Abstract
Summary
Background
The Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation was developed using both CKD and non-CKD patients to potentially replace the Modification of Diet in Renal Disease (MDRD) equation that was derived with only CKD patients. The objective of our study was to compare the accuracy of the MDRD and CKD-EPI equations for estimating GFR in a large group of patients having GFR measurements for diverse clinical indications.
Design, setting, participants, and measurements
A cross-sectional study was conducted of patients who underwent renal function assessment for clinical purposes by simultaneous measurements of serum creatinine and estimation of GFR using the MDRD and CKD-EPI equations and renal clearance of iothalamate (n = 5238).
Results
Bias compared with measured GFR (mGFR) varied for each equation depending on clinical presentation. The CKD-EPI equation demonstrated less bias than the MDRD equation in potential kidney donors (−8% versus −18%) and postnephrectomy donors (−7% versus −15%). However, the CKD-EPI equation was slightly more biased than the MDRD equation in native CKD patients (6% versus 3%), kidney recipients (8% versus 1%), and other organ recipients (9% versus 3%). Among potential kidney donors, the CKD-EPI equation had higher specificity than the MDRD equation for detecting an mGFR <60 ml/min per 1.73 m2 (98% versus 94%) but lower sensitivity (50% versus 70%).
Conclusions
Clinical presentation influences the estimation of GFR from serum creatinine, and neither the CKD-EPI nor MDRD equation account for this. Use of the CKD-EPI equation misclassifies fewer low-risk patients as having reduced mGFR, although it is also less sensitive for detecting mGFR below specific threshold values used to define CKD stages.
Introduction
Multiple equations are now widely used to estimate the GFR using serum creatinine values. Indeed, among adults younger than 70 years, organizations such as the National Kidney Foundation and the American Society of Nephrology currently recommend reporting estimated GFR (eGFR) using the Modification of Diet in Renal Disease (MDRD) equation with every serum-creatinine measurement, as long as the eGFR is less than 60 ml/min per 1.73 m2. However, this equation was developed using data from a study population with chronic kidney disease (CKD), and subsequent studies have demonstrated that the MDRD equation significantly underestimates GFR among individuals with normal kidney function (1–8). In addition, the extent to which the equation can be applied to other population groups, such as persons older than 70 years, is not clear.
Recently, the CKD Epidemiology Collaboration (CKD-EPI) equation was developed to address known limitations of the MDRD equation (9,10) using a mixed multicenter sample of approximately 70% subjects with known CKD or at high risk for it (e.g., transplant recipients) and only 30% with low risk for CKD (e.g., kidney donors). Thus, the CKD-EPI equation was designed to address to reduce the tendency of the MDRD equation to underestimate GFR when applied to healthy individuals. Indeed, it performed better than the MDRD equation when validated in a multicenter sample of 3896 subjects with a similar ratio of high-risk and low-risk subjects (9,10). Because the CKD-EPI equation appears less biased at lower GFR, automated reporting of eGFR calculated with it has been advocated even when the value is >60 ml/min per 1.73 m2 (11).
Therefore, the objectives of our study were the following: (1) to determine the performance of the MDRD and CKD-EPI equations for estimating GFR across different patient populations of varied clinical presentation in a large single-center sample of 5238 patients and (2) to assess the performance of these equations across the full adult age spectrum, including those >70 years old.
Materials and Methods
Patient Population
From June 1, 2007, to January 29, 2010, all adult patients who underwent an outpatient iothalamate clearance at the Mayo Clinic in Rochester, MN, were identified (n = 7713). All of the patients in this cohort underwent simultaneous serum creatinine and renal iothalamate clearance measurements. As part of the iothalamate clearance test, the clinical indication for the test, demographics, height, and weight were all documented in a laboratory database. Clinical indications were divided into the following categories: potential kidney donor, postnephrectomy kidney donors, native chronic kidney disease, kidney transplant recipient, and nonkidney organ transplant recipient. Patients who had received both a kidney and nonkidney allograft were classified as kidney transplant recipients. The clinical rationale for measurement of GFR for each of the patient populations was as follows: potential donors always undergo GFR measurement by iothalamate as part of a protocol evaluation. Postnephrectomy donors and transplant recipients undergo GFR measurement as part of a protocol follow-up evaluation. For the CKD patient population, the decision to measure GFR was made by the treating physician. All of the patients in the study population who underwent GFR assessment were stable outpatients and in steady state in relation to GFR.
Patients who underwent renal function assessment for the purpose of chemotherapy dosing, paraplegic and quadriplegic patients, patients known to have neurogenic bladder, patients under the age of 18 years, dialysis patients, and amputees were all excluded from the study (n = 2475). These patients were identified based on notes recorded in the laboratory database as well as a thorough review of medical records. For any subject who had multiple GFR measurements, only the first was used in the analysis. Our final study population totaled 5238 patients. The Mayo Clinic institutional review board approved this study.
