Abstract
Summary
Two new potential pharmacologic therapies for recurrent stone disease are described. The role of hyperuricosuria in promoting calcium stones is controversial with only some but not all epidemiologic studies demonstrating associations between increasing urinary uric acid excretion and calcium stone disease. The relationship is supported by the ability of uric acid to “salt out” (or reduce the solubility of) calcium oxalate in vitro. A randomized, controlled trial of allopurinol in patients with hyperuricosuria and normocalciuria was also effective in preventing recurrent stones. Febuxostat, a nonpurine inhibitor of xanthine oxidase (also known as xanthine dehydrogenase or xanthine oxidoreductase) may have advantages over allopurinol and is being tested in a similar protocol, with the eventual goal of determining whether urate-lowering therapy prevents recurrent calcium stones. Treatments for cystinuria have advanced little in the past 30 years. Atomic force microscopy has been used recently to demonstrate that effective inhibition of cystine crystal growth is accomplished at low concentrations of l-cystine methyl ester and l-cystine dimethyl ester, structural analogs of cystine that provide steric inhibition of crystal growth. In vitro, l-cystine dimethyl ester had a significant inhibitory effect on crystal growth. The drug's safety and effectiveness will be tested in an Slc3a1 knockout mouse that serves as an animal model of cystinuria.
Introduction
The current pharmacologic therapies for prevention of recurrent kidney stones all are relatively ancient (1). Only a handful of drugs are commonly used today, none of which is less than 30 years old. For prevention of recurrent calcium stones, available therapies include the thiazides (chlorthalidone, hydrochlorothiazide, and indapamide), potassium citrate, and allopurinol. Differences between pharmacologic therapy for calcium oxalate and calcium phosphate stones are not clearly delineated. Uric acid stones are treated with urinary alkalinization, usually with potassium citrate and less frequently with sodium citrate, and allopurinol has only a secondary role. For cystine stones, alkalinization as for uric acid stones and thiol therapy with tiopronin and d-penicillamine are appropriate.
This review briefly considers two novel topics: Febuxostat for calcium stones and new inhibitors of cystine crystallization. Other pharmacologic therapies are in development; these are simply two topics of current interest to the author.
Febuxostat
Approximately one third of patients with recurrent calcium stones have hyperuricosuria as one of their urinary risk factors (2). Increasing the urate concentration of experimental solutions effectively halves the amount of oxalate required to provoke calcium oxalate crystallization and increases the size of particles deposited (3). The pathophysiology of this relationship has been attributed to the ability of uric acid to “salt out” calcium oxalate. Salting out is simply the ability of an electrolyte, in this case uric acid, to reduce the solubility of a nonelectrolyte, in this case calcium oxalate (4). For the purposes of this definition, nonelectrolytes and electrolytes are salts that have low and high solubilities, respectively.
This phenomenon is distinct from epitaxy, whereby one crystal forms on another. (Although sodium urate can lead to crystallization of calcium oxalate in vitro, such an effect has not been demonstrated to occur in human urine and is now considered unimportant in explaining the ability of hyperuricosuria to promote calcium stone disease.)
Although these in vitro data and phenomena are interesting, the epidemiologic data demonstrating that hyperuricosuria is an important risk factor for calcium stones is less compelling with variable results and conclusions. Most recently, three well characterized cohorts of prospectively followed participants were studied with regard to 24-hour uric acid excretion as a risk for kidney stone formation (5). In 3350 men and women, 2237 of whom had a history of nephrolithiasis, urinary uric acid had a significant inverse association with stone formation in men, a marginal inverse association with risk in younger women, and no association in older women.
