 2004). Indeed, the role of Bcl-2 family members in Drosophila in apoptosis is relatively minor (Sevrioukov et al., 2007). In contrast, the Hid/Grim/Reaper gene products that derepress the IAP proteins that block caspases through the ubiquitin proteosome pathway, are potent inducers of fly cell apoptosis (Bader and Steller, 2009). Recent work in flies links mitochondrial fission and fusion processes to Hid, Grim and Reaper, as well as to the Bcl-2 family members Buffy and DEBCL and to apoptosis. As derepressors of cytosolic IAP, there had been no explanation why Hid (Haining et al., 1999), Grim (Claveria et al., 2002) and Reaper (Olson et al., 2003) localize to mitochondria until recent work has linked Reaper to the mitochondrial fusion machinery. Although caution should be taken in interpreting these results due to the expression in heterologous cells of fly proteins that lack known mammalian homologues, Reaper expressed in HeLa cells localizes to the OMM and inhibits mitochondrial fusion inducing mitochondrial fragmentation. Reminiscent of mammalian proapoptotic Bcl-2 family members (Nechushtan et al., 2001a), Reaper forms concentrated foci on mitochondria (Thomenius et al., 2011). Overexpression in HeLa cells of Mfn2, a dynamin family member that mediates mitochondrial fusion, changes Reaper localization from foci to more evenly coat mitochondria suggesting they interact, consistent with data that a peptide of Reaper can co-immunoprecipitate with Xenopus Mfn2 (Thomenius et al., 2011). Mitochondrial localization, mitochondrial fragmentation activity and Mfn2 interaction required the GH3 domain of Reaper indicating that Reaper binds Mfn2 on mitochondria through the GH3 domain that is required to induce apoptosis (Thomenius et al., 2011). Alternatively or additionally, mitochondrial Hid may recruit Reaper to mitochondria (Sandu et al. 2010). Interestingly, mitochondrial Reaper and Hid can recruit IAPs to mitochondria where they may induce IAP autoubiquitination and proteosomal degradation. Mitochondria fragment during apoptosis in Drosophila (Goyal et al., 2007) (Abdelwahid et al., 2007) possibly through Reaper interaction with mitofusin and inhibition of mitochondria fusion (Thomenius et al., 2011). Preventing this mitochondrial fragmentation by inhibition of Drp1 prevents death of fly cells in vitro and in vivo (Abdelwahid et al., 2007; Goyal et al., 2007).
Apoptosis in the worm
CED-9, the sole multidomain Bcl-2 family member in C. elegans, is localized to the mitochondria (Chen et al., 2000). Although it primarily inhibits apoptosis through the binding and sequestration of the caspase activator, CED-4, CED-9 can promote apoptosis in weak loss of function CED-3 caspase mutants (Hengartner and Horvitz, 1994). As in mammalian and Drosophila cells, mitochondria fragment in the worm during apoptosis and this fragmentation is dependent on CED-9 and the BH3-only protein, EGL-1 (Jagasia et al., 2005). Although there is no evidence of cytochrome c release or MOMP in the worm, inhibition of mitochondrial fragmentation by expression of a dominant negative inhibitor of Drp1 prevents 20% of the normal developmental cell death in C. elegans (Jagasia et al., 2005).