Iothalamate Clearance, Serum Creatinine, and eGFR
GFR was measured by renal clearance of nonradiolabeled iothalamate (mGFR) as described previously (12). The test was performed under a standardized protocol. The patients were fasted and underwent testing early in the day to minimize the effects of diet and diurnal variation on GFR. They were orally hydrated with water to ensure adequate urine flows for accurate measurement. In brief, the patients underwent testing via subcutaneous injection of nonradiolabeled iothalamate after oral hydration with four to six glasses of water. Two hours later, renal clearance of iothalamate was measured over 45 to 60 minutes. Plasma and urine iothalalamate concentrations were measured by capillary electrophoresis on a Beckman MDQ capillary electrophoresis analyzer. GFR was expressed per 1.73 m2 by multiplying the measured value by 1.73 and dividing by body surface area. Serum or plasma creatinine values were obtained using an isotope dilution mass spectrometry–traceable Roche enzymatic method (Roche P or D Modular or Roche Cobas C501 with Roche Creatininase Plus assay; Roche Diagnostics, Indianapolis, IN) using a blood sample obtained within 24 hours of the study. GFR was estimated using both the revised MDRD equation for isotope dilution mass spectrometry–traceable creatinine assays (13) and the newer CKD-EPI equation (9).
GFR measured by iothalamate clearance may be imprecise because of within-individual measurement error, and this will negatively affect the assessment of the MDRD and CKD-EPI equation performance by analyses that are influenced by precision such as R2, root mean square error, SD of bias, and P30%. However, iothalamate clearance was the gold standard used to develop both of these equations and by definition provides unbiased estimates for assessing the performance of these equations. Furthermore, Mayo Clinic patients who had iothalamate clearances between 1996 and 2003 by the methods described here were part of the data set used to develop the CKD-EPI equation (9).
Statistical Analyses
Both the CKD-EPI and MDRD equations were developed using least-squares regression, which was intended to achieve no bias between log mGFR and log eGFR across levels of eGFR in the development sample. Therefore, in this study mGFR was plotted against eGFR on a log-log scale to assess bias in a manner that was consistent with the original approach used to derive the equations. Separate regression lines were drawn for postnephrectomy kidney donors and potential kidney donors. The other three patient populations (kidney transplant recipients, CKD patients, and nonkidney transplant patients) were grouped together, and a single regression line was drawn to represent these three groups because the regression lines for these three groups were virtually identical. The resulting three regression lines representing the different patient populations were compared with assess deviation (bias) relative to the line of identity.
For each stage of eGFR (<15, 15 to 29, 30 to 44, 45 to 59, 60 to 89, and ≥90 ml/min per 1.73 m2), the percentage of patients with an mGFR in the same stage was assessed for each of the five patient populations. Because both equations contain the same four variables (serum creatinine, age, gender, and race), the analyses focused on comparing bias rather than relative precision. Bias was assessed two different ways: (1) percentage bias, calculated as Σ[(eGFR − mGFR)/mGFR]/n and (2) bias on a logarithmic scale, calculated and presented as a percentage: exp[Σ(log eGFR − log mGFR)/n] − 1. Bias was compared by age group (<40, 40 to 69, and ≥70 years) and gender with each equation in each patient population. Bias estimates were also plotted against age by using a smoother function (lambda = 1000,000) to depict the difference between eGFR and mGFR in each patient population. To assess the diagnostic accuracy of each equation depending on the screening setting, the sensitivity and specificity for an mGFR <60 ml/min per 1.73 m2 (common threshold for CKD) (14) or <80 ml/min per 1.73 m2 (common threshold for donor rejection) (15) in potential kidney donors was calculated. All of the statistical analyses were performed using JMP, version 8.02 (SAS Institute, Cary, NC).
Results
Description of Patient Population
There were 5238 patients identified with both an iothalamate renal clearance result and a plasma or serum creatinine available within 24 hours of the test. The characteristics of the study population are summarized in Table 1. The population consisted of five major groups divided by clinical indication for testing: potential kidney donors (n = 583), postnephrectomy kidney donors (n = 97), native CKD (n = 2324), kidney transplant recipients (n = 1375), and recipients of organs other than kidneys (n = 859). Of all of the patients in our study, 19% (n = 870) were aged 70 years and older; however, most of these had known CKD or a high-risk condition (transplant) for CKD (n = 856), whereas relatively few were potential kidney donors (n = 7) or postnephrectomy kidney donors (n = 7). The cohort was largely Caucasian (89%) with only a small fraction identified as African American (1.9%, n = 98).
Table 1.
