Abstract
Gene therapy holds promise for the treatment of many inherited and acquired diseases of the eye. Successful ocular to targeted cells with minimal toxicity. A major gene therapy interventions depend on challenge is to overcome both intracellular and extracellular barriers associated with ocular gene delivery. Numerous viral and nonviral vectors were explored to improve transfection efficiency. Among nonviral delivery systems, polymeric vectors have gained significant attention in recent years owing to their nontoxic and non-immunogenic nature. Polyplexes or nanoparticles can be prepared by interaction of cationic polymers with DNA, which facilitate cellular uptake, endolysosomal escape and nuclear entry through active mechanisms. Chemical modification of these polymers allows for the generation of flexible delivery vectors with desirable properties. In this article several synthetic and natural polymeric systems utilized for ocular gene delivery are discussed.
The eye is an immune-privileged organ and amenable to gene therapy. It is readily accessible for therapeutic administration, along with measurement of therapeutic response. Ocular gene therapy has potential for the treatment of many acquired and inherited diseases of the eye. Successful gene therapy interventions depend on efficient gene transfer to targeted cells to provide stable and prolonged gene expression with minimal toxicity. A major challenge is to address both intracellular and extracellular barriers associated with ocular gene delivery [1]. Over the last decade numerous viral and nonviral delivery systems were introduced to improve gene transfer to various ocular tissues. Viral vectors such as adenovirus, recombinant adeno-associated virus, lentivirus and retrovirus have been evaluated in ocular cell lines and animal models [2,3]. Viral vectors improve cellular uptake, intracellular trafficking and provide higher gene expression over a longer period. Recombinant adeno-associated virus-based delivery of RPE65 cDNA to dogs by subretinal injection restored retinal function and provided stable long-term gene expression for more than 3 years. This result was further translated into clinical trials in humans [4–6]. However, viral vectors generally suffer from safety issues such as muta-genesis and toxicity [7]. It often requires repeated administration leading to immunogenicity and acute inflammatory responses that depend on the type of viral vectors utilized for the treatment. In addition, the process entails high production costs [7–10]. These limitations have prompted a need to develop a nonviral delivery system that has high biosafety and low cytotoxicity. Nonviral delivery approaches involve administration of naked DNA and other physicochemical methods to deliver nucleic acids to ocular tissues. Physical methods such as gene gun, electroporation, iontophoresis, microinjections and ultrasound-mediated gene delivery can be promising strategies for local gene transfer. However, the invasive nature of such approaches reduces patient compliance for effective therapy [11]. For example, the gene gun method requires bombardment of plasmid DNA (pDNA)-coated gold or tungsten particles with high-voltage electric discharge for successful gene transfer, which can transiently destabilize or damage local ocular tissues [12]. Approaches based on utilization of nonviral vectors are comparatively less invasive and do not exhibit antigen-specific immune and inflammatory responses after ocular administration [12]. Chemical methods mainly involve the use of cationic lipids or polymers to prepare delivery vectors. These cationic carriers condense DNA via electrostatic interactions to form lipoplexes or polyplexes. While the transfection efficiency of cationic nonviral vectors is still low compared with viral vectors, various modifications are possible to improve efficacy of these carriers.
In this article we have described various ocular barriers that hinder the gene transfer process. Moreover, we have summarized the applications of polymeric vectors in ocular gene delivery.
Barriers for ocular gene therapy
Nucleic acids are high molecular weight (MW), negatively charged hydrophilic molecules. For topical gene delivery, precorneal tear clearance due to blinking and lacrimal drainage is a major barrier [13]. The presence of extracellular endo-nucleases in the tear film can cleave polynucleotides on the ocular surface [14]. In addition, systemic absorption of topically applied drugs via conjunctival and nasal blood capillaries decreases the ocular bioavailability of nucleic acids [15]. Various barriers present at the anterior and posterior segments of the eye that restrict the entry of naked DNA or viral and nonviral vectors are summarized in the following sections (Figures 1 & 2).
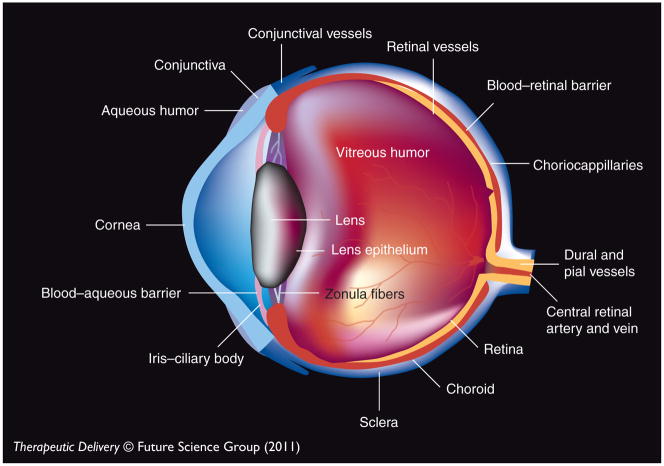
Figure 1.
Eye structure.
