Abstract
A stereocontrolled synthesis of a complex pentacycle embodying the molecular architecture of the cortistatin class of natural products was achieved from the (+)-Hajos-Parrish ketone. The cornerstone of our approach is a hypervalent iodine induced tandem intramolecular oxidative dearomatization and nitrile oxide cycloaddition. The manner in which these ring formations were orchestrated has yielded a rather concise strategy for synthesis.
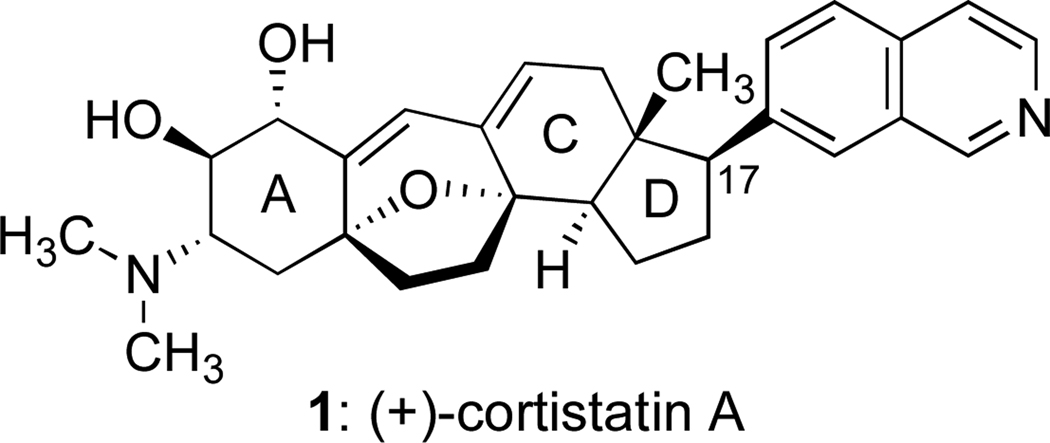
Since the discovery of the antiangiogenic properties of fumagillin and TNP-470 by Folkman and coworkers,1 there has been an active interest in the identification of new natural products and drug-like small molecules that are capable of inhibiting the proliferation of endothelial cells in vitro and tumor-induced angiogenesis in vivo. Inhibitors of this process are of considerable interest as potential therapeutic agents because unregulated angiogenesis can contribute to the growth and metastasis of malignant tumors and inflammatory diseases.2 In three publications, Kobayashi and coworkers described the intricate structures of eleven rearranged steroidal alkaloids of which several members are potent and selective inhibitors of endothelial cell growth.3 These compounds were isolated from the Indonesian marine sponge Corticium simplex and aptly named the cortistatins. Cortistatin A (1) (Figure 1), the parent and most active member of this family, inhibits human umbilical vein endothelial (HUVEC) cells with an IC50 of 1.8 nM.3 A remarkable aspect of cortistatin A is that it suppresses HUVEC cell growth without killing the cells;4 cortistatin A is thus cytostatic, not cytotoxic. As of this writing, the biomolecular basis for the promising biological activity of this natural product is unknown.
Figure 1.
The molecular structure of (+)-cortistatin A (1).
All of the cortistatins share a unique pentacyclic core structure and differ mostly in the nature of the substituent attached to C-17 (steroid numbering) and/or the degree of functionalization found in ring A. The rigid core framework of the cortistatins, with its seven-membered B ring and challenging tetrahydrofuran heterocycle, has inspired a growing number of creative undertakings in the area of chemical synthesis. The laboratories of Baran,5 Nicolaou and Chen,6 and Shair7 have achieved impressive syntheses of the full structure of cortistatin A (1), while the groups of Sarpong,8 Hirama,9 Danishefsky,10 Gung,11 Yang,12 Kobayashi,13 and Magnus14 have described conceptually interesting approaches to key elements of the cortistatin structure. Our laboratory was also drawn to the problem of synthesizing the polycyclic ring system that distinguishes the cortistatin class. In the course of dealing with this problem, we discovered that the reagent [bis(acetoxy)iodo]benzene is capable of triggering a productive double annulation that serves the synthesis by establishing three required bonds and two ring systems. Our construction of the pentacyclic core framework of the cortistatins featuring this hypervalent iodine-mediated transformation is described in this report.
