Abstract
Summary
Background and objectives
Chronic kidney disease (CKD) is prevalent in minority populations and racial/ethnic differences in survival are incompletely understood.
Design, setting, participants, & measurements
Secondary analysis of Kidney Early Evaluation Program participants from 2000 through 2008 with CKD, not on dialysis, and without previous kidney transplant was performed. Self-reported race/ethnicity was categorized into five groups: non-Hispanic white, African American, Asian, American Indian/Alaska Native, and Hispanic. CKD was defined as a urinary albumin to creatinine ratio of ≥30 mg/g among participants with an estimated GFR (eGFR) ≥60 ml/min per 1.73 m2 or an eGFR of <60 ml/min per 1.73 m2. The outcome was all-cause mortality. Covariates used were age, sex, obesity, diabetes, hypertension, albuminuria, baseline eGFR, heart attack, stroke, smoking, family history, education, health insurance, geographic region, and year screened.
Results
19,205 participants had prevalent CKD; 55% (n = 10,560) were White, 27% (n = 5237) were African American, 9% (n = 1638) were Hispanic, 5% (n = 951) were Asian, and 4% (n = 813) were American Indian/Alaska Native. There were 1043 deaths (5.4%). African Americans had a similar risk of death compared with Whites (adjusted Hazard Ratio (AHR) 1.07, 95% CI 0.90 to 1.27). Hispanics (AHR 0.66, 95% CI 0.50 to 0.94) and Asians (AHR 0.63, 95% CI 0.41 to 0.97) had a lower mortality risk compared with Whites. In contrast, American Indians/Alaska Natives had a higher risk of death compared with Whites (AHR 1.41, 95% CI 1.08 to 1.84).
Conclusions
Significant differences in mortality among some minority groups were found among persons with CKD detected by community-based screening.
Introduction
Chronic kidney disease (CKD) affects over 10% of the U.S. population and is a burgeoning public health problem (1–3). Considerable racial and ethnic disparities exist in the prevalence of CKD and end-stage renal disease (ESRD) (4–6). Additionally, CKD has been consistently shown to be an independent risk factor for mortality (7,8).
Few studies have evaluated the association of race/ethnicity with mortality among those with CKD not on dialysis (9). Some studies show a survival advantage among blacks compared with whites with very late stage CKD (10) or among those insured (11). Others have found a similar adjusted mortality for blacks, whites, and Hispanics with CKD, although subgroup analyses revealed a higher mortality for younger blacks in comparison with whites (12). Overall mortality for blacks is higher than that for whites in the general population (13–15). In contrast, among the Hispanic population, there is the finding of lower mortality despite a greater number of risk factors in what has been termed the “Hispanic Paradox” (16). This has been shown among Hispanic patients with CKD as well (17). Few studies have looked at the American Indian/Alaska Native or Asian populations (18–20).
Using a large racially/ethnically diverse screened study population from the Kidney Early Evaluation Program (KEEP), we investigated the associations between race/ethnicity and risk for all-cause mortality among those with prevalent CKD. We then adjusted for important factors that might confound any differences observed. We further categorized participants as having early (stages 1 to 2) or later (stages 3 to 5) CKD, according to the current recommended staging system, and repeated analyses within each stratum (21). Understanding racial/ethnic differences in mortality among those with CKD can help focus future research and may lead to the targeted development of novel prevention and management strategies to improve outcomes.
Materials and Methods
KEEP Study Population
KEEP is a free, community-based, voluntary screening program designed to detect CKD, to identify individuals at increased risk for kidney disease, and to encourage follow-up care (22). The KEEP definition for increased risk for kidney disease is persons with either a personal diagnosis of diabetes or hypertension, or with a family history of diabetes, hypertension, or kidney disease. KEEP screenings are conducted in urban and rural locations throughout the United States through each state's local National Kidney Foundation affiliate (22). Officially launched nationwide in August 2000, KEEP has screened more than 128,000 participants.