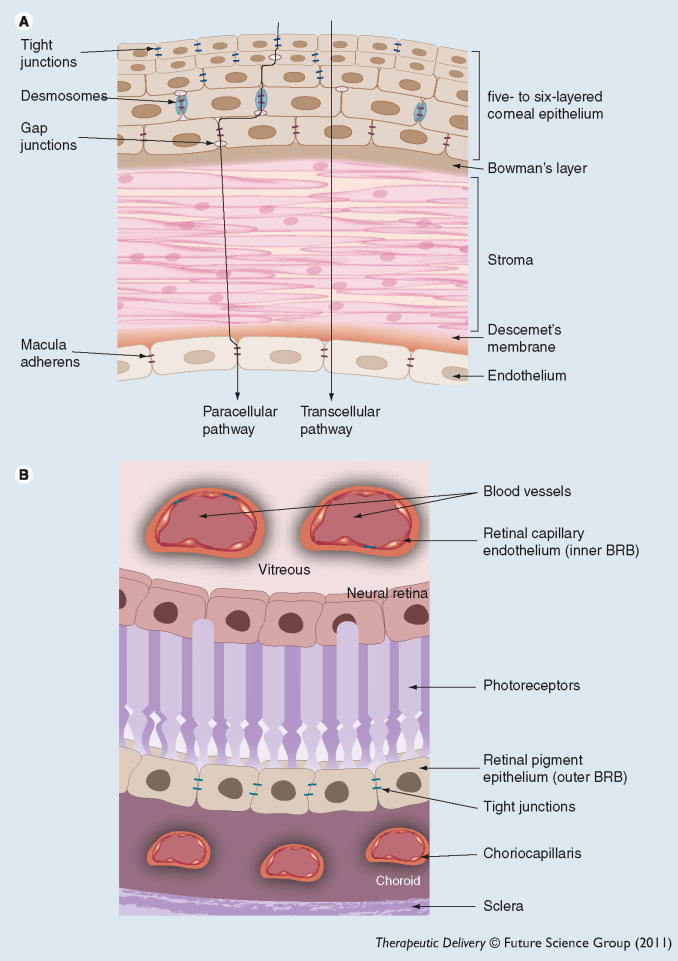
Figure 2. Ocular barriers.
(A) Corneal barrier; (B) BRB.
BRB: Blood–retinal barrier.
Barriers to corneal gene therapy
Since 1994, numerous studies have been conducted in several animal models to evaluate corneal gene therapy for corneal graft rejection [16], anterior and stromal dystrophies [17], corneal neovascularization [18], herpes simplex keratitis [19] and corneal scarring [20].
The cornea is an avascular and transparent tissue that reflects light and protects the eye from external pathogens. The adult human cornea is approximately 0.5–0.7 mm thick. The cornea is a good target tissue to evaluate gene therapy potential owing to its easy accessibility, immune-privileged nature and relatively simple histological structure [21]. Outcome of corneal gene therapy can be easily monitored in live animals with fluorescent gene markers. In addition, ex vivo nucleic acid delivery to the cornea offers several advantages as it can be preserved for several weeks in an ex vivo culture [3]. However, several anatomical and physiological barriers hinder the success of corneal gene therapy. The cornea primarily consists of five layers: a stratified epithelium, Bowman’s membrane, thick collagenous stroma, Descemet’s membrane and a monolayer of endothelium (Figure 2A). The outermost corneal epithelium is composed of six to seven layers of stratified epithelial cells with tight junctions between cells [22]. These tight junctions act as a barrier for the transport of polar macromolecules through a paracellular route [23]. Kamata et al. failed to transfect the cornea by administering an eye drop solution of an adenoviral vector expressing Escherichia coli β-galactosidase (AxCALacZ) in the mouse model. The tight junction of epithelial and Bowman’s membrane restricted the adenoviral invasion [24]. Permeation of drug molecules via a paracellular pathway mainly depends on intercellular pore size. Small molecules can diffuse readily relative to macromolecules. Physiological pH pores of corneal epithelium are negatively charged, which influences permeation of both positively and negatively charged molecules [25]. Moreover, epithelial tight junctions restrict the delivery of genes into the stroma. Various penetration-enhancing agents such as ethylenediaminetetraacetic acid (EDTA), capable of opening the tight junctions, have been investigated for corneal epithelial gene delivery [26]. An attempt to deliver genes to the stroma, using polymeric micelles containing eye drops required pretreatment of the cornea with EDTA in mice and rabbit models [27]. In addition, various nano-carriers such as nanoparticles and micelles have been evaluated for corneal gene delivery [27,28].
The stroma is composed of extracellular matrix and keratocyes. It consists of 90% water and mainly provides thickness to the cornea. The stroma does not hinder the transport of hydrophilic small and large molecules. Gene delivery to the stroma has been achieved by intrastromal injection. Sometimes electroporation and ultrasound alone or in combination with injection is required for successful stromal gene delivery [29].
The corneal endothelium is the innermost monolayer and is approximately 5 μm thick. It is a leaky barrier positioned between the stroma and aqueous humor. Owing to its inaccessibility from the ocular surface, gene delivery to the endothelium requires direct injection into the anterior chamber. Numerous viral and nonviral vectors were investigated for corneal endothelial gene delivery, but a successful noninvasive approach has not yet been reported [30,31]. An adenovirus-mediated gene-transfer study by Kamata et al. suggested that endothelium or Descemet’s membrane can restrict gene expression in the stroma and cornea [24]. The investigators reported that gene expression was restricted to endothelial cells after the injection of an adenoviral vector into the anterior chamber of a mouse eye.
Conjunctiva is a thin mucous membrane that covers the inner surface of the eyelids (palpebral conjunctiva), anterior sclera (bulbar conjunctiva) and is folded at the fornix (fornix conjunctiva). The conjunctival epithelium is two to three layers thick. It possesses a 20-times larger surface area with higher pore density and more paracellular space in comparison to the cornea. Due to poor corneal permeation, large hydrophilic molecules such as proteins, peptides and nucleic acids prefer a noncorneal route (via conjunctiva) to enter intraocular tissues. However, apical surface of conjunctival epithelial cells restricts the entry of macromolecules. In addition, bulbar conjunctiva prevents the entry of topically applied drugs to the inner region of the eye via a noncorneal route. A highly vascularized layer under the conjunctival epithelium, termed substantia propria, is mainly responsible for systemic drug clearance [22,25].