Bcl-2 family proteins and mitochondrial dynamics
The link between mitochondrial fission and apoptosis has been furthered strengthened by several studies that show Bcl-2 family members alter mitochondrial dynamics even in healthy cells (Delivani et al., 2006; Karbowski et al., 2006; Rolland et al., 2009; Tan et al., 2008). Overexpression of Bcl-2 family proteins CED-9 and Bcl-xL can induce mitochondrial fusion in mammalian cells (Delivani et al., 2006) and in C. elegans (Rolland et al., 2009; Yamaguchi et al., 2008). Bcl-xL can also accelerate mitochondrial fission in mammalian neurons thereby, accelerating mitochondrial dynamics (Berman et al., 2009). The proapoptotic Bax and Bak, although having the opposite effect on cell viability relative to Bcl-xL, are required for the normal rate of mitochondrial fusion in cells (Karbowski et al., 2006). Recent work also shows that in a cell free system of mitochondrial fusion, recombinant Bax can stimulate the process specifically through Mfn2 and not Mfn1 (Hoppins et al., 2011). Tethering Bax with disulfides to hinder conformational change abolishes the mitochondrial fusion activity indicating that at least subtle conformational changes are involved. Promotion of mitochondrial fusion may be a consequence of direct interaction between Bcl-2 family members and Mfn1/2 (Brooks et al., 2007; Delivani et al., 2006; Rolland et al., 2009; Cleland et al., 2010) although how mitofusins may be activated remains unclear. Recent work links Bcl-2 family members to mitochondrial dynamics also in Drosophila, during fly oogenesis. Loss of the fly Bcl-2 family members, Buffy and/or DEBCL, disrupts nurse cell death, which occurs after the nurse cells transfer some of their cytosol and organelles into the developing oocyte. Buffy and/or DEBCL loss also increase mitochondrial network formation and mitochondrial clumping in the surviving cells, suggesting that in certain tissues in the fly, Bcl-2 family members affect mitochondrial dynamics upstream of apoptosis (Tanner et al., 2011). Thus, links between Bcl-2 family members and mitochondrial dynamics have been identified in mammals, insects and roundworms. How the effects of the Bcl-2 family members on mitochondrial dynamics in healthy cells may relate to mitochondrial fragmentation during apoptosis (subsequent to Bcl-2 family member conformational change and insertion into mitochondrial membranes) is an important question that remains to be resolved.
Concluding remarks
Over the past decade, our understanding of the mechanism of action of Bcl-2 family members has expanded significantly. The 3D structure of many members of the family, in solution, has been solved and the mechanism by which these proteins interact is better understood, even though some controversies remain as to how BH3-only proteins regulate multidomain Bcl-2 family members. Importantly, the lipid composition and structural organization of the OMM has emerged as one of the central players in the regulation of Bcl-2 family member activity. However, the precise 3D structure that many Bcl-2 proteins adopt in this membrane is still unknown. Without this essential information, it is impossible to understand precisely how MOMP occurs. The challenge for the coming years will be to solve the structure of membrane-embedded key Bcl-2 family members and to understand how the lipid composition and structure of the OMM controls activation of Bcl-2 family members and MOMP.
Recent work from several laboratories has also unraveled new functions for many pro-and antiapoptotic members of the Bcl-2 family, besides their role in apoptosis. Although our review has been focused on the involvement of Bcl-2 family members in mitochondrial dynamics, it is clear that these proteins play additional roles in other cellular processes. These functions, and new ones that may be discovered in the future, will certainly help decipher the complex role Bcl-2 family members play in life and death of the cell.
Acknowledgments