Screening Protocol
Screening data were collected on participant demographic characteristics, personal and family medical history, and health behaviors (22). One-time systolic and diastolic BP measurements and height and weight measurements were obtained (22). Blood and urine specimens were collected and processed to determine serum creatinine, fasting blood glucose, and urine albumin levels (22). Serum creatinine values for KEEP participants were calibrated against values measured at the Cleveland Clinic Research Laboratory using the Roche enzymatic assay. Subsequently, eGFR using the original (raw) serum creatinine value was recalculated using the four-variable Modification of Diet in Renal Disease (MDRD) Study equation with the newly calibrated serum creatinine values (23,24). Spot urine specimens were collected and tested semiquantitatively for urine albumin levels, using Micral assay (Roche Pharmaceuticals, Indianapolis) until September 2001. Since then, they have been analyzed with the Clinitek assay (Bayer Diagnostics, Tarrytown, PA) (22).
CKD-Albuminuria and Reduced eGFR
Albuminuria, or early stage CKD, is defined as a urinary albumin to creatinine ratio (ACR) of ≥30 mg/g among participants with eGFR ≥60 ml/min per 1.73 m2, corresponding to CKD stages 1 to 2 according to current Kidney Disease Outcomes Quality Initiative guidelines (21). Reduced eGFR, or later stage CKD, is defined as an eGFR of <60 ml/min per 1.73 m2, equivalent to CKD stages 3 to 5 (21).
CKD Study Population
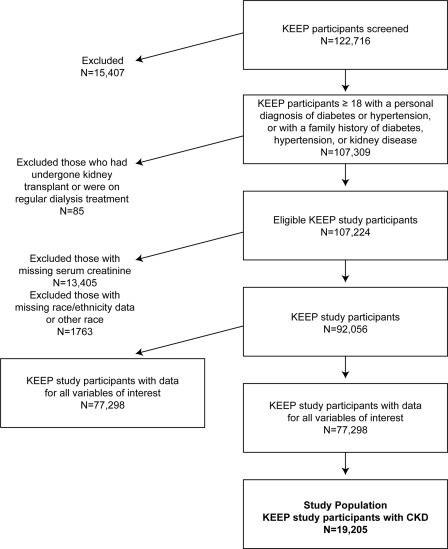
In this study, we evaluated only those participants screened from August 2000 to December 2008 (total n = 122,716). Of these, we included those who were aged at least 18 years, with a self-reported diagnosis of diabetes or hypertension, or with a family history of diabetes, hypertension, or kidney disease (n = 107,309). Participants who had undergone kidney transplant or were on regular dialysis treatment were excluded from this analysis (n = 85), leaving an eligible KEEP study population of 107,224 participants. Subsequently, we excluded 13,405 participants with missing serum creatinine and 1763 participants with missing race/ethnicity data or who self-identified as non-Hispanic/other race. Of the 92,056 participants remaining, a total of 77,298 participants (84%) had data for all variables of interest. Next from that group, 25% (n = 19,205) had CKD as defined above, thus constituting our study population (Figure 1).
Figure 1.
Flow diagram illustrating how the cohort was built for analyses for this study.
Predictor Variables and Covariates
Race and ethnicity information was obtained by self-report at the time of KEEP screening and was categorized into five racial/ethnic groups: non-Hispanic white, African American, Asian, American Indian/Alaska Native, and Hispanic. Persons of Hispanic origin may be of any race. The other four racial categories, white, African American, Asian, American Indian/Alaska Native, did not include persons who reported Hispanic origin. In the paper we use the terms whites and non-Hispanic whites, and blacks and African Americans, interchangeably.
Potential covariates were determined a priori as characteristics well described to influence CKD and mortality risk: age, sex, obesity, diabetes, hypertension, and smoking; family history of diabetes, hypertension, or kidney disease; educational level and presence of health insurance; and geographic region. Age was determined by self-reported date of birth at the time of screening. Obesity was defined as body mass index ≥30 kg/m2. Diabetes was defined as a history of diabetes (self-report or retinopathy), use of medications to treat diabetes, or fasting blood glucose level ≥126 mg/dl or nonfasting blood glucose level ≥200 mg/dl in the absence of self-report or medication use (25). Hypertension was defined by participant self-report or by systolic BP ≥130 mmHg or diastolic BP ≥80 mmHg since all participants in this analysis had CKD (21).
Smoking status was self-reported and categorized as current/former versus never. Family history of diabetes, hypertension, and kidney disease were determined by participant self-report of having a first-degree relative with the condition. Educational level was self-reported and dichotomized as less than high school or high school equivalent and higher. Health insurance status was determined by the participant's report at the time of screening. To account for potential regional differences in CKD by race/ethnicity, we categorized the United States into the four defined census geographic regions: Northeast, Midwest, South, and West (26).
