Abstract
Summary
Background and objectives
We assessed the activation of the oxidative stress pathway in patients with IgA nephropathy (IgAN), while evaluating the classic marker of the disease (galactose-deficient serum IgA1).
Design, setting, participants, & measurements
Sera from 292 patients and 69 healthy controls from Italy and the United States were assayed for advanced oxidation protein products (AOPPs), free sulfhydryl groups on albumin (SH-Alb), and IgA1 with galactose-deficient hinge-region O-glycans (Gd-IgA1). Gd-IgA1 was detected by binding to Helix aspersa agglutinin (HAA) and expressed as total Gd-IgA1 or as degree of galactose deficiency relative to a standard Gd-IgA1 myeloma protein (%HAA).
Results
Sera from IgAN patients showed higher levels of Gd-IgA1, %HAA, and AOPPs, but lower levels of SH-Alb in comparison to that from healthy controls. Serum levels of AOPPs significantly correlated with serum Gd-IgA1 and %HAA. The relationship between these biomarkers and clinical features at sampling and during follow-up was assessed in 62 patients with long-term follow-up. AOPPs and %HAA correlated with proteinuria at sampling and independently associated with subsequent proteinuria. Levels of AOPPs correlated with rate of decline in renal function after sampling. The combination of a high level of AOPPs and a high level of %HAA associated with decline in estimated GFR.
Conclusions
Serum levels of aberrantly glycosylated IgA1 are elevated and oxidative stress pathways are activated in patients with IgAN; the intensity of the stress correlated with expression and progression of the disease. We speculate that oxidative stress may modulate the nephrotoxicity of aberrantly glycosylated IgA1 in IgAN.
Introduction
IgA nephropathy (IgAN) is characterized by a dysregulation of the immune system, with aberrantly glycosylated polymeric IgA1 in the circulation and mesangial deposits. This aberrant IgA1 has galactose-deficient O-glycans (1,2). Normally, a N-acetylgalactosamine (GalNAc) residue is attached to a serine or threonine in the IgA1 hinge region of the heavy chain and the structure is extended by the addition of galactose (Gal) to form a Galβ1,3GalNAc disaccharide. In patients with IgAN, activities of key enzymes involved in this process are altered, leading to production of an increased number of carbohydrate side chains that lack Gal.
IgA1 isolated from sera of patients with IgAN contains elevated levels of Gal-deficient IgA1 (Gd-IgA1) within immune complexes. These complexes stimulate cultured human mesangial cells (3–5), resulting in activation of oxidative stress pathways (6–8). Evidence of increased oxidative stress has been noted in renal tissue of patients with IgAN (9) as well as in their sera and/or erythrocytes (i.e., increased levels of lipoperoxide or malondialdehyde and reduced activity of superoxide dismutase, catalase, and glutathione peroxidase) (10,11). Moreover, increased serum levels of another marker of oxidative stress, advanced oxidation protein products (AOPPs), have been recently associated with proteinuria and disease progression in patients with IgAN (12). Although the serum level of Gd-IgA1 is considered a disease marker for IgAN, its unique role in inducing renal pathology or disease progression has been questioned. Notably, levels of Gd-IgA1 are commonly elevated in healthy first-degree relatives without clinical evidence of renal injury (13). In patients with IgAN, the specific degree of Gal deficiency of O-glycans on serum IgA1 remains constant over long intervals (2). Serum levels of Gd-IgA1 are increased in patients with severe IgAN who progressed to end-stage renal failure and renal transplantation. Nonetheless, because the levels overlap between patients grouped by recurrence of IgAN in the grafted kidney, altered IgA1 O-glycosylation alone is insufficient to trigger disease development and progression (14). Indeed, 4% to 16% of apparently healthy individuals in the general population have IgA deposits in their glomeruli (15,16), although it is unknown whether these deposits consist of Gd-IgA1. Therefore, we have postulated that the pathogenesis of IgAN requires multiple hits in the setting of an increased serum level of Gd-IgA1 to become fully manifest (17). The aim of this study was to investigate the potential of circulating Gd-IgA1 and measures of oxidative stress as risk markers for development and progression of IgAN.
We measured serum Gd-IgA1 and AOPPs and free sulfhydryl groups on serum albumin (SH-Alb) in large cohorts of patients with IgAN from Italy and the United States. In a representative subgroup, we assessed the correlations of these parameters with proteinuria and renal clearance function at sampling as well as with renal function decline and proteinuria during the subsequent follow-up. We found that although both an increased proportion of Gd-IgA1 in the total serum IgA1 and markers of oxidative stress were associated with clinical activity of IgAN, the stronger risk marker of progression was the serum level of AOPPs.
Materials and Methods
Patients
We assayed serum samples from 292 patients with IgAN followed by centers in Turin, Italy, and New York. Patients with Henoch–Schönlein nephritis, systemic lupus erythematosus, celiac disease, chronic liver disease, or diabetes were excluded. Seventy-nine patients were Italian, all of Caucasian ethnicity, and 213 patients were from the United States (83% Caucasians, 7% African Americans, and 10% Asians). Healthy controls were selected from healthy blood donors of the same areas from which patients were recruited (northern Italy and Atlantic coast of the United States); sera from 69 healthy individuals from Italy and from the United States, matched for ethnicity and sex ratios of the patients, were used. All serum samples were stored at −20°C before analyses.
For 230 patients, clinical data were incomplete; therefore, we did not include them in further statistical analyses.