We thank Drs Megan Cleland and Soojay Banerjee for help with artwork. JCM is supported by the Swiss National Foundation (subsidy 31003A-124968/1), Oncosuisse and the Geneva Department of Education. RJY is supported by the Intramural Program of the NINDS, NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelwahid E, Yokokura T, Krieser RJ, Balasundaram S, Fowle WH, White K. Mitochondrial disruption in Drosophila apoptosis. Dev Cell. 2007;12:793–806. doi: 10.1016/j.devcel.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, Bernasconi L, Bernard A, Mermod JJ, Mazzei G, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- Ardail D, Privat JP, Egret-Charlier M, Levrat C, Lerme F, Louisot P. Mitochondrial contact sites. Lipid composition and dynamics. J Biol Chem. 1990;265:18797–18802. [PubMed] [Google Scholar]
- Bader M, Steller H. Regulation of cell death by the ubiquitin-proteasome system. Curr Opin Cell Biol. 2009;21:878–884. doi: 10.1016/j.ceb.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basanez G, Nechushtan A, Drozhinin O, Chanturiya A, Choe E, Tutt S, Wood KA, Hsu Y, Zimmerberg J, Youle RJ. Bax, but not Bcl-xL, decreases the lifetime of planar phospholipid bilayer membranes at subnanomolar concentrations. Proc Natl Acad Sci U S A. 1999;96:5492–5497. doi: 10.1073/pnas.96.10.5492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SB, Chen YB, Qi B, McCaffery JM, Rucker EB, 3rd, Goebbels S, Nave KA, Arnold BA, Jonas EA, Pineda FJ, et al. Bcl-x L increases mitochondrial fission, fusion, and biomass in neurons. J Cell Biol. 2009;184:707–719. doi: 10.1083/jcb.200809060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Classen M, Padmavathi PV, Ishikawa T, Zeth K, Steinhoff HJ, Bordignon E. Molecular details of Bax activation, oligomerization, and membrane insertion. J Biol Chem. 2010;285:6636–6647. doi: 10.1074/jbc.M109.081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks C, Wei Q, Feng L, Dong G, Tao Y, Mei L, Xie ZJ, Dong Z. Bak regulates mitochondrial morphology and pathology during apoptosis by interacting with mitofusins. Proc Natl Acad Sci USA. 2007;104:11649–11654. doi: 10.1073/pnas.0703976104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, Meflah K, Vallette FM, Juin P. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Cassidy-Stone A, Chipuk JE, Ingerman E, Song C, Yoo C, Kuwana T, Kurth MJ, Shaw JT, Hinshaw JE, Green DR, et al. Chemical inhibition of the mitochondrial division dynamin reveals its role in Bax/Bak-dependent mitochondrial outer membrane permeabilization. Dev Cell. 2008;14:193–204. doi: 10.1016/j.devcel.2007.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Hersh BM, Conradt B, Zhou Z, Riemer D, Gruenbaum Y, Horvitz HR. Translocation of C. elegans CED-4 to nuclear membranes during programmed cell death. Science. 2000;287:1485–1489. doi: 10.1126/science.287.5457.1485. [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol. 2003;160:189–200. doi: 10.1083/jcb.200211046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- Chernomordik LV, Kozlov MM. Mechanics of membrane fusion. Nature structural & molecular biology. 2008;15:675–683. doi: 10.1038/nsmb.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Christenson E, Merlin S, Saito M, Schlesinger P. Cholesterol effects on BAX pore activation. J Mol Biol. 2008;381:1168–1183. doi: 10.1016/j.jmb.2008.06.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cipolat S, Rudka T, Hartmann D, Costa V, Serneels L, Craessaerts K, Metzger K, Frezza C, Annaert W, D'Adamio L, et al. Mitochondrial rhomboid PARL regulates cytochrome c release during apoptosis via OPA1-dependent cristae remodeling. Cell. 2006;126:163–175. doi: 10.1016/j.cell.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Claveria C, Caminero E, Martinez AC, Campuzano S, Torres M. GH3, a novel proapoptotic domain in Drosophila Grim, promotes a mitochondrial death pathway. Embo J. 2002;21:3327–3336. doi: 10.1093/emboj/cdf354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland MM, Norris KL, Karbowski M, Wang C, Suen DF, Jiao S, George NM, Luo X, Li Z, Youle RJ. Bcl-2 family interaction with the mitochondrial morphogenesis machinery. Cell Death Differ. 