Additional covariates included were albuminuria, cardiovascular comorbidity, and baseline eGFR as they are important and could influence CKD and mortality risk. Albuminuria status was categorized as microalbuminuria (30 to 299 mg/g) and macroalbuminuria (≥300 mg/g). Cardiovascular comorbidities were defined as self-report of a history of heart attack or stroke. Our results did not change and so we present the fully adjusted models.
Mortality
Our outcome variable was all-cause mortality, determined using a previously validated multilevel tracking system by the Chronic Disease Research Group at Minneapolis Medical Research Foundation, Hennepin County Medical Center. This system is capable of using name and social security number data and incident ESRD patient records with cross-checks against the U.S. Medicare database and the Social Security Administration Death Files (27). To calculate death rates, we defined follow-up time from the KEEP screening date until censoring at May 31, 2009, or the date of death.
Statistical Analyses
We used a complete-case analysis approach in which we analyzed participants with available data for all covariates of interest. Of the 92,056 participants available, a total of 77,298 participants (84%) had data for all variables of interest. Demographics were similar for included and excluded participants, except that participants excluded because of missing values (n = 14,758) had slightly higher prevalence of diabetes (31% versus 28%) and hypertension (60% versus 54%). Of the 14,758 excluded, 44% self-reported their race/ethnicity as white, 34% as African American, 14% as Hispanic, 5% as Asian, and 3% as American Indian/Alaska Native; relative to the overall KEEP registry, African Americans and Hispanics were disproportionately excluded.
We compared demographics and clinical characteristics by race/ethnicity using ANOVA or chi-squared analyses as appropriate. We calculated mortality rates (per 1000 person-years) by race/ethnicity for all participants with CKD, and then separately for early and later stage CKD. Additionally, to explore the effect of age, we dichotomized age <65 versus ≥65 years and calculated the death rates by race/ethnicity for all participants with CKD for these subgroups.
We conducted multivariate Cox proportional hazards regression analyses to determine the association of race/ethnicity with all-cause mortality among all participants with CKD, and then separately among those with early and later stage CKD. All covariates were included in the multivariate analyses.
Statistical analyses were performed with the SAS statistical package (release 9.1; SAS Institute Inc., Cary, NC). Cleveland Clinic Institutional Review Board (IRB) approved this study. The Hennepin County Medical Center IRB approved the KEEP program, including research protocol, process of obtaining informed consent, and data management procedures related to KEEP.
Results
Of the 19,205 KEEP participants from 2000 through 2008 with prevalent CKD, 55% (n = 10,560) self-reported their race/ethnicity as white, 27% (n = 5237) as African American, 9% (n = 1638) as Hispanic, 5% (n = 951) as Asian, and 4% (n = 813) as American Indian/Alaska Native. The overall mean age was 60 years, most participants were women, the vast majority had hypertension, and nearly half had diabetes (Table 1). Whites were the oldest group on average, American Indian/Alaska Natives had highest prevalence of obesity and diabetes, and African Americans had the highest hypertension prevalence. Whites were more likely to be included because of low eGFR and the other groups more likely to be included because of albuminuria.
Table 1.