For 62 patients, complete clinical data were available, including demographics, past medical history, serum creatinine, proteinuria at the time of study, and serial clinical assessments (serum creatinine and proteinuria at least three times over an interval longer than 3 years; Table 1) over a mean follow-up of 4.46 (range 3 to 10) years. Estimated GFR (eGFR) was calculated using the four-variable Modification of Diet in Renal Disease formula for adults (18) and the Schwartz formula for children younger than 18 years of age (19). Rate of decline in renal clearance function was determined by fitting a straight line through available data for eGFR using the principle of least squares for each patient (Table 1).
Table 1.
Demographics of the healthy controls and demographics, clinical history, and clinical features of the subgroup of 62 patients with IgA nephropathy provided with complete data set
| Healthy controls | |
| number | 69 |
| gender (men/women) | 48/21 (70%/30%) |
| ethnicity (Caucasian/African American/Asian) | 62/3/4 (90%/4%/6%) |
| IgAN patients | |
| number | 62 |
| gender (men/women) | 44/18 (71%/29%) |
| ethnicity (Caucasian/African American/Asian) | 256/15/21 (88%/5%/7%) |
| pediatric at time of biopsy (<18 years) | 47% |
| At sampling | |
| time elapsed since renal biopsy (years) | 4.3 (IQR 2.4 to 7.5) |
| age (years) | 18.7 (IQR 13.7 to 22.3) |
| eGFR (ml/min per 1.73 m2) | 100.6 (IQR 85.7 to 119.4) |
| stage 1, 2, 3, 4, 5 CKD (DOQI) | 66%, 23%, 8%, 3%, 0% |
| proteinuria (g/d) | 1.03 (IQR 0.31 to 1.59) |
| Follow-up | |
| duration of follow-up (years) | 4.46 ± 1.53 |
| 50% decline in renal function | No patient |
| stage 1, 2, 3, 4, 5 CKD (DOQI) at the end of follow-up | 61%, 26%, 9%, 2.4%, 1.6% |
| rate of renal function decline (ml/min per 1.73 m2/yr) | −0.75 ± 6.1 |
| time-average proteinuria (g/d) | 0.73 (IQR 0.3 to 1.7) |
In these patients, we assessed the relationships between the investigated biomarkers and clinical features at sampling and during follow-up. Values are expressed as mean ± SD or median (IQR). Calculations of eGFR are detailed in Materials and Methods. CKD stages were determined based on eGFR according to KDOQI guidelines. CKD, chronic kidney disease; eGFR, estimated GFR; IQR, interquartile range.
Proteinuria was measured with a 24-hour collection; in children, results were corrected for body surface area, to homogenize units of measurement for patients of various ages (20). An average proteinuria (21) was determined for each 1-year block during follow-up; the average of all 1-year measurements was defined as time-average proteinuria (TA-proteinuria).
Determination of Serum IgA and Gd-IgA1 by ELISA
Serum total IgA and Gd-IgA1 were measured as described previously (22). Briefly, ELISA plates were coated with an F(ab′)2 fragment of goat IgG anti-human IgA (Jackson ImmunoResearch, Inc., West Grove, PA), 1 μg/ml. Bound IgA was detected with a biotin-labeled F(ab′)2 fragment of goat IgG anti-human IgA (Biosource, Camarillo, CA). Binding was measured after the addition of avidin-horseradish peroxidase conjugate (ExtrAvidin; Sigma-Aldrich, St. Louis, MO); the reaction was developed with the peroxidase chromogenic substrate o-phenylenediamine (OPD)-H2O2 (Sigma-Aldrich), and absorbance at 490 nm was measured (EL312 Bio-Kinetics microplate reader, Bio-Tek Instruments Inc., Winooski, VT). The standard for IgA consisted of a pool of normal human sera (Binding Site, Birmingham, UK) previously calibrated for Ig isotype concentrations, including that of IgA1.
To measure serum total Gd-IgA1, 96-well plates (MaxiSorp; Nunc, Thermo Fisher Scientific, Rochester, NY) were coated with the F(ab′)2 fragment of goat anti-human IgA (Jackson ImmunoResearch), 3 μg/ml. Plates were blocked with 2% BSA (Sigma-Aldrich) in PBS with 0.05% Tween 20. Diluted samples were added and incubated overnight at 4°C. Captured IgA was subsequently desialylated by treatment for 3 hours at 37°C with 10 mU/ml neuraminidase from Vibrio cholerae (Roche, Indianapolis, IN) in 10 mM sodium acetate buffer, pH 5. Samples were incubated for 3 hours at 37°C with GalNAc-specific biotinylated HAA lectin (Sigma-Aldrich) diluted 1:500. Bound lectin was detected with avidin-horseradish peroxidase conjugate. Naturally Gal-deficient IgA1 myleoma protein purified from plasma of a patient with IgA1 (Ale) multiple myeloma was used as the standard for Gd-IgA1. (This standard differs from the previously published standard; see references 13,23.) Results were expressed as relative Gal deficiency of IgA1 (percentage of standard Gd-IgA1 myeloma protein; %HAA) or as total amount of Gd-IgA1 (U/ml).