2010;18:235–247. doi: 10.1038/cdd.2010.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czabotar PE, Colman PM, Huang DC. Bax activation by Bim? Cell Death Differ. 2009;16:1187–1191. doi: 10.1038/cdd.2009.83. [DOI] [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985;822:1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Dejean LM, Martinez-Caballero S, Guo L, Hughes C, Teijido O, Ducret T, Ichas F, Korsmeyer SJ, Antonsson B, Jonas EA, et al. Oligomeric Bax is a component of the putative cytochrome c release channel MAC, mitochondrial apoptosis-induced channel. Mol Biol Cell. 2005;16:2424–2432. doi: 10.1091/mbc.E04-12-1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delivani P, Adrain C, Taylor RC, Duriez PJ, Martin SJ. Role for CED-9 and Egl-1 as regulators of mitochondrial fission and fusion dynamics. Mol Cell. 2006;21:761–773. doi: 10.1016/j.molcel.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Desagher S, Osen-Sand A, Nichols A, Eskes R, Montessuit S, Lauper S, Maundrell K, Antonsson B, Martinou J-C. Bid-induced Conformational Change of Bax Is Responsible for Mitochondrial Cytochrome c Release during Apoptosis. J Cell Biol. 1999;144:891–901. doi: 10.1083/jcb.144.5.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, Kluck RM. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, Neutzner A, Tjandra N, Youle RJ. Bcl-x(L) Retrotranslocates Bax from the Mitochondria into the Cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epand RF, Martinou JC, Montessuit S, Epand RM, Yip CM. Direct evidence for membrane pore formation by the apoptotic protein Bax. Biochem Biophys Res Commun. 2002;298:744–749. doi: 10.1016/s0006-291x(02)02544-5. [DOI] [PubMed] [Google Scholar]
- Epand RF, Schlattner U, Wallimann T, Lacombe ML, Epand RM. Novel lipid transfer property of two mitochondrial proteins that bridge the inner and outer membranes. Biophysical journal. 2007;92:126–137. doi: 10.1529/biophysj.106.092353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskes R, Desagher S, Antonsson B, Martinou JC. Bid induces the oligomerization and insertion of Bax into the outer mitochondrial membrane. Mol Cell Biol. 2000;20:929–935. doi: 10.1128/mcb.20.3.929-935.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estaquier J, Arnoult D. Inhibiting Drp1-mediated mitochondrial fission selectively prevents the release of cytochrome c during apoptosis. Cell Death Differ. 2007;14:1086–1094. doi: 10.1038/sj.cdd.4402107. [DOI] [PubMed] [Google Scholar]
- Frank S, Gaume B, Bergmann-Leitner ES, Leitner WW, Robert EG, Catez F, Smith CL, Youle RJ. The role of dynamin-related protein 1, a mediator of mitochondrial fission, in apoptosis. Dev Cell. 2001;1:515–525. doi: 10.1016/s1534-5807(01)00055-7. [DOI] [PubMed] [Google Scholar]
- Gallenne T, Gautier F, Oliver L, Hervouet E, Noel B, Hickman JA, Geneste O, Cartron PF, Vallette FM, Manon S, et al. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009;185:279–290. doi: 10.1083/jcb.200809153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Saez AJ, Chiantia S, Salgado J, Schwille P. Pore formation by a Bax-derived peptide: effect on the line tension of the membrane probed by AFM. Biophysical journal. 2007;93:103–112. doi: 10.1529/biophysj.106.100370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Saez AJ, Coraiola M, Serra MD, Mingarro I, Muller P, Salgado J. Peptides corresponding to helices 5 and 6 of Bax can independently form large lipid pores. FEBS J. 2006;273:971–981. doi: 10.1111/j.1742-4658.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, Tu HC, Kim H, Cheng EH, Tjandra N, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebert N, Joshi AS, Kutik S, Becker T, McKenzie M, Guan XL, Mooga VP, Stroud DA, Kulkarni G, Wenk MR, et al. Mitochondrial cardiolipin involved in outer-membrane protein biogenesis: implications for Barth syndrome. Curr Biol. 2009;19:2133–2139. doi: 10.1016/j.cub.2009.10.