Abstract
Summary
Recent population studies have found symptomatic kidney stone formers to be at increased risk for chronic kidney disease (CKD). Although kidney stones are not commonly identified as the primary cause of ESRD, they still may be important contributing factors. Paradoxically, CKD can be protective against forming kidney stones because of the substantial reduction in urine calcium excretion. Among stone formers, those with rare hereditary diseases (cystinuria, primary hyperoxaluria, Dent disease, and 2,8 dihydroxyadenine stones), recurrent urinary tract infections, struvite stones, hypertension, and diabetes seem to be at highest risk for CKD. The primary mechanism for CKD from kidney stones is usually attributed to an obstructive uropathy or pyelonephritis, but crystal plugs at the ducts of Bellini and parenchymal injury from shockwave lithotripsy may also contribute. The historical shift to less invasive surgical management of kidney stones has likely had a beneficial impact on the risk for CKD. Among potential kidney donors, past symptomatic kidney stones but not radiographic stones found on computed tomography scans were associated with albuminuria. Kidney stones detected by ultrasound screening have also been associated with CKD in the general population. Further studies that better classify CKD, better characterize stone formers, more thoroughly address potential confounding by comorbidities, and have active instead of passive follow-up to avoid detection bias are needed.
Introduction
Intuitively, one might expect kidney stones to be a risk factor for chronic kidney disease (CKD). One of the roles of the kidney is to excrete metabolic wastes such as calcium and oxalate at supersaturated concentrations yet prevent precipitation of crystals. Thus, stone formation could be considered a form of “impaired kidney function” and a sign of a diseased kidney. In patients presenting with symptomatic kidney stones, it is standard of care to obtain a serum creatinine level and assess for acute kidney injury (AKI) from an obstructive uropathy. After surgical or conservative management to clear the stone, the serum creatinine level will often return to baseline with resolution of the AKI. The long-term implications of a kidney stone on health of the kidney are less clear. Are stone formers at increased risk for CKD, and, if so, which types of stone disease are associated with increased risk? Are stone formers at increased risk for ESRD? Which mechanisms link kidney stones to CKD? Do asymptomatic (incidental) stones convey the same risk for CKD as symptomatic stones? This last question is of particular importance when evaluating potential living kidney donors. This review presents available literature that addresses these questions.
We performed an Ovid Medline search (1950 to August 2010) to identify articles to review. English articles that contained the words “kidney/renal stone(s)/calculi/calculus” or “nephrolithiasis/urolithiasis” were merged with articles that contained the words “chronic kidney disease” or “chronic renal failure” or “chronic renal insufficiency” or “dialysis.” We further reviewed the abstracts from articles identified with this query for relevant articles. We identified additional articles by reviewing reference lists and by communicating with experts in the field. One review article, published in 2001, evaluated the risk for CKD in stone formers (1). Thus, much of the focus in this review is on relevant literature in the past decade.
Kidney Stones as Risk Factors for CKD
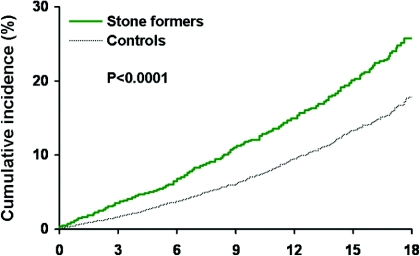
Several studies have evaluated the risk for CKD among stone formers. However, population-based prospective cohort studies with active follow-up are lacking. A population-based historical cohort study assessed the risk for CKD over several decades among all stone formers in Olmsted County, MN. Stone formers were identified by diagnostic codes, and CKD was identified by two separate criteria: (1) Diagnostic codes and (2) elevated serum creatinine levels (or estimated GFR [eGFR] <60 ml/min per 1.73 m2) sustained for ≥3 months. Compared with matched control subjects, stone formers had a 51% to 68% increased risk for CKD by diagnostic codes (Figure 1) and a 25% to 44% increased risk for CKD by elevated serum creatinine levels. The stronger magnitude of association with CKD by diagnostic codes could be from an association with proteinuria (one of the diagnostic codes) that was not captured with serum creatinine or from detection bias with diagnostic codes. Importantly, there was an increased risk for CKD with kidney stones when analysis was based on follow-up of all or when analysis was limited to patients who had follow-up serum creatinine levels. Furthermore, the increased risk for CKD with kidney stones was not explained by several other risk factors for CKD, such as diabetes, hypertension, and obesity, that are common in stone formers (2). Hippisley-Cox et al. (3) recently performed a large population-based historical cohort study and found that women stone formers in England and Wales had a 27% increased adjusted risk for moderate to severe CKD (eGFR <45 ml/min per 1.73 m2 or CKD diagnosis). An association was not seen in men; however, only 0.7% of the cohort had a diagnosis of stone former, much less than the 5% prevalence usually reported for kidney stones (4). A limitation of these studies was reliance on diagnostic codes to adjust for comorbidities because some comorbidities, particularly metabolic syndrome, are not adequately detected with diagnostic codes.
Figure 1.
Risk for a clinical diagnosis of CKD between stone formers and control subjects in Olmsted County. X-axis is years. Reprinted from reference 2, with permission.
