Abstract
Summary
Background and objectives
Cystatin C is used increasingly as a biomarker of renal function; however, cystatin C assays are not standardized. Our objective was to compare cystatin C results within the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study over time and in repeated measures to evaluate for assay drift.
Design, setting, participants, & measurements
Serum samples were obtained at baseline (visit 1 [V1], 2000 to 2002) and follow-up (visit 2 [V2], 2003 to 2005; visit 3 [V3], 2006 to 2008) and were assayed in 2006 (V1), 2007 to 2008 (V2), and 2010 (V3) in the same laboratory.
Results
Mean cystatin C levels measured using the Dade–Behring assay decreased over time in subjects, with measures at all three visits (V1: 0.80 ± 0.19 [0.42 to 3.41], V2: 0.75 ± 0.22 [0.39 to 3.77], and V3: 0.69 ± 0.22 [0.39 to 3.79]). Cystatin C values were lower in V1 and V2 samples remeasured in 2010 (mean differences −0.13 ± 0.04 and −0.08 ± 0.04, P < 0.0001 for both). Correlations for original and re-run values were strong for V1 (r = 0.99) and V2 (r = 0.99). Deming regression equations and Bland–Altman plots suggest a systematic shift in the values over time.
Conclusions
Systematic shifts in cystatin C levels, which can be corrected by regression adjustment, occurred in our laboratory in samples measured in 2006 and 2007 to 2008 as compared with 2010. Assay standardization and measurement reliability for cystatin C must be addressed.
Introduction
Cystatin C is used increasingly as a biomarker of renal function (1–5) and cardiovascular disease risk and death (6–11). However, cystatin C assays are not standardized and this presents challenges for comparing cystatin C within a study over time and between studies using different laboratories. Moreover, assay reproducibility over time and between laboratories is of utmost importance for use as a longitudinal measure of renal function research and clinical care. It has been previously noted that an approximate 15% drift in the Dade–Behring cystatin C assay has occurred over the past decade (12); however, we are unaware of published data documenting this assay drift over time. We have measured cystatin C in the Coronary Artery Calcification in Type 1 Diabetes (CACTI) study in the same clinical laboratory at three times points between 2006 and 2010, and this brief report documents systematic shifts in results in cystatin C results over this time period. The purposes of this report are to disseminate this information on the assay drift over time and justify use of regression equations to standardize these measurements within the CACTI study.
Study Population and Methods
Study Population.
The data presented in this report were collected as part of the baseline examination of 1416 participants in the CACTI study who were 19 to 56 years of age (13). At baseline, all participants were asymptomatic for coronary artery disease and had no history of coronary artery bypass graft, coronary angioplasty, or unstable angina. Participants with type 1 diabetes (T1D) generally had been diagnosed when <30 years of age or had positive antibodies or a clinical course consistent with T1D. Participants completed a baseline examination between March 2000 and April 2002. A more detailed description of the study and baseline characteristics of this cohort has been published (14). The second and third study visits followed the same protocol and occurred between 2003 and 2005 and 2006 and 2008, respectively. Data are included on all subjects who had cystatin C measured at all three visits. All participants provided informed consent, and the Colorado Multiple Institutional Review Board approved the study.
Serum samples in the CACTI study were obtained at baseline (visit 1 [V1], 2000 to 2002) and follow-up (visit 2 [V2], 2003 to 2005; visit 3 [V3], 2006 to 2008) and stored at −80°C. Cystatin C was assayed as batches in baseline samples in 2006 (June 16 to July 9, 2006), V2 samples in 2007 to 2008 (December 27, 2007, to January 14, 2008), and in V3 samples in 2010 (July 10 to November 2, 2010) in the University of Colorado Hospital Clinical Laboratory using the commercially available Dade–Behring assay following package insert instructions on a Prospec (V1 and V2) and then a BNII instrument (V3). A subset of available samples from V1 (n = 189) and V2 (n = 195) was re-run in 2010 concurrent with V3 samples. Additionally, 61 samples from V3 were assayed for a second time 4 months (November 2, 2010) after cystatin C was first measured at V3 to ensure consistency within our laboratory and to investigate the possible effect of an additional freeze-thaw cycle.
Statistical Analyses
Paired t tests, Pearson correlations, Deming regression equations, and Bland–Altman plots were used to compare original and re-run values. Deming regression is a preferred method for comparing two analytic methods or the same method at different time points because it allows for measurement error in the x- and y-axes, does not assume measurement error is normally distributed, and is less influenced by outliers. A P value <0.05 was considered statistically significant, and SAS version 9.2 was used for data analysis. Values are presented as mean ± SD [min to max].