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George NM, Evans JJ, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain M, Mathai JP, McBride HM, Shore GC. Endoplasmic reticulum BIK initiates DRP1-regulated remodelling of mitochondrial cristae during apoptosis. Embo J. 2005;24:1546–1556. doi: 10.1038/sj.emboj.7600592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giam M, Huang DC, Bouillet P. BH3-only proteins and their roles in programmed cell death. Oncogene. 2008;27 Suppl 1:S128–S136. doi: 10.1038/onc.2009.50. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Metcalfe AD, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J Cell Biol. 2000;149:431–446. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvez F, Schug ZT, Houtkooper RH, MacKenzie ED, Brooks DG, Wanders RJ, Petit PX, Vaz FM, Gottlieb E. Cardiolipin provides an essential activating platform for caspase-8 on mitochondria. J Cell Biol. 2008;183:681–696. doi: 10.1083/jcb.200803129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal G, Fell B, Sarin A, Youle RJ, Sriram V. Role of mitochondrial remodeling in programmed cell death in Drosophila melanogaster. Dev Cell. 2007;12:807–816. doi: 10.1016/j.devcel.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haining WN, Carboy-Newcomb C, Wei CL, Steller H. The proapoptotic function of Drosophila Hid is conserved in mammalian cells. Proc Natl Acad Sci U S A. 1999;96:4936–4941. doi: 10.1073/pnas.96.9.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasler SL. Cholesterol, Cardiolipin, and Mitochondria Permeabilisation. Anticancer Agents Med Chem. 2011 doi: 10.2174/187152012800228724. [DOI] [PubMed] [Google Scholar]
- Hengartner MO, Horvitz HR. Activation of C. elegans cell death protein CED-9 by an amino-acid substitution in a domain conserved in Bcl-2. Nature. 1994;369:318–320. doi: 10.1038/369318a0. [DOI] [PubMed] [Google Scholar]
- Hoppins S, Edlich F, Cleland MM, Banerjee S, McCaffery JM, Youle RJ, Nunnari J. The soluble form of Bax regulates mitochondrial fusion via MFN2 homotypic complexes. Mol Cell. 2011;41:150–160. doi: 10.1016/j.molcel.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppins S, Lackner L, Nunnari J. The machines that divide and fuse mitochondria. Annual review of biochemistry. 2007;76:751–780. doi: 10.1146/annurev.biochem.76.071905.090048. [DOI] [PubMed] [Google Scholar]
- Hovius R, Lambrechts H, Nicolay K, de Kruijff B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim Biophys Acta. 1990;1021:217–226. doi: 10.1016/0005-2736(90)90036-n. [DOI] [PubMed] [Google Scholar]
- Ishihara N, Nomura M, Jofuku A, Kato H, Suzuki SO, Masuda K, Otera H, Nakanishi Y, Nonaka I, Goto Y, et al. Mitochondrial fission factor Drp1 is essential for embryonic development and synapse formation in mice. Nat Cell Biol. 2009;11:958–966. doi: 10.1038/ncb1907. [DOI] [PubMed] [Google Scholar]
- Iverson SL, Enoksson M, Gogvadze V, Ott M, Orrenius S. Cardiolipin is not required for Bax-mediated cytochrome c release from yeast mitochondria. J Biol Chem. 2003;8:8. doi: 10.1074/jbc.M305020200. [DOI] [PubMed] [Google Scholar]
- Jagasia R, Grote P, Westermann B, Conradt B. DRP-1-mediated mitochondrial fragmentation during EGL-1-induced cell death in C. elegans. Nature. 2005;433:754–760. doi: 10.1038/nature03316. [DOI] [PubMed] [Google Scholar]
- James DI, Parone PA, Mattenberger Y, Martinou JC. hFis1, a novel component of the mammalian mitochondrial fission machinery. J Biol Chem. 2003;278:36373–36379. doi: 10.1074/jbc.M303758200. [DOI] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, Santel A, Fuller M, Smith CL, Youle RJ. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Norris KL, Cleland MM, Jeong SY, Youle RJ. Role of Bax and Bak in mitochondrial morphogenesis. Nature. 2006;443:658–662. doi: 10.1038/nature05111. [DOI] [PubMed] [Google Scholar]