Besides cohort studies, several other population-based studies have assessed for an association between kidney stones and CKD. Gillen et al. (5) used the Third National Health and Nutrition Examination Survey (NHANES III) of the US population to compare eGFR between the 6% who reported a history of kidney stones and the 94% who did not report a history of kidney stones. Among individuals who were overweight or obese, a history of kidney stones was associated with an eGFR that was 3.4 ml/min per 1.73 m2 lower after multivariable adjustment. A similar association in normal-weight individuals was not evident. Another population-based survey in Thailand found the 5% with a reported history of kidney stones to have an increased adjusted risk for CKD (odds ratio [OR] 2.7) (6). However, the definition of CKD included microhematuria, in addition to the more standard criteria that require an eGFR <60 ml/min per 1.73 m2 or albuminuria. Therefore, it is unclear the extent that microhematuria caused by direct stone injury confounded this association. Vupputuri et al. (7) found that patients who had CKD and were identified by diagnostic codes and elevated serum creatinine levels were more likely to report a history of kidney stones on telephone interview when compared with matched community control subjects (OR 1.9). Interestingly, the association was strongest in individuals without hypertension and in individuals who were identified by CKD diagnostic codes for interstitial nephritis or diabetic nephropathy. Because cases were identified via hospital record review, they may have had more unidentified comorbidities than control subjects did.
Kidney Stones as Risk Factors for ESRD
Epidemiologic association studies usually seek to identify risk factors that predict a disease. The situation is arguably reversed when assessing kidney stones (a symptomatic disease) as a predictor for a largely asymptomatic disease/risk factor (CKD) that does not always progress to clinically important outcomes. Thus, it is of particular interest also to evaluate kidney stones as a predictor for clinically important outcomes related to CKD, in particular ESRD. In their cohort study, Hippisley-Cox et al. (3) reported an increased risk for ESRD with women (hazard ratio 2.1) but not men stone formers. The Olmsted County cohort study did not find evidence of increased risk for ESRD with stone formers, but the study had relatively few events (2). Stankus et al. (8) surveyed 300 black hemodialysis patients for a history of kidney stones and compared findings with the 5341 black individuals who participated in NHANES III. The likelihood of self-reported past kidney stones was higher for patients with ESRD than for the population control subjects (8% versus 3%). Of the 25 patients with ESRD and past kidney stones, only five had a stone episode within 5 years of starting dialysis and only two had ESRD that was primarily attributed to the stone disease.
It is important to distinguish stone formers in whom stones are the primary cause of ESRD from those in whom they are a contributing risk factor but not the primary cause. Jungers et al. (9) specifically investigated ESRD cases that had been attributed to kidney stones by reviewing the case histories of 1391 consecutive patients with ESRD in France. Forty-five (3.2%) had ESRD attributed primarily or exclusively to kidney stones, with struvite stones in 19, calcium stones in 12, uric acid stones in eight, and rare hereditary stones in six (four primary hyperoxaluria, two cystinuria). Tosetto et al. (10) identified a history of kidney stones in only 3.2% of 1901 patients who had ESRD and were on hemodialysis, two thirds of whom (2.1%) had ESRD attributed to the kidney stones. The US Renal Data System reports only 0.2% (908 or 546,878) of all incident patients with ESRD from 2004 through 2008 had kidney stones as the primary cause, although these data are based on the ESRD Medical Evidence form (CMS 2728) and not chart review (11).
The study by Jungers et al. (9) found that 40% of stone formers who develop ESRD had a solitary functioning kidney before developing ESRD. Worcester et al. (12) evaluated the cause of a solitary functioning kidney among 115 stone formers (3.5% of the patients seen in a stone clinic). The top three causes of loss for function in one kidney were staghorn calculi or high stone burden (29%), infection (23%), and ureteral obstruction (21%), whereas surgery was responsible for kidney loss in only 8%. There was a historical shift in the surgical management of kidney stones from open lithotomy to less invasive shockwave lithotripsy (SWL) and endourology, and this has likely had a beneficial impact on ESRD risk in stone formers.
Although kidney stones are predictive of future CKD, paradoxically, there is reason to believe that CKD is protective against formation of kidney stones. When GFR declines, there is an associated fall in urine calcium excretion (13), an important risk factor for stone formation. Indeed, evidence suggests that stone recurrence rates may be lower in stone formers with a reduced GFR (14). Therefore, and ironically, if kidney stones lead to CKD, which in turn improves stone disease, then underrecognition of the contribution of kidney stones to the development of ESRD may occur.
Before the development of ESRD, it is often assumed that CKD, regardless of cause, leads to a higher risk for cardiovascular morbidity and mortality. Whether this is true in stone formers who develop CKD requires further study. In Olmsted County, increased risk for mortality among stone formers was not evident (2). However, an increased risk for myocardial infarction among stone formers was detected, although this was not explained by CKD in stone formers (15).
Risk for CKD by Stone Type
There is evidence that the risk for CKD varies by stone type, but more studies are needed. Population-based studies often lack the granular detailed data to characterize stone type because many stone formers never have their stones analyzed or urine chemistries evaluated, and, even if so, this information often is not available in the databases available for study. Saucier et al. (16) studied community stone formers in Olmsted County, MN, and identified 53 who developed CKD and were matched with 106 who did not develop CKD. Hypertension, diabetes, six or more urinary tract infections, allopurinol therapy, and struvite stone type were identified as risk factors for CKD. The association with allopurinol could either reflect treatment of hyperuricosuria for stone prevention or treatment of hyperuricemia secondary to CKD. Only half of the participants had stone type determined, and even fewer had urine chemistry data. Number of stone episodes, surgical procedures, and stone passage symptoms were not associated with CKD, although there was limited statistical power in this study (16).