With many small and inadequately controlled trials in the literature suggesting that reducing uric acid excretion might reduce calcium stone recurrence, only one adequate randomized, controlled trial has demonstrated this effect (6). The hypothesis was that allopurinol, an inhibitor of xanthine oxidase (also known recently as xanthine dehydrogenase or xanthine oxidoreductase) would decrease the rate of recurrent calcium oxalate calculi in patients with hyperuricosuria (>800 mg/d in men, >750 mg/d in women) and normocalciuria (<300 mg/d in men, <250 mg/d in women). The patients were randomly assigned to either allopurinol 100 mg three times a day or placebo. Recurrent stone outcomes were determined either by recording of stone passage or by comparison of plain radiographs taken at yearly intervals with the baseline film. Allopurinol reduced uric acid excretion by >400 mg/d, whereas the placebo group had a variable reduction of 0 to 100 mg/d. Although both groups had a reduction in stone events, new stones occurred in 18 patients receiving placebo and nine receiving allopurinol, and the allopurinol group had a significantly longer time before recurrence.
This study exists in the context of the heterogeneity of epidemiologic data that fail to demonstrate strongly that hyperuricosuria is a risk factor for calcium stones. It has therefore been suggested that the effect of allopurinol on calcium stone incidence is actually not related to its ability to lower uric acid production (5,7). Its effects could include other effects of xanthine oxidase inhibition, as the enzyme has been linked to oxidative stress and production of free radicals (8). How these pleiotropic effects of xanthine oxidase and its inhibition are linked to stone formation remain speculative and unproved.
One interesting question is whether xanthine oxidase inhibition to reduce uric acid excretion is effective only in calcium stone formers with normocalciuria and not in patients with hypercalciuria. Review of this literature demonstrates a paucity of evidence. In one frequently cited article, a nonrandomized trial, 31 patients were treated with allopurinol, and 43 patients served as untreated controls for recurrent calcium stones (9). Overall, patients who were treated with allopurinol had a recurrence rate of 49% (15 of 31), whereas the control patients, who were not treated with allopurinol, had a recurrence rate of 40% (17 of 43). In other words, allopurinol had no effect on the aggregated group. Five of six with both hyperuricosuria and hypercalciuria had an episode of stone recurrence at 2 years, whereas only two of eight with hyperuricosuria and normocalciuria did; regardless of uric acid excretion, the patients with hypercalciuria had a 64% recurrence rate, whereas patients with normocalciuria had a 40% recurrence rate. This small, nonrandomized trial with smaller subgroups cannot serve as an adequate basis for concluding that urate-lowering therapy is not efficacious in the presence of hypercalciuria. In a review of the efficacy of allopurinol for calcium stones, Ettinger (7) tabulated 15 articles that either did or did not prescribe the drug in a selective manner, that is, excluding hypercalciuria. The studies are mostly small and nonrandomized. He concluded, “Treatment has not been systematically evaluated for patients (with) hyperuricosuria combined with hypercalciuria. The school of selective treatment would suggest thiazide and allopurinol.” However, this combination is thought to lead to a higher rate of allopurinol hypersensitivity, perhaps via increased reabsorption of the metabolite oxypurinol (10). Many such cases have occurred when allopurinol was given to treat asymptomatic hyperuricemia developing from thiazide use.
Febuxostat is a newer xanthine oxidase inhibitor and, unlike allopurinol, is not a purine analog. It received approval by the Food and Drug Administration in 2009 for the long-term management of hyperuricemia in patients with gout. Its efficacy in reducing serum uric acid concentration and urinary uric acid excretion may be superior to allopurinol, although the two have not been properly tested head-to-head because allopurinol has not been titrated past 300 mg/d in studies performed in the United States (11–13). Whereas excretion of allopurinol is mainly via the kidneys, febuxostat is largely metabolized by the liver. It may therefore be particularly useful for urate lowering in patients with chronic kidney disease, a category that is not necessarily relevant to stone disease. Studies have concluded that the 80-mg dosage does not require adjustment in patients with impaired GFR, although the data for patients with estimated GFR <30 ml/min per 1.73 m2 are limited (14,15). Febuxostat is currently used mostly in patients with allopurinol allergy or hypersensitivity and in patients who fail to achieve serum uric acid targets with allopurinol (13). In stone formers being treated for gout and hyperuricemia, it might be preferable, in the allopurinol-intolerant patient, to uricosuric agents.