Patient demographics by group
| Overall (n = 5238) | Potential Kidney Donors (n = 583) | Postnephrectomy Kidney Donors (n = 97) | Native CKD Patients (n = 2324) | Kidney Recipients (n = 1375) | Other Organ Recipients (n = 859) | |
|---|---|---|---|---|---|---|
| Mean age (SD) | 56.1 (14.8) | 45.4 (12.1) | 53.2 (10.9) | 59.8 (15.4) | 54.1 (13.9) | 57.1 (12.2) |
| Age <40 years, n (%) | 765.0 (15%) | 188.0 (32%) | 9.0 (9%) | 265.0 (11%) | 219.0 (16%) | 84.0 (10%) |
| Age >70 years, n (%) | 984.0 (19%) | 7.0 (1%) | 8.0 (8%) | 660.0 (28%) | 198.0 (14%) | 111.0 (13%) |
| Male, n (%) | 2900.0 (55%) | 256.0 (44%) | 36.0 (37%) | 1307.0 (56%) | 787.0 (57%) | 514.0 (60%) |
| African American race, n (%) | 98.0 (2%) | 16.0 (3%) | 2.0 (2%) | 48.0 (2%) | 22.0 (2%) | 10.0 (1%) |
| Mean height, cm (SD) | 171.5 (10.2) | 171.8 (9.7) | 170.9 (8.8) | 171.2 (10.3) | 171.5 (10.4) | 172.2 (10.1) |
| Mean weight, kg (SD) | 85.6 (21.4) | 82.8 (16.5) | 81.2 (18.8) | 87.1 (22.2) | 86.2 (22.3) | 82.8 (20.3) |
| Mean body mass index, kg/m2 (SD) | 29.0 (6.4) | 28.0 (4.7) | 27.7 (5.3) | 29.6 (6.7) | 29.2 (6.6) | 27.8 (5.8) |
| Mean serum creatinine concentration, mg/dl (SD) | 1.64 (1.06) | 0.91 (0.17) | 1.21 (0.26) | 2.04 (1.37) | 1.48 (0.62) | 1.36 (0.52) |
| Mean creatinine clearance, ml/min per 1.73 m2 (SD) | 64.6 (31.4) | 101.8 (21.6) | 70.4 (20.2) | 56.1 (32.4) | 62.7 (23.6) | 65.1 (28.3) |
| Mean eGFR by MDRD equation, ml/min per 1.73 m2 (SD) | 51.8 (25.2) | 79.7 (15.4) | 54.8 (13.1) | 43.9 (26.9) | 50.4 (18.0) | 55.8 (22.4) |
| Mean eGFR by CKD-EPI equation, ml/min per 1.73 m2 (SD) | 55.2 (27.2) | 88.7 (16.3) | 60.1 (15.6) | 45.8 (28.3) | 53.9 (18.9) | 59.3 (23.4) |
| Mean mGFR, ml/min per 1.73 m2 (SD) | 55.9 (29.7) | 98.9 (20.1) | 65.8 (17.4) | 46.2 (29.5) | 52.3 (19.5) | 57.3 (24.1) |
| Percentage of eGFR by MDRD equation within 30% of mGFR | 77.6% | 75.8% | 82.5% | 75.2% | 80.1% | 80.6% |
| Percentage of eGFR by CKD-EPI equation within 30% of mGFR | 78.4% | 88.7% | 93.8% | 75.0% | 78.4% | 78.5% |
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease; eGFR, estimated GFR; mGFR, measured GFR.
Performance of the MDRD and CKD-EPI Equations in Estimating GFR among Various Patient Populations
Table 2 lists the percentages of patients with mGFR correctly classified into the same eGFR stage by the CKD-EPI equation or MDRD equation. Except for stage 1 (eGFR >90 ml/min per 1.73 m2), the CKD-EPI equation generally performed better than the MDRD equation for classifying patients into the correct stage. However, both the CKD-EPI and MDRD equations incorrectly classified at least 50% of patients with an eGFR of 45 to 59 ml/min per 1.73 m2, potential kidney donors with an eGFR 60 to 89 ml/min per 1.73 m2, postnephrectomy kidney donors with an eGFR 30 to 44 ml/min per 1.73 m2, or kidney recipients with an eGFR >90 ml/min per 1.73 m2.
Table 2.
Percentage of patients with mGFR in the same eGFR range calculated using the CKD-EPI equation and MDRD equation
| eGFR range | Equation | Patient Group |
||||
|---|---|---|---|---|---|---|
| Potential Kidney Donors (n = 583) | Postnephrectomy Kidney Donors (n = 97) | Native CKD (n = 2324) | Kidney Recipients (n = 1375) | Other Organ Recipients (n = 859) | ||
| >90 | CKD-EPI | 86% | 80% | 63% | 31% | 55% |
| MDRD | 90% | 100% | 66% | 41% | 64% | |
| 60 to 89 | CKD-EPI | 50% | 85% | 58% | 55% | 54% |
| MDRD | 38% | 77% | 51% | 62% | 55% | |
| 45 to 59 | CKD-EPI | 27% | 30% | 46% | 42% | 49% |
| MDRD | 15% | 18% | 40% | 44% | 46% | |
| 30 to 44 | CKD-EPI | 31% | 58% | 54% | 59% | |
| MDRD | 27% | 46% | 50% | 55% | ||
| 15 to 29 | CKD-EPI | 68% | 69% | 57% | ||
| MDRD | 67% | 63% | 51% | |||
| <15 | CKD-EPI | 72% | 80% | 71% | ||
| MDRD | 72% | 75% | 57% | |||
CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease; eGFR, estimated GFR; mGFR, measured GFR.