Barriers to retinal gene therapy
Over the last decade, progress has been made on gene therapy of chronic posterior segment diseases such as age-related macular degeneration, retinitis pigmentosa, cytomegalovirus retinitis and proliferative vitreoretinopathy. Retinal diseases mainly affect the retinal pigment epithelium (RPE), neural retina, photoreceptor cells and microvasculature of the retina or choroid. Desirable levels of nucleic acids cannot be achieved in the retinal tissues by topical application owing to permeation barriers described in the previous section. After systemic administration a small fraction of the administered dose can reach the posterior segment because of blood–ocular barriers. In addition, systemic administration requires very high doses of polynucleotides, which may lead to several adverse effects in nontargeted tissues [32]. Intravitreal and subretinal injections are necessary for retinal gene delivery as they provide direct access of DNA or viral/nonviral vectors to the retinal tissues. However, due to short intravitreal half-lives, repeated administration of DNA or siRNA is required to maintain stable gene expression [33,34]. Repeated injections are associated with high risks of endo-phthalmitis, retinal detachment and lens damage [25]. Subretinal injection of viral vectors packaged with DNA has shown encouraging results in animal models [4,35], but this administration route is not always preferred owing to its invasive nature. Therefore, nonviral vector-mediated gene delivery through intravitreal injection may be effective in producing stable gene expression in retinal tissues. However, comprehensive understanding of various posterior segment barriers is required for the development of nonviral vectors. The nature of these barriers is discussed in the following sections.
Vitreous as a barrier
Vitreous is a transparent gel-like material composed of two major structural components: collagen (300 μg/ml) and hyaluronan (65–400 μg/ml). 3D networks of collagen fibrils are crosslinked with proteoglycan filaments containing negatively charged glycosaminoglycans (GAG). The collagen interfibrillar space is filled with a dense network of negatively charged hyaluronan, chondroitin and heparan sulfate proteoglycans [36]. This complex network may immobilize nonviral vectors. In addition, negatively charged GAG and other proteoglycans can bind to positively charged nanocarriers. Ex vivo studies performed by Pitkanen et al. and Peeters et al. confirmed with cationic vectors that vitreous can act as a barrier for gene delivery [37,38]. Pitkanen et al. reported the blockage of gene expression in the RPE cells due to immobilization of cationic nanocarriers (polyplexes and cationic liposome) in the proteoglycan matrix or interactions with the negatively charged GAG [37]. Peeters et al. observed the aggregation of cationic lipoplexes in the vitreous due to a reduction in the zeta potential of nanocarriers through interactions with GAG. These nanocarriers, upon destabilization, become immobilized in vitreous gel that can obstruct intracellular trafficking and/or cellular uptake [38]. Moreover, movement of naked DNA can be hindered by vitreous structure [34]. Size and charge are the two major factors that limit the movement of gene carriers in the vitreous. Apart from vitreous, extracellular GAG, which is present in different parts of the retina, can also alter the trafficking of lipo- and polyplexes [39].
Blood–retinal barrier
The retina is comprised of two main layers: RPE and neural retina. The blood–retinal barrier (BRB) is composed of retinal endothelial cells (inner-BRB) and RPE (outer-BRB). RPE is the outermost monolayer of the retina between the neural retina and choroid, which regulates the entry of solutes from choriocapillaris into the retina. Tight junctions encircle each RPE cell and prevent the permeation of molecules from systemic circulation to the retina as well as from vitreous into systemic circulation. Inner-BRB restricts the paracellular transport of hydrophilic molecules and large proteins by forming intercellular tight junctions. Neural retina is a soft multilayered tissue composed of different cell types. Among these layers, the internal- and external-limiting membranes and the interphotoreceptor matrix, rich in GAG, offer significant resistance. Studies performed by Pitkänen et al. suggested that the neural retina can also act as a barrier for transport of cationic vectors to the RPE following intravitreal injection [39]. The investigators measured ex vivo permeability of cationic polymeric carriers (polyethylenimine [PEI]–DNA and poly L-lysine [PLL]–DNA complexes), liposomal carriers (1,2-dioleyl-3-trimethylammonium-propane DNA complexes) and macromolecules (FITC-Dextran) of various molecular size across the neural retina into the RPE cells in the bovine eye model. The results revealed that the effect of positive charge is more pronounced than size of the molecule for regulating permeation through the neural retina [39].
Blood–aqueous barrier
The blood–aqueous barrier (BAB) is an anterior segment barrier formed by endothelial cells of the iris and nonpigmented cells of the ciliary epithelium. BAB regulates nonspecific entry of foreign substances from blood circulation to the aqueous humor. However, the BAB is less effective than the BRB, primarily due to the leaky nature of the ciliary epithelium.
Polymeric vectors in ocular gene delivery
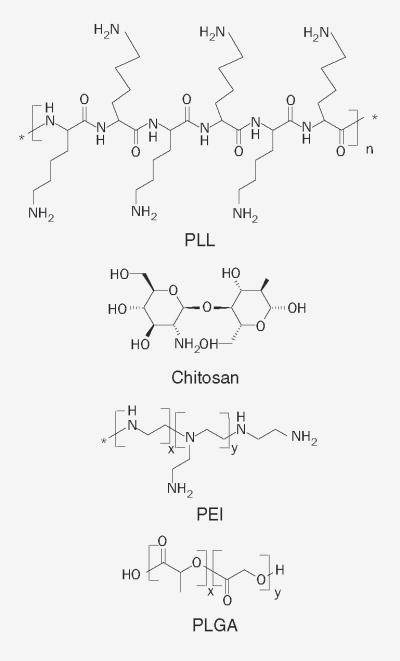
Synthetic and natural polymer-mediated gene delivery has gained attention in recent years. Large-scale production and chemical modification of polymeric vectors is feasible. Polymeric vectors are very effective where low therapeutic levels of nucleic acids are required over a short duration. The choice of delivery vectors is often based on the disease type, administration route and targeted tissue [40,41]. For effective gene delivery, polymer-based vectors must fulfill various criteria while overcoming different ocular barriers. Delivery vectors should provide optimum stability of the gene construct and deliver genetic cargo to the targeted cells. In this regard, cationic polymers can easily interact with DNA to form polyplexes or condense large-size DNA to form nanoparticles. These vectors avoid DNA degradation by limiting exposure to various enzymes. Small-sized, positively charged polymeric carriers facilitate cell binding, internalization, endolysosomal escape and nuclear entry through active mechanisms [12,42]. Cellular uptake of various polymer–DNA complexes mainly occurs by phagocytosis, pinocytosis and adsorptive- or receptor-mediated endocytosis. Surface modification of polymers with a targeting moiety can further enhance receptor-mediated endocytosis and nuclear entry [43,44]. Commonly investigated polymers for ocular gene delivery include PLL, chitosan, PEI, polylactide and polylactic-co-glycolic acid. The chemical structures of these polymers are shown in Figure 3. Development of in vivo delivery carriers that can overcome major ocular barriers and efficiently reach the targeted tissue is always challenging. Various in vivo polymeric gene delivery carriers evaluated in the last decade are summarized in Table 1.