On the basis of the possibility that the drug could be as efficacious as or superior to allopurinol, I helped Takeda design a study to test the hypothesis that febuxostat, like allopurinol, could reduce hyperuricosuria and recurrent calcium stones. The study has completed enrollment at a number of kidney stone prevention programs and urology practices throughout the United States. Its title is “Febuxostat Versus Allopurinol or Placebo in Subjects with Hyperuricosuria and Calcium Oxalate Stones” (ClinicalTrials.gov identifier NCT01077284). The preliminary design seeks essentially to replicate the allopurinol study of Ettinger et al. (6). Patients with a history of calcium stones and at least one 3-mm stone in place and with hyperuricosuria (>700 mg/d) and normocalciuria (<4 mg/kg) will be randomly assigned to one of three groups: febuxostat 80 mg/d, allopurinol 200 or 300 mg/d (depending on GFR), or placebo. Hyperuricemia is not required. The outcome is uric acid excretion and assessment of stones on computed tomography at 6 months. Although this period of time is likely too short to demonstrate a change in stone outcomes, the uric acid excretion results may serve as the basis for a possible longer study sufficient to replicate the findings of Ettinger (6). The study will generate data on the magnitude of reduction of uricosuria by this dosage of febuxostat.
In a potential longer phase 3 study, with a primary outcome of stone burden as determined by computed tomography, febuxostat could be compared with allopurinol. Because allopurinol is available as a generic medication, it is significantly less expensive. Febuxostat may be more efficacious if greater reductions in serum uric acid correlate with lower urinary uric acid (11,12) and if that in turn correlates with less stone disease activity. Whereas allopurinol is associated with an incidence of rash of 2% and rare life-threatening hypersensitivity reactions (10), febuxostat seems thus far to be associated with fewer hypersensitivity events, with only two reported to Takeda via the Food and Drug Administration's Medwatch (Patricia Macdonald, RN, personal communication). Liver function abnormalities are seen at a slightly higher rate than in placebo-treated patients. Other potential advantages of febuxostat, of unproven clinical significance thus far, is that, unlike allopurinol, it inhibits both the oxidized and reduced forms of xanthine oxidase and has fewer effects on other enzymes involved in purine and pyrimidine metabolism. It might also be worthwhile to test urate-lowering therapy with or without thiazides in patients with hypercalciuria and hyperuricosuria. Both allopurinol and febuxostat may be better tolerated than thiazides in many patients (16) and may have additional cardiovascular benefits related to either reduction of uric acid or inhibition of xanthine oxidase (17). The limitation to using allopurinol more widely because of the purported lack of efficacy in hypercalciuria and the risk for hypersensitivity when co-administered with thiazides leaves open the possibility of a range of studies with febuxostat.
New Therapies for Cystinuria
Cystinuria is an autosomal recessive disorder that occurs as the result of mutations in one of the two genes that code for the proteins that constitute the cystine and basic amino acid transporter expressed in the proximal tubule (18). The result is failure to reabsorb filtered cystine, a poorly soluble amino acid that crystallizes in the distal tubules and forms large and recurrent stones.
Treatments for cystinuria have essentially not changed for more than 30 years (19). Fluids to increase urine volume and dilute excreted cystine are considered critical. Dietary therapy, which remains untested in actually changing stone activity, includes sodium and animal protein restriction. Reduction of sodium intake is associated with a reduction in cystine excretion, although the basis for this effect is not clear (20,21); the cystine transporter in the proximal tubule is not a sodium-dependent transporter. Restriction of animal protein ingestion facilitates urinary alkalinization to increase cystine solubility and reduces intake of the cystine precursor methionine, reducing urinary cystine excretion (22). Pharmacologic therapies are twofold. Urinary alkalinization increases cystine solubility and is achieved with administration of citrate or bicarbonate. Because cystine is a dimer of cysteine linked by a disulfide bridge, thiols, whether d-penicillamine or tiopronin, can break the disulfide bridge and form a more soluble complex with monomeric cysteine. Captopril, although also a thiol, is ineffective because its appearance in the urine is insufficient to have a meaningful effect.