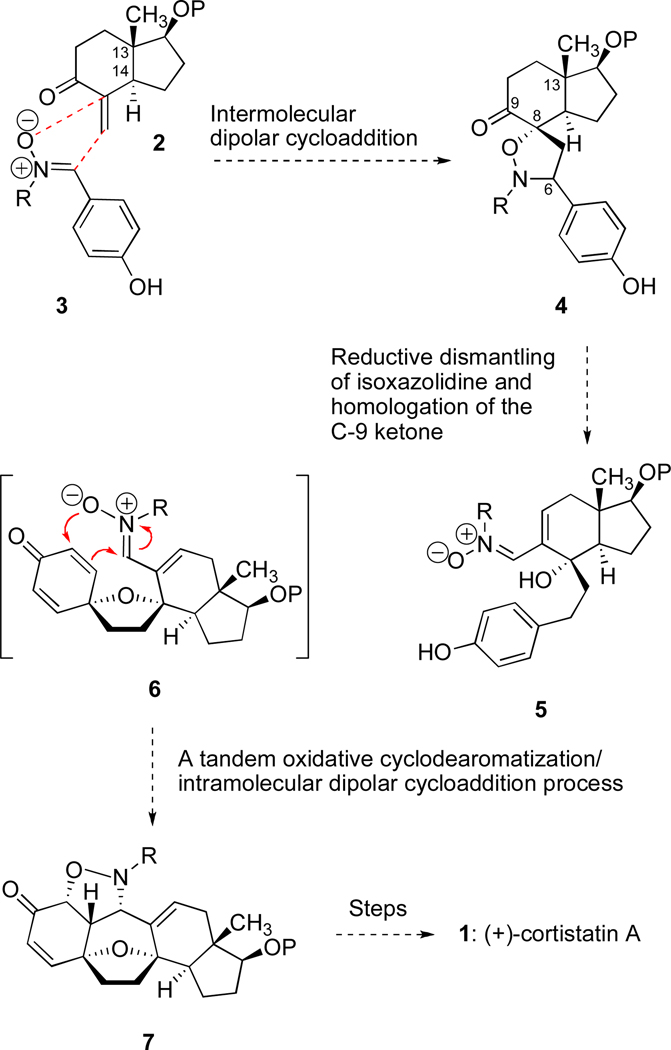
In the design phase, we envisioned that the pentacyclic core of cortistatin A might arise from three independent cyclization events, two of which would be nitrone/alkene [3+2] dipolar cycloadditions. The first dipolar cycloaddition would accomplish a merger of fragments (2 + 3 → 4, Scheme 1) and simultaneously construct the crowded C8–O bond as well as the C6–C7 bond. After dismantling the newly formed isoxazolidine ring system in 4 by an unprecedented type of double reduction,15 we would then elaborate complex nitrone 5 as a prelude to the key transformation of the synthesis. In principle, a structure of the type 5 could be directly transformed to a cortistatin-like pentacycle (cf. 7) by an oxidative cyclodearomatization with interception of the resulting dienone by an intramolecular nitrone/alkene cycloaddition (see 5 → 6 → 7); an analysis of a molecular model of 6 suggested that such a cycloaddition should be diastereoface-selective and facile owing to an enforced proximity of the nitrone moiety and one of the alkenes of the cyclic dienone. If successful, this one laboratory operation would fashion two carbon–oxygen bonds as well as the central B-ring of the cortistatin system. From an intermediate of type 7, a synthesis of (+)-cortistatin A (1) would require a reductive cleavage of the isoxazolidine N–O bond, elimination of the amino function, functionalization of the newly desymmetrized A ring, and the introduction of the C-17 isoquinoline system.
