Abstract
Recent studies have suggested that cortical activation can be measured using event-related augmentation of gamma-oscillations in humans. We determined how commonly and differentially gamma-oscillations (50-150 Hz) were modulated by three distinct word-association tasks during extraoperative electrocorticography monitoring in a patient with focal epilepsy who underwent epilepsy surgery. He was auditorily presented names of common foods (e.g.: apple) during each task. He was instructed to overtly verbalize the color (e.g.: red) of each given food during the first association task, the taste (e.g.: sweet) during the second task, and the texture (e.g.: crunchy) during the third task. All three word-association tasks commonly elicited significant augmentation of gamma-oscillations in the superior temporal gyrus, the middle temporal gyrus and inferior frontal gyrus, as well as the pre- and post-central gyri. The food-texture association task specifically elicited significant gamma-augmentation in the supramarginal gyrus. This preliminary study generated the hypothesis that word-association tasks may supplement functional language mapping using electrical stimulation. Differential gamma-augmentation in the supramarginal gyrus might be attributed to a larger workload required in the food-texture association task compared to the remaining two tasks.
Keywords: pediatric epilepsy surgery, subdural EEG recording, electrocorticography (ECoG), event-related synchronization, functional cortical mapping
INTRODUCTION
The general principles of epilepsy surgery include: complete removal of the epileptogenic zone and preservation of the eloquent areas [1]. Recent studies have suggested that recording of event-related gamma-oscillations on electrocorticography (ECoG) is useful to localize the cortical sites involved in language tasks [2-7]; in short, augmentation of gamma-oscillations is considered to represent cortical activation [8]. Taking advantage of a unique opportunity to monitor neuronal activities using intracranial ECoG recording, we determined whether three distinct word-association tasks similarly or differentially modulated gamma-oscillations in human cerebral cortex.
METHODS
The study was performed on a 17-year-old right-handed male with a diagnosis of non-lesional focal epilepsy. Phase-I presurgical evaluation lateralized the epileptogenic zone to the left hemisphere. Video-EEG recording did not show interictal spikes but demonstrated ictal discharges maximally involving the left temporal area. Brain MRI was normal. Verbal Comprehension Index was 109, and the Wada test suggested left hemisphere dominance for speech.
The patient underwent chronic implantation of subdural electrodes on the left hemisphere (total number of electrodes: 112), in order to determine the presumed epileptogenic zone and eloquent cortical regions. During extraoperative ECoG monitoring, he completed three word-association tasks. The details are described in the supplementary document on the website (Supplementary Document S1). He was auditorily presented a total of 40 names of common foods (e.g.: apple) during each task. He was instructed to overtly verbalize the color (e.g.: red) of each given food during the first association task, the taste (e.g.: sweet) during the second task, and the texture (e.g.: crunchy) during the third task.
Each ECoG trial was transformed into the time-frequency domain, and we determined ‘when’ and ‘where’ gamma-oscillations (50-150 Hz) were augmented. The time-frequency analysis used in the present study was previously validated [5-13]. The measures of interest in the present study included: a percent change of the amplitude of gamma-oscillations relative to that during the reference period (i.e.: the resting baseline) as well as statistical significance of task-related augmentation of gamma-oscillations. The reaction time was also measured for each association task. The methodological details are described in the supplementary document on the website (Supplementary Document S1).
RESULTS
The mean reaction time was 2.0 sec (standard deviation [SD]: 0.9 sec) in the food-color association task, 2.3 sec (SD: 0.9 sec) in the food-taste association task, and 2.6 sec (SD: 1.2 sec) in the food-texture association task. Post-hoc analysis with one-way ANOVA demonstrated that the reaction time significantly differed between the food-color and food-texture association tasks alone (p=0.04).