Baseline demographic characteristics of participants with chronic kidney disease, by race/ethnicity, Kidney Early Evaluation Program, 2000 through 2008
| Characteristics | White (n = 10,560) | African American (n = 5237) | Hispanic (n = 1638) | Asian (n = 957) | American Indian/Alaska Native (n = 813) | Pa |
|---|---|---|---|---|---|---|
| Age [years], meanb | 65 (14) | 59 (14) | 56 (15) | 61 (13) | 58 (16) | <0.01 |
| Female, % | 69 | 73 | 68 | 57 | 74 | <0.01 |
| Obesity, % | 43 | 57 | 48 | 24 | 59 | <0.01 |
| Diabetes, % | 40 | 42 | 44 | 45 | 51 | <0.01 |
| Hypertension, % | 89 | 92 | 84 | 86 | 83 | <0.01 |
| Microalbuminuria, %c | 33 | 55 | 54 | 51 | 53 | <0.01 |
| Macroalbuminuria, %c | 4 | 6 | 8 | 6 | 11 | <0.01 |
| eGFR <60, % | 78 | 56 | 55 | 60 | 58 | <0.01 |
| Baseline eGFR, meanb | 56 (18) | 68 (28) | 69 (28) | 64 (24) | 67 (29) | <0.01 |
| Heart attack, %c | 12 | 9 | 8 | 8 | 11 | <0.01 |
| Stroke, %c | 8 | 8 | 7 | 7 | 8 | 0.33 |
| Current or former smoker, % | 44 | 43 | 36 | 31 | 56 | <0.01 |
| Family history of, % | ||||||
| diabetes | 52 | 62 | 65 | 51 | 74 | <0.01 |
| hypertension | 74 | 82 | 72 | 71 | 71 | <0.01 |
| kidney disease | 18 | 22 | 21 | 15 | 25 | <0.01 |
| High school education or higher, % | 88 | 81 | 63 | 80 | 73 | <0.01 |
| Health insurance, yes, % | 92 | 83 | 63 | 80 | 72 | <0.01 |
| Region,d % | ||||||
| Northeast | 25 | 18 | 20 | 30 | 12 | <0.01 |
| Midwest | 17 | 16 | 9 | 6 | 21 | |
| South | 44 | 64 | 49 | 14 | 36 | |
| West | 14 | 2 | 23 | 50 | 31 |
Note: total, n = 19,205. The racial categories did not include persons who reported Hispanic origin, and persons of Hispanic origin may be of any race. eGFR, estimated glomerular filtration rate (ml/min per 1.73 m2).
χ2, except for mean age, for which analysis of variance was used.
Mean ± SD.
Excluding missing values for percentage.
Northeast: Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Vermont; Midwest: Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin; South: Alabama, Arkansas, Delaware, District of Columbia, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, and West Virginia; West: Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, Nevada, New Mexico, Oregon, Utah, Washington, and Wyoming.
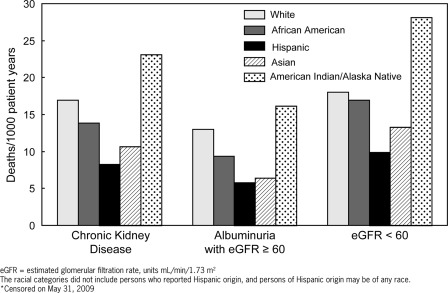
Among our study population with CKD, 1043 deaths (23 per 1000 person-years) occurred, of which 214 deaths were among those with albuminuria (16 per 1000 person-years) and 829 deaths (28 per 1000 person-years) were among those with a reduced eGFR <60 ml/min per 1.73 m2. American Indians/Alaska Natives had the highest unadjusted death rates compared with all other race/ethnic groups in each category of CKD (Figure 2). Conversely, Hispanics had the lowest death rates in each CKD category (Figure 2). In subgroup analyses, results were similar. For the <65 years subgroup, deaths per 1000-person years was 7 for whites, 7 for African Americans, 4 for Hispanics, 6 for Asians, and 14 for American Indian/Alaska Natives. For the ≥65 years subgroup, deaths per 1000-person years was 26 for whites, 26 for African Americans, 18 for Hispanics, 17 for Asians, and 40 for American Indian/Alaska Natives.
Figure 2.
Racial/ethnic differences in death rates among participants with chronic kidney disease, Kidney Early Evaluation Program, 2000 through 2009.
African Americans had a similar risk of death compared with whites in each CKD category after adjustment for age, sex, clinical and socioeconomic factors, geographic region, and year screened (Table 2). However, American Indian/Alaska Natives had a 43% increased hazard ratio risk of death compared with whites after adjustment (Table 2). Results were similar for any CKD and early versus later CKD in each group except among Asians where there was a significantly lower risk of death among those with albuminuria (Table 2). In a final sensitivity analysis limiting the analyses to those with eGFR <45 (n = 3063), our results were essentially unchanged for African Americans (hazard ratio [HR] 1.00, 95% confidence interval [CI] 0.73 to 1.37), Asians (HR 0.43, 95% CI 0.18 to 1.01), and American Indian/Alaska Natives (HR 1.37, 95% CI 0.87 to 2.16). For Hispanics the association was weakened (HR 0.87, 95% CI 0.50 to 1.54).
Table 2.