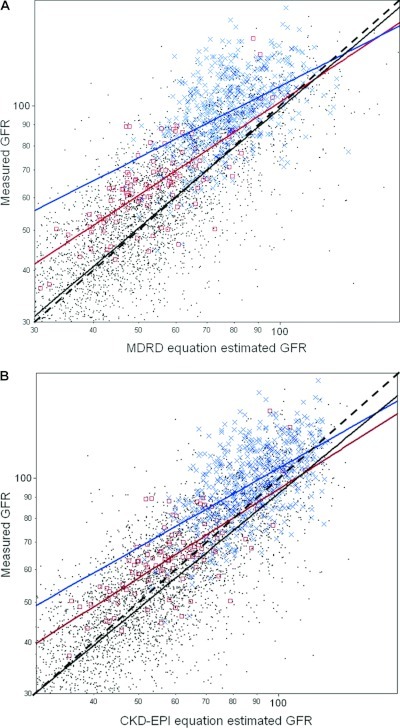
mGFR was plotted against eGFR calculated using the CKD-EPI equation (Figure 1A) and against eGFR by the MDRD equation (Figure 1B). When the regression line of mGFR versus eGFR is compared with the line of identity, it is apparent that the MDRD equation is relatively unbiased for native CKD and transplant recipients, but the CKD-EPI equation slightly overestimated GFR in these groups. The regression lines demonstrated bias for both equations among potential kidney donors and postnephrectomy kidney donors with underestimation of GFR, particularly at lower levels of eGFR. However, in the clinically important range of eGFR 50 to 70 ml/min per 1.73 m2, the CKD-EPI equation underestimated GFR to a lesser degree.
Figure 1.
GFR measured by iothalamate clearance (mGFR) is plotted against GFR estimated by the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation (Panel A) and the Modification of Diet in Renal Disease (MDRD) equation (Panel B) on a log-log scale for 5238 patients. The black dashed line represents the line of identity. With either equation, the blue regression line for potential kidney donors (blue ×) and the red regression line for postnephrectomy kidney donors (red □) show systematic deviation (underestimation) from the line of identity. With the CKD-EPI equation, the black solid regression line for native CKD and transplant recipients (black dots) also shows systemic deviation (overestimation) from the line of identity.
Next, the percentage bias between eGFR and mGFR was stratified by clinical indication for GFR testing (Table 3). The CKD-EPI equation underestimated mGFR to a lesser degree than the MDRD equation among potential kidney donors and postnephrectomy kidney donors. However, the CKD-EPI equation still underestimated mGFR among potential kidney donors with an eGFR <90 ml/min per 1.73 m2 and postnephrectomy kidney donors with an eGFR <60 ml/min per 1.73 m2 (−13% or more). The CKD-EPI equation also overestimated GFR more than the MDRD equation among those with CKD, kidney recipients, and other organ recipient populations. Both the CKD-EPI and MDRD equations substantially overestimated GFR (16% or more) among these patient populations when the eGFR was >90 ml/min per 1.73 m2. Repeating the analysis with percentage bias based on logarithmic eGFR (Table 3), the CKD-EPI equation showed less bias in the CKD, kidney recipient, and other organ recipient populations.
Table 3.
Percentage bias and bias on a logarithmic scale (95% confidence interval) for eGFR calculated using the CKD-EPI equation and MDRD equation with mGFR by iothalamate renal clearance in different populations
| eGFR range (ml/min/1.73 m2) | Equation | Potential Kidney Donors | Postnephrectomy Kidney Donors | Native CKD | Kidney Recipients | Other Organ Recipients |
|---|---|---|---|---|---|---|
| Percentage bias | ||||||
| overall | CKD-EPI | −8% (−10%, −7%) | −7% (−11%, −4%) | 6% (4%, 8%) | 8% (6%, 10%) | 9% (7%, 12%) |
| MDRD | −18% (−19%, −16%) | −15% (−18%, −12%) | 3% (2%, 5%) | 1% (0%, 3%) | 3% (1%, 6%) | |
| >90 | CKD-EPI | −3% (−5%, −1%) | −4% (−34%, 26%) | 16% (11%, 21%) | 26% (19%, 34%) | 18% (13%, 24%) |
| MDRD | −8% (−11%, −5%) | −17% (−49%, 14%) | 18% (11%, 25%) | 36% (22%, 51%) | 18% (11%, 29%)) | |
| 60 to 89 | CKD-EPI | −13% (−15%, −11%) | 2% (−4%, 8%) | 7% (3%, 11%) | 14% (11%, 16%) | 11% (7%, 14%) |
| MDRD | −20% (−22%, −19%) | −3% (−12%, 6%) | 1% (−3%, 5%) | 6% (3%, 9%) | 3% (0%, 7%) | |
| 45 to 59 | CKD-EPI | −16% (−25%, −7%) | −13% (−17%, −8%) | 7% (2%, 13%) | 3% (1%, 5%) | 9% (3%, 15%) |