Measurement of AOPPs
Serum AOPPs levels were measured (24) by spectrophotometry on a microplate reader (MR 5000; Dynatech, Paris) calibrated with chloramine-T solutions (Sigma-Aldrich) which, in the presence of potassium iodide, absorb light at 340 nm. In test wells, 200 μl of serum diluted 1:5 in PBS was placed on a microtiter plate (Becton Dickinson Labware, Lincoln Park, NJ) and 20 μl of acetic acid was added. In standard wells, 20 μl of acetic acid was added to 200 μl of chloramine-T solution (0 to 500 μmol/L). Ten microliters of 1.16 mol/L potassium iodide (Sigma-Aldrich) followed by 20 μl of acetic acid were added immediately before reading. Absorbance of the reaction mixture was read at 340 nm on the microplate reader against a blank containing 200 μl of PBS, 10 μl of potassium iodide, and 20 μl of acetic acid. Because chloramine-T absorbance at 340 nm was linear within the range of 0 to 100 μmol/L, AOPP concentrations were expressed as μmol/L of chloramine-T equivalents.
Measurement of Albumin-Free SH Groups
Any decrease in titratable free SH for a given protein is an indirect marker of oxidative stress (25). Synthesis of SH-specific cyanines, their validation, and methods for their application have been detailed (26). Samples from patients and controls were normalized for protein content (CBB silver colloidal) and solubilized in 8 M urea, 4% wt/vol CHAPS, 10 mM Tris-HCl, pH 8.8, 1 mM EDTA, and 5 mM TBP. Labeling with iodoacetamide cyanines C3NIASO3 and C5NIASO3 dyes was done in the same solution, at concentrations of each cyanine varying from 10 to 400 pmol of dye per mg of proteins, for 1 hour, and the Cy-SH reaction was quenched with 65 mM DTE for 1 hour at room temperature in a dark box and gentle shaker. Specificity of the reaction for SH residues was assessed by competition with 150 mM unlabeled iodoacetamide. Determination of SH-labeled proteins was done with SDS-PAGE in 8 to 16 T% polyacrylamide gels (27). Results were expressed as arbitrary units (AU) corresponding to the fluorescence intensity at Ex532/Em555 corrected for the concentration of albumin.
Statistical Analyses
For each test, values within the 90th percentile for healthy controls were considered normal.
The t test and Mann–Whitney test were used to compare normally and not normally distributed variables, respectively; similarly, Pearson and Spearman tests were used to correlate variables.
Rate of decline in renal function for each patient was determined by fitting a straight line through the eGFR data points according to least squares and used in linear regression models. To analyze data using receiver operating characteristic (ROC) curves, eGFR slope was dichotomized in two groups: positive when ≥0 ml/min per 1.73 m2/yr or negative when <0 ml/min per 1.73 m2/yr.
The relationships between biomarkers and TA-proteinuria (dichotomized in 2 groups using 1 g/d as the cutoff point) were tested using ROC curves. Multiple linear regressions were used to determine whether biomarkers were independent of each other.
On the basis of AOPPs and %HAA, we divided patients into three categories, those with (1) normal AOPPs and normal %HAA, (2) abnormal AOPPs or %HAA, or (3) abnormal AOPPS and %HAA. Relationships between these categories and eGFR slope were assessed using the trend test. The same method was applied for AOPPs and GdIgA1 or Sh-Alb.
All P values were two-tailed and those <0.05 were considered significant. Confidence intervals (CI) were calculated at the 95% level. Analyses were performed using SPSS software (version 16, SPSS Inc., Chicago, IL).
Results
Circulating Levels of Gd-IgA1 and Markers of Oxidative Stress
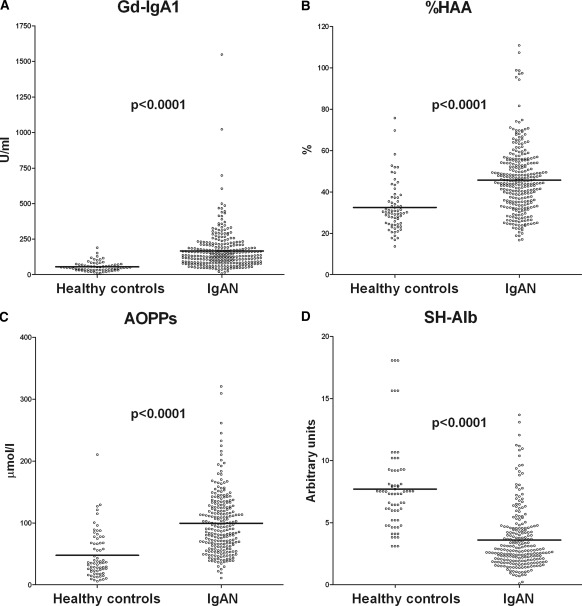
Sera from the 292 patients with IgAN contained, in comparison with those from healthy controls, higher levels of total IgA (3.62 ± 1.86 versus 1.79 ± 0.93 mg/ml, P < 0.0001) and Gd-IgA1, both as total levels (Gd-IgA1: 183.4 ± 156.4 versus 56.1 ± 33.8 U/ml, P < 0.0001) and as a relative degree of IgA1 Gal deficiency (%HAA: 49.6% ± 15.8% versus 32.5% ± 11.8%, P < 0.0001) (Figure 1, A and B). IgAN patients had higher serum AOPP levels (103.9 ± 49.10 versus 47.8 ± 37.5 μmol/L, P < 0.0001) and lower amounts of SH-Alb (3.9 ± 2.6 versus 7.7 ± 3.5 AU, P < 0.0001) when compared with healthy controls (Figure 1, C and D).
Figure 1.