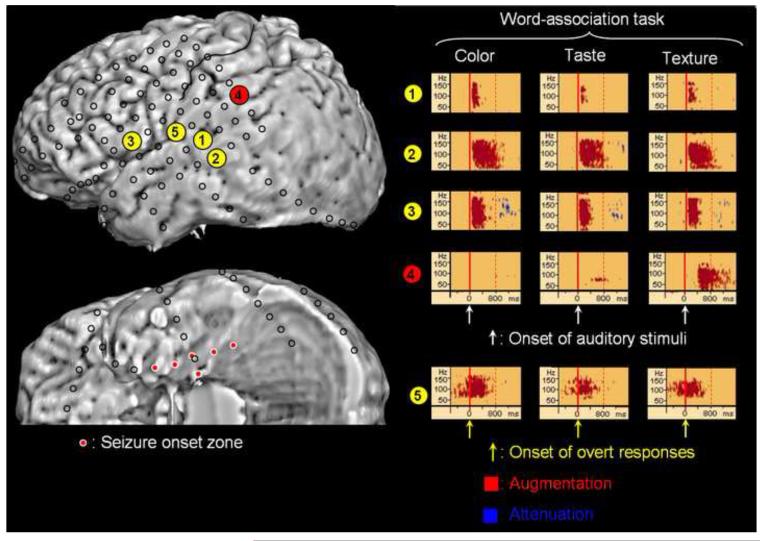
The areas showing gamma-augmentation commonly elicited by all three word-association tasks included: (i) the superior temporal gyrus following auditory presentation of the name of food, (ii) the middle temporal gyrus and inferior frontal gyrus following presentation of auditory stimuli and prior to overt responses, and (iii) the pre- and post-central gyri immediately prior to and during overt responses (Figure 1). On the other hand, the food-texture association task differentially elicited gamma-augmentation in the supramarginal gyrus 400-1,200 msec after the onset of auditory stimuli but prior to the onset of overt responses.
Figure 1. The results of time-frequency analysis.
The results of time-frequency analysis relative to the onset of auditory stimuli are as follows. All three word-association tasks commonly elicited significant gamma-augmentation (50-150 Hz) at electrode #1 in the superior temporal gyrus approximately at +100 to +200 msec, at electrode #2 in the middle temporal gyrus at +100 to +800 msec, and at electrode #3 in the inferior frontal gyrus at +100 to +400 msec. Gamma-augmentation at electrode #3 was followed by brief gamma-attenuation. The food-texture association task specifically elicited gamma-augmentation at electrode #4 in the supramarginal gyrus at +400 to +1,200 msec. Since the mean reaction time was 2.6 sec in the food-texture association task, gamma-augmentation at electrode #4 in the supramarginal gyrus was estimated to occur prior to the onset of overt responses. According to the time-frequency analysis relative to the onset of overt responses, all three word-association tasks commonly elicited significant gamma-augmentation at electrode #5 in the inferior Rolandic area at -300 to +500 msec. The detailed methods to decide significance of gamma-augmentation are described in the supplementary document on the website (Supplementary Document S1).
Electrical neurostimulation of electrode #2 elicited receptive aphasia and that of electrode #5 elicited movement of the throat. Neurostimulation of electrodes #1, #3, and #4 failed to elicit apparent clinical symptoms.
Ictal subdural ECoG recording showed that both interictal spikes and the seizure onset were confined to the medial temporal region (Figure 1). The patient underwent left anterior temporal lobectomy while language-related cortex indicated by electrical neurostimulation and task-related gamma-oscillations was preserved. Pathologic assessment of the resected tissue suggested the presence of microdysgenesis and gliosis in the left medial temporal lobe. He has been seizure-free without apparent speech deficits (follow-up: 14 months).
DISCUSSION
Significance of gamma-augmentation similarly elicted by three word-association tasks
Word association tasks require (i) perception of acoustic-phonetic features of a given stimulus, (ii) semantic comprehension of a given word, (iii) retrieval of an adjective phrase relevant to the task, and (iv) execution of overt responses. In the present study, all three word-association tasks commonly elicited gamma-augmentation in the left superior temporal gyrus, middle temporal gyrus, inferior frontal gyrus and pre- and post-central gyri. The results are consistent with the generally-accepted notion derived from functional MRI studies of healthy adults [14,15] and from ECoG studies of patients with epilepsy [2,5,11,16,17]; it has been suggested that (i) the left superior temporal gyrus plays a central role in acoustic-phonetic perception, (ii) the left middle temporal gyrus in semantic comprehension, (iii) the left inferior frontal gyrus in retrieval of a relevant word, and (iv) the pre- and post-central gyri in execution of overt responses. The time-frequency analysis in the present study demonstrated significant gamma-augmentation at sites where neurostimulation failed to elicit a clinical symptom (Figure 1). We have previously reported that our time-frequency ECoG analysis showed significant gamma-augmentation in areas larger than the eloquent areas suggested by electrical neurostimulation [5]. Further studies of a large population using post-operative neuropsychological measures are warranted to determine the clinical significance of discrepancy between gamma-measures on ECoG and neurostimulation data.