Adjusted hazards ratios of risk of death for participants with chronic kidney disease, by race/ethnicity, Kidney Early Evaluation Program, 2000 through 2009
| Race/Ethnicity | Adjusteda Hazards Ratio (95% CI) |
||
|---|---|---|---|
| CKD (n = 19,205) | Albuminuria and eGFR ≥60 (n = 6068) | eGFR <60 (n = 13,137) | |
| White | 1.00 | 1.00 | 1.00 |
| African American | 1.07 (0.90 to 1.27) | 1.08 (0.78 to 1.49) | 1.03 (0.84 to 1.27) |
| Hispanic | 0.66 (0.50 to 0.94) | 0.52 (0.27 to 1.01) | 0.70 (0.45 to 1.07) |
| Asian | 0.63 (0.41 to 0.97) | 0.44 (0.20 to 0.99)b | 0.71 (0.41 to 1.20) |
| American Indian/Alaska Native | 1.41 (1.08 to 1.84)b | 1.22 (0.73 to 2.03) | 1.37 (0.99 to 1.89) |
Note: KEEP censored on May 31, 2009. CKD, chronic kidney disease; CI, confidence interval; eGFR, estimated glomerular filtration rate (ml/min per 1.73 m2); KEEP, Kidney Early Evaluation Program.
Adjusted for age, sex, obesity, diabetes mellitus, hypertension, albuminuria (macroalbuminuria only for albuminuria and eGFR ≥60 group), baseline eGFR, heart attack, stroke, smoking status, family history of diabetes mellitus, family history of hypertension, family history of CKD, education level, presence of health insurance, region where screened, and year screened.
P < 0.05.
Discussion
We examined differences in mortality by race/ethnicity among those with prevalent CKD detected at a community-based voluntary screening. We found that risk of death was similar among African Americans, lower for Hispanics and Asians, and considerably higher for American Indians/Alaska Natives compared with that of whites. This pattern persisted in the subgroup analyses among those <65 and ≥65 years of age. A similar pattern of racial/ethnic differences in mortality risk existed among those with albuminuria and eGFR ≥60 ml/min per 1.73 m2, or early stage CKD, and for those with eGFR <60 ml/min per 1.73 m2, or later stage CKD. These differences persisted, even after adjustment for possible confounders such as age, sex, obesity, diabetes, hypertension, education, insurance status, as well as region and year screened. Our age-stratified death rates were consistent with our adjusted results. Given that the racial/ethnic minority and the CKD populations in the United States are growing, our study underscores the importance of having a national CKD surveillance system to track and elucidate patterns of disease, understand mechanisms of disease progression, and help create programs to improve outcomes for all (28).
Mortality was highest among the American Indian/Alaska Native population with CKD, even after adjustment for their greater risk factor burden. Exact reasons for this higher mortality risk are unclear. One possibility is an increased burden of a multitude of chronic diseases, such as obesity, diabetes, cardiovascular disease, and liver disease (29,30). Importantly, the American Indian/Alaska Native population is made up of over 500 federally recognized tribes, and there are substantial regional differences in the prevalence of diseases (19, 31–34). We attempted to account for obesity, diabetes, and hypertension in our adjustments; however, we cannot account for severity of disease, all comorbidities, or all regional differences. Thus, it may be that certain subgroups of this broad population are driving the higher mortality that we cannot capture in our analyses.
We found a lower mortality among Hispanics with CKD. This is consistent with the literature on the existence of a “Hispanic paradox,” describing their high chronic disease risk factor profile yet lower mortality compared with whites (16,35–36). Recently, a first-ever life expectancy report for the U.S. Hispanic population was released, showing higher life expectancy compared with whites and blacks (37). Additionally, among an insured Hispanic population with CKD, Peralta et al. found a higher risk of development of ESRD and a lower mortality compared with whites (17). Hispanics with ESRD on dialysis have generally had better prognosis than their white counterparts (38–40). We were unable to look at subgroups within the Hispanic population, and there may be heterogeneity in findings based upon country of origin (41).