Figure 3.
Polymers utilized in ocular gene delivery.
Table 1.
Various polymeric vectors utilized for in vivo ocular gene delivery.
| Polymeric carrier | Delivery site | Target tissue | Model | Year | Ref. |
|---|---|---|---|---|---|
| PEG–PLL | Intravitreal and subretinal injection | RPE cells and photoreceptor population | Mouse | 2006 | [55] |
| Hyaluronan–chitosan nanoparticle | Topical application | Corneal and conjunctival cells | Rabbit | 2008 | [28] |
| PLGA nanoparticles | Intravitreal injection | RPE cells | Rat | 2005 | [98] |
| PEG–PLA microparticles | Subretinal injection | Photoreceptor layer | Rat | 2010 | [102] |
| ODN–PEI complexes | Intravitreal injection | Superficial and inner retinal layers | Rat | 2006 | [84] |
| PEO–PPO–PEO micelles | Topical application | Anterior and posterior segment tissues | Mouse and rabbit | 2007 | [27] |
| Dehydrated plasmid–PEI complexes | corneal stromal pocket | Cornea | Rats | 2005 | [85] |
ODN–PEI: Oligonucleotide–polyethylenimine; PEG–PLA: Polyethylene glycol–polylactic acid; PEG–PLL: Polyethylene glycol–poly L-lysine; PEO–PPO–PEO: Poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide); PLGA: Poly(lactide-co-glycolide); RPE: Retinal pigment epithelium.
Poly L-lysine
Poly L-lysine is one of the most widely studied polymers in gene delivery. It is a biodegradable and biocompatible liner polymer with primary ε-amino groups that remain positively charged at physiological pH. PLL can interact and condense DNA to form polymer–DNA complexes called polyplexes [45]. PLL can condense with DNA to form small particles with a charge balance that facilitates cellular entry. Moreover, PLL serves as a nuclear import signal that facilitate the entry of pDNA into the nucleus and protects DNA degradation in the nucleus [43,46]. Studies by Garcia-Valenzuela et al. reported transfection of pDNA-carrying reporter genes to the retinal ganglionic cells [47]. This report compared gene transfection efficiency of the plasmid solution and PLL–DNA complex after administration at the intact axon terminal in the adult Wistar rat. PLL–DNA complexes were condensed to form 20 nm compact structures, which demonstrated higher transfection levels of extrinsic DNA for a longer duration compared with a plasmid solution [47].
Studies carried out by Mannisto et al. suggested that the gene transfer capacity of PLL–DNA complexes depends upon various physicochemical properties of polymers, such as MW and shape [48]. A linear PLL of high MW (20 kDa) can bind efficiently to DNA in comparison to low MW PLL (2.9 kDa) and branched PLL G3 (third generation; 1 kDa). Similarly, high-MW PLL–DNA complexes demonstrated higher in vitro transfection efficiency into the RPE cells. The orientation of amines in branched PLL is less favorable for DNA binding whereas linear PLL (20 kDa), with higher lysine residues in the structure, provide flexibility for DNA condensation. In addition, investigators observed that both liner PLL (20 kDa) and branched PLL (93.4 kDa) with a lower polymer:DNA charge ratio (+/−) of 2:1 condensed to form spherical complexes of sizes ranging from 20 nm to 2 μm in diameter. Complexes of linear and branched PLL with a charge ratio of 2:1–4:1 showed positive zeta-potentials, which indicate complete binding of positively charged amine groups of PLL to the phosphate groups of DNA. However, due to poor condensation in the case of PLL G3, pDNA was rendered at the surface that had provided a negative charge and formed toroidal- and rod-shaped complexes [48]. It is clear from these observations that physicochemical properties of PLL determine DNA binding efficiency. PLL has lower transfection efficiency than PEI because of poor endosomal escape and inadequate dissociation of DNA from the PLA–DNA complexes [49–51]. PLL complexes show aggregation and rapid binding with red blood cells and plasma proteins [52]. Cotransfection of cationic PLL with endosomolytic agents can improve transfection efficacy. One approach for increasing transfection efficiency and circulation time of PLL vectors is to conjugate or coat them with polyethylene glycol (PEG). Several other approaches, such as introduction of histidine residues in the backbone of PLL and conjugation of the targeting moiety, are attempting to improve efficiency of these carriers [51,53].