Serum levels of galactose-deficient IgA1 and markers of oxidative stress. Serum levels of Gal-deficient IgA1, as total amount ([A] Gd-IgA1) or as a relative degree of galactose deficiency (percentage of standard Gal-deficient IgA1 myeloma protein) ([B] %HAA); serum levels of markers of oxidative stress, i.e., advanced oxidation protein products ([C] AOPPs) and albumin containing free SH groups ([D] SH-Alb) in 69 healthy controls and in 292 patients with IgAN. P = significance of the difference between patients and healthy controls. AOPPs, advanced oxidation protein products; Gd-IgA1, galactose-deficient IgA1; %HAA, % Helix aspersa agglutinin; SH-Alb, free sulfhydryl groups on albumin.
Values for Gd-IgA1 and %HAA correlated (r2 = 0.32, P < 0.0001). Levels of total IgA correlated with levels of Gd-IgA1 (r2 = 0.67, P < 0.001). Levels of AOPPs correlated with those of %HAA (r = 0.24, P = 0.01), which are independent of total IgA. Levels of AOPPs and SH-Alb did not correlate.
Correlation with Clinical Data
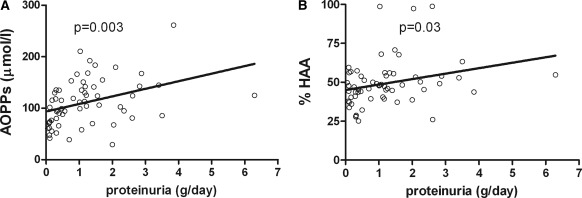
The subgroup of 62 patients with complete clinical data had, as did the whole cohort of 292 cases, levels of Gd-IgA1, %HAA, and AOPPs higher than those of the healthy controls (144.8 ± 79.3 versus 56.1 ± 33.8 U/ml, P < 0.0001; 49.4% ± 14.4% versus 32.5% ± 11.8%, P < 0.0001; and 111.1 ± 45.2 versus 47.8 ± 37.5 μmol/L, P < 0.0001, respectively) and lower levels of SH-Alb (4.7 ± 2.3 versus 7.7 ± 3.5 AU, P < 0.0001). No correlation was detected between markers of oxidative stress or serum Gd-IgA1 level and eGFR, gender, or age at time of sampling. AOPPs and %HAA correlated with proteinuria at sampling (r = 0.38, P = 0.003 and r = 0.25, P = 0.03, respectively; Figure 2, A and B), whereas an inverse relationship with SH-Alb did not reach statistical significance.
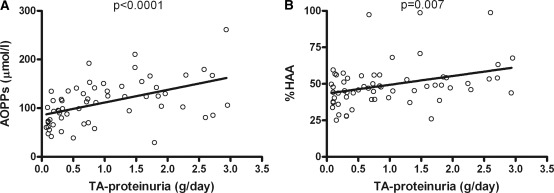
Figure 2.
Correlation between proteinuria and markers of oxidative stress or galactose deficient IgA1. Correlation between proteinuria at sampling (g/d) and serum levels of AOPPs (A) or serum Gal-deficient IgA1 expressed as percentage of a standard Gal-deficient IgA1 (Ale) myeloma protein ([B] %HAA) in 62 patients with IgAN. AOPPs, advanced oxidation protein products; %HAA, % Helix aspersa agglutinin.
Patients with abnormal levels of AOPPs, %HAA, and SH-Alb had greater proteinuria at sampling and TA-proteinuria over follow-up than did patients with normal levels of these biomarkers (Table 2).
Table 2.
Proteinuria at sampling and time-average proteinuria calculated over follow-up subsequent to sampling in patients with abnormal circulating levels of AOPPs, SH-Alb, %HAA, or Gd-IgA1
| Proteinuria at sampling (g/d) | P | Time-average proteinuria (g/d) | P | |
|---|---|---|---|---|
| AOPP abnormal (30 patients) | 1.2 (0.8 to 1.8) | 0.01 | 1.1 (0.7 to 1.8) | 0.02 |
| normal (32 patients) | 0.4 (0.1 to 1.4) | 0.3 (0.1 to 0.8) | ||
| SH-Alb abnormal (25 patients) | 1.2 (0.5 to 2.7) | 0.02 | 1.1 (0.3 to 1.9) | 0.03 |
| normal (37 patients) | 0.8 (0.3 to 1.4) | 0.6 (0.2 to 1.3) | ||
| %HAA abnormal (25 patients) | 1.3 (0.5 to 2.4) | 0.02 | 1.0 (0.3 to 2.4) | 0.03 |
| normal (37 patients) | 0.7 (0.3 to 1.2) | 0.6 (0.2 to 1.4) | ||
| Gd-IgA1 abnormal (42 patients) | 1.1 (0.3 to 2.0) | 0.86 | 0.8 (0.2 to 1.8) | 0.88 |
| normal (20 patients) | 0.6 (0.3 to 1.3) | 0.6 (0.3 to 1.2) |
Abnormal values were those exceeding the 90th percentile for healthy controls for AOPPs, %HAA, and Gd-IgA1, and those lower than 10th percentile for healthy controls for SH-Alb. AOPPs, advanced oxidation protein products; Gd-IgA1, galactose-deficient IgA1; %HAA, % Helix aspersa agglutinin; SH-Alb, free sulfhydryl groups on albumin.