We did not find mortality differences among African Americans and whites in this screened cohort, similar to a population level study which also found no difference in the overall cohort, although black individuals who were younger than 65 years were distinct and found to be 78% more likely to die than white individuals (12). Our study was consistent with findings from an insured population (11). Although there are similarities between the NHANES and KEEP populations, KEEP is an enriched sample by nature of the target population for screening (27). Observed socioeconomic differences, such as education level and health insurance status, between African Americans and whites are narrower in KEEP than in society at large. Over 80% of African Americans in KEEP reported having health insurance; our findings thus may indicate that racial differences in CKD outcomes could narrow or disappear with equalization of socioeconomic status and access to care. Nonetheless, African Americans with CKD do have an elevated ESRD risk that is incompletely understood (4).
We found a significantly lower risk of death among Asians compared with whites, especially among those with early CKD. Prior studies found that overall Asians have a similar or better life expectancy than whites (9). Additionally, there is heterogeneity in the Asian population by country of origin that is not accounted for in our analyses (20). It is unclear if our findings are unique to CKD or due to the Asian population's lower risk overall.
There are clear strengths to this study, such as its inclusion of Asian and American Indian/Alaska Native populations, its national scope and perspective, its contemporary status, extending 2000 through 2008, and the participants being almost universally insured. However, there are also several limitations. We cannot determine causality or account for changes in CKD status or risk factors over time. In KEEP, as with other epidemiologic studies, CKD diagnosis is made on a single sample, rather than the repeated measures as currently recommended in clinical practice. Our cohort is derived from a group of voluntary, screened participants, of which more than 68% were women. We categorized participants according to their self-identified race/ethnicity status into one of five major racial/ethnic categories; however, the groups themselves are heterogeneous and we cannot account for differences in subgroups among them. We did not have complete data on all KEEP participants from which the study population was derived. There could also be an effect of misclassification of early CKD as determination was made by a single urine sample. Our outcome is all-cause mortality, and we do not have access to cause-specific mortality or other relevant outcomes. At this time, we have inadequate data to evaluate the incidence and survival of ESRD among KEEP participants; however, in prior studies racial/ethnic minorities with ESRD have had better survival than whites with ESRD (42–44).
Using a large, diverse screened population, we found racial/ethnic differences in the risk of death among those with CKD. Future studies are needed to explore these differences among racial/ethnic subgroups in more depth, such as influences of neighborhood or census tract, racial/ethnic heterogeneity, disease progression, and disease-specific mortality.
Disclosures
The Kidney Early Evaluation Program (KEEP) is a program of the National Kidney Foundation, Inc., and is supported by Amgen, Abbott, Novartis, Siemens, Genentech, Genzyme, Nephroceuticals, Pfizer, LifeScan, and Suplena. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Acknowledgments
Part of this material was presented in abstract form at the annual meeting of the American Society of Nephrology; November 18 through 21, 2010; Denver, CO.
Dr. Jolly's work was supported by an NIH Diversity Supplement 3U01HL064244-09S1. Dr. Shlipak's work was supported by the American Heart Association Established Investigator Award, and R01 DK 066488 from NIDDK. Dr. Norris is supported in part by the NIH Grants U54 RR026138 and P20 MD00182.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1. Coresh J, Selvin E, Stevens LA, Manzi J, Kusek JW, Eggers P, Van Lente F, Levey AS: Prevalence of chronic kidney disease in the United States. JAMA 298: 2038–2047, 2007 [DOI] [PubMed] [Google Scholar]
- 2. Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Schoolwerth AC, Engelgau MM, Hostetter TH, Rufo KH, Chianchiano D, McClellan WM, Warnock DG, Vinicor F: Chronic kidney disease: A public health problem that needs a public health action plan. Prev Chronic Dis 3: A57, 2006 [PMC free article] [PubMed] [Google Scholar]