| MDRD | −25% (−30%, −21%) | −18% (−22%, −15%) | −1% (−4%, 3%) | −2% (−4%, 0%) | 3% (−2%, 8%) | |
| 30 to 44 | CKD-EPI | −15% (−20%, −10%) | 5% (1%, 8%) | 4% (1%, 7%) | 4% (−1%, 9%) | |
| MDRD | −19% (−23%, −15%) | 2% (−2%, 6%) | −2% (−5%, 1%) | −1% (−5%, 4%) | ||
| 15 to 29 | CKD-EPI | 5% (1%, 8%) | 11% (3%, 19%) | 1% (−7%, 9%) | ||
| MDRD | 5% (2%, 9%) | 5% (−2%, 11%) | 3% (−7%, 13%) | |||
| <15 | CKD-EPI | 0% (−7%, 7%) | 7% (−10%, 24%) | 7% (−29%, 43%) | ||
| MDRD | 2% (−5%, 9%) | 3% (−13%, 20%) | −6% (−31%, 19%) | |||
| Bias on a logarithmic scale | ||||||
| overall | CKD-EPI | −10% (−11%, −9%) | −9% (−12%, −5%) | 1% (−1%, 2%) | 4% (3%, 6%) | 5% (3, 7%) |
| MDRD | −19% (−20%,−18%) | −16% (−19%, −13%) | −2% (−3%, −1%) | −2% (−3%, −1%) | −1% (−3%, 1%) | |
| >90 | CKD-EPI | −4% (−6%, −2%) | −7% (−32%, 29%) | 11% (7%, 15%) | 23% (16%, 30%) | 15% (11%, 20%) |
| MDRD | −9% (−12%, −6%) | −18% (−45%, 22%) | 12% (7%, 18%) | 31% (19%, 44%) | 15% (9%, 22%) | |
| 60 to 89 | CKD-EPI | −14% (−16%, −13%) | 1% (−5%, 7%) | 2% (1%, 5%) | 11% (8%, 13%) | 7% (4%, 11%) |
| MDRD | −22% (−23%, −20%) | −5% (−14%, 4%) | −3% (−6%, −1%) | 3% (1%, 6%) | 0% (−3%, 3%) | |
| 45 to 59 | CKD-EPI | −17% (−25%, −9%) | −14% (−18%, −9%) | 1% (−2%, 4%) | 0% (−2%, 2%) | 4% (0%, 7%) |
| MDRD | −26% (−21%, −22%) | −19% (−23%, −16%) | −5% (−8%, −2%) | −5% (−7%, −3%) | −2% (−5%, 1%) | |
| 30 to 44 | CKD-EPI | −15% (−20%, −10%) | −1% (−3%, 2%) | 0% (−2%, 3%) | 0% (−4%, 4%) | |
| MDRD | −19% (−23%, −15%) | −3% (−6%, −1%) | −6% (−8%, −3%) | −5% (−8%, −1%) | ||
| 15 to 29 | CKD-EPI | −1% (−3%, 2%) | 5% (−2%, 12%) | −4% (−11%, 4%) | ||
| MDRD | −1% (−3%, 2%) | −1% (−6%, 5%) | −3% (−10%, 6%) | |||
| <15 | CKD-EPI | −7% (−11%, −3%) | 3% (−11%, 20%) | 1% (−29%, 43%) | ||
| MDRD | −6% (−10%, −1%) | 0% (−14%, 15%) | −10% (−33%, 22%) |
The percentage bias was calculated as follows: {Σ [(eGFR − mGFR)/mGFR × 100%]}/n. The percentage bias on a logarithmic scale was calculated as follows: exp[Σ (log eGFR − log mGFR)/n] − 1. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease; eGFR, estimated GFR; mGFR, measured GFR.
Effect of Age on the Performance of Equations to Estimate GFR
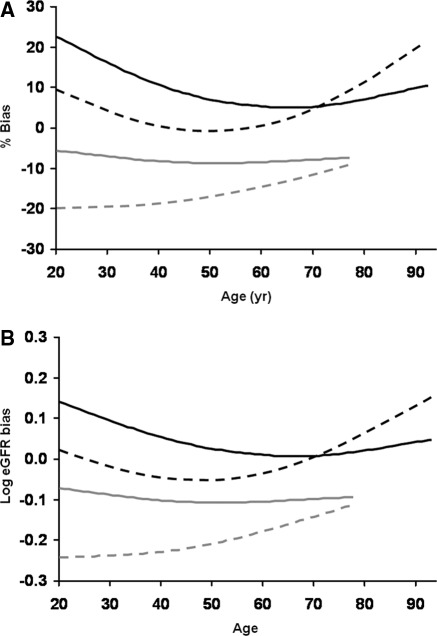
The percentage bias (Figure 2A) and bias on a logarithmic scale (Figure 2B) were separately calculated for each equation across the age spectrum. Potential kidney donors and postnephrectomy kidney donors were grouped together, as were native CKD and transplant recipients, because equations performed similarly within these groups. Among the native CKD patients and transplant recipients, the CKD-EPI equation tended to overestimate GFR more in younger adults, whereas the MDRD equation tended to overestimate GFR more in older adults (Table 4). The percentage bias for each equation was also evaluated by gender. Once again potential kidney donors and postnephrectomy kidney donors were grouped together, as were native CKD, kidney transplant recipients, and other organ transplant recipients. The differential effects of gender on bias were minimal (Table 5).
Figure 2.
Bias with estimated GFR (eGFR) by age (years). Mean percentage bias (eGFR − mGFR)/mGFR (Panel A) and mean bias calculated for log eGFR − log mGFR (Panel B) are both depicted. The CKD-EPI equation is represented by the solid smoother curves (lambda = 1000,000), and the MDRD equation is represented by the dashed smoother curves. Native CKD and transplant recipients (n = 4558) are represented by the black curves, and the potential kidney donors and postnephrectomy kidney donors (n = 680) are represented by the gray curves.
Table 4.