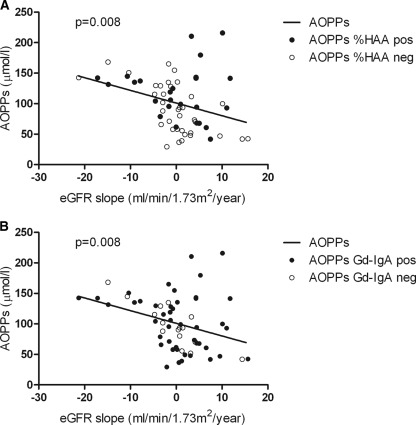
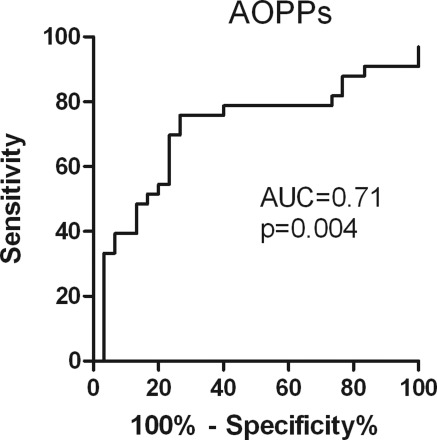
Levels of AOPPs correlated with rate of decline in renal function (r = −0.33, P = 0.008; Figure 3), whereas no such correlation was observed with levels of SH-Alb, total Gd-IgA1, or %HAA. In the ROC analysis (Figure 4), the best cutoff value for AOPPs was 100.7 μmol/L, yielding a sensitivity of 76% and specificity of 73% in predicting eGFR slope.
Figure 3.
Correlation between loss of eGFR and AOPPs. Correlation between eGFR slope (ml/min per 1.73 m2/yr) calculated over the follow-up after sampling and serum levels of AOPPs. Sera positive for %HAA (A) or Gd-IgA1 (B) are indicated with closed circles; sera negative for %HAA (A) or Gd-IgA1 (B) are indicated with open circles. AOPPs, advanced oxidation protein products; eGFR, estimated GFR; Gd-IgA1, galactose-deficient IgA1; %HAA, % Helix aspersa agglutinin.
Figure 4.
Predictive value of AOPPs on eGFR loss. ROC analysis of the predictive value of circulating levels of AOPPs at sampling on eGFR slope over the follow-up interval (positive outcome: eGFR slope ≥0 ml/min per 1.73 m2/yr, negative outcome eGFR slope <0 ml/min per 1.73 m2/yr). AOPPs, advanced oxidation protein products; AUC, area under the curve; eGFR, estimated GFR; ROC, receiver operating characteristic.
The association of increased levels of AOPPs and high %HAA was a risk marker of loss of eGFR (trend test P = 0.01; Figure 5); also the association of high values of AOPPs and decreased levels of SH-Alb was a risk marker of loss of eGFR (trend test P = 0.002).
Figure 5.
Predictive value of the combination of AOPPs and galactose-deficient IgA1 on eGFR loss. On the basis of AOPPs levels (A) and %HAA (B), the patients were divided into three categories: those with normal AOPPs and normal %HAA, those with abnormal AOPPs or %HAA, and those with abnormal AOPPs and %HAA; the relationship between these categories and eGFR was assessed by trend test. AOPPs, advanced oxidation protein products; AUC, area under the curve; eGFR, estimated GFR; %HAA, % Helix aspersa agglutinin; ROC, receiver operating characteristic.
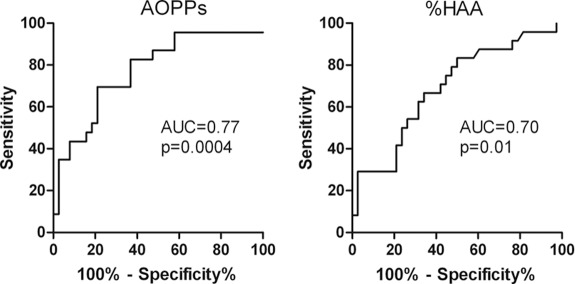
TA-proteinuria correlated with eGFR slope (r = −0.348, P < 0.05) and also with AOPPs (r = 0.49, P < 0.0001; Figure 6A) and %HAA (r = 0.29, P = 0.007; Figure 6B), but not with SH-Alb. At multivariate analysis, serum levels of AOPPs and %HAA were associated with TA-proteinuria over the follow-up interval after sampling, independently of each other (B = 0.09, P < 0.0001 and B = 0.016, P = 0.01, respectively). Because TA-proteinuria >1 g/d is a significant risk factor for progression (21), we used this value to dichotomize TA-proteinuria for ROC analysis (Figure 7); the best cutoff value was 121.5 μmol/L for AOPPs (sensitivity 70% and specificity 79%) and 48% for %HAA (sensitivity 67% and specificity 66%). Patients with AOPPs >121.5 μmol/L had higher TA-proteinuria compared with those with AOPPs <121.5 mol/L (1.41 ± 0.74 versus 0.69 ± 0.82, P = 0.001).
Figure 6.
Correlation between time-average proteinuria and AOPPs or galactose-deficient IgA1. Correlation between time-average proteinuria (TA-proteinuria) over the follow-up interval after sampling and serum levels of AOPPs (A) and %HAA (B). AOPPs, advanced oxidation protein products; %HAA, % Helix aspersa agglutinin; TA, time-average.
Figure 7.
Predictive value of AOPPs and galactose-deficient IgA1 on time-average proteinuria. Receiver operating characteristic curve analysis of the predictive value of circulating levels of AOPPs and %HAA at sampling on time-average proteinuria (TA-proteinuria) over the follow-up interval (positive outcome: TA-proteinuria <1 g/d, negative outcome TA-proteinuria ≥1 g/d). AOPPs, advanced oxidation protein products; AUC, area under the curve; Gd-IgA1, galactose-deficient IgA1; %HAA, % Helix aspersa agglutinin; TA, time-average.