Scheme 1.
A plan for synthesizing (+)-cortistatin A (1) featuring two nitrone/alkene cycloadditions and an oxidative cyclodearomatization. R = alkyl group; P = protecting group.
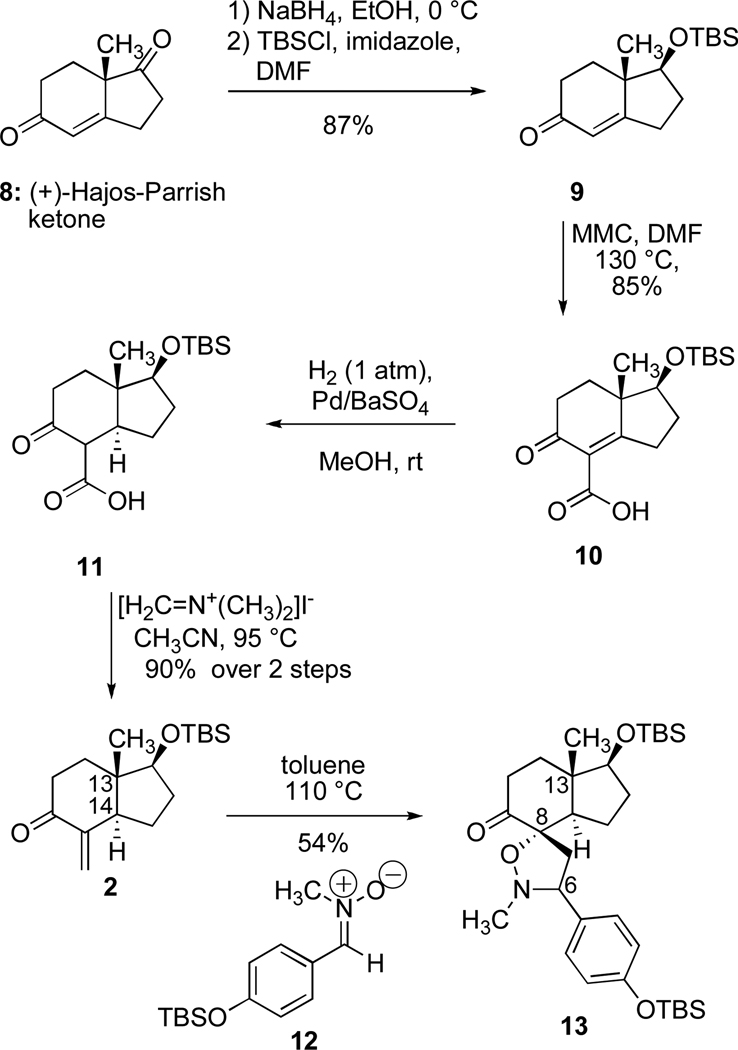
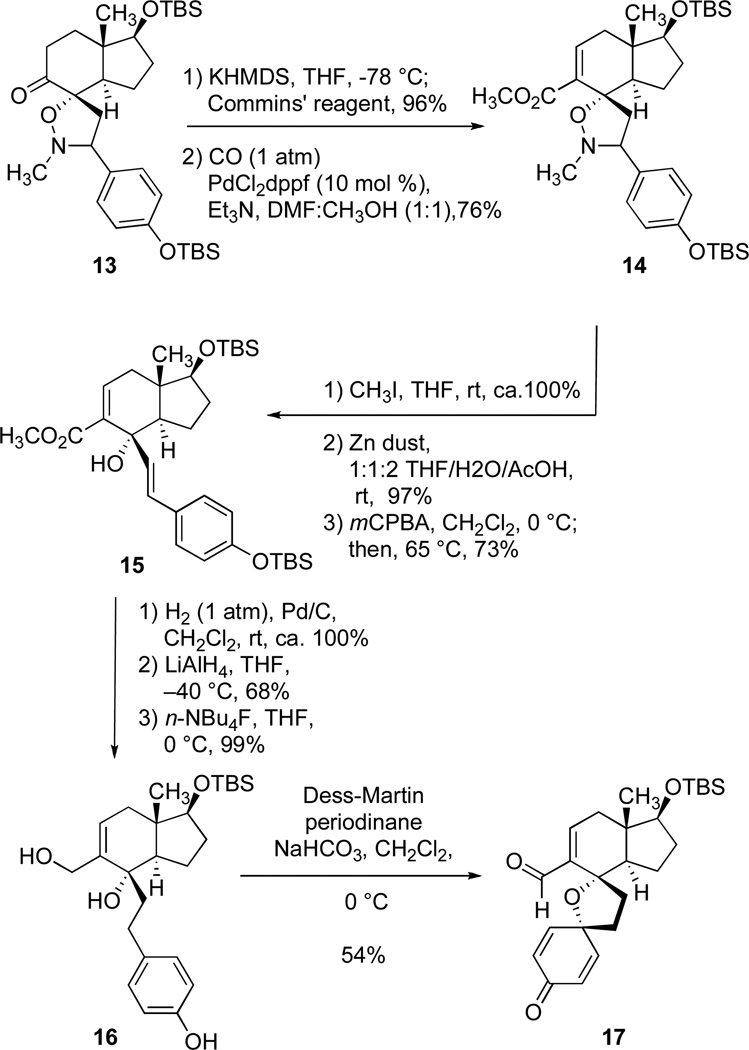
At the outset, we favored the idea of constructing the cortistatin core structure on the foundation provided by the (+)-Hajos-Parrish ketone (8) (Scheme 2).16 This popular building block for asymmetric synthesis17 comprises two rings, both of which are expressed in the structures of the cortistatins, and furnishes the methyl-bearing, quaternary stereogenic center corresponding to position 13 in the goal system. This one stereocenter was expected to direct the stereochemical development of the entire effort. Thus, from (+)-Hajos-Parrish ketone (8), key intermediate 2 (P = Sit-BuMe2) was fashioned by an improved method18 through the following sequence of reactions (Scheme 2): (1) a site-selective reduction of the unconjugated ketone in 8 with sodium borohydride, (2) protection of the resulting secondary alcohol in the form of a tert-butyldimethylsilyl ether, (3) carboxylation of the alpha position of the enone system in 9 with methyl magnesium carbonate (Stiles’s reagent), (4) hydrogenation of the alkene in compound 10 to establish the C-14 stereogenic center, and (5) introduction of the exocyclic alkene found in 2 by reaction of compound 11 with Eschenmoser’s salt19 with concomitant loss of carbon dioxide. Literature precedent20 provided a basis for our hope that the newly formed enone 2 would display the desired sense of regioselectivity in a union with a nitrone of type 3 (Scheme 1). A thermally-induced, [3+2] dipolar cycloaddition of nitrone 1221 with enone 2 was, in fact, completely regioselective and afforded isoxazolidine 13 (Scheme 3); this union also exhibited complete diastereoface selectivity owing to the disposition of the angular methyl group at C-13 (cortistatin numbering) as well as the bias provided by the configuration at C-14 in dipolarophile 2. 