Percentage bias (95% confidence interval) of eGFR calculated using the CKD-EPI equation and MDRD equation with mGFR by iothalamate renal clearance by age group
| Equation | Age Group | Potential Kidney Donors | Postnephrectomy Kidney Donors | Native CKD | Kidney Recipients | Other Organ Recipients |
|---|---|---|---|---|---|---|
| CKD-EPI | <40 years | −8% (−10%, −5%) | a | 18% (10%, 27%) | 12% (8%, 16%) | 14% (7%, 21%) |
| 40 to 69 years | −9% (−11%, −7%) | −9% (−13%, −5%) | 4% (1%, 6%) | 7% (5%, 8%) | 9% (6%, 12%) | |
| >70 years | a | a | 5% (2%, 8%) | 10% (5%, 14%) | 8% (1%, 14%) | |
| MDRD | <40 years | −19% (−22%, −17%) | a | 8% (0%, 16%) | 1% (−3%, 4%) | 2% (−5%, 8%) |
| 40 to 69 years | −17% (−19%, −16%) | −17% (−20%, −13%) | 0% (−2%, 2%) | 0% (−2%, 1%) | 3% (0%, 5%) | |
| >70 years | a | a | 9% (5%, 12%) | 11% (6%, 15%) | 8% (1%, 15%) |
The percentage bias was calculated as follows: {Σ [(eGFR − mGFR)/mGFR]}/n. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease; eGFR, estimated GFR.
Group contained <10 persons to estimate bias.
Table 5.
Percentage bias (95% confidence interval) of eGFR calculated using the CKD-EPI equation and MDRD equation with mGFR by iothalamate renal clearance by gender
| Equation | Gender | <40 years old |
40 to 69 years old |
≥70 years old |
|||
|---|---|---|---|---|---|---|---|
| Before and after Kidney Donation | CKD and Transplant Recipients | Before and after Kidney Donation | CKD and Transplant Recipients | Before and after Kidney Donation | CKD and Transplant Recipients | ||
| CKD-EPI | Male | −6.9% (−10.6%, −3.2%) | 19.2% (13.2%, 25.3%) | −9.5% (−11.8%, −7.3%) | 6.7% (4.8%, 8.7%) | a | 7.4% (4.0%, 10.7%) |
| Female | −7.2% (−10.6%, −3.9%) | 11.0% (4.5%, 17.6%) | −8.6% (−10.6%, −6.7%) | 5.4% (3.7%, 7.1%) | a | 4.4% (1.2%, 7.4%) | |
| MDRD | Male | −17.8% (−21.4%,−14.2%) | 8.9% (3.3%, 14.5%) | −16.3% (−18.5%, −15.8%) | 1.0% (−0.7%, 2.6%) | a | 11.5% (7.9%, 15.0%) |
| Female | −19.6% (−22.8%, −16.4%) | −1.5% (−7.6%, 4.5%) | −17.6% (−19.5%, −15.8%) | −0.4% (−2.3%, 1.5%) | a | 5.3% (2.2%, 8.5%) | |
The percentage bias was calculated as follows: {Σ [(eGFR − mGFR)/mGFR × 100%]}/n. CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; MDRD, Modification of Diet in Renal Disease; eGFR, estimated GFR; mGFR, measured GFR.
Group contained <10 persons to estimate bias.
Ability of Estimating Equations to Detect Reduced mGFR
Only 2% (n = 10) of the potential kidney donors had a mGFR <60 ml/min per 1.73 m2, whereas 17% (n = 97) had an mGFR <80 ml/min per 1.73 m2. Among potential kidney donors (persons without known CKD), the CKD-EPI equation had 50% sensitivity and 98% specificity for detecting an mGFR <60 ml/min per 1.73 m2 (current definition of CKD stage 3), whereas the MDRD equation had 70% sensitivity and 94% specificity for detecting an mGFR <60 ml/min per 1.73 m2. The CKD-EPI equation had 71% sensitivity and 76% specificity for detecting an mGFR <80 ml/min per 1.73 m2 (a GFR threshold many centers currently use to exclude kidney donation), whereas the MDRD equation had 89% sensitivity and 48% specificity. For any GFR threshold below 90 ml/min per 1.73 m2, there was a consistent trade-off between increased specificity with the CKD-EPI equation versus increased sensitivity with the MDRD equation.
Discussion
This study demonstrates that performance of the CKD-EPI equation depends on the clinical presentation of the patient, as has been widely recognized when the MDRD equation is used (16). However, the CKD-EPI equation demonstrates less negative bias than the MDRD equation among persons without kidney disease. This is likely due to a higher average muscle mass among healthy persons who were purposefully used in the cohort used to develop the CKD-EPI equation. Perhaps there is a trade-off: the CKD-EPI equation overestimates GFR among patients with CKD or at high risk for CKD (e.g., transplant recipients) (17). Including healthy persons in the CKD-EPI cohort also explains the resulting trade-off between higher specificity than the MDRD equation for detecting a low mGFR, at the cost of lower sensitivity. The bias with each equation depends on age with substantial overestimation of GFR in the youngest and oldest age groups in both CKD and transplant recipient populations.