Discussion
This study investigated, for the first time, the relative contribution of circulating Gd-IgA1 and a pro-oxidant milieu on the expression of disease activity and progression in patients with IgAN. The major conclusion is that although both biomarkers are associated with IgAN and with clinical activity of disease, systemic oxidative stress, as denoted by circulating AOPPs, was the stronger risk marker of disease progression. Notably, progression was more likely when the %HAA was also high.
AOPPs are a family of oxidized, di-tyrosine-containing protein compounds that are generated by monocyte activation, myeloperoxidase release, and contact with reactive oxygen species (28). In addition to being markers of oxidative protein damage, AOPPs play an important role as effector molecules, by activating monocytes and triggering respiratory burst and inflammation (29), inducing vascular endothelial dysfunction and accelerating atherosclerosis by activating endothelial cells (30). AOPPs also act on resident renal cells to increase synthesis of TGF-β1 and matrix components that may lead to renal fibrosis (31). AOPPs induce podocyte depletion and proteinuria when injected into experimental animals and when added in very low concentrations to podocyte cultures (32). The cascade of intracellular signaling events elicited by AOPPs leads to nuclear translocation of nuclear factor kappa B (NF-κB) and synthesis of pro-inflammatory and prosclerotic mediators in podocytes. The series of events finally induces p53-dependent apoptosis that likely plays a critical role in promoting proteinuria and accelerating glomerulosclerosis (32,33). It is difficult to state whether the association we found between signs of oxidative stress and proteinuria in patients with IgAN is causative (due to an effect of AOPPs on podocytes leading to development of proteinuria) or simply indicates a phase of clinical activity and potential progression of renal disease. The observational nature of this study precludes determination of a causal relationship between higher serum levels of AOPPs and progressive renal injury.
In spite of the important pathologic implication of AOPPs, we still lack a clear structural characterization of these molecules. Albumin is likely a major target of oxidative stress (25,28,34,35), as its thiol groups are almost always targeted in oxidation processes. Hence, SH-Alb gives a quantification of available SH-buffering groups.
In patients with IgAN, we detected high levels of AOPPs and low amounts of SH-Alb; each finding correlated with severity of proteinuria. These associations were observed also in patients with preserved renal function, thereby suggesting oxidative stress early in the clinical course of IgAN. The presence of enhanced oxidative stress may not be unique to patients with IgAN, and additional studies including such patients are necessary to examine this point. However, the aim of our study was to look for interplay between aberrantly glycosylated IgA and pro-oxidative milieu, rather than demonstrating specificity of these findings with respect to patients with other glomerular diseases.
We confirmed the presence of an increased level of Gd-IgA1 in the circulation of the patients with IgAN and examined this biomarker as a risk marker of progression. Some weak correlations were indeed found with proteinuria, albeit at levels of significance lower than those for the markers of oxidative stress. When we considered the prognostic value of both series of biomarkers, only the serum level of AOPPs strongly correlated with decline in renal function. However, the combination of a high AOPP level with a high %HAA was a strong risk marker of progression. Aberrantly glycosylated IgA1 can activate transcription factor NF-κB in mononuclear cells (7). We recently reported increased NF-κB nuclear translocation in peripheral blood mononuclear cells of patients with IgAN, particularly during phases of clinical activity (36). AOPPs can activate NF-κB also in mesangial cells (31). NF-κB activation triggers transcription of inducible nitric oxide synthase (iNOS), a powerful mediator of oxidative stress (37). Circulating mononuclear cells as well as resident renal cells are also targets of angiotensin II and TGF-β1, both capable of triggering generation of intracellular superoxide and initiating the oxidative stress pathway (38). Increased serum levels of markers of oxidative stress may derive from circulating cells as well as resident renal cells, sharing receptors and activating common pathways of inflammatory response (39).
In summary, levels of Gd-IgA1 and signs of oxidative stress are increased in the circulation of patients with IgAN in comparison with healthy controls. Progression to reduced renal function was predicted by sustained proteinuria during follow-up, but not by proteinuria at sampling, likely because half the cases had stable or even improved renal function during the follow-up interval in spite of initially high proteinuria. An increased serum level of AOPPs was the only biomarker that strongly associated with renal function decline and proteinuria during follow-up. Combining a high serum level of aberrantly glycosylated IgA1 with a high serum level of AOPPs strengthened the association. These data suggest that the nephrotoxicity of Gd-IgA1 in patients with IgAN may be enhanced in the presence of systemic oxidation and support the hypothesis that the intensity of the oxidative stress alters expression and progression of this common renal disease.