The thiols, however, are not particularly well tolerated and are associated with a significant incidence of allergy; gastrointestinal intolerance; and a broad variety of adverse effects that include elevation in hepatic enzymes, blood dyscrasias, skin disorders such as elastosis perforans serpiginosa, and proteinuria with membranous nephropathy (23). Tiopronin is better tolerated than d-penicillamine, with 30.6% and 69.4%, respectively, having reactions that required cessation of therapy in one nonrandomized study (23); however, adverse reactions to tiopronin were extremely common. The basis for the toxicity of thiols in general might result from the ability of thiols to produce superoxide radicals and hydrogen peroxide. Thiols can auto-oxidize to disulphides, whereas the latter can be reduced to thiols, and this potential for redox cycling can potentially produce these toxic byproducts (24). The reaction of thiol drugs with cystine might be a model for their interactions with disulfide bridge–containing proteins throughout the body. The basis for their use in various other diseases includes their ability both to induce autoimmunity and inhibit T and B cells and to inhibit various enzymes including metalloproteinases. Other approaches to the treatment of cystinuria would therefore be welcome.
Ward and his team in the Department of Chemistry at New York University recently reported a potential alternative approach to the prevention of cystine kidney stones based on crystal growth inhibition (25). The technique that allows crystal growth and the effect of inhibitors to be measured is atomic force microscopy (AFM) (26). This technique provides for in situ visualization of the early stages of growth of organic crystals in liquids. The result is the ability to observe nucleation events and topographic features of growing crystals that play an important role in crystal growth. It is then possible to quantify the velocity of growth under varying conditions and in the presence of changing concentrations of putative inhibitors. Real-time in situ AFM of l-cystine during growth in aqueous solutions containing l-cystine revealed expanding steps generating hexagonal hillocks in a spiral growth pattern.
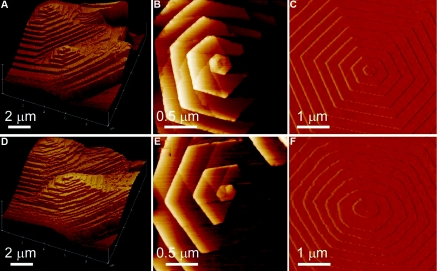
Examination of the crystal structure of l-cystine suggested several potential inhibitors of crystal growth. Among several that were tested, the most effective were l-cystine dimethyl ester (L-CDME) and l-cystine methylester (L-CME). These molecules serve as structural mimics of l-cystine in which one carboxylate group is replaced by a methylester group (L-CME), or in the symmetrical form, both carboxylates are replaced by methylester groups (L-CDME). Using these inhibitors resulted in roughening of the otherwise highly linear growing step edges and rounding of the hillock corners seen by AFM (25) (Figure 1). The step velocity decreased with increasing L-CDME concentration, becoming negligible above 30 mg/L. The inhibitor effect was reversible, with the rate of growth returning to the original value once the aqueous solution containing L-CDME was replaced with one containing only l-cystine. L-CDME was significantly more efficacious in inhibiting crystal growth than L-CME. Importantly, for consideration of whether L-CDME could be useful in people with cystinuria, the concentrations at which L-CDME was effective at a concentration of only 2 mg/L. Because l-cystine stone formers generally have urinary l-cystine concentrations ranging from 1 to 4 mM, comparable with the concentrations used for the AFM studies. Therefore, L-CDME concentrations near 2 mg/L (<0.01 mM), at which inhibition of l-cystine growth was highly effective, may be adequate for therapeutic effect.