In kidney transplant recipients, nonkidney transplant recipients, and CKD patient populations, the CKD-EPI equation was more biased than the MDRD equation with greater overestimation of GFR. In addition, both equations underestimated GFR among potential kidney donors and postnephrectomy kidney donors, although to a lesser extent with the CKD-EPI equation. The results obtained using the MDRD equation confirm findings by multiple previous studies, because it has previously been observed that the MDRD equation significantly underestimates GFR among individuals with normal kidney function (1–5). A recent study in 96 healthy adults also found less underestimation of GFR with the CKD-EPI than the MDRD equation (18). Although including low-risk individuals in the cohort used to develop the CKD-EPI equation did attenuate underestimation of GFR in low-risk populations and improve specificity for detecting an mGFR <60 ml/min per 1.73 m2 as compared with the MDRD equation, it did so at the expense of overestimating GFR when used in CKD patients and transplant recipients and reduced sensitivity for detecting an mGFR <60 ml/min per 1.73 m2. Therefore, it seems that improved performance in the healthier populations was obtained at the expense of slightly reduced performance in more diseased populations. However, in certain circumstances this may be a clinical advantage, because serum-creatinine measurements are often made in low-risk populations for screening purposes.
A recent study by White et al. (19) compared eGFR by both equations to mGFR among kidney transplant recipients. Bias for the CKD-EPI was found to be lower than the four-variable MDRD study equation, with a mean of −9.3 versus −4.5 ml/min per 1.73 m2 for the MDRD and CKD-EPI equations, respectively. This finding is somewhat different than our results, which demonstrate a bias of 1% in kidney transplant recipients using the MDRD equation and a bias of 8% using the CKD-EPI equation (Table 3). However, White et al. (19) used plasma clearance of 99mTc-diethylenetriamine pentaacetic acid measure GFR, whereas renal iothalamate clearance was used in our study. Our findings with respect to the nephrectomized donors are similar to those recently reported by Tent et al. (20) and Lane et al. (21), showing that both the CKD-EPI and MDRD equations have a similar percentage bias before and after kidney donation, with the CKD-EPI equation demonstrating less overall negative bias than the MDRD equation in these groups (20,21).
Our analyses show that GFR using the MDRD equation and the CKD-EPI equation is overestimated in those >70 years, patients with CKD, and transplant recipients. Indeed, efforts to develop an equation that uses serum creatinine and is accurate in both high-risk and low-risk populations may not be possible without better surrogates than demographics to estimate creatinine generation (muscle mass) (22). However, it is important to recognize that eGFR by either equation remains clinically useful, even in these populations, because serum creatinine alone more severely overestimates GFR, especially among the very elderly with CKD. Therefore, individual laboratories may want to broaden age restrictions for reporting eGFR, specifically to include those >70 years old, because our data suggest that it retains reasonable utility in this group.
Although our data confirm that the CKD-EPI equation is more accurate among patients without CKD, it still underestimates GFR in these low-risk populations. Furthermore, improved specificity for an mGFR less than 60 ml/min per 1.73 m2 is at the expense of decreased sensitivity. Importantly, neither the MDRD equation (89% sensitivity and 48% specificity) nor CKD-EPI equation (71% sensitivity and 76% specificity) may be deemed accurate enough for identifying a GFR less than 80 ml/min per 1.73 m2, a common threshold to exclude patients from kidney donation. Tent et al. (20) made similar observations and also recommended against using an eGFR as the sole measure to approve or exclude potential kidney donors, without considering other clinical indicators of CKD. Results of other laboratory tests, such as biochemical and or microscopic urinalysis, may be helpful in determining whether patients with reduced GFR are eligible to donate.
In conclusion, despite great strides in standardizing laboratory measurement of serum creatinine and refinement of equations, estimates of GFR based upon serum creatinine remain an unsatisfactory test for renal function. Importantly, clinical presentation is vital to the interpretation of eGFR because both equations are strongly influenced by patient category. In particular, otherwise healthy individuals with an eGFR just below 60 ml/min per 1.73 m2 by either equation will likely have higher levels of mGFR. Therefore, interpretation of eGFR derived using either equation requires caution, in particular when the eGFR is >60 ml/min per 1.73 m2. In addition, given the problems with using serum creatinine to estimate GFR, other endogenous markers, such as cystatin C (23) or beta trace protein (24,25), that are less influenced by muscle mass may be helpful as confirmatory tests to identify patients with true reductions in GFR.
Disclosures
None.
Acknowledgments
We thank Barbara Rauvola, supervisor of the Renal Function Laboratory, for gracious assistance accumulating the data used for this study. The data in this study have been presented as an abstract at the American Society of Nephrology annual meeting in November 2010 (abstract TH-FC084). This work was supported in part by Grant K23 DK078229, National Institute of Diabetes and Digestive and Kidney Diseases (to A.D.R.).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, Measured GFR as “Gold Standard”—All that Glitters Is Not Gold? on pages 1813–1814.