Pegylation of PLL molecules enhanced stabilization of complexes and also raised in vitro transfection efficiency in RPE cells [48]. PEG could sterically stabilize the carriers, which results in smaller sized complexes with a narrow particle size distribution. Pegylation in general does not change the DNA binding capacity of PLL; however, total amount of PEG in the carrier can change DNA condensation [48]. Two probable mechanisms for enhanced transfection efficiency of pegylated PLL are: an increase in endosomal leakage that results in rapid release of DNA or PLL–DNA complexes from the endosomes; and an increase in diffusion of complexes within the cytoplasm. Moreover, pegylation may enhance cellular uptake by inducing fusion of nanocarriers with membrane phospholipids [54]. Farjo et al. prepared neutral DNA nanoparticles of 25 nm in size with a PEG-conjugated 30mer lysine peptide [55]. Trifluoroacetate and acetate were utilized to condense DNA through a counter-ion effect. To evaluate cell-specific transfection efficiency, nanoparticles were administered by intravitreal and subretinal injections to adult wild-type mice. Substantial expression of encapsulated EGFP was observed in the lens, retina, sclera, choroid and RPE after intravitreal injection. Fluorescence microscopic examination revealed the presence of EGFP in the retinal ganglionic cells, cornea and trabecular meshwork. Trifluoroacetate–GFP nanoparticles were transported to the inner plexiform layer, probably due to their ellipsoidal shape. Subretinal injection of nanoparticles produced higher EGFP expression in the photoreceptor cell population, retina, sclera, choroid, RPE and inner nuclear layer relative to intravitreal injection. Injection of naked pDNA via both routes generated minimal expression in ocular cells. PEG–lysine nanoparticles containing compacted DNA can safely transfer genes in various ocular tissues without causing cellular infiltration or inflammation [55]. Another study demonstrated the therapeutic application of PEG–lysine nano-particles in the treatment of hereditary ocular disease [56]. All these findings suggest therapeutic applications of PLL polymers and modified PLL nanoparticles in ocular gene therapy.
Chitosan
Chitosan is a natural polycationic polymer obtained by deacetylation of chitin. Several reports summarized the unique properties of chitosan for ocular drug delivery [57,58]. Each deacetylated subunit of chitosan backbone carries a primary amine group that is positively charged in an acidic medium. In a neutral or alkaline medium, chitosan binds to DNA via hydrophobic- and hydrogen-bonding interactions. Chitosan complexes can be designed to deliver therapeutic macromolecules such as proteins, DNA and siRNA [59]. High-charge density, biodegradability and relatively low cytotoxicity render chitosan a promising vector for gene delivery [60]. Chitosan could enhance the penetration of molecules by modulating the tight junctions of the corneal epithelial cells in a reversible manner. It was reported by various researchers that chitosan alters the paracellular and transcellular pathway without disrupting the cellular integrity [61,62]. However, poor solubility and inefficient endosomal escape due to low buffering capacity can reduce its transfection efficiency relative to dendrimers, PEI and other lipoidal carriers [63]. Chitosan can form a self-assembling complex with DNA in the size range of 150–500 nm. The size of the complex depends on the MW of polymer and the chitosan:DNA ratio [64].
Transfection efficiency of a chitosan-based vector depends on various parameters such as MW, degree of deacetylation (DD), pH of the transfection medium and stoichiometry of the chitosan–DNA complex [65–67]. Optimum combination of MW and DD in chitosan regulates the gene transfer capacity of chitosan–DNA nanoparticles [60,68]. In a particular study, Huang et al. prepared chitosan–DNA nanoparticles with different MW of chitosan (213, 98, 48, 17 and 10 kDa) and various DD (88, 61 and 46%) [60]. The investigators obtained maximum DNA loading in nanoparticles at a chitosan:DNA ratio of 6:1, irrespective of MW and DD. This study reported that chitosan with MW of 10 kD and 88% DD, and MW of 213 kD and 46% DD showed poor capacity to condense DNA and exhibited lower nanoparticles uptake by A549 cells. By contrast, highest condensation, cell uptake and gene expression were observed with chitosan of MW 213 kD with 88% DD. The report suggested that high-MW chitosan carrying a higher density of amino groups can form more compact nanoparticles, which in turn result in a slower release of DNA from the chitosan matrix [60].
Chitosan displays excellent physicochemical properties such as film-forming capacity and mucoadhesive binding that are complimentary in topical drug delivery. However, chitosan complexes have not been explored much for ocular gene delivery. One assumption is that chitosan forms complexes with nucleic acids by simple electrostatic interaction that can be overcome by other anionic ligands naturally present in the body, such as GAGs and heparin, which can result in premature release of therapeutic genes [69]. Furthermore, direct injection of chitosan in the ocular tissues can induce immune responses [70]. Chitosan nanoparticles can partly overcome these shortcomings and can be explored for delivery of genes on the ocular surface [71]. Chitosan nanoparticles can be advantageous for gene delivery as the particle preparation method avoids use of organic solvent and sonication that prevents decontamination of nucleic acids [72]. Gene transfer ability of these nanocarriers was significantly improved by chemical modification of chitosan or addition of new material in the composition of nanocarriers.
Fuente et al. proposed new nanocarriers comprised of chitosan and hyaluronic acid (HA) for ocular gene therapy [28]. HA is a normal constituent of the ocular tissues and can be utilized for the treatment of corneal diseases owing to its mucoadhesive nature [28,73]. In addition, HA can target the CD44 receptors expressed on the ocular surfaces [74]. de la Fuente et al. evaluated in vitro cellular uptake, cytotoxicity and transfection efficiency of model pDNA encapsulated HA–chitosan nanoparticles in normal human conjunctival (IOBA-NHC) and human corneal epithelial cell lines [75]. Cytotoxicity results revealed that cell viability was inversely proportional to the concentration of HA in nanoparticles. Transfection efficiencies of nanoparticles depended on the MW of chitosan used for nano-particle preparation. Presence of an additional mucin layer on the surface of IOBA-NHC cell line acted as a barrier for nanoparticle uptake that resulted in low transfection levels in comparison to the human corneal epithelial cell-line [75].