- 4. United States Renal Data System: USRDS 2009 Annual Data Report, Bethesda, MD, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, 2009 [Google Scholar]
- 5. Jolly SE, Burrows NR, Chen SC, Li S, Jurkovitz CT, Narva AS, Norris KC, Shlipak MG: Racial and ethnic differences in albuminuria in individuals with estimated GFR greater than 60 mL/min/1.73 m2: Results from the Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 55: S15–S22, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Norris K, Nissenson AR: Race, gender, and socioeconomic disparities in CKD in the United States. J Am Soc Nephrol 19: 1261–1270, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Weiner DE, Tighiouart H, Amin MG, Stark PC, MacLeod B, Griffith JL, Salem DN, Levey AS, Sarnak MJ: Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: A pooled analysis of community-based studies. J Am Soc Nephrol 15: 1307–1315, 2004 [DOI] [PubMed] [Google Scholar]
- 9. Kalantar-Zadeh K, Kovesdy CP, Derose SF, Horwich TB, Fonarow GC: Racial and survival paradoxes in chronic kidney disease. Nat Clin Pract Nephrol 3: 493–506, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Kovesdy CP, Anderson JE, Derose SF, Kalantar-Zadeh K: Outcomes associated with race in males with nondialysis-dependent chronic kidney disease. Clin J Am Soc Nephrol 4: 973–978, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Derose SF, Rutkowski MP, Levin NW, Liu IL, Shi JM, Jacobsen SJ, Crooks PW: Incidence of end-stage renal disease and death among insured African Americans with chronic kidney disease. Kidney Int 76: 629–637, 2009 [DOI] [PubMed] [Google Scholar]
- 12. Mehrotra R, Kermah D, Fried L, Adler S, Norris K: Racial differences in mortality among those with CKD. J Am Soc Nephrol 19: 1403–1410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Levine RS, Foster JE, Fullilove RE, Fullilove MT, Briggs NC, Hull PC, Husaini BA, Hennekens CH: Black-white inequalities in mortality and life expectancy, 1933–1999: Implications for healthy people 2010. Public Health Rep 116: 474–483, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mensah GA, Mokdad AH, Ford ES, Greenlund KJ, Croft JB: State of disparities in cardiovascular health in the United States. Circulation 111: 1233–1241, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Murray CJ, Kulkarni SC, Michaud C, Tomijima N, Bulzacchelli MT, Iandiorio TJ, Ezzati M: Eight Americas: Investigating mortality disparities across races, counties, and race-counties in the United States. PLoS Med 3: e260, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rewers M, Shetterly SM, Hoag S, Baxter J, Marshall J, Hamman RF: Is the risk of coronary heart disease lower in Hispanics than in non-Hispanic whites? The San Luis Valley Diabetes Study. Ethn Dis 3: 44–54, 1993 [PubMed] [Google Scholar]
- 17. Peralta CA, Shlipak MG, Fan D, Ordonez J, Lash JP, Chertow GM, Go AS: Risks for end-stage renal disease, cardiovascular events, and death in Hispanic versus non-Hispanic white adults with chronic kidney disease. J Am Soc Nephrol 17: 2892–2899, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Pavkov ME, Bennett PH, Knowler WC, Krakoff J, Sievers ML, Nelson RG: Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA 296: 421–426, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Narayanan ML, Schraer CD, Bulkow LR, Koller KR, Asay E, Mayer AM, Raymer TW: Diabetes prevalence, incidence, complications and mortality among Alaska Native people 1985–2006. Int J Circumpolar Health 69: 236–252, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Hall YN, Hsu CY: New insights into the epidemiology of chronic kidney disease in US Asians and Pacific Islanders. Curr Opin Nephrol Hypertens 15: 264–269, 2006 [DOI] [PubMed] [Google Scholar]
- 21. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 22. Brown WW, Peters RM, Ohmit SE, Keane WF, Collins A, Chen SC, King K, Klag MJ, Molony DA, Flack JM: Early detection of kidney disease in community settings: The Kidney Early Evaluation Program (KEEP). Am J Kidney Dis 42: 22–35, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F: Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 25. National Kidney Foundation's Kidney Early Evaluation Program (KEEP) 2009 Annual Data Report. Am J Kidney Dis 55 [3 Suppl 2]: S40–S153, 2010 [DOI] [PubMed] [Google Scholar]
- 26. United States Census Bureau The Four Census Regions. Available at: http://www.census.gov/geo/www/us_regdiv.pdf Accessed October 14, 2010