22 This union effectively established the challenging and crowded C8–O bond and the C6–C7 bond. With the C-8 oxygen atom suitably protected in the form of an isoxazolidine ring, we elected to homologate the C-9 ketone (Scheme 3). This was achieved by a low temperature enolization of ketone 13 with in situ triflation of the enolate oxygen atom with the Comins reagent,23 followed by a palladium-catalyzed, carbonylative ester formation; this two stage sequence was effective and gave rise to methyl ester 14. While efforts to reduce both the N–O bond and the unneeded benzylic C–N bond in the context of 13 with reducing metals or by hydrogenolysis were complicated by side product formation and/or decomposition, it was possible to achieve these objectives by the following sequence: (1) a quantitative methylation of the isoxazolidine nitrogen atom in 14 to give the corresponding quaternary ammonium salt, (2) a high-yielding cleavage of the N–O bond with zinc dust, and (3) an oxidation of the dimethylamino group with meta-chloroperbenzoic acid to set the stage for a mild Cope elimination24 of the resulting tertiary amine N-oxide. This three-step sequence of reactions produced compound 15 in an excellent overall yield, and, from that vantage point, we could arrive at triol 16 by a hydrogenation of the styryl double bond in 15, a complete reduction of the methyl ester function with lithium aluminum hydride, and a selective, fluoride-induced desilylation of the phenolic silyl ether. Interestingly, when compound 16 was exposed to two equivalents of Dess-Martin periodinane, the primary, allylic alcohol was oxidized to the corresponding aldehyde and the tertiary alcohol and phenol functions participated in an oxidative cyclodearomatization reaction; this double oxidation process yielded tetracyclic aldehyde 17, and, while we are unaware of the ordering of the two oxidation events, this structural change was most welcome. We suspect that our supply of Dess-Martin periodinane may have contained 2-iodoxybenzoic acid (IBX), which is the direct precursor to the Dess-Martin periodinane and an agent known to effect oxidative dearomatizations.25
Scheme 2.