Figure 1.
(A and B) Real-time in situ AFM images of an l-cystine crystal, acquired 12 minutes apart. A pair of hexagonal hillocks generated by two closely spaced dislocations serve as landmarks. (C and D) AFM images of a single dislocation center of l-cystine (C) and d-cystine (D) crystal during growth. (E and F) AFM images of a hexagonal growth hillock on the face of l-cystine before (E) and after (F) addition of L-CDME (5 mg/L; 0.02 mM), revealing roughening of the steps as a result of step pinning. Images were acquired in aqueous solutions containing 2 mM l-cystine. AFM, atomic force microscopy. Reprinted, from reference 25, with permission.
The advantage of these inhibitors over the thiols in use today is that at the low concentrations at which they seem effective, they may have a better profile of adverse effects and be better tolerated. They may also be more effective. As structural mimics of l-cystine, they interfere with crystallization by providing a steric inhibitory effect rather than the chemical reaction of disulfide exchange offered by tiopronin and d-penicillamine.
However, the use of L-CDME is not without potential risk. Interestingly, incubation of LLC-PK1 cells, a model of proximal tubular epithelium, with L-CDME leads to lysosomal accumulation of cystine (27). The cells have impaired solute transport, oxygen utilization and substrate utilization (28–30). This loading with cystine serves as a model for cystinosis, an autosomal recessive disorder of lysosomal cystine accumulation as a result of abnormal cystine transport. Cystinosis is characterized by the Fanconi syndrome and frequently the development of kidney failure. The use of L-CDME as a treatment of cystinuria would then depend on its efficacy at dosages low enough to lead to inhibitory urine concentrations while not causing lysosomal accumulation and mimicking cystinosis. It may therefore be desirable to use AFM to find other possible inhibitors of cystine crystal growth to avoid this possible adverse effect. It is of course also possible that other molecules could be shown to be more effective than L-CDME.
The further development and testing of L-CDME and its congeners is being undertaken by Dr. Amrik Sahota. Cystinuria has been replicated in three mouse models in which the mouse genes Slc3a1 and Slc7a9 are mutated, leading to a reasonable facsimile of the human disorder (31–33). In collaboration with Dr. Ward, Dr. Sahota and his co-workers are administering L-CDME to cystinuric mice and using micro-computed tomography to assess efficacy. Densitometric analysis of x-ray images has been successfully used to assess quantitatively stone growth in response to treatment of cystinuric mice with d-penicillamine (32). If L-CDME is effective and safe in knockout mice, then the eventual goal would be to study the drug or its congeners in humans with cystinuria. Ensuring that the dosages used to inhibit urinary stone formation are not associated with lysosomal accumulation of cystine as in cystinosis will be an important part of the safety monitoring.
Disclosures
Dr. Goldfarb is a consultant for Takeda. Takeda was not involved with the concept or writing of the manuscript and did not approve its contents.
Acknowledgments
The study of febuxostat described is funded by Takeda. Pat MacDonald, RN, of Takeda has been particularly supportive and enthusiastic in going forth with this project. Dr. Michael Ward's atomic force microscopy studies were supported primarily by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01-DK068551, the NYU Molecular Design Institute, and the Advanced Photon Source, ChemMatCARS Sector 15, which is principally supported by the National Science Foundation/U.S. Department of Energy (CHE-0535644). The Rare Kidney Stone Consortium is funded by NIDDK and Office of Rare Disease Research via the Rare Disease Clinical Research Network, grant 1U54DK083908-01.