Disclosures
None.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health (DK082753, DK078244, DK075868, DK080301, DK077279, DK071802, and DK061525), by the grant A273 Ricerca scientifica applicata Regione Piemonte, and by Associazione Infanzia Nefropatica (Turin).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Mestecky J, Tomana M, Moldoveanu Z, Julian BA, Suzuki H, Matousovic K, Renfrow MB, Novak L, Wyatt RJ, Novak J: Role of aberrant glycosylation of IgA1 molecules in the pathogenesis of IgA nephropathy. Kidney Blood Press Res 31: 29–37, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Coppo R, Feehally J, Glassock RJ: IgA nephropathy at two score and one. Kidney Int 77: 181–186, 2010 [DOI] [PubMed] [Google Scholar]
- 3. Moura IC, Arcos-Fajardo M, Sadaka C, Leroy V, Benhamou M, Novak J, Vrtovsnik F, Haddad E, Chintalacharuvu KR, Monteiro RC: Glycosylation and size of IgA1 are essential for interaction with mesangial transferrin receptor in IgA nephropathy. J Am Soc Nephrol 15: 622–634, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Amore A, Cirina P, Conti G, Brusa P, Peruzzi L, Coppo R: Glycosylation of circulating IgA in patients with IgA nephropathy modulates proliferation and apoptosis of mesangial cells. J Am Soc Nephrol 12: 1862–1871, 2001 [DOI] [PubMed] [Google Scholar]
- 5. Novak J, Tomana M, Matousovic K, Brown R, Hall S, Novak L, Julian BA, Wyatt RJ, Mestecky J: IgA1-containing immune complexes in IgA nephropathy differentially affect proliferation of mesangial cells. Kidney Int 67: 504–513, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Gómez-Guerrero C, González E, Hernando P, Ruiz-Ortega M, Egido J: Interaction of mesangial cells with IgA and IgG immune complexes: A possible mechanism of glomerular injury in IgA nephropathy. Contrib Nephrol 104: 127–137, 1993 [DOI] [PubMed] [Google Scholar]
- 7. Gómez-Guerrero C, López-Franco O, Suzuki Y, Sanjuán G, Hernández-Vargas P, Blanco J, Egido J: Nitric oxide production in renal cells by immune complexes: Role of kinases and nuclear factor-kappaB. Kidney Int 62: 2022–2034, 2002 [DOI] [PubMed] [Google Scholar]
- 8. Chen HC, Guh JY, Chang JM, Lai YH: Differential effects of circulating IgA isolated from patients with IgA nephropathy on superoxide and fibronectin production of mesangial cells. Nephron 88: 211–217, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Suzuki D, Miyata T, Saotome N, Horie K, Inagi R, Yasuda Y, Uchida K, Izuhara Y, Yagame M, Sakai H, Kurokawa K: Immunohistochemical evidence for an increased oxidative stress and carbonyl modification of proteins in diabetic glomerular lesions. J Am Soc Nephrol 10: 822–832, 1999 [DOI] [PubMed] [Google Scholar]
- 10. Chen JX, Zhou JF, Shen HC: Oxidative stress and damage induced by abnormal free radical reactions and IgA nephropathy. J Zhejiang Univ Sci B 6: 61–68, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Vas T, Wagner Z, Jenei V, Varga Z, Kovács T, Wittmann I, Schinzel R, Balla G, Balla J, Heidland A, Nagy J: Oxidative stress and non-enzymatic glycation in IgA nephropathy. Clin Nephrol 64: 343–351, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Descamps-Latscha B, Witko-Sarsat V, Nguyen-Khoa T, Nguyen AT, Gausson V, Mothu N, Cardoso C, Noël LH, Guérin AP, London GM, Jungers P: Early prediction of IgA nephropathy progression: Proteinuria and AOPP are strong prognostic markers. Kidney Int 66: 1606–1612, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Gharavi AG, Moldoveanu Z, Wyatt RJ, Barker CV, Woodford SY, Lifton RP, Mestecky J, Novak J, Julian BA: Aberrant IgA1 glycosylation is inherited in familial and sporadic IgA nephropathy. J Am Soc Nephrol 19: 1008–1014, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coppo R, Amore A, Chiesa M, Lombardo F, Cirina P, Andrulli S, Passerini P, Conti G, Peruzzi L, Giraudi R, Messina M, Segoloni G, Ponticelli C: Serological and genetic factors in early recurrence of IgA nephropathy after renal transplantation. Clin Transplant 21: 728–737, 2007 [DOI] [PubMed] [Google Scholar]
- 15. Waldherr R, Rambousek M, Duncker WD, Ritz E: Frequency of mesangial IgA deposits in non-selected autopsy series. Nephrol Dial Transplant 4: 943–946, 1989 [DOI] [PubMed] [Google Scholar]
- 16. Suzuki K, Honda K, Tanabe K, Toma H, Nihei H, Yamaguchi Y: Incidence of latent mesangial IgA deposition in renal allograft donors in Japan. Kidney Int 63: 2286–2294, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Suzuki H, Fan R, Zhang Z, Brown R, Hall S, Julian BA, Chatham WW, Suzuki Y, Wyatt RJ, Moldoveanu Z, Lee JY, Robinson J, Tomana M, Tomino Y, Mestecky J, Novak J: Aberrantly glycosylated IgA1 in IgA nephropathy patients is recognized by IgG antibodies with restricted heterogeneity. J Clin Invest 119: 1668–1677, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 19. Work DF, Schwartz GJ: Estimating and measuring glomerular filtration rate in children. Curr Opin Nephrol Hypertens 17: 320–325, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Coppo R, Troyanov S, Camilla R, Hogg RJ, Cattran DC, Cook HT, Feehally J, Roberts IS, Amore A, Alpers CE, Barratt J, Berthoux F, Bonsib S, Bruijn JA, D'Agati V, D'Amico G, Emancipator SN, Emma F, Ferrario F, Fervenza FC, Florquin S, Fogo AB, Geddes CC, Groene HJ, Haas M, Herzenberg AM, Hill PA, Hsu SI, Jennette JC, Joh K, Julian BA, Kawamura T, Lai FM, Li LS, Li PK, Liu ZH, Mezzano S, Schena FP, Tomino Y, Walker PD, Wang H, Weening JJ, Yoshikawa N, Zhang H, Working Group of the International IgA Nephropathy Network and the Renal Pathology Society: The Oxford IgA nephropathy clinicopathological classification is valid for children as well as adults. Kidney Int 77: 921–927, 2010 [DOI] [PubMed] [Google Scholar]