Synthesis of isoxazolidine 13.
DMF = N,N’-dimethyl formamide; MMC = Methyl magnesium carbonate; TBS = tert-butyldimethylsilyl.
Scheme 3.
Synthesis of tetracyclic dienone 17.
KHMDS = potassium hexamethylsilylazide; THF = tetrahydrofuran; DMF = N,N’-dimethyl formamide; rt = room temperature; mCPBA = meta-chloroperoxybenzoic acid; TBS = tert-butyldimethylsilyl; dppf = 1,1’bis(diphenylphosphino)ferrocene
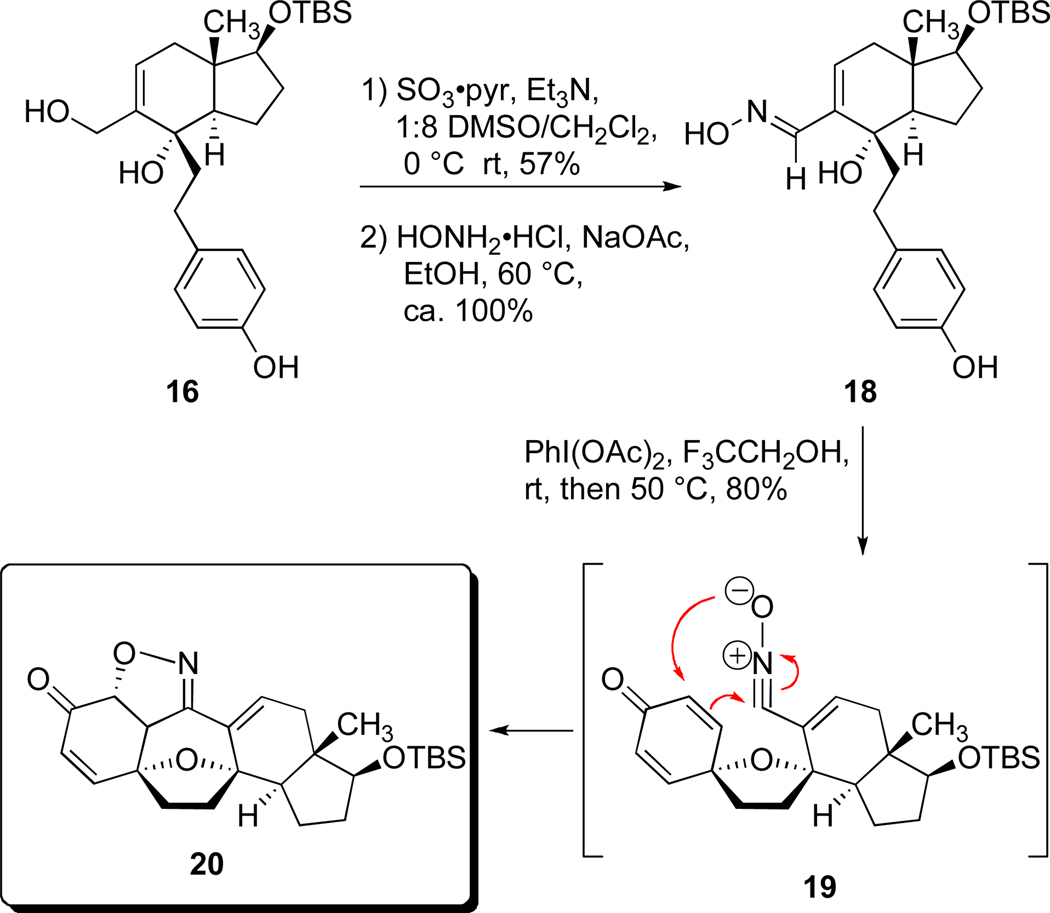
With four of the five rings in place, our inclination was to transform the aldehyde function of 17 to the corresponding nitrone for a final [3+2] dipolar cycloaddition. As matters transpired, attempts to achieve condensation reactions between 17 and N-alkyl hydroxyl amines were complicated by competing 1,4-conjugate additions to both the enal moiety and the cross-conjugated dienone. While these difficulties could not be circumvented, they did encourage a return to opportunities provided by the chemistry of triol 16. Specifically, we sought a means to incorportate a dipole or its equivilent into the precursor to the dearomatization. Conversion of the triol 16 to the tetracycle 17 inspired us to seek conditions to oxidatively access a reactive dipole concomitantly with cyclodearomatization. Eventually, this concept led to the discovery of the novel construction shown in Scheme 4.
Scheme 4.
Synthesis of cortistatin pentacycle 20 featuring tandem oxidative cyclizations.
Pyr = pyridine; DMSO = dimethylsulfoxide; Ph = C6H5; TBS = tert-butyldimethylsilyl.
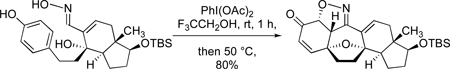
The Parikh-Doering method26 permitted an oxidation of the primary allylic alcohol moiety of 16 to the corresponding enal, after which a quantitative condensation between the latter compound and hydroxyl amine hydrochloride under the conditions shown gave rise to oxime diol 18. At this juncture in our effort, we encountered the recent, fascinating report by the Ciufolini laboratory demonstrating that bis(acetoxy)iodobenzene is effective at converting