- Katsov K, Muller M, Schick M. Field theoretic study of bilayer membrane fusion. I. Hemifusion mechanism. Biophysical journal. 2004;87:3277–3290. doi: 10.1529/biophysj.103.038943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaled AR, Kim K, Hofmeister R, Muegge K, Durum SK. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci U S A. 1999;96:14476–14481. doi: 10.1073/pnas.96.25.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Zhao Y, Ding WX, Shin JN, He X, Seo YW, Chen J, Rabinowich H, Amoscato AA, Yin XM. Bid-cardiolipin interaction at mitochondrial contact site contributes to mitochondrial cristae reorganization and cytochrome C release. Mol Biol Cell. 2004;15:3061–3072. doi: 10.1091/mbc.E03-12-0864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, Schneider G, Luers GH, Schrader M. Peroxisome elongation and constriction but not fission can occur independently of dynamin-like protein 1. J Cell Sci. 2004;117:3995–4006. doi: 10.1242/jcs.01268. [DOI] [PubMed] [Google Scholar]
- Kozlovsky Y, Kozlov MM. Membrane fission: model for intermediate structures. Biophysical journal. 2003;85:85–96. doi: 10.1016/S0006-3495(03)74457-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. doi: 10.1152/physrev.00013.2006. [DOI] [PubMed] [Google Scholar]
- Kutuk O, Letai A. Regulation of Bcl-2 family proteins by posttranslational modifications. Curr Mol Med. 2008;8:102–118. doi: 10.2174/156652408783769599. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, Newmeyer DD. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Kvansakul M, Yang H, Fairlie WD, Czabotar PE, Fischer SF, Perugini MA, Huang DC, Colman PM. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 2008;15:1564–1571. doi: 10.1038/cdd.2008.83. [DOI] [PubMed] [Google Scholar]
- Lalier L, Cartron PF, Olivier C, Loge C, Bougras G, Robert JM, Oliver L, Vallette FM. Prostaglandins antagonistically control Bax activation during apoptosis. Cell Death Differ. 2011;18:528–537. doi: 10.1038/cdd.2010.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarou M, Stojanovski D, Frazier AE, Kotevski A, Dewson G, Craigen WJ, Kluck RM, Vaux DL, Ryan MT. Inhibition of Bak activation by VDAC2 is dependent on the Bak transmembrane anchor. J Biol Chem. 2010;285:36876–36883. doi: 10.1074/jbc.M110.159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Lucken-Ardjomande S, Montessuit S, Martinou JC. Bax activation and stress-induced apoptosis delayed by the accumulation of cholesterol in mitochondrial membranes. Cell Death Differ. 2008;15:484–493. doi: 10.1038/sj.cdd.4402280. [DOI] [PubMed] [Google Scholar]
- Lutter M, Fang M, Luo X, Nishijima M, Xie X, Wang X. Cardiolipin provides specificity for targeting of tBid to mitochondria. Nat Cell Biol. 2000;2:754–761. doi: 10.1038/35036395. [DOI] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ. Which came first, the cytochrome c release or the mitochondrial fission? Cell Death Differ. 2006;13:1291–1295. doi: 10.1038/sj.cdd.4401985. [DOI] [PubMed] [Google Scholar]
- Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O'Reilly LA, Adams JM, Strasser A, Lee EF, Fairlie WD, et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol. 2009;186:355–362. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill RA, Dagda RK, Dickey AS, Cribbs JT, Green SH, Usachev YM, Strack S. Mechanism of Neuroprotective Mitochondrial Remodeling by PKA/AKAP1. PLoS Biol. 2011;9:e1000612. doi: 10.1371/journal.pbio.1000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu T, Liu Q, Tocilj A, Watson M, Shore G, Gehring K. The X-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell. 2006;24:677–688. doi: 10.1016/j.molcel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, Manstein DJ, Bossy-Wetzel E, Basanez G, Meda P, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Lamensdorf I, Yoon S-H, Youle RJ. Bax and Bak Coalesce into Novel Mitochondria-associated Clusters during Apoptosis. J Cell Biol. 2001a;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Lamensdorf I, Yoon SH, Youle RJ. Bax and Bak coalesce into novel mitochondria-associated clusters during apoptosis. J Cell Biol. 2001b;153:1265–1276. doi: 10.1083/jcb.153.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson MR, Holley CL, Gan EC, Colon-Ramos DA, Kaplan B, Kornbluth S. A GH3-like domain in reaper is required for mitochondrial localization and induction of IAP degradation. J Biol Chem. 2003;278:44758–44768. doi: 10.1074/jbc.M308055200. [DOI] [PubMed] [Google Scholar]