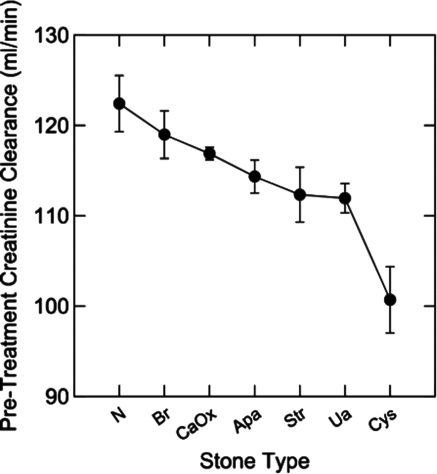
Stone formers who present to specialty clinics are likely to have more severe disease than those in the general population but also have better characterized stone disease because of a more comprehensive evaluation. Worcester et al. (17) reviewed urinary creatinine clearance data from 1856 stone formers with stone analyses at the University of Chicago and compared the findings with 153 normal individuals (adjusted for age, gender, and weight). Cysteine stone formers had a much lower creatinine clearance than other types of stone formers or nonstone patients. Uric acid, calcium oxalate, apatite, and struvite stone formers also had lower creatinine clearance than normal individuals (Figure 2). When examined in regard to diseases that cause kidney stones, those with stones from bowel disease, intestinal bypass for obesity, and renal tubular acidosis also had lower creatinine clearance than normal individuals (17).
Figure 2.
Pretreatment urinary creatinine clearance in normal control subjects (N) and different types of stone formers: Brushite (Br), calcium oxalate (CaOx), apatite (Apa), struvite (Str), uric acid (Ua), and cystine (Cys). Reprinted from reference 17, with permission.
Putative Mechanisms for CKD in Stone Formers
Kidney stones could potentially lead to CKD via multiple pathways. Obstructive uropathy from a stone can cause acute injury but could also produce irreversible chronic injury. The mechanism by which any obstructive uropathy causes CKD is not completely understood. Renal vasoconstriction and inflammation can occur in response to increased intratubular pressure (18–20). The resulting fall in renal perfusion normalizes intratubular pressure (21) but can also cause ischemia. If this ischemia persists long enough, glomerulosclerosis, tubular atrophy, and interstitial fibrosis occur (22). Complete unilateral obstruction for 24 hours in a rat model leads to irreversible loss of function in 15% of the nephrons of the affected kidney (23). Therefore, if kidney stones caused recurrent episodes of obstructive uropathy, then there could be substantial loss of functional nephrons. Initially, the remaining functional nephrons may hypertrophy and hyperfilter to compensate and cause no change in the overall GFR. However, long-term, these “overworked” nephrons may fail, leading to detectable CKD (24).
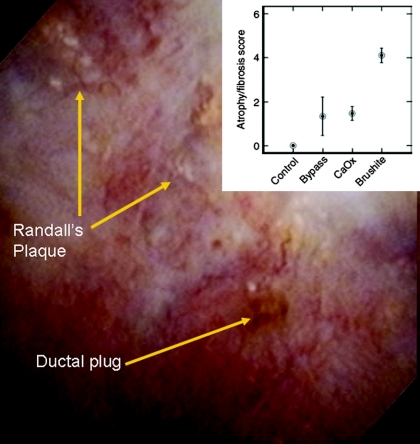
Some pathways leading to CKD may be specific to certain stone types. Among predominantly calcium phosphate stone formers, approximately one third (5% overall) are brushite stone formers. Evan et al. (25,26) studied stone formers with renal biopsies and video imaging during a percutaneous nephrolithotomy and found plugs at the ducts of Bellini in brushite stone formers as well as intestinal bypass stone formers. However, glomerulosclerosis, tubular atrophy, and interstitial fibrosis were particularly common among brushite stone formers (Figure 3) (27). Because there are only approximately 100 ducts of Bellini in each kidney (each represents the confluence of many collecting ducts), the investigators hypothesized that obstruction of these ducts could explain the increased chronic parenchymal injury (nephrosclerosis) on the cortical biopsy. Hypercalciuria and SWL treatments are common in brushite stone formers, and it is possible that these factors contributed to the development of CKD (28).
Figure 3.
A stone obstructing the duct of Bellini in a brushite stone former during a percutaneous nephrolithotomy. (Inset) Tubular atrophy and fibrosis score on cortical biopsy by stone type. Reprinted from reference 27, with permission.
Extracorporeal SWL is widely used to treat renal stones <2 cm in diameter (29). SWL has been shown in animal models to induce parenchymal injury that increases with the number of shocks, with level of energy, and with smaller kidneys. There is also an acute reduction in GFR and renal blood flow from vasoconstriction (30,31). Evaluation of treated kidneys with magnetic resonance imaging, excretory urogram, and nuclear scintigraphy reveals that 74% of patients have abnormal findings consistent with a renal contusion from the SWL. However, a long-term effect of SWL on kidney function has not been shown. Eassa et al. (32) found no change in eGFR at approximately 4 years of follow-up or in the differential renal function of the treated kidney as assessed by nuclear scintigraphy. Even longer term follow-up suggests that the risk for an elevated serum creatinine level is not increased with SWL compared with percutaneous nephrolithotomy or conservative management (33). Hypertension has been described as a complication of SWL in many (34,35) but not all (32,36,37) studies. A recent population-based study did not find an increased risk with developing hypertension, suggesting that this complication is not common with most stone formers who undergo SWL (38). Pretreatment with low-energy shockwaves seems to be protective against renal injury from the high-energy shockwaves needed to treat stones (39).