aldoximes into nitrile oxides and that such an oxidation may be conducted in tandem with an oxidative dearomatization of a phenol.27 Encouraged by this precedent we set out to fashion the cortistatin tetrahydrofuran ring by an intramolecular oxidative dearomatization in tandem with an intramolecular dipolar cycloaddition. Two oxidations and two ring formations would establish the core skeleton of the cortistatins. The outcome of the pivotal oxidation event was most gratifying because we discovered that exposure of compound 18 to bis(acetoxy)iodobenzene in trifluoroethanol directly produced compound 20, a substance possessing the full pentacyclic core architecture of cortistatin A. Compound 20 was the only diastereoisomer isolated. This productive and rather efficient transformation comprises three individual steps: (1) an ether ring formation via oxidative dearomatization, (2) an oxidation of the oxime function to a transient nitrile oxide (see 19), and (3) a final intramolecular [3+2] dipolar cycloaddition of 19 to yield compound 20. A significant level of target-relevant complexity is produced in this single laboratory operation, which complements the report of Ciufolini and coworkers by showing that double cyclizations are also possible by the action of bis(acetoxy)iodobenzene on structurally complex oxime phenols.
In summary, a stereocontrolled synthesis of a complex pentacycle embodying the molecular architecture of the cortistatin class of natural products was achieved from the (+)-Hajos-Parrish ketone. The approach described herein utilizes a phenol as a latent cortistatin A-ring, two dipolar cycloaddition events, and an oxidative cyclodearomatization. The manner in which these ring formations were orchestrated has yielded a rather concise strategy for synthesis. Our current aim is to leverage this chemistry in syntheses of the full structure of cortistatin A as well as new and potentially biologically active compounds sharing its unique structural features.