References
- 1. Bertolatus JA, Goddard L: Evaluation of renal function in potential living kidney donors. Transplantation 71: 256–260, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Lin J, Knight EL, Hogan ML, Singh AK: A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol 14: 2573–2580, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Pierrat A, Gravier E, Saunders C, Caira MV, Ait-Djafer Z, Legras B, Mallie JP: Predicting GFR in children and adults: A comparison of the Cockcroft-Gault, Schwartz, and modification of diet in renal disease formulas. Kidney Int 64: 1425–1436, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Rule AD, Gussak HM, Pond GR, Bergstralh EJ, Stegall MD, Cosio FG, Larson TS: Measured and estimated GFR in healthy potential kidney donors. Am J Kidney Dis 43: 112–119, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Vervoort G, Willems HL, Wetzels JF: Assessment of glomerular filtration rate in healthy subjects and normoalbuminuric diabetic patients: Validity of a new (MDRD) prediction equation. Nephrol Dial Transplant 17: 1909–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Rule AD, CF: The new Mayo Clinic equation for estimating glomerular filtration rate. Ann Intern Med 142: 679–681, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Poggio ED, Wang X, Greene T, Van Lente F, Hall PM: Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 16: 459–466, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Stevens LA, Manzi J, Levey AS, Chen J, Deysher AE, Greene T, Poggio ED, Schmid CH, Steffes MW, Zhang YL, Van Lente F, Coresh J: Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis 50: 21–35, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Stevens LA, Schmid CH, Zhang YL, Coresh J, Manzi J, Landis R, Bakoush O, Contreras G, Genuth S, Klintmalm GB, Poggio E, Rossing P, Rule AD, Weir MR, Kusek J, Greene T, Levey AS: Development and validation of GFR-estimating equations using diabetes, transplant and weight. Nephrol Dial Transplant 25: 449–457, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stevens LA, Schmid CH, Greene T, Zhang YL, Beck GJ, Froissart M, Hamm LL, Lewis JB, Mauer M, Navis GJ, Steffes MW, Eggers PW, Coresh J, Levey AS: Comparative performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations for estimating GFR levels above 60 mL/min/1.73 m2. Am J Kidney Dis 56: 486–495, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wilson DM, Bergert JH, Larson TS, Liedtke RR: GFR determined by nonradiolabeled iothalamate using capillary electrophoresis. Am J Kidney Dis 30: 646–652, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F: Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
- 14. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis, 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 15. Poggio ED, Rule AD, Tanchanco R, Arrigain S, Butler RS, Srinivas T, Stephany BR, Meyer KH, Nurko S, Fatica RA, Shoskes DA, Krishnamurthi V, Goldfarb DA, Gill I, Schreiber MJ, Jr: Demographic and clinical characteristics associated with glomerular filtration rates in living kidney donors. Kidney Int 75: 1079–1087, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rule AD, Larson TS, Bergstralh EJ, Slezak JM, Jacobsen SJ, Cosio FG: Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and in chronic kidney disease. Ann Intern Med 141: 929–937, 2004 [DOI] [PubMed] [Google Scholar]
- 17. Rule AD, Rodeheffer RJ, Larson TS, Burnett JC, Jr., Cosio FG, Turner ST, Jacobsen SJ: Limitations of estimating glomerular filtration rate from serum creatinine in the general population. Mayo Clin Proc 81: 1427–1434, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Soares AA, Eyff TF, Campani RB, Ritter L, Weinert LS, Camargo JL, Silveiro SP: Performance of the CKD Epidemiology Collaboration (CKD-EPI) and the Modification of Diet in Renal Disease (MDRD) Study equations in healthy South Brazilians. Am J Kidney Dis 55: 1162–1163, 2010 [DOI] [PubMed] [Google Scholar]
- 19. White CA, Akbari A, Doucette S, Fergusson D, Knoll GA: Estimating glomerular filtration rate in kidney transplantation: Is the new chronic kidney disease epidemiology collaboration equation any better? Clin Chem 56: 474–477, 2010 [DOI] [PubMed] [Google Scholar]
- 20. Tent H, Rook M, Stevens LA, van Son WJ, van Pelt LJ, Hofker HS, Ploeg RJ, van der Heide JJ, Navis G: Renal function equations before and after living kidney donation: A within-individual comparison of performance at different levels of renal function. Clin J Am Soc Nephrol 5: 1960–1968, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lane BR, Demirjian S, Weight CJ, Larson BT, Poggio ED, Campbell SC: Performance of the chronic kidney disease-epidemiology study equations for estimating glomerular filtration rate before and after nephrectomy. J Urol 183: 896–901, 2010 [DOI] [PubMed] [Google Scholar]
- 22. Rule AD, Bailey KR, Schwartz GL, Khosla S, Lieske JC, Melton LJ, 3rd: For estimating creatinine clearance measuring muscle mass gives better results than those based on demographics. Kidney Int 75: 1071–1078, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simonsen O, Grubb A, Thysell H: The blood serum concentration of cystatin C (gamma-trace) as a measure of the glomerular filtration rate. Scand J Clin Lab Invest 45: 97–101, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Hoffmann A, Nimtz M, Conradt HS: Molecular characterization of beta-trace protein in human serum and urine: a potential diagnostic marker for renal diseases. Glycobiology 7: 499–506, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Melegos DN, Grass L, Pierratos A, Diamandis EP: Highly elevated levels of prostaglandin D synthase in the serum of patients with renal failure. Urology 53: 32–37, 1999 [DOI] [PubMed] [Google Scholar]