Moreover, this study evaluated interactions and transfection efficiency of nanoparticles on the cornea and conjunctiva after topical application to a rabbit eye [28]. The results confirmed nanoparticle localization into corneal and conjunctival cells by confocal microscopy. The investigators suggested that nanoparticles might have crossed the epithelia by a transcellular pathway due to interaction of HA and chitosan with CD44 receptors on the ocular surface. HA tend to degrade inside the cell by hyaluronidases and other enzymes whereas chitosan remains stable for longer periods of time. Possibly due to this reason, intracellular nanoparticle assimilation and gradual degradation was noticed. Moreover, model plasmid-loaded HA–chitosan nanoparticles achieved transfection levels in the cornea and the conjunctiva for up to 7 days [28].
PEI
Polyethylenimine is a cationic polymer with high charge density. It exists in branched and linear forms that are commonly synthesized utilizing an aziridine monomer. Synthesis of branched polymer is performed by acid catalyzed polymerization of aziridine whereas linear PEI is synthesized by either polymerization of aziridine at a lower temperature or by hydrolysis of poly-(2-propyl-2-oxazoline) under acidic conditions [76]. PEI of different MW contains primary, secondary and tertiary amino groups in a 1:2:1 ratio and possess a strong buffering effect due to pH-dependent protonation of amino groups. A change in pH from 7 to 5 raises the protonation of amino groups from 20 to 45% [77]. PEI has been widely explored by numerous investigators as a gene-delivery vector. The proton-sponge effect of PEI is responsible for efficient gene transfer, which evades lysosomal degradation by rupture of endosomal vesicle prior to fusion. This mechanism was confirmed with the observation that the concentration of chloride ions increase in the polyplexes containing an endocytotic vesicle, which leads to lysis of the endosome. Another investigation confirms this hypothesis by utilizing an N-quaternized derivative of PEI that demonstrated twofold lower transfection efficiency relative to PEI. The researchers attributed lower transfection efficiency to reduced binding affinity of highly charged N-quaternized PEI with DNA in comparison to PEI [78].
Transfection efficiency of branched PEI depends upon MW, charge density and degree of branching [79,80]. In addition, experimental conditions also regulate the gene-transfer process. High-MW branched PEI exhibited superior transfection efficiency in comparison to lower MW derivatives. However, branched PEI suffers from the limitation of reduced cell viability are therefore, low MW (5–48 kDa) branched PEI are preferred. Other factors, such as N:P ratio and particle size, also influence gene delivery. A higher N:P ratio in general improves cellular uptake due to the presence of more positively charged amino groups [81]. Linear PEI in the presence of salt forms large particles in the nanometer to micrometer range. It was reported that transfection efficiency of linear PEI was higher in comparison to branched PEI [82]. Urtti et al. evaluated the role of cell cycle on PEI- or PLL-mediated gene transfer in human RPE cell line (D407) [49]. PEI and PLL exhibited different transfection efficiency in synchronized cells. PEI is well known for its excellent buffering capacity whereas PLL has poor buffering capacity in the endosomal pH range. Despite this fact the investigators observed a less-pronounced difference in expression levels between PLL- and PEI-mediated gene transfer at G1 phase in comparison to other phases of the cell cycle. It was hypothesized that changes in cytoplasm or endocytotic vesicle may be responsible for differential transfection. Polyplexes-mediated gene transfer was believed to be independent of mitotic activity owing to its inherent nuclear uptake capability. However, these studies suggest cell-cycle dependent uptake and transfection of PEI–DNA or PLL–DNA complexes [49]. Successful retinal delivery of PEI–DNA complexes depends upon circumvention of the vitreous and neural retina [37,39]. Most of the intravitreally administered cationic polyplexes do not reach the RPE due to poor permeation through the vitreous and neural retina. Positively charged PEI has limited mobility in the vitreous. It was reported that PEI of 25 kDa complexed with DNA demonstrated significant decrease in cellular uptake by RPE in the presence of neural retina [39]. These results suggest that the cationic charge of PEI limits its delivery to RPE. However, the effect of PEI MW was not elucidated in these studies.
Reinisalo et al. studied the freeze–dried complex of PEI–DNA for transfection of well-differentiated retinoblastoma-derived WERI-Rb1 cell [83]. The researchers performed reverse transfection with PEI of a MW of 25,000 (PEI-25) and 75,000 (PEI-75). It was reported that the freeze–dried PEI–DNA complex retained biological activity even after several months of storage and promoted cell adhesion. PEI of different MWs displayed variable effects in the presence of PLL/laminin as a substrate. For example, PEI-75 demonstrated enhanced transfection efficiency in the presence of substrate, although gene-transfer capability of PEI-25 was reduced. This study reported higher transfection efficiency with high MW PEI. However, earlier reverse transfection studies were performed with surface-modified low-MW PEI. It was observed that air-dried complexes of DNA–PEI exhibited low reporter activity after 1 day, in contrast to freeze–dried complexes. Authors suggested that PEI could be utilized for the detection of weak promoters and for reverse transfection of postmitotic retinal cells, which are difficult to transfect.
In another investigation by Santos et al., well-defined structures of ODN–PEI complexes in the form of spheroid nanoparticles were examined for TGβ-2 downregulation activity [84]. ODN–PEI complexes produced aggregation in water due to predominant electrostatic interactions, whereas in HEPES buffer saline smaller size particles were observed. The authors attributed higher transfection of ODN–PEI complexes prepared in HEPES buffer saline to size and morphology of the complexes. In vitro uptake in rat muller glial cells demonstrated 40% down-regulation of TGβ-2 mRNA and 47% reduction in protein expression. It was suggested that dissociation of ODN–PEI complexes is important for efficient gene transfection. Studies performed for 72 h demonstrated downregulation, whereas at 24 h no gene expression was observed due to slower dissociation of ODN–PEI complexes. Intravitreal injection of ODN–PEI complexes in rats showed gene transfection in superficial and inner retinal layers [84]. These findings suggest the effects of preparation medium on the size and morphology of ODN–PEI complexes, which ultimately determine the transfection efficiency of the polymeric vectors [84].