Supplementary Material
Acknowledgment
This work was supported by the National Institute of General Medical Sciences (GM065483), a Focused Giving Award from Johnson and Johnson, and Princeton University. Additionally, we would like to thank Merck Research Labs for fellowship support of J.L.F and the Princeton University Council on Science and Technology for fellowship support for C.S.J.
Footnotes
Supporting Information Available: Experimental procedures and full characterization data for all new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Ingber D, Fujita T, Kishimoto S, Sudo K, Kanamaru T, Brem H, Folkman J. Nature. 1990;348:555. doi: 10.1038/348555a0. [DOI] [PubMed] [Google Scholar]
- 2.(a) Folkman J. New Engl. J. Med. 1971;285:1182. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]; (b) Folkman J. New Engl. J. Med. 1995;333:1757. doi: 10.1056/NEJM199512283332608. [DOI] [PubMed] [Google Scholar]; (c) Folkman J. Nat. Med. 1995;1:27. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]; (d) Giannis A, Rübsam F. Angew. Chem. Int. Ed. Engl. 1997;36:588. Angew. Chem. 1997, 109. [Google Scholar]; (e) Taunton J. Chem. Biol. 1997;4:493. doi: 10.1016/s1074-5521(97)90320-3. [DOI] [PubMed] [Google Scholar]
- 3.(a) Aoki S, Watanabe Y, Sanagawa M, Setiawan A, Kotoku N, Kobayashi M. J. Am. Chem. Soc. 2006;128:3148. doi: 10.1021/ja057404h. [DOI] [PubMed] [Google Scholar]; (b) Aoki S, Watanabe Y, Tanabe D, Setiawan A, Arai M, Kobayashi M. Tetrahedron Lett. 2007;48:4485. [Google Scholar]; (c) Watanabe Y, Aoki S, Tanabe D, Setiawan A, Kobayashi M. Tetrahedron. 2007;63:4074. [Google Scholar]
- 4.Aoki S, Watanabe Y, Tanabe D, Arai M, Suna H, Miyamoto K, Tsujibo H, Tsujikawa K, Yamamoto H, Kobayashi M. Bioorg. Med. Chem. 2007;15:6758. doi: 10.1016/j.bmc.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 5.Shenvi RA, Guerrero CA, Shi J, Li CC, Baran PS. J. Am. Chem. Soc. 2008;130:7241. doi: 10.1021/ja8023466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Nicolaou KC, Sun Y-P, Peng X-S, Polet D, Chen DY–K. Angew. Chem. Int. Ed. 2008;47:7310. doi: 10.1002/anie.200803550. [DOI] [PubMed] [Google Scholar]; (b) Nicolaou KC, Peng X-S, Sung Y-P, Polet D, Zou B, Shik CL, Chen DDY–K. J. Am. Chem. Soc. 2009;131:10587. doi: 10.1021/ja902939t. [DOI] [PubMed] [Google Scholar]
- 7.Lee HM, Nieto-Oberhuber C, Shair MD. J. Am. Chem. Soc. 2008;130:16864. doi: 10.1021/ja8071918. [DOI] [PubMed] [Google Scholar]
- 8.Simmons EM, Hardin AR, Guo X, Sarpong R. Angew. Chem. Int. Ed. 2008;47:6650. doi: 10.1002/anie.200802203. [DOI] [PubMed] [Google Scholar]
- 9.Yamashita S, Iso K, Hirama M. Org. Lett. 2008;10:3413. doi: 10.1021/ol8012099. [DOI] [PubMed] [Google Scholar]
- 10.(a) Dai M, Danishefsky S. J. Tetrahedron Lett. 2008;49:6610. doi: 10.1016/j.tetlet.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dai M, Wang Z, Danishefsky SJ. Tetrahedron Lett. 2008;49:6613. doi: 10.1016/j.tetlet.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Dai M, Danishefsky A. Heterocycles. 2009;77:157. [Google Scholar]
- 11.Craft DT, Gung BW. Tetrahedron Lett. 2008;49:5931. doi: 10.1016/j.tetlet.2008.07.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu L, Gao Y, Che C, Wu N, Wang DZ, Li CC, Yang Z. Chem. Commun. 2009:662. doi: 10.1039/b817376a. [DOI] [PubMed] [Google Scholar]
- 13.Kotoku N, Sumii Y, Hayashi T, Kobayashi M. Tetrahedron Lett. 2008;49:7078. [Google Scholar]
- 14.Magnus P, Littich R. Org. Lett. 2009;11:3938. doi: 10.1021/ol901537n. [DOI] [PubMed] [Google Scholar]
- 15.N-O reduction attended by benzylic C-N hydrogenolysis is an unprecedented transformation.