Unique pathways may lead to CKD among struvite and uric acid stone formers. Chronic pyelonephritis from an infected struvite stone will lead to inflammation and eventual destruction of renal parenchyma (40). Struvite stones can lead to ESRD either from obstructive nephropathy with staghorn calculi or from recurrent pyelonephritis (41). The urease enzyme and resultant high urine pH caused by microorganisms in struvite stone formers may also contribute to kidney injury. Large staghorn calculi may also cause papillary necrosis (1). The risk for CKD in uric acid stone formers may be related to the concurrent hyperuricemia often seen in addition to the stones themselves. Uric acid crystals can deposit in the interstitium leading to inflammation (similar to gout) followed by interstitial fibrosis and CKD (42). Several general population studies have identified serum uric acid as an independent risk factor for incident CKD but did not adjust for kidney stones (43,44). Alternatively, diabetic nephropathy may link uric acid stones to CKD, given the increased risk for uric acid stones in patients with diabetes (45).
CKD in Rare Hereditary Stone Formers
Patients with rare hereditary forms of kidney stones that cause marked excretion of minerals important in stone formation, including primary hyperoxaluria, cystinuria, Dent disease, and adenine phosphoribosyltransferase (APRT) deficiency, are an important subgroup of stone formers. Patients with these disorders experience recurring stones often starting in childhood and are at high risk for CKD. ESRD is common in primary hyperoxaluria, Dent disease, and APRT deficiency, with usually less aggressive CKD in cystinuria. Cystinuria is also the most common of the rare hereditary kidney stone diseases.
Patients with primary hyperoxaluria have deficiencies of hepatic enzymes important in the metabolic pathways for detoxification of glyoxylate. Deficiency of either alanine glyoxylate aminotransferase (type 1) or glyoxylate hydroxypyruvate reductase (type 2) results in marked overproduction of oxalate by the liver. Recently, a third genetic cause of primary hyperoxaluria was also identified, although the mechanism of oxalate overproduction in this subgroup has not yet been elucidated (46). Among patients with primary hyperoxaluria, urine oxalate excretion rates are typically two to eight times the upper limit of normal, such that the urine is markedly supersaturated for calcium oxalate. ESRD may occur as early as the first 6 months of life or may not occur until mid-adulthood. By the sixth decade of life, 90% of those with type 1 primary hyperoxaluria will have reached ESRD (47).
Cystinuria is an autosomal recessive disorder as a result of abnormal cystine transport in gut and kidney. Marked cystinuria is the hallmark of the disease. Because cystine is relatively insoluble in urine, cystine crystals and stones develop, most often beginning in childhood. The clinical course is characterized by recurring stone formation and repeated surgical procedures for extraction of stones from the urinary tract. Crystal plug obstruction of the ducts of Bellini occurs with increased glomerulosclerosis and interstitial fibrosis in the cortex (48). By adulthood, GFR may be reduced and CKD is more common than found in the usual stone former (49,50).
APRT deficiency, occurring as an autosomal recessive trait, leads to marked overproduction of 2,8 dihydroxyadenine by many body tissues, leading to excess excretion of this relatively insoluble compound in urine. ESRD is a widely recognized complication (51). Dent disease (X-linked recessive nephrolithiasis), caused by mutations of the CLCN5 chloride channel, is associated with variable degrees of hypercalciuria and proximal renal tubule dysfunction (low molecular weight proteinuria) with CKD and ESRD occurring in affected males, usually between the ages of 15 and 45 years (52). Recently, it was observed that mutations in the gene associated with Lowe syndrome (OCRL1) can also present with a Dent disease phenotype (53,54).
Each of these inherited inborn errors of metabolism produces high concentrations of poorly soluble mineral salts in the urine, favoring the formation of crystals and kidney stones. The resulting crystals can be incorporated into the kidney parenchyma and incite an inflammatory reaction. Direct cell toxicity from high concentrations of molecules such as oxalate, injurious effects of crystals (either direct or related to a secondary inflammatory response), crystal plug obstruction of the ducts of Bellini, and consequences of stone formation such as ureter obstruction, repeated procedures, or infection are all potential factors for the development of CKD and eventual ESRD. It is unclear which of these factors is most injurious. It is also unclear whether the same factors are responsible for CKD in each of the four diseases. If these diseases are recognized early and treated effectively, then CKD may be preventable. However, delay in diagnosis is the rule because of lack of familiarity with these diseases and availability of diagnostic tests.