- 27. McCullough PA, Li S, Jurkovitz CT, Stevens LA, Wang C, Collins AJ, Chen SC, Norris KC, McFarlane SI, Johnson B, Shlipak MG, Obialo CI, Brown WW, Vassalotti JA, Whaley-Connell AT: CKD and cardiovascular disease in screened high-risk volunteer and general populations: The Kidney Early Evaluation Program (KEEP) and National Health and Nutrition Examination Survey (NHANES) 1999–2004. Am J Kidney Dis 51: S38–S45, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Saran R, Hedgeman E, Plantinga L, Burrows NR, Gillespie BW, Young EW, Coresh J, Pavkov M, Williams D, Powe NR: Establishing a national chronic kidney disease surveillance system for the United States. Clin J Am Soc Nephrol 5: 152–161, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Native American Health Care Disparities Briefing Executive Summary, Office of the General Counsel, U.S. Commission on Civil Rights, February 2004 [Google Scholar]
- 30. Trends in Indian Health. Rockville, MD, Indian Health Service, Department of Health and Human Services, 1999 [Google Scholar]
- 31. United States Census Bureau, The American Indian and Alaska Native Populations: Census 2000 Brief, U.S. Department of Commerce Economics and Statistics Administration, February 2002 [Google Scholar]
- 32. Jolly SE, Noonan CJ, Roubideaux YD, Goldberg JH, Ebbesson SO, Umans JG, Howard BV: Albuminuria among Alaska Natives–findings from the Genetics of Coronary Artery Disease in Alaska Natives (GOCADAN) study. Nephron Clin Pract 115: c107–c113, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robbins DC, Knowler WC, Lee ET, Yeh J, Go OT, Welty T, Fabsitz R, Howard BV: Regional differences in albuminuria among American Indians: An epidemic of renal disease. Kidney Int 49: 557–563, 1996 [DOI] [PubMed] [Google Scholar]
- 34. Stidley CA, Shah VO, Scavini M, Narva AS, Kessler D, Bobelu A, MacCluer JW, Welty TK, Zager PG: The Zuni kidney project: A collaborative approach to an epidemic of kidney disease. J Am Soc Nephrol 14: S139–S143, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Liao Y, Cooper RS, Cao G, Kaufman JS, Long AE, McGee DL: Mortality from coronary heart disease and cardiovascular disease among adult U.S. Hispanics: Findings from the National Health Interview Survey (1986 to 1994). J Am Coll Cardiol 30: 1200–1205, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Sorlie PD, Backlund E, Johnson NJ, Rogot E: Mortality by Hispanic status in the United States. JAMA 270: 2464–2468, 1993 [PubMed] [Google Scholar]
- 37. Arias E: United States life tables by Hispanic origin. National Center for Health Statistics. Vital Health Stat 2(152): 1–33, 2010 [PubMed] [Google Scholar]
- 38. Frankenfield DL, Rocco MV, Roman SH, McClellan WM: Survival advantage for adult Hispanic hemodialysis patients? Findings from the end-stage renal disease clinical performance measures project. J Am Soc Nephrol 14: 180–186, 2003 [DOI] [PubMed] [Google Scholar]
- 39. Pugh JA, Tuley MR, Basu S: Survival among Mexican-Americans, non-Hispanic whites, and African-Americans with end-stage renal disease: The emergence of a minority pattern of increased incidence and prolonged survival. Am J Kidney Dis 23: 803–807, 1994 [DOI] [PubMed] [Google Scholar]
- 40. Murthy BV, Molony DA, Stack AG: Survival advantage of Hispanic patients initiating dialysis in the United States is modified by race. J Am Soc Nephrol 16: 782–790, 2005 [DOI] [PubMed] [Google Scholar]
- 41. Hunt KJ, Resendez RG, Williams K, Haffner SM, Stern MP, Hazuda HP: All-cause and cardiovascular mortality among Mexican-American and non-Hispanic White older participants in the San Antonio Heart Study- evidence against the “Hispanic paradox”. Am J Epidemiol 158: 1048–1057, 2003 [DOI] [PubMed] [Google Scholar]
- 42. Frankenfield DL, Krishnan SM, Ashby VB, Shearon TH, Rocco MV, Saran R: Differences in mortality among Mexican-American, Puerto Rican, and Cuban-American dialysis patients in the United States. Am J Kidney Dis 53: 647–657, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Powe NR: Reverse race and ethnic disparities in survival increase with severity of chronic kidney disease: What does this mean? Clin J Am Soc Nephrol 1: 905–906, 2006 [DOI] [PubMed] [Google Scholar]
- 44. Frankenfield DL, Roman SH, Rocco MV, Bedinger MR, McClellan WM: Disparity in outcomes for adult Native American hemodialysis patients? Findings from the ESRD Clinical Performance Measures Project, 1996 to 1999. Kidney Int 65: 1426–1434, 2004 [DOI] [PubMed] [Google Scholar]