Results
Unexpectedly, mean cystatin C values were lower over time in subjects (n = 984) with measurements at all three visits (V1: 0.80 ± 0.19 [0.42 to 3.41], V2: 0.75 ± 0.21 [0.39 to 3.77], and V3: 0.69 ± 0.22 [0.39 to 3.79]; Table 1). As expected, cystatin C levels were higher in subjects with T1D as compared with nondiabetics (non-DM) at all three visits. Estimated GFR using the serum creatinine-based Chronic Kidney Disease Epidemiology Collaboration equation (15) decreased over time in T1D (V1: 69.6 ± 14.9 ml/min per 1.73 m2, V2: 69.3 ± 15.0 ml/min per 1.73 m2, and V3: 67.4 ± 14.4 ml/min per 1.73 m2) and non-DM subjects (V1: 67.8 ± 10.7 ml/min per 1.73 m2, V2: 67.5 ± 11.0 ml/min per 1.73 m2, and V3: 65.7 ± 10.7 ml/min per 1.73 m2).
Table 1.
Mean cystatin C by visit in all subjects with three completed values
| Visit | T1D (n = 449) | non-DM (n = 535) | All (n = 984) |
|---|---|---|---|
| 1 | 0.81 ± 0.25 [0.51 to 3.41] | 0.78 ± 0.10 [0.42 to 1.14] | 0.80 ± 0.18 [0.42 to 3.41] |
| 2 | 0.78 ± 0.28 [0.46 to 3.77] | 0.73 ± 0.10 [0.39 to 1.38] | 0.75 ± 0.21 [0.39 to 3.77] |
| 3 | 0.72 ± 0.30 [0.39 to 3.79] | 0.66 ± 0.11 [0.41 to 1.41] | 0.69 ± 0.22 [0.39 to 3.79] |
Mean ± SD [min to max]. T1D, type 1 diabetes; non-DM, nondiabetics.
Cystatin C values were lower in V1 (n = 189) and V2 (n = 195) samples remeasured in 2010 (mean differences −0.13 ± 0.04 and −0.08 ± 0.04, P < 0.0001 for both; Table 2). At V3, 61 samples were re-assayed within 4 months of the first V3 measurement and were very similar (mean difference = 0.004 ± 0.048, P = 0.49), indicating that our university clinical laboratory has currently reproducible methods and there was no effect of an additional freeze-thaw cycle on results. Correlations between original and re-run values were strong for V1 and V2 (r = 0.99 and r = 0.99, respectively, P < 0.0001, for both).
Table 2.
Mean cystatin C re-runs by visit
| Visit | Original | Re-Run | Difference | P |
|---|---|---|---|---|
| 1 (n = 189) | 0.82 ± 0.29 [0.51 to 3.41] | 0.70 ± 0.27 [0.42 to 3.15] | 0.13 ± 0.04 [0.06 to 0.30] | <0.01 |
| 2 (n = 195) | 0.79 ± 0.35 [0.51 to 4.17] | 0.71 ± 0.34 [0.43 to 3.89] | 0.08 ± 0.04 [−0.04 to 0.28] | <0.01 |
Mean ± SD [min to max] or for difference mean ± SD [min to max].
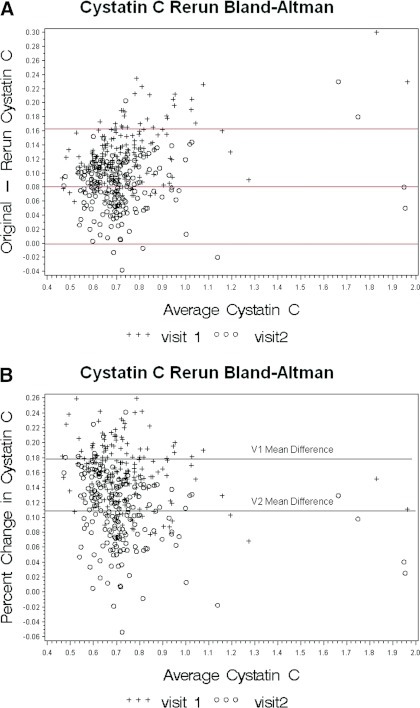
A systematic shift occurred in cystatin C values between V1 (2006) and 2010 and between V2 (2007 to 2008) and 2010 on the basis of Deming regression equations (V1: y = −0.069745 + xV1(0.93043), and V2: y = −0.050875 + xV2(0.96211) and Bland–Altman plots; Figure 2, A and B). However, the difference between the two slopes (V1 − remeasure and V2 − remeasure) was of borderline significance (P = 0.061), and we present individual equations because it is not clear if there was a shift between 2006 and 2007 to 2008. No differences existed by T1D status in the difference in cystatin C levels measured at V1 and V2 and repeat measures.
Figure 2.