Significance of gamma-augmentation differentially elicted by the food-texture association task
In the present study, the patient was given same series of auditory stimuli in a pseudorandom order during each task, while the way of word-association was different across the three tasks. Why did the food-texture association task specifically elicit significant gamma-augmentation in the left supramarginal gyrus? It was shown that lexical semantic judgment, phoneme judgment, auditory semantic-naming, picture-naming and verb generation tasks elicited gamma-augmentation in the left supramarginal gyrus in some subjects in previous studies [2,4-6]. Lesion studies have reported association between loss of function of the left supramarginal gyrus and conduction aphasia characterized by good auditory comprehension, fluent speech production, relatively poor speech repetition, frequent phonemic errors in production, and naming difficulties [18,19]. A study of 14 healthy adults using functional MRI showed that auditory presentation of real words, compared to pseudowords, elicited larger cortical activation involving the left supramarginal gyrus during a semantic decision task [20]. Yet, these observations do not explain the mechanism of gamma-augmentation specifically elicited by the food-texture association task.
Behavioral data in the present study demonstrated that the food-texture association task required the longest reaction time. Gamma-augmentation in the left supramarginal gyrus occurred following those in the left middle temporal or inferior frontal gyrus (Figure 1). Thus, differential gamma-augmentation in the left supramarginal gyrus might be attributed to a larger workload required in the food-texture association task compared to the remaining two tasks. A previous study of six healthy adults using magnetoencephalography showed that larger cortical activation was elicited in the left supramarginal gyrus by a language task requiring larger workload compared to an easier task [21]. In that study, subjects were instructed (i) to covertly simply read a presented word in the easier task and (ii) to change a presented word to the past tense in their minds in the more demanding task; the degree of cortical activation was estimated using measurement of attenuation of oscillations at 25-50 Hz [8].
Methodological considerations
There are several factors potentially altering the results of time-frequency analysis in the present study. The number of trials in each task could have influenced the results of ECoG analyses. More electrode sites potentially could have shown gamma-augmentation reaching significance, if a larger number of trials were administered to the patient and if all tasks were successfully completed. Due to the limited time frame with the patient room being quiet, we recognized that a larger number of trials were difficult to assign to the patient. The patient had a diagnosis of focal epilepsy arising from the left medial temporal region. We are aware that the seizure onset and surrounding areas could be dysfunctional; interpretation and generalization of our results must be made cautiously and should be compared to observations from studies of healthy humans using noninvasive diagnostic modalities such as functional MRI. Phenytoin was administered intravenously prior to neurostimulation in the present study. Phenytoin, a sodium channel blocker, may affect the findings of neurostimulation, and failure to elicit clinical symptoms by neurostimulation could be partially attributed to the acute effect of phenytoin given prior to neurostimulation [22].
Supplementary Material
ACKNOWLEDGEMENT
This work was supported by NIH grants NS47550 and NS64033 (to E. Asano). We are grateful to Harry T. Chugani, M.D., Elizabeth A. Leleszi, M.D., Ruth Roeder, R.N., M.S., Carol Pawlak, R.EEG/EP.T, and the staff of the Division of Electroneurodiagnostics at Children’s Hospital of Michigan, Wayne State University for the collaboration and assistance in performing the studies described above.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCE
- [1].Rosenow F, Lüders H. Presurgical evaluation of epilepsy. Brain. 2001;124:1683–1700. doi: 10.1093/brain/124.9.1683. [DOI] [PubMed] [Google Scholar]
- [2].Sinai A, Bowers CW, Crainiceanu CM, Boatman D, Gordon B, Lesser RP, Lenz FA, Crone NE. Electrocorticographic high gamma activity versus electrical cortical stimulation mapping of naming. Brain. 2005;128:1556–1570. doi: 10.1093/brain/awh491. [DOI] [PubMed] [Google Scholar]
- [3].Tanji K, Suzuki K, Delorme A, Shamoto H, Nakasato N. High-frequency gamma-band activity in the basal temporal cortex during picture-naming and lexical-decision tasks. J Neurosci. 2005;25:3287–3293. doi: 10.1523/JNEUROSCI.4948-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Basirat A, Sato M, Schwartz JL, Kahane P, Lachaux JP. Parieto-frontal gamma band activity during the perceptual emergence of speech forms. Neuroimage. 2008;42:404–413. doi: 10.1016/j.neuroimage.2008.03.063. [DOI] [PubMed] [Google Scholar]