A dehydrated complex of PEI with plasmid expressing basic FGF was evaluated in the treatment of corneal angiogenesis. Researchers reported that corneal neovascularization was dose dependent after application of dehydrated complexes of PEI in rats [85]. Earlier investigation by Nguyen et al. demonstrated that transferrin-conjugated PEI were effective for in vitro transfection of GFP in the corneal endothelial cells isolated from New Zealand White rabbits. However, they did not observe similar GFP expression in the ex vivo culture [86]. In another investigation, adult human retinal ganglion cells were successfully transfected with GFP utilizing a DNA–PEI complex [87]. It was reported that a decade-old, fully differentiated human retinal neuron can be easily transfected with PEI [87]. White et al. performed PEI-mediated gene transfection in Y79 retinoblastoma cells utilizing an adenofection technique [88]. In this method, PEI was utilized to couple pDNA with adenovirus. Researchers reported that PEI-mediated adenofection promoted adherence of cells and higher promoter activity in retinal cells relative to nonretinal cells [88]. Fatta et al. evaluated the effect of PEI on oligonucleotide encapsulation inside the polymeric microspheres [89]. They observed increased encapsulation efficiency from 67 to 83% upon increasing N:P from 15 to 45. In addition, increased porosity of the microspheres was observed. The burst release was lowered at an N:P ratio of 15. Investigators reported enhanced nuclear localization of oligonucleotides following microencapsulation of the PEI complex [89].
Polyglycolic acid, polylactic acid & copolymers poly(lactide-co-glycolide)
Synthetic polyesters such as polyglycolic acid (PGA), polylactic acid (PLA), and their copolymers (poly(lactide-co-glycolide) [PLGA]) are widely explored for the delivery of small molecules. These polymers exhibit bulk erosion. The degradation rate of these polymers depends upon MW and lactide:glycolide ratio in the copolymer. Drug release rate can be modulated by changing the degradation rate of polymeric carriers [90]. The degradation products, lactic acid and glycolic acid, are eliminated from the body via the Krebs cycle in the form of water and carbon dioxide [91]. Various in vitro [92,93] and in vivo [94,95] studies demonstrated the biocompatible nature of PLA or PLGA nanocarriers in ocular tissues. Nanoparticles prepared with low MW PLGA/PLA exhibited higher loading and better transfection efficiency of pDNA in comparison with high-MW PLGA/PLA [96]. Plasmid encapsulation mainly occurred through ionic interaction and depended upon the surface charge of the polymeric carriers. In addition, in vitro release of nanoparticles showed continuous delivery of pDNA for up to 2 weeks without any modification of the functional activity of the plasmid [96]. Studies done by Panyam et al. suggested that PLGA nanoparticles can rapidly escape from the endolysosomal compartment through the reversal of surface charge in the acidic environment [97]. Bejjani et al. reported the internalization of PLGA nanoparticles encapsulated with GFP plasmid or red nuclear fluorescent protein plasmid into RPE cells after intravitreal injection in rats [98]. In vivo expression levels of plasmid protein in RPE cells were higher than in vitro expression possibly due to the intense phagocytosis and non-diving nature of the RPE cells. The expression of proteins were detected on the fourth day after the single intravitreal injection and sustained for 14 days. However, there are several drawbacks associated with the use of hydrophobic polymer-based nanocarriers in gene delivery. As a result of the hydrophobic surface, PLGA–PLA nano-particles are prone to serum protein binding. Entrapment of hydrophilic DNA into the hydrophobic PLGA/PLA polymer is often difficult. Moreover, slower release of DNA from the nano-particles does not provide therapeutic levels at the target sites [91,99]. Modification or conjugation of PLA or PLGA with PEG improved the efficacy of nanocarriers. Potential use of PEG–PLA nano-particles for the gene delivery was suggested by many investigators [91,100]. PEG conjugation can improve the hydrophilicity of PLA nanoparticles that increases the affinity of polymer with DNA and results in higher nucleic acids loading inside the particles. In addition, PEG may provide a favorable microenvironment for the protein or gene molecules enabling long-term storage [91]. Conjugation of nuclear localization signal peptide on the surface of PLGA nanospheres was also reported to enhance in vitro transfection efficiency [101]. Rafat et al. reported sustained delivery of transactivator of transcription-enhanced green fluorescent fusion protein (Tat-EGFP) to the photoreceptor layer of the retina for at least 2 months after single subretinal injection of PEG–PLA microparticles to the rats [102]. Tat-EGFP-encapsulated microparticles exhibited a typical biphasic in vitro release profile. Results from these studies suggest that nanocarriers prepared from biodegradable polymers such as PLA or PLGA are promising for the delivery of genes to various ocular tissues. However, so far, only a few reports are available regarding the applications of polyester-based nanocarriers for ocular gene therapy. Recently, Zhang et al. evaluated PLGA nanoparticles encapsulating shRNA-expressing pDNA to downregulate the expression of the HIF-1α gene in the posterior segment of rat eyes in the treatment of choroidal neovascularization [103]. The investigators reported that pshHIF-1α containing nanoparticles were able to silence the expression of HIF-1α at the third day after single intravitreal injection. PLGA nanoparticles released pDNA in a sustained manner and did not exhibit any toxic effect to the RPE cells and photoreceptors.