Bland–Altman plot of (A) mean cystatin C versus the difference between initial and repeat cystatin C measures and (b) mean cystatin C versus the percent difference between initial and repeat cystatin C measures.
Discussion
Systematic shifts in cystatin C levels, corrected by regression adjustment, occurred in our laboratory in samples measured in 2006 and 2007 to 2008 as compared with 2010. Cystatin C assay values declined from the baseline visit to V2 and again from V2 to V3 in the CACTI cohort. This decline occurred in patients with T1D and in non-DM controls. Of note, in T1D subjects, the minimum values decreased, further indicating a shift in the assay, whereas the maximum values increased, consistent with decreasing GFR in the cohort as would be expected with increased T1D duration.
The analysis of paired samples that were run at the time of the original assay (V1 in 2006 and V2 in 2007 to 2008) and then again in July of 2010 confirm that there has been a significant downward shift in the reported cystatin C values. This shift is evident in patients with T1D and in non-DM controls. The correlation between the original values and the re-run values is excellent, with correlation coefficients of 0.99 for V1 and 0.99 for V2. Plots of original versus re-run cystatin C values show a strong linear relationship between original and re-run values (Figure 1). The Bland–Altman plots of the mean value (original and re-run) versus the difference (Figure 2A) and percent differences (Figure 2B) show that the difference remains the same regardless of the original value, but there is an apparent shift of values such that most of the original values are higher than the re-run values at V2, whereas at baseline this is true for all samples.
Figure 1.
Correlation of initial cystatin C measurement and repeated cystatin C measurement. (V1) measured between June 16 and July 9, 2006; (V2) measured between December 27, 2007, and January 14, 2008; and (V3) measured between July 10 and November 2, 2010, including repeat measures of V1 and V2 samples.
Previous research suggests that cystatin C measures have little variation because of storage time or freeze-thaw cycles (1,16,17). A review of potential applications of cystatin C in research and clinical decision-making has been published (18); standardization of cystatin C measurements—a common issue with any analyte that gains widespread usage—should be a priority. Efforts have previously been focused on assay calibration for serum creatinine (19,20) and for other analytes of research and clinical importance, such as hemoglobin A1c (21). To our knowledge, this is the first report to document a drift in the Dade–Behring assay for cystatin C as performed in a university clinical laboratory, although this has been noted in the proceedings of a recent National Institutes of Health (NIH) working group meeting (12). Possible explanations could be the materials used for calibration and a change in Dade–Behring instrumentation (12)—both consistent with the data we present from CACTI.
In conclusion, one implication of these data is that over the time period of this study, uncorrected cystatin C would overestimate renal function by approximately 10% to 15% and thereby obscure what would have been clinically relevant changes in renal function. Knowledge of this has important implications if cystatin C has been used for clinical care or research over this time period. In our data, we demonstrate that the shift was systematic and therefore amenable to standardization with a regression equation for future analysis of longitudinal data, although this equation may not be applicable to other research studies or clinical scenarios. The difference in the slope of the Deming regression equations at V1 and V2 approached statistical significance, and we present individual equations for better precision. As cystatin C is used increasingly, issues of assay standardization and measurement reliability must be addressed.
Disclosures
None.
Acknowledgments
Support for this study was provided by the NIH National Heart Lung and Blood Institute grant (R01 HL61753, HL79611) and the Diabetes Endocrinology Research Center Clinical Investigation Core (P30 DK57516). The study was performed at the Adult General Clinical Research Center at the University of Colorado–Denver Health Sciences Center supported by NIH-M01-RR00051, at the Barbara Davis Center for Childhood Diabetes, and at Colorado Heart Imaging Center in Denver, Colorado. D.M.M. was supported by grant K23 DK075360. Part of this material was presented in abstract form at the American Heart Association Epidemiology conference; March 23 through 25, 2011; Atlanta, GA.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Laterza OF, Price CP, Scott MG: Cystatin C: An improved estimator of glomerular filtration rate? Clin Chem 48: 699–707, 2002 [PubMed] [Google Scholar]