- [5].Brown EC, Rothermel R, Nishida M, Juhász C, Muzik O, Hoechstetter K, Sood S, Chugani HT, Asano E. In vivo animation of auditory-language-induced gamma-oscillations in children with intractable focal epilepsy. Neuroimage. 2008;41:1120–1131. doi: 10.1016/j.neuroimage.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Edwards E, Nagarajan SS, Dalal SS, Canolty RT, Kirsch HE, Barbaro NM, Knight RT. Spatiotemporal imaging of cortical activation during verb generation and picture naming. Neuroimage. 2010 doi: 10.1016/j.neuroimage.2009.12.035. doi:10.1016/j.neuroimage.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu M, Wisneski K, Schalk G, Sharma M, Roland J, Breshears J, Gaona C, Leuthardt EC. Electrocorticographic Frequency Alteration Mapping for Extraoperative Localization of Speech Cortex. Neurosurgery. 2010 doi: 10.1227/01.NEU.0000345352.13696.6F. doi: 10.1227/01.NEU.0000345352.13696.6F. [DOI] [PubMed] [Google Scholar]
- [8].Crone NE, Sinai A, Korzeniewska A. High-frequency gamma oscillations and human brain mapping with electrocorticography. Prog Brain Res. 2006;159:275–295. doi: 10.1016/S0079-6123(06)59019-3. [DOI] [PubMed] [Google Scholar]
- [9].Fukuda M, Nishida M, Juhász C, Muzik O, Sood S, Chugani HT, Asano E. Short-latency median-nerve somatosensory-evoked potentials and induced gamma-oscillations in humans. Brain. 2008;131:1793–1805. doi: 10.1093/brain/awn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Asano E, Nishida M, Fukuda M, Rothermel R, Juhász C, Sood S. Differential visually-induced gamma-oscillations in human cerebral cortex. Neuroimage. 2009;45:477–489. doi: 10.1016/j.neuroimage.2008.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Fukuda M, Rothermel R, Juhász C, Nishida M, Sood S, Asano E. Cortical gamma-oscillations modulated by listening and overt repetition of phonemes. Neuroimage. 2010;49:2735–2745. doi: 10.1016/j.neuroimage.2009.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nagasawa T, Rothermel R, Juhász C, Fukuda M, Nishida M, Akiyama T, Sood S, Asano E. Cortical gamma-oscillations modulated by auditory-motor tasks -intracranial recording in patients with epilepsy- Hum Brain Mapp. 2010 doi: 10.1002/hbm.20963. doi: 10.1002/hbm.20963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fukuda M, Juhász C, Hoechstetter K, Sood S, Asano E. Somatosensory-related gamma-, beta- and alpha-augmentation precedes alpha- and beta-attenuation in humans. Clin Neurophysiol. 2010;121:366–375. doi: 10.1016/j.clinph.2009.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Belin P, Zatorre RJ, Lafaille P, Ahad P, Pike B. Voice-selective areas in human auditory cortex. Nature. 2000;403:309–312. doi: 10.1038/35002078. [DOI] [PubMed] [Google Scholar]
- [15].Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Springer JA, Kaufman JN, Possing ET. Human temporal lobe activation by speech and nonspeech sounds. Cereb Cortex. 2000;10:512–528. doi: 10.1093/cercor/10.5.512. [DOI] [PubMed] [Google Scholar]
- [16].Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Brazier Award-winning article, 2001. Clin Neurophysiol. 2001;112:565–582. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- [17].Towle VL, Yoon HA, Castelle M, Edgar JC, Biassou NM, Frim DM, Spire JP, Kohrman MH. ECoG gamma activity during a language task: differentiating expressive and receptive speech areas. Brain. 2008;131:2013–2027. doi: 10.1093/brain/awn147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rothi LJ, McFarling D, Heilman KM. Conduction aphasia, syntactic alexia, and the anatomy of syntactic comprehension. Arch Neurol. 1982;39:272–275. doi: 10.1001/archneur.1982.00510170014004. [DOI] [PubMed] [Google Scholar]
- [19].Damasio AR. Aphasia. N Engl J Med. 1992;326:531–539. doi: 10.1056/NEJM199202203260806. [DOI] [PubMed] [Google Scholar]
- [20].Xiao Z, Zhang JX, Wang X, Wu R, Hu X, Weng X, Tan LH. Differential activity in left inferior frontal gyrus for pseudowords and real words: an event-related fMRI study on auditory lexical decision. Hum Brain Mapp. 2005;25:212–221. doi: 10.1002/hbm.20105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ihara A, Hirata M, Sakihara K, Izumi H, Takahashi Y, Kono K, Imaoka H, Osaki Y, Kato A, Yoshimine T, Yorifuji S. Gamma-band desynchronization in language areas reflects syntactic process of words. Neurosci Lett. 2003;339:135–138. doi: 10.1016/s0304-3940(03)00005-3. [DOI] [PubMed] [Google Scholar]
- [22].Chen R, Samii A, Caños M, Wassermann EM, Hallett M. Effects of phenytoin on cortical excitability in humans. Neurology. 1997;49:881–883. doi: 10.1212/wnl.49.3.881. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.