In recent years, different non-ionic block copolymers have been explored for non-invasive topical gene delivery formulating micellar eye drops. Micelle carriers composed of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) are known for their stability against various enzymes and ability to avoid blood–serum interaction. Liaw et al. evaluated PEO–PPO–PEO polymeric micelles for in vivo gene transfer to mouse and rabbit eyes [26]. Micelles containing cytomegalovirus LacZ plasmid were administered topically three times a day and after 2–3 days intense levels of β-galactosidase expression were detected in the conjunctiva, choroid sclera, iris and tendon of the lateral rectus muscles, whereas low levels of expression were detected in other ocular tissues such as cornea, anterior chamber, vitreous body and RPE. The presence of reporter gene was detected for up to 5 days in the conjunctiva, sclera and iris. The polymeric micelles were biocompatible with the ocular tissues, which did not exhibit any evidence of cellular inflammation, toxicity and cytological disruption in the experimental eyes. Furthermore, in order to enhance transfection efficiency, ocular tissues were treated with EDTA and cytochalasin B before the application of plasmid-containing micelles. Pretreatment with EDTA and cytochalasin B enhanced the transfection levels in the conjunctiva and sclera of the nude mouse and iris of the rabbit eye by opening the tight junctions of the cornea and improving the paracellular transport of polymeric micelles [26]. In another study, PEO–PPO–PEO polymeric micelles in conjunction with cornea-specific promoters (keratin 12 [K12] and keratocan [Kera3.2]) were evaluated for in vivo corneal and stromal gene delivery in nude mice and rabbits [27]. The polymeric micelles were stable against in vitro DNase I treatment. Lac Z expression was limited to the cornea after topical administrations of pK12–Lac Z-containing polymeric micelles. Lac Z expression in stroma was significantly enhanced in both animal species after administration of pKera3.2–Lac Z-containing polymeric micelles after pretreatment of cornea with EDTA. Arginine–glycine–aspartic acid (RGD) peptide is a known endocytotic inhibitor and pretreatment with RGD peptide decreased the β-galactosidase activity, which suggested that internalization of micelles may be regulated by endocytosis [27]. Other biodegradable block copolymers such as PLGA–PEG–PLGA [104], PEG–PLGA–PEG [105] were also studied to improve gene transfection in various other tissues [100,106]. However, their role in ocular gene delivery is yet to be explored.
Future perspective
In the last decade, development of nonviral methods for ocular gene delivery has made significant progress in animal models. Among nonviral approaches, polymeric vector-based approaches hold enormous future potential. However, several issues such as efficient gene transfer, reproducibility and stable gene expression need to be addressed before translation of polymeric vectors into clinical therapy. Future studies are required for better understanding of ocular barriers that regulate the transfection kinetics and intracellular distribution of polyplexes. These findings will also assist in development of polymeric vectors utilizing non-ionic and block copolymers. Furthermore, novel strategies involving encapsulation of polyplexes could overcome the shortcoming of cellular toxicity associated with the use of a high concentration of cationic polymers. In addition, issues regarding short intravitreal half-life and transient gene expression could be addressed with nanoencapsulation strategies. The design of new polymers with improved chemistry might be helpful in enhancing gene transfer to the targeted site. Moreover, cell-targeting approaches could avoid potential adverse effects. All these approaches are anticipated to improve ocular gene therapy, and better understanding of the structure–activity relationship of synthetic polymers with transfection efficiency will enable future translation of these vectors to clinical trials.
Executive summary.
Barriers for ocular gene therapy
Anatomical and physiological barriers present at anterior and posterior segments of the eye restrict gene transfer to various ocular tissues.
Polymeric vectors such as polyplexes, nanoparticles and micelles were successful to some extent in overcoming the ocular barriers.
Polymeric vectors in ocular gene delivery
Polymeric carriers are biocompatible, biodegradable and nonimmunogenic.
Polymers such as poly L-lysine, polyethylenimine, poly(lactide-co-glycolide) and chitosan exhibit negligible cytotoxicity to the ocular tissues. Therefore, with their repeated administration is possible to achieve sustained levels of gene expression.
Cationic polymers by electrostatic interaction spontaneously form nanosized complexes with nucleic acids, such a simple chemistry is suitable for overcoming drawbacks associated with large-scale production of viral vectors. Compacted DNA nanoparticles are effective in delivering genes to various ocular tissues.
Although polymeric vectors have their own limitations, transfection capabilities of these vectors are not comparable to viral vectors, chemical modifications such as pegylation and conjugation of the targeting moiety are always possible to enhance efficacy by delivering the nucleic acids to the targeted ocular cells and tissues.
Key Terms
- Lipoplexes or polyplexes
Complexes of cationic lipid and cationic polymers with the DNA are called lipoplexes and polyplexes respectively. These complexes facilitate the cellular entry of DNA molecule and protect it from degradation
- Transfection efficiency
The effectiveness of non-viral delivery systems to transfer gene to the eukaryote cell
- Pegylation
Covalent conjugation of polyethylene glycol (PEG) to a drug, protein or polymeric carrier, which reduces the immunogenicity, increases the circulation time and provide hydrophilicity to a hydrophobic carrier or drug molecule
- EGFP
A mutant of green fluorescent protein with relatively higher brightness
- N:P ratio
Ratio of moles of amine groups of cationic polymers to the phosphate groups of DNA, which influences the transfection efficiency and cytotoxicity of polyplexes
- Adenofection
Complexation of adenovirus with plasmid DNA mediated by polyethylenimine
Footnotes
For reprint orders, please contact reprints@future-science.com
Financial & competing interests disclosure
This work was supported by NIH grants RO1 EY09171–16 and RO1 EY10659–14. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Bibliography
- 1.Wu TL, Ertl HC. Immune barriers to successful gene therapy. Trends Mol Med. 2009;15:32–39. doi: 10.1016/j.molmed.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Chaum E, Hatton MP. Gene therapy for genetic and acquired retinal diseases. Surv Ophthalmol. 2