- 2. Dharnidharka VR, Kwon C, Stevens G: Serum cystatin C is superior to serum creatinine as a marker of kidney function: A meta-analysis. Am J Kidney Dis 40: 221–226, 2002 [DOI] [PubMed] [Google Scholar]
- 3. Buysschaert M, Joudi I, Wallemacq P, Hermans MP. Performance of serum cystatin-C versus serum creatinine in subjects with type 1 diabetes. Diab Care 26:1320, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Filler G, Bokenkamp A, Hofmann W, Le BT, Martinez-Bru C, Grubb A: Cystatin C as a marker of GFR—History, indications, and future research. Clin Biochem 38: 1–8, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Tan GD, Lewis AV, James TJ, Altmann P, Taylor RP, Levy JC: Clinical usefulness of cystatin C for the estimation of glomerular filtration rate in type 1 diabetes: Reproducibility and accuracy compared with standard measures and iohexol clearance. Diab Care 25: 2004–2009, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Maahs DM, Ogden LG, Kretowski A, Snell-Bergeon JK, Kinney GL, Berl T, Rewers M: Serum cystatin C predicts progression of subclinical coronary atherosclerosis in persons with type 1 diabetes mellitus. Diabetes 56: 2774–2779, 2007 [DOI] [PubMed] [Google Scholar]
- 7. Koenig W, Twardella D, Brenner H, Rothenbacher D: Plasma concentrations of cystatin C in patients with coronary heart disease and risk for secondary cardiovascular events: More than simply a marker of glomerular filtration rate. Clin Chem 51: 321–327, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Luc G, Bard JM, Lesueur C, Arveiler D, Evans A, Amouyel P, Ferrieres J, Juhan-Vague I, Fruchart JC, Ducimetiere P: Plasma cystatin-C and development of coronary heart disease: The PRIME study. Atherosclerosis 185: 375–380, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Shlipak MG, Sarnak MJ, Katz R, Fried LF, Seliger SL, Newman AB, Siscovick DS, Stehman-Breen C: Cystatin C and the risk of death and cardiovascular events among elderly persons. N Engl J Med 352: 2049–2060, 2005 [DOI] [PubMed] [Google Scholar]
- 10. Shlipak MG, Katz R, Sarnak MJ, Fried LF, Newman AB, Stehman-Breen C, Seliger SL, Kestenbaum B, Psaty B, Tracy RP, Siscovick DS: Cystatin C and prognosis for cardiovascular and kidney outcomes in elderly persons without chronic kidney disease. Ann Intern Med 145: 237–246, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Shlipak MG, Wassel Fyr CL, Chertow GM, Harris TB, Kritchevsky SB, Tylavsky FA, Satterfield S, Cummings SR, Newman AB, Fried LF: Cystatin C and mortality risk in the elderly: The Health, Aging, and Body Composition study. J Am Soc Nephrol 17: 254–261, 2006 [DOI] [PubMed] [Google Scholar]
- 12. National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) National Kidney Disease Education Program (NKDEP) Laboratory Working Group Meeting American Association of Clinical Chemistry Annual Meeting, Chicago, IL, July 22, 2009 [Google Scholar]
- 13. Maahs DM, Kinney GL, Wadwa P, Snell-Bergeon JK, Dabelea D, Hokanson J, Ehrlich J, Garg S, Eckel RH, Rewers MJ: Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population. Diab Care 28: 301–306, 2005 [DOI] [PubMed] [Google Scholar]
- 14. Dabelea D, Kinney G, Snell-Bergeon JK, Hokanson JE, Eckel RH, Ehrlich J, Garg S, Hamman RF, Rewers M: Effect of type 1 diabetes on the gender difference in coronary artery calcification: A role for insulin resistance?: The Coronary Artery Calcification in Type 1 Diabetes (CACTI) Study. Diabetes 52: 2833–2839, 2003 [DOI] [PubMed] [Google Scholar]
- 15. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, III, Feldman HI, Kusek JW, Eggers P, Van LF, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Finney H, Newman DJ, Gruber W, Merle P, Price CP. Initial evaluation of cystatin C measurement by particle-enhanced immunonephelometry on the Behring nephelometer systems (BNA, BN II). Clin Chem 43: 1016–1022, 1997 [PubMed] [Google Scholar]
- 17. Erlandsen EJ, Randers E, Kristensen JH: Evaluation of the Dade–Behring N latex cystatin C assay on the Dade–Behring nephelometer II system. Scand J Clin Lab Invest 59: 1–8, 1999 [DOI] [PubMed] [Google Scholar]
- 18. Shlipak MG: Cystatin C: Research priorities targeted to clinical decision making. Am J Kidney Dis 51: 358–361, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Murthy K, Stevens LA, Stark PC, Levey AS: Variation in the serum creatinine assay calibration: A practical application to glomerular filtration rate estimation. Kidney Int 68: 1884–1887, 2005 [DOI] [PubMed] [Google Scholar]
- 20. Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van LF: Expressing the Modification of Diet in Renal Disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Geistanger A, Arends S, Berding C, Hoshino T, Jeppsson JO, Little R, Siebelder C, Weykamp C: Statistical methods for monitoring the relationship between the IFCC reference measurement procedure for hemoglobin A1c and the designated comparison methods in the United States, Japan, and Sweden. Clin Chem 54: 1379–1385, 2008 [DOI] [PubMed] [Google Scholar]