- 21. Reich HN, Troyanov S, Scholey JW, Cattran DC: Toronto Glomerulonephritis Registry: Remission of proteinuria improves prognosis in IgA nephropathy. J Am Soc Nephrol 18: 3177–3183, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Suzuki H, Moldoveanu Z, Hall S, Brown R, Vu HL, Novak L, Julian BA, Tomana M, Wyatt RJ, Edberg JC, Alarcón GS, Kimberly RP, Tomino Y, Mestecky J, Novak J: IgA1-secreting cell lines from patients with IgA nephropathy produce aberrantly glycosylated IgA1. J Clin Invest 118: 629–639, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moldoveanu Z, Wyatt RJ, Lee JY, Tomana M, Julian BA, Mestecky J, Huang WQ, Anreddy SR, Hall S, Hastings MC, Lau KK, Cook WJ, Novak J: Patients with IgA nephropathy have increased serum galactose-deficient IgA1 levels. Kidney Int 71: 134–138, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Descamps-Latscha B, Witko-Sarsat V, Nguyen-Khoa T, Nguyen AT, Gausson V, Mothu N, London GM: Advanced oxidation protein products as risk factors for atherosclerotic cardiovascular events in nondiabetic predialysis patients. Am J Kidney Dis 45: 39–47, 2005 [DOI] [PubMed] [Google Scholar]
- 25. Himmelfarb J, McMonagle E: Albumin is the major plasma protein target of oxidant stress in uremia. Kidney Int 60: 358–363, 2001 [DOI] [PubMed] [Google Scholar]
- 26. Bruschi M, Grilli S, Candiano G, Fabbroni S, Della Ciana L, Petretto A, Santucci L, Urbani A, Gusmano R, Scolari F, Ghiggeri GM: New iodo-acetamido cyanines for labeling cysteine thiol residues. A strategy for evaluating plasma proteins and their oxido-redox status. Proteomics 9: 460–469, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685, 1970 [DOI] [PubMed] [Google Scholar]
- 28. Witko-Sarsat V, Friedlander M, Nguyen Khoa T, Capeillère-Blandin C, Nguyen AT, Canteloup S, Dayer JM, Jungers P, Drüeke T, Descamps-Latscha B: Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol 161: 2524–2532, 1998 [PubMed] [Google Scholar]
- 29. Capeillère-Blandin C, Gausson V, Descamps-Latscha B, Witko-Sarsat V: Biochemical and spectrophotometric significance of advanced oxidized protein products. Biochim Biophys Acta 1689: 91–102, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Guo ZJ, Niu HX, Hou FF, Zhang L, Fu N, Nagai R, Lu X, Chen BH, Shan YX, Tian JW, Nagaraj RH, Xie D, Zhang X: Advanced oxidation protein products activate vascular endothelial cells via a RAGE-mediated signalling pathway. Antioxid Redox Signal 10: 1699–1712, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wei XF, Zhou QG, Hou FF, Liu BW, Liang M: Advanced oxidation protein products induce mesangial cell perturbation through PKC-dependent activation of NADPH oxidase. Am J Physiol Renal Physiol 296: F427–F437, 2009 [DOI] [PubMed] [Google Scholar]
- 32. Zhou LL, Hou FF, Wang GB, Yang F, Xie D, Wang YP, Tian JW: Accumulation of advanced oxidation protein products induces podocyte apoptosis and deletion through NADPH-dependent mechanisms. Kidney Int 76: 1148–1160, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Liu Y: Advanced oxidation protein products: A causative link between oxidative stress and podocyte depletion. Kidney Int 76: 1125–1127, 2009 [DOI] [PubMed] [Google Scholar]
- 34. Terawaki H, Yoshimura K, Hasegawa T, Matsuyama Y, Negawa T, Yamada K, Matsushima M, Nakayama M, Hosoya T, Era S: Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int 66: 1988–1993, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Mera K, Anraku M, Kitamura K, Nakajou K, Maruyama T, Tomita K, Otagiri M: Oxidation and carboxy methyl lysine-modification of albumin: Possible involvement in the progression of oxidative stress in hemodialysis patients. Hypertens Res 28: 973–980, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Coppo R, Camilla R, Alfarano A, Balegno S, Mancuso D, Peruzzi L, Amore A, Dal Canton A, Sepe V, Tovo P: Upregulation of the immunoproteasome in peripheral blood mononuclear cells of patients with IgA nephropathy. Kidney Int 75: 536–541, 2009 [DOI] [PubMed] [Google Scholar]
- 37. Cuzzocrea S: Role of nitric oxide and reactive oxygen species in arthritis. Curr Pharm Des 12: 3551–3570, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Yatabe J, Sanada H, Yatabe MS, Hashimoto S, Yoneda M, Felder RA, Jose PA, Watanabe T: Angiotensin II type 1 receptor blocker attenuates the activation of ERK and NADPH oxidase by mechanical strain in mesangial cells in the absence of angiotensin II. Am J Physiol Renal Physiol 296: F1052–F1060, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shah SV, Baliga R, Rajapurkar M, Fonseca VA: Oxidants in chronic kidney disease. J Am Soc Nephrol 18: 16–28, 2007 [DOI] [PubMed] [Google Scholar]