I appreciate the thoughtful suggestions of Dr. Amrik Sahota.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1. Moe OW, Pearle MS, Sakhaee K: Pharmacotherapy of urolithiasis: Evidence from clinical trials. Kidney Int 79: 385–392, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ettinger B: Hyperuricosuric calcium stone disease and mixed stones. In: Kidney Stones: Medical and Surgical Management, edited by Coe FL, Favus MJ, Pak CY, Parks JH, Preminger GM. Philadelphia, Lippincott-Raven, 1996, pp 851–858 [Google Scholar]
- 3. Ryall RL, Grover PK, Marshall VR: Urate and calcium stones: Picking up a drop of mercury with one's fingers? Am J Kidney Dis 17: 426–430, 1991 [DOI] [PubMed] [Google Scholar]
- 4. Grover PK, Marshall VR, Ryall RL: Dissolved urate salts out calcium oxalate in undiluted human urine in vitro: Implications for calcium oxalate stone genesis. Chem Biol 10: 271–278, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Curhan GC, Taylor EN: 24-h uric acid excretion and the risk of kidney stones. Kidney Int 73: 489–496, 2008 [DOI] [PubMed] [Google Scholar]
- 6. Ettinger B, Tang A, Citron JT, Livermore B, Williams T: Randomized trial of allopurinol in the prevention of calcium oxalate calculi. N Engl J Med 315: 1386–1389, 1986 [DOI] [PubMed] [Google Scholar]
- 7. Ettinger B: Does hyperuricosuria play a role in calcium oxalate lithiasis? J Urol 141: 738–741, 1989 [DOI] [PubMed] [Google Scholar]
- 8. Guthikonda S, Sinkey C, Barenz T, Haynes WG: Xanthine oxidase inhibition reverses endothelial dysfunction in heavy smokers. Circulation 107: 416–421, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Fellstrom B, Backman U, Danielson BG, Holmgren K, Johansson G, Lindsjo M, Ljunghall S, Wikstrom B: Allopurinol treatment of renal calcium stone disease. Br J Urol 57: 375–379, 1985 [DOI] [PubMed] [Google Scholar]
- 10. Arellano F, Sacristan JA: Allopurinol hypersensitivity syndrome: A review. Ann Pharmacother 27: 337–343, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, Streit J, Joseph-Ridge N: Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 353: 2450–2461, 2005 [DOI] [PubMed] [Google Scholar]
- 12. Schumacher HR, Jr, Becker MA, Wortmann RL, MacDonald PA, Hunt B, Streit J, Lademacher C, Joseph-Ridge N: Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: A 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 59: 1540–1548, 2008 [DOI] [PubMed] [Google Scholar]
- 13. Jansen TL, Richette P, Perez-Ruiz F, Tausche AK, Guerne PA, Punzi L, Leeb B, Barskova V, Uhlig T, Pimentao J, Zimmermann-Gorska I, Pascual E, Bardin T, Doherty M: International position paper on febuxostat. Clin Rheumatol 29: 835–840, 2010 [DOI] [PubMed] [Google Scholar]
- 14. Mayer MD, Khosravan R, Vernillet L, Wu JT, Joseph-Ridge N, Mulford DJ: Pharmacokinetics and pharmacodynamics of febuxostat, a new non-purine selective inhibitor of xanthine oxidase in subjects with renal impairment. Am J Ther 12: 22–34, 2005 [DOI] [PubMed] [Google Scholar]
- 15. Hoshide S, Takahashi Y, Ishikawa T, Kubo J, Tsuchimoto M, Komoriya K, Ohno I, Hosoya T: PK/PD and safety of a single dose of TMX-67 (febuxostat) in subjects with mild and moderate renal impairment. Nucleosides Nucleotides Nucleic Acids 23: 1117–1118, 2004 [DOI] [PubMed] [Google Scholar]