- 16. Hajos ZG, Parrish DR. J. Org. Chem. 1974;39:1615. (b) For preparation of the Hajos-Parrish ketone, see: Org. Synth. 1990;7:363.
- 17.For discussions of the utility of the Hajos-Parrish ketone for the enantioselective synthesis of steroids see: Chapelon A-S, Moralèda D, Rodriguez R, Ollivier C, Santelli M. Tetrahedron. 2007;63:11511.
- 18.During the course of our studies we developed a scalable and reliable synthesis of 2. Experimental details of the synthesis of this intermediate are provided in the supporting information. For the first synthesis of 2, see: Isaacs RCA, Di Grandi MJ, Danishefsky SJ. J. Org. Chem. 1993;58:3938.
- 19.Schreiber J, Maag H, Hashimoto N, Eschenmoser A. Angew. Chem. Int. 1971;10:330. [Google Scholar]
- 20.(a) Padwa A, Burgess EM, Gingrich HL, Roush DM. J. Org. Chem. 1982;47:786. [Google Scholar]; (b) Padwa A, Fisera L, Koehler KF, Rodriguez A, Wong GSK. J. Org Chem. 1984;49:276. [Google Scholar]
- 21.Nitrone 12 was easily prepared by a condensation of N-methylhydroxylamine hydrochloride with the commerially available p-tert-butyldimethylsiyloxybenzaldehyde. See supporting information for detailed procedures.
- 22.Isoxazolidine 13 was isolated as an inconsequential mixture of C-6 diastereoisomers. Both diastereoisomers exhibited nOe correlations with the angular methyl.
- 23.Comins DL, Dehghani A. Tetrahedron Letters. 1992;33:6299. [Google Scholar]
- 24.(a) Cope AC, Foster TT, Towle PH. J. Am.Chem. Soc. 1949;71:3929. [Google Scholar]; (b) Cope AC, Trumbull ER. Org. React. 1960;11:317. [Google Scholar]
- 25.(a) Quideau S, Lyvinec G, Marguerit M, Bathany K, Ozanne-Beaudenon A, Buffeteau T, Cavagnat D, Chenede A. Angew. Chem. 2009;121:4675. doi: 10.1002/anie.200901039. [DOI] [PubMed] [Google Scholar]; (b) Ozanne A, Pouységu L, Depernet D, Francois B, Quideau S. Org. Lett. 2003;5:2903–2906. doi: 10.1021/ol0349965. [DOI] [PubMed] [Google Scholar]; (c) Magdziak D, Rodriguez AA, Van De Water RW, Pettus TRR. Org. Lett. 2002;4:205–288. doi: 10.1021/ol017068j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Parikh JP, Doering WE. J. Am. Chem. Soc. 1967;89:5505. [Google Scholar]
- 27.Mendelsohn BA, Lee S, Kim S, Teyssier F, Aulakh VS, Ciufolini MA. Org. Lett. 2009;11:1539. doi: 10.1021/ol900194v. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.