Asymptomatic Stone Formers
With the increased use of computed tomography (CT) and other radiographic imaging, incidental asymptomatic kidney stones often are detected without pain or gross hematuria being present. Are these patients at increased risk for CKD? Lorenz et al. (55) recently found that 11% of potential kidney donors at the Mayo Clinic have at least one kidney stone. Although the finding of a radiographic stone did not lead to exclusion from donation in most (76%), kidney stone was the most common radiographic kidney abnormality and the radiographic kidney abnormality that contributed most to nondonation (39% of exclusions). Strang et al. (56) found that the increased sensitivity of CT angiograms compared with other imaging methods led to twice as many potential kidney donors being excluded for radiographic kidney abnormalities (including stones).
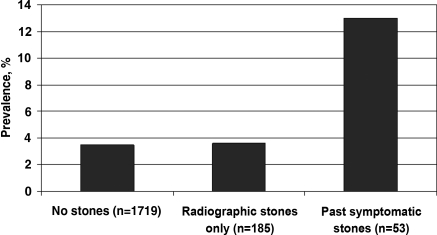
In a transplant program in which stone formers with severe disease were excluded from donor evaluations, potential donors with a history of symptomatic stones that were evaluated were still more likely to have albuminuria than those without stones (Figure 4) (57). However, albuminuria was not more common in potential donors with asymptomatic radiographic stones. Notably, potential donors with past symptomatic stones had larger radiographic stones (median 4 mm) than potential donors without past symptomatic stones (median 0.5 mm). Focal renal scarring was more prevalent among both asymptomatic and past-symptomatic stone formers than potential donors with no stones. Overall, these data suggest that asymptomatic stone formers may carry a lower risk for CKD than those with past symptomatic stones. However, outcome studies of asymptomatic stone formers who donate a kidney would better inform patients and clinicians during donor evaluations (58).
Figure 4.
Prevalence of albuminuria among nonstone formers, asymptomatic stone formers, and past symptomatic stone formers who present to donate a kidney (57). The prevalence of albuminuria (>30 mg/24 h) was increased in past symptomatic stone formers compared with potential donors without past symptomatic stones (P < 0.001).
Chen et al. (59) assessed predictors of prevalent CKD in a random sample of the Chinese adult population. This study uniquely evaluated all 2554 participants with a renal ultrasound to detect kidney stones. Ultrasound can typically detect kidney stones that are >5 mm (whereas CT scanning can detect stones as small as 0.5 to 1 mm). Sonographic kidney stones were found in 56 participants (2.2%) and were associated with an eGFR <60 ml/min per 1.73 m2 in multivariable analysis (OR 2.6). Past symptomatic kidney stone events were not reported; thus, it is unclear whether the risk for a reduced eGFR differed between symptomatic and asymptomatic stone formers (59).
Study Design Challenges
Several study design limitations hamper risk estimates of CKD among stone formers. First, many studies do not make the distinction between AKI and CKD. Assessment of the kidney function after treating the acute obstruction is needed, ideally at several time points. Second, stone formers seen in urology and nephrology referral clinics are likely to have more severe stone disease than those in the general population. Identifying stone formers in the general population can be a challenge because validation of stone former status among those identified by diagnostic code or survey can be difficult. Many kidney stones are identified as incidental findings on CT or ultrasound, and the clinical conditions that lead to imaging studies rather than the kidney stone itself may contribute to risk for CKD. Third, the urine dipstick or urinalysis used to help diagnose kidney stones (hematuria) is also a test to diagnose CKD (proteinuria). Therefore, there may be a bias in historical studies that rely on passive detection of CKD through clinical care. This is further complicated by follow-up serum creatinine testing being more common among stone formers than control subjects (2). Fourth, recent studies often used an eGFR <60 ml/min per 1.73 m2 to identify CKD. This threshold captures the normal age-related decline in GFR in addition to the loss of GFR from a disease such as kidney stones. Studies are needed to determine whether kidney stones lead to a GFR lower than expected with normal aging. This is better determined using age-specific eGFR thresholds or even an elevated serum creatinine level (60). Fifth, some studies have used microscopic hematuria as part of the composite definition of CKD (in addition to albuminuria and reduced GFR). This is problematic because kidney stones themselves cause hematuria that is not glomerular in origin, and thus, CKD is not detected. Finally, thorough and accurate assessment of potential confounders, particularly medications and comorbidities, has been inadequate in most studies.
Conclusions
Several studies have found stone formers to be at increased risk for CKD and ESRD, but more research is needed. There may be significant heterogeneity in the risk for CKD, and better characterization of the stone types and clinical factors that identify stone formers at most risk for developing CKD are needed. In particular, do common calcium stone formers with infrequent episodes of stone passage and asymptomatic stone formers have increased risk for CKD? There are effective dietary and medical treatments to prevent stone formation and growth, and aggressively treating stone formers at increased risk for CKD and ESRD may be indicated. Whether stone formers can be approved as kidney donors is a common dilemma. A clearer understanding of the risk for CKD risk among kidney stone formers would obviously have an impact on this determination. Further studies, particularly prospective studies with active instead of passive follow-up, are needed to understand the relationship between kidney stones and CKD.
Disclosures
None.