- 16. Huen SC, Goldfarb DS: Adverse metabolic side effects of thiazides: Implications for patients with calcium nephrolithiasis. J Urol 177: 1238–1243, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Keenan RT, Pillinger MH: Hyperuricemia, gout, and cardiovascular disease: An important “muddle.” Bull NYU Hosp Jt Dis 67: 285–290, 2009 [PubMed] [Google Scholar]
- 18. Chillaron J, Font-Llitjos M, Fort J, Zorzano A, Goldfarb DS, Nunes V, Palacin M: Pathophysiology and treatment of cystinuria. Nat Rev Nephrol 6: 424–434, 2010 [DOI] [PubMed] [Google Scholar]
- 19. Mattoo A, Goldfarb DS: Cystinuria. Semin Nephrol 28: 181–191, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Rodriguez LM, Santos F, Malaga S, Martinez V: Effect of a low sodium diet on urinary elimination of cystine in cystinuric children. Nephron 71: 416–418, 1995 [DOI] [PubMed] [Google Scholar]
- 21. Goldfarb DS, Coe FL, Asplin JR: Urinary cystine excretion and capacity in patients with cystinuria. Kidney Int 69: 1041–1047, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Rodman JS, Blackburn P, Williams JJ, Brown A, Pospischil MA, Peterson CM: The effect of dietary protein on cystine excretion in patients with cystinuria. Clin Nephrol 22: 273–278, 1984 [PubMed] [Google Scholar]
- 23. Pak CY, Fuller C, Sakhaee K, Zerwekh JE, Adams BV: Management of cystine nephrolithiasis with alpha-mercaptopropionylglycine. J Urol 136: 1003–1008, 1986 [DOI] [PubMed] [Google Scholar]
- 24. Munday R: Toxicity of thiols and disulphides: Involvement of free-radical species. Free Radic Biol Med 7: 659–673, 1989 [DOI] [PubMed] [Google Scholar]
- 25. Rimer JD, An Z, Zhu Z, Lee MH, Goldfarb DS, Wesson JA, Ward MD: Crystal growth inhibitors for the prevention of L-cystine kidney stones through molecular design. Science 330: 337–341, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hillier AC, Ward MD: Atomic force microscopy of the electrochemical nucleation and growth of molecular crystals. Science 263: 1261–1264, 1994 [DOI] [PubMed] [Google Scholar]
- 27. Moran A, Ben-Nun A, Potashnik R, Bashan N: Renal cells in culture as a model for cystinosis. J Basic Clin Physiol Pharmacol 1: 357–372, 1990 [DOI] [PubMed] [Google Scholar]
- 28. Foreman JW, Benson L: Effect of cystine loading and cystine dimethylester on renal brushborder membrane transport. Biosci Rep 10: 455–459, 1990 [DOI] [PubMed] [Google Scholar]
- 29. Foreman JW, Benson LL: Effect of cystine loading on substrate oxidation by rat renal tubules. Pediatr Nephrol 4: 236–239, 1990 [DOI] [PubMed] [Google Scholar]
- 30. Foreman JW, Bowring MA, Lee J, States B, Segal S: Effect of cystine dimethylester on renal solute handling and isolated renal tubule transport in the rat: A new model of the Fanconi syndrome. Metabolism 36: 1185–1191, 1987 [DOI] [PubMed] [Google Scholar]
- 31. Ercolani M, Sahota A, Schuler C, Yang M, Evan AP, Reimer D, Barone JG, Tischfield JA, Levin RM: Bladder outlet obstruction in male cystinuria mice. Int Urol Nephrol 42: 57–63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Font-Llitjos M, Feliubadalo L, Espino M, Cleries R, Manas S, Frey IM, Puertas S, Colell G, Palomo S, Aranda J, Visa J, Palacin M, Nunes V: Slc7a9 knockout mouse is a good cystinuria model for antilithiasic pharmacological studies. Am J Physiol Renal Physiol 293: F732–F740, 2007 [DOI] [PubMed] [Google Scholar]
- 33. Peters T, Thaete C, Wolf S, Popp A, Sedlmeier R, Grosse J, Nehls MC, Russ A, Schlueter V: A mouse model for cystinuria type I. Hum Mol Genet 12: 2109–2120, 2003 [DOI] [PubMed] [Google Scholar]