- Parone PA, James DI, Da Cruz S, Mattenberger Y, Donze O, Barja F, Martinou JC. Inhibiting the mitochondrial fission machinery does not prevent Bax/Bak-dependent apoptosis. Mol Cell Biol. 2006;26:7397–7408. doi: 10.1128/MCB.02282-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polcic P, Su X, Fowlkes J, Blachly-Dyson E, Dowhan W, Forte M. Cardiolipin and phosphatidylglycerol are not required for the in vivo action of Bcl-2 family proteins. Cell Death Differ. 2005;12:310–312. doi: 10.1038/sj.cdd.4401566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian S, Wang W, Yang L, Huang HW. Structure of transmembrane pore induced by Bax-derived peptide: evidence for lipidic pores. Proc Natl Acad Sci U S A. 2008;105:17379–17383. doi: 10.1073/pnas.0807764105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland SG, Lu Y, David CN, Conradt B. The BCL-2-like protein CED-9 of C. elegans promotes FZO-1/Mfn1,2- and EAT-3/Opa1-dependent mitochondrial fusion. J Cell Biol. 2009;186:525–540. doi: 10.1083/jcb.200905070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roucou X, Rostovtseva T, Montessuit S, Martinou JC, Antonsson B. Bid induces cytochrome c-impermeable Bax channels in liposomes. Biochem J. 2002;363:547–552. doi: 10.1042/0264-6021:3630547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SS, Ehrlich AM, Craigen WJ, Hajnoczky G. VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria. EMBO Rep. 2009;10:1341–1347. doi: 10.1038/embor.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvesen GS, Abrams JM. Caspase activation - stepping on the gas or releasing the brakes? Lessons from humans and flies. Oncogene. 2004;23:2774–2784. doi: 10.1038/sj.onc.1207522. [DOI] [PubMed] [Google Scholar]
- Sandu C, Ryoo HD, Steller H. Drosophila IAP antagonists form multimeric complexes to promote cell death. J Cell Biol. 2010;190:1039–1052. doi: 10.1083/jcb.201004086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer B, Quispe J, Choudhary V, Chipuk JE, Ajero TG, Du H, Schneiter R, Kuwana T. Mitochondrial Outer Membrane Proteins Assist Bid in Bax-mediated Lipidic Pore Formation. Mol Biol Cell. 2009 doi: 10.1091/mbc.E08-10-1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schinzel A, Kaufmann T, Borner C. Bcl-2 family members: integrators of survival and death signals in physiology and pathology [corrected] Biochim Biophys Acta. 2004;1644:95–105. doi: 10.1016/j.bbamcr.2003.09.006. [DOI] [PubMed] [Google Scholar]
- Schlame M, Rua D, Greenberg ML. The biosynthesis and functional role of cardiolipin. Prog Lipid Res. 2000;39:257–288. doi: 10.1016/s0163-7827(00)00005-9. [DOI] [PubMed] [Google Scholar]
- Schlesinger PH, Gross A, Yin XM, Yamamoto K, Saito M, Waksman G, Korsmeyer SJ. Comparison of the ion channel characteristics of proapoptotic BAX and antiapoptotic BCL-2. Proc Natl Acad Sci U S A. 1997;94:11357–11362. doi: 10.1073/pnas.94.21.11357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schug ZT, Gottlieb E. Cardiolipin acts as a mitochondrial signalling platform to launch apoptosis. Biochim Biophys Acta. 2009;1788:2022–2031. doi: 10.1016/j.bbamem.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Sevrioukov EA, Burr J, Huang EW, Assi HH, Monserrate JP, Purves DC, Wu JN, Song EJ, Brachmann CB. Drosophila Bcl-2 proteins participate in stress-induced apoptosis, but are not required for normal development. Genesis. 2007;45:184–193. doi: 10.1002/dvg.20279. [DOI] [PubMed] [Google Scholar]