Acknowledgments
Investigators that contributed to this work were supported by the National Institutes of Heath (NIH), including the O'Brien Urology Research Center at Mayo Clinic P50 DK083007 (A.D.R., A.E.K., and J.C.L.), K23 DK078229 (A.D.R.), and the Rare Kidney Stone Consortium U54 DK083908 (J.C.L.), a part of NIH Rare Diseases Clinical Research Network, and are funded by the National Institute of Diabetes and Digestive Kidney Diseases and the NIH Office of Rare Diseases Research.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Gambaro G, Favaro S, D'Angelo A: Risk for renal failure in nephrolithiasis. Am J Kidney Dis 37: 233–243, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Rule AD, Bergstralh EJ, Melton LJ, 3rd, Li X, Weaver AL, Lieske JC: Kidney stones and the risk for chronic kidney disease. Clin J Am Soc Nephrol 4: 804–811, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hippisley-Cox J, Coupland C: Predicting the risk of chronic kidney disease in men and women in England and Wales: Prospective derivation and external validation of the QKidney Scores. BMC Fam Pract 11: 49, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stamatelou KK, Francis ME, Jones CA, Nyberg LM, Curhan GC: Time trends in reported prevalence of kidney stones in the United States: 1976–1994. Kidney Int 63: 1817–1823, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Gillen DL, Worcester EM, Coe FL: Decreased renal function among adults with a history of nephrolithiasis: A study of NHANES III. Kidney Int 67: 685–690, 2005 [DOI] [PubMed] [Google Scholar]
- 6. Ingsathit A, Thakkinstian A, Chaiprasert A, Sangthawan P, Gojaseni P, Kiattisunthorn K, Ongaiyooth L, Vanavanan S, Sirivongs D, Thirakhupt P, Mittal B, Singh AK: Prevalence and risk factors of chronic kidney disease in the Thai adult population: Thai SEEK study. Nephrol Dial Transplant 25: 1567–1575, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Vupputuri S, Soucie JM, McClellan W, Sandler DP: History of kidney stones as a possible risk factor for chronic kidney disease. Ann Epidemiol 14: 222–228, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Stankus N, Hammes M, Gillen D, Worcester E: African American ESRD patients have a high pre-dialysis prevalence of kidney stones compared to NHANES III. Urol Res 35: 83–87, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Jungers P, Joly D, Barbey F, Choukroun G, Daudon M: ESRD caused by nephrolithiasis: Prevalence, mechanisms, and prevention. Am J Kidney Dis 44: 799–805, 2004 [PubMed] [Google Scholar]
- 10. Tosetto E, Graziotto R, Artifoni L, Nachtigal J, Cascone C, Conz P, Piva M, Dell'Aquila R, De Paoli Vitali E, Citron L, Nalesso F, Antonello A, Vertolli U, Zagatti R, Lupo A, D'Angelo A, Anglani F, Gambaro G: Dent's disease and prevalence of renal stones in dialysis patients in Northeastern Italy. J Hum Genet 51: 25–30, 2006 [DOI] [PubMed] [Google Scholar]
- 11. Collins AJ, Foley RN, Herzog C, Chavers B, Gilbertson D, Ishani A, Kasiske B, Liu J, Mau LW, McBean M, Murray A, St Peter W, Guo H, Gustafson S, Li Q, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Dalleska F, Daniels F, Dunning S, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L: US Renal Data System 2010 Annual Data Report. Am J Kidney Dis 57[Suppl 1]: A8, e1–e526, 2011 [DOI] [PubMed] [Google Scholar]
- 12. Worcester E, Parks JH, Josephson MA, Thisted RA, Coe FL: Causes and consequences of kidney loss in patients with nephrolithiasis. Kidney Int 64: 2204–2213, 2003 [DOI] [PubMed] [Google Scholar]
- 13. Craver L, Marco MP, Martínez I, Rue M, Borràs M, Martín ML, Sarró F, Valdivielso JM, Fernández E: Mineral metabolism parameters throughout chronic kidney disease stages 1–5: Achievement of K/DOQI target ranges. Nephrol Dial Transplant 22: 1171–1176, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Marangella M, Bruno M, Cosseddu D, Manganaro M, Tricerri A, Vitale C, Linari F: Prevalence of chronic renal insufficiency in the course of idiopathic recurrent calcium stone disease: Risk factors and patterns of progression. Nephron 54: 302–306, 1990 [DOI] [PubMed] [Google Scholar]
- 15. Rule AD, Roger VL, Melton LJ, 3rd, Bergstralh EJ, Li X, Peyser PA, Krambeck AE, Lieske JC: Kidney stones associate with increased risk for myocardial infarction. J Am Soc Nephrol 21: 1641–1644, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Saucier NA, Sinha MK, Liang KV, Krambeck AE, Weaver AL, Bergstralh EJ, Li X, Rule AD, Lieske JC: Risk factors for CKD in persons with kidney stones: A case-control study in Olmsted County, Minnesota. Am J Kidney Dis 55: 61–68, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Worcester EM, Parks JH, Evan AP, Coe FL: Renal function in patients with nephrolithiasis. J Urol 176: 600–603, discussion 603, 2006 [DOI] [PubMed] [Google Scholar]
- 18. Klahr S: New insights into the consequences and mechanisms of renal impairment in obstructive nephropathy. Am J Kidney Dis 18: 689–699, 1991 [DOI] [PubMed] [Google Scholar]
- 19. Hwang SJ, Haas M, Harris HW, Jr, Silva P, Yalla S, Sullivan MR, Otuechere G, Kashgarian M, Zeidel ML: Transport defects of rabbit medullary thick ascending limb cells in obstructive nephropathy. J Clin Invest 91: 21–28, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ophascharoensuk V, Giachelli CM, Gordon K, Hughes J, Pichler R, Brown P, Liaw L, Schmidt R, Shankland SJ, Alpers CE, Couser WG, Johnson RJ: Obstructive uropathy in the mouse: Role of osteopontin in interstitial fibrosis and apoptosis. Kidney Int 56: 571–580, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Gaudio KM, Siegel NJ, Hayslett JP, Kashgarian M: Renal perfusion and intratubular pressure during ureteral occlusion in the rat. Am J Physiol 238: F205–F209, 1980 [DOI] [PubMed] [Google Scholar]
- 22. Tanner GA, Evan AP: Glomerular and proximal tubular morphology after single nephron obstruction. Kidney Int 36: 1050–1060, 1989 [DOI] [PubMed] [Google Scholar]
- 23. Bander SJ, Buerkert JE, Martin D, Klahr S: Long-term effects of 24-hr unilateral ureteral obstruction on renal function in the rat. Kidney Int 28: 614–620, 1985 [DOI] [PubMed] [Google Scholar]
- 24. Brenner BM: Hemodynamically mediated glomerular injury and the progressive nature of kidney disease. Kidney Int 23: 647–655, 1983 [DOI] [PubMed] [Google Scholar]
- 25. Evan A, Lingeman J, Coe FL, Worcester E: Randall's plaque: Pathogenesis and role in calcium oxalate nephrolithiasis. Kidney Int 69: 1313–1318, 2006 [DOI] [PubMed] [Google Scholar]
- 26. Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M: Randall's plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. J Clin Invest 111: 602–605, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Evan AP, Lingeman JE, Coe FL, Shao Y, Parks JH, Bledsoe SB, Phillips CL, Bonsib S, Worcester EM, Sommer AJ, Kim SC, Tinmouth WW, Grynpas M: Crystal-associated nephropathy in patients with brushite nephrolithiasis. Kidney Int 67: 576–591, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Krambeck AE, Handa SE, Evan AP, Lingeman JE: Profile of the brushite stone former. J Urol 184: 1367–1371, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lotan Y, Pearle MS: Economics of stone management. Urol Clin North Am 34: 443–453, 2007 [DOI] [PubMed] [Google Scholar]
- 30. Willis LR, Evan AP, Connors BA, Blomgren P, Fineberg NS, Lingeman JE: Relationship between kidney size, renal injury, and renal impairment induced by shock wave lithotripsy. J Am Soc Nephrol 10: 1753–1762, 1999 [DOI] [PubMed] [Google Scholar]
- 31. Evan AP, Willis LR, Lingeman JE, McAteer JA: Renal trauma and the risk of long-term complications in shock wave lithotripsy. Nephron 78: 1–8, 1998 [DOI] [PubMed] [Google Scholar]
- 32. Eassa WA, Sheir KZ, Gad HM, Dawaba ME, El-Kenawy MR, Elkappany HA: Prospective study of the long-term effects of shock wave lithotripsy on renal function and blood pressure. J Urol 179: 964–968, discussion 968–969, 2008 [DOI] [PubMed] [Google Scholar]
- 33. Krambeck AE, LeRoy AJ, Patterson DE, Gettman MT: Long-term outcomes of percutaneous nephrolithotomy compared to shock wave lithotripsy and conservative management. J Urol 179: 2233–2237, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Krambeck AE, Gettman MT, Rohlinger AL, Lohse CM, Patterson DE, Segura JW: Diabetes mellitus and hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol 175: 1742–1747, 2006 [DOI] [PubMed] [Google Scholar]
- 35. Janetschek G, Frauscher F, Knapp R, Hofle G, Peschel R, Bartsch G: New onset hypertension after extracorporeal shock wave lithotripsy: Age related incidence and prediction by intrarenal resistive index. J Urol 158: 346–351, 1997 [DOI] [PubMed] [Google Scholar]
- 36. Sato Y, Tanda H, Kato S, Ohnishi S, Nakajima H, Nanbu A, Nitta T, Koroku M, Akagashi K, Hanzawa T: Shock wave lithotripsy for renal stones is not associated with hypertension and diabetes mellitus. Urology 71: 586–591, discussion 591–592, 2008 [DOI] [PubMed] [Google Scholar]
- 37. Elves AW, Tilling K, Menezes P, Wills M, Rao PN, Feneley RC: Early observations of the effect of extracorporeal shockwave lithotripsy on blood pressure: A prospective randomized control clinical trial. BJU Int 85: 611–615, 2000 [DOI] [PubMed] [Google Scholar]
- 38. Krambeck AE, Rule AD, Li X, Bergstralh EJ, Gettman MT, Lieske JC: Shock wave lithotripsy is not predictive of hypertension among community stone formers at long-term followup. J Urol 185: 164–169, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Willis LR, Evan AP, Connors BA, Handa RK, Blomgren PM, Lingeman JE: Prevention of lithotripsy-induced renal injury by pretreating kidneys with low-energy shock waves. J Am Soc Nephrol 17: 663–673, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Larsson PA, Cano M, Grenabo L, Brorson JE, Hedelin H, Pettersson S, Johansson SL: Morphological lesions of the rat urinary tract induced by inoculation of mycoplasmas and other urinary tract pathogens. Urol Int 44: 210–217, 1989 [DOI] [PubMed] [Google Scholar]
- 41. Gupta M, Bolton DM, Gupta PN, Stoller ML: Improved renal function following aggressive treatment of urolithiasis and concurrent mild to moderate renal insufficiency. J Urol 152: 1086–1090, 1994 [DOI] [PubMed] [Google Scholar]
- 42. Johnson RJ, Kivlighn SD, Kim YG, Suga S, Fogo AB: Reappraisal of the pathogenesis and consequences of hyperuricemia in hypertension, cardiovascular disease, and renal disease. Am J Kidney Dis 33: 225–234, 1999 [DOI] [PubMed] [Google Scholar]
- 43. Weiner DE, Tighiouart H, Elsayed EF, Griffith JL, Salem DN, Levey AS: Uric acid and incident kidney disease in the community. J Am Soc Nephrol 19: 1204–1211, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Obermayr RP, Temml C, Gutjahr G, Knechtelsdorfer M, Oberbauer R, Klauser-Braun R: Elevated uric acid increases the risk for kidney disease. J Am Soc Nephrol 19: 2407–2413, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lieske JC, de la Vega LS, Gettman MT, Slezak JM, Bergstralh EJ, Melton LJ, 3rd, Leibson CL: Diabetes mellitus and the risk of urinary tract stones: A population-based case-control study. Am J Kidney Dis 48: 897–904, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Belostotsky R, Seboun E, Idelson GH, Milliner DS, Becker-Cohen R, Rinat C, Monico CG, Feinstein S, Ben-Shalom E, Magen D, Weissman I, Charon C, Frishberg Y: Mutations in DHDPSL are responsible for primary hyperoxaluria type III. Am J Hum Genet 87: 392–399, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lieske JC, Monico CG, Holmes WS, Bergstralh EJ, Slezak JM, Rohlinger AL, Olson JB, Milliner DS: International registry for primary hyperoxaluria. Am J Nephrol 25: 290–296, 2005 [DOI] [PubMed] [Google Scholar]
- 48. Evan AP, Coe FL, Lingeman JE, Shao Y, Matlaga BR, Kim SC, Bledsoe SB, Sommer AJ, Grynpas M, Phillips CL, Worcester EM: Renal crystal deposits and histopathology in patients with cystine stones. Kidney Int 69: 2227–2235, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Assimos DG, Leslie SW, Ng C, Streem SB, Hart LJ: The impact of cystinuria on renal function. J Urol 168: 27–30, 2002 [PubMed] [Google Scholar]
- 50. Worcester EM, Coe FL, Evan AP, Parks JH: Reduced renal function and benefits of treatment in cystinuria vs other forms of nephrolithiasis. BJU Int 97: 1285–1290, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Edvardsson V, Palsson R, Olafsson I, Hjaltadottir G, Laxdal T: Clinical features and genotype of adenine phosphoribosyltransferase deficiency in Iceland. Am J Kidney Dis 38: 473–480, 2001 [DOI] [PubMed] [Google Scholar]
- 52. Ludwig M, Utsch B, Monnens LA: Recent advances in understanding the clinical and genetic heterogeneity of Dent's disease. Nephrol Dial Transplant 21: 2708–2717, 2006 [DOI] [PubMed] [Google Scholar]
- 53. Bockenhauer D, Bokenkamp A, van't Hoff W, Levtchenko E, Kist-van Holthe JE, Tasic V, Ludwig M: Renal phenotype in Lowe syndrome: A selective proximal tubular dysfunction. Clin J Am Soc Nephrol 3: 1430–1436, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bokenkamp A, Bockenhauer D, Cheong HI, Hoppe B, Tasic V, Unwin R, Ludwig M: Dent-2 disease: A mild variant of Lowe syndrome. J Pediatr 155: 94–99, 2009 [DOI] [PubMed] [Google Scholar]
- 55. Lorenz EC, Vrtiska TJ, Lieske JC, Dillon JJ, Stegall MD, Li X, Bergstralh EJ, Rule AD: Prevalence of renal artery and kidney abnormalities by computed tomography among healthy adults. Clin J Am Soc Nephrol 5: 431–438, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Strang AM, Lockhart ME, Kenney PJ, Amling CL, Urban DA, El-Galley R, Burns JR, Colli JL, Hammontree LN, Kolettis PN: Computerized tomographic angiography for renal donor evaluation leads to a higher exclusion rate. J Urol 177: 1826–1829, 2007 [DOI] [PubMed] [Google Scholar]
- 57. Lorenz EC, Lieske JC, Vrtiska TJ, Krambeck AE, Li X, Bergstralh EJ, Melton LJ, 3rd, Rule AD: Clinical characteristics of potential kidney donors with asymptomatic kidney stones. Nephrol Dial Transplant February 1, 2011. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Strang AM, Lockhart ME, Amling CL, Kolettis PN, Burns JR: Living renal donor allograft lithiasis: A review of stone related morbidity in donors and recipients. J Urol 179: 832–836, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Chen N, Wang W, Huang Y, Shen P, Pei D, Yu H, Shi H, Zhang Q, Xu J, Lv Y, Fan Q: Community-based study on CKD subjects and the associated risk factors. Nephrol Dial Transplant 24: 2117–2123, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Poggio ED, Rule AD: A critical evaluation of chronic kidney disease: Should isolated reduced estimated glomerular filtration rate be considered a ‘disease’? Nephrol Dial Transplant 24: 698–700, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]