- Shamas-Din A, Brahmbhatt H, Leber B, Andrews DW. BH3-only proteins: Orchestrators of apoptosis. Biochim Biophys Acta. 2011 doi: 10.1016/j.bbamcr.2010.11.024. [DOI] [PubMed] [Google Scholar]
- Sheridan C, Delivani P, Cullen SP, Martin SJ. Bax- or Bak-induced mitochondrial fission can be uncoupled from cytochrome C release. Mol Cell. 2008;31:570–585. doi: 10.1016/j.molcel.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. doi: 10.1038/nrm2952. [DOI] [PubMed] [Google Scholar]
- Tan FJ, Husain M, Manlandro CM, Koppenol M, Fire AZ, Hill RB. CED-9 and mitochondrial homeostasis in C. elegans muscle. J Cell Sci. 2008;121:3373–3382. doi: 10.1242/jcs.032904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner EA, Blute TA, Brachmann CB, McCall K. Bcl-2 proteins and autophagy regulate mitochondrial dynamics during programmed cell death in the Drosophila ovary. Development. 2011;138:327–338. doi: 10.1242/dev.057943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrones O, Antonsson B, Yamaguchi H, Wang HG, Liu Y, Lee RM, Herrmann A, Basanez Gb. Lipidic pore formation by the concerted action of pro-apoptotic BAX and tBID. J Biol Chem. 2004;279:30081–30091. doi: 10.1074/jbc.M313420200. [DOI] [PubMed] [Google Scholar]
- Thomenius M, Freel CD, Horn S, Krieser R, Abdelwahid E, Cannon R, Balasundaram S, White K, Kornbluth S. Mitochondrial fusion is regulated by Reaper to modulate Drosophila programmed cell death. Cell Death Differ. 2011 doi: 10.1038/cdd.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, et al. SLP-2 is required for stress-induced mitochondrial hyperfusion. Embo J. 2009;28:1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaux DL. Apoptogenic factors released from mitochondria. Biochim Biophys Acta. 2011;1813:546–550. doi: 10.1016/j.bbamcr.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Wakabayashi J, Zhang Z, Wakabayashi N, Tamura Y, Fukaya M, Kensler TW, Iijima M, Sesaki H. The dynamin-related GTPase Drp1 is required for embryonic and brain development in mice. J Cell Biol. 2009;186:805–816. doi: 10.1083/jcb.200903065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, Smith E, Verdine GL, Korsmeyer SJ. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Wasiak S, Zunino R, McBride HM. Bax/Bak promote sumoylation of DRP1 and its stable association with mitochondria during apoptotic cell death. J Cell Biol. 2007;177:439–450. doi: 10.1083/jcb.200610042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermann B. Mitochondrial fusion and fission in cell life and death. Nat Rev Mol Cell Biol. 2010;11:872–884. doi: 10.1038/nrm3013. [DOI] [PubMed] [Google Scholar]
- Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta. 2011;1813:521–531. doi: 10.1016/j.bbamcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Yamaguchi R, Lartigue L, Perkins G, Scott RT, Dixit A, Kushnareva Y, Kuwana T, Ellisman MH, Newmeyer DD. Opa1-mediated cristae opening is Bax/Bak and BH3 dependent, required for apoptosis, and independent of Bak oligomerization. Mol Cell. 2008;31:557–569. doi: 10.1016/j.molcel.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model? A case study on melittin pores. Biophysical journal. 2001;81:1475–1485. doi: 10.1016/S0006-3495(01)75802-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yethon JA, Epand RF, Leber B, Epand RM, Andrews DW. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J Biol Chem. 2003;278:48935–48941. doi: 10.1074/jbc.M306289200. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Zaltsman Y, Shachnai L, Yivgi-Ohana N, Schwarz M, Maryanovich M, Houtkooper RH, Vaz FM, De Leonardis F, Fiermonte G, Palmieri F, et al. MTCH2/MIMP is a major facilitator of tBID recruitment to mitochondria. Nat Cell Biol. 2010;12:553–562. doi: 10.1038/ncb2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhu W, Lapolla SM, Miao Y, Shao Y, Falcone M, Boreham D, McFarlane N, Ding J, Johnson AE, et al. Bax forms an oligomer via separate, yet interdependent, surfaces. J Biol Chem. 285:17614–17627. doi: 10.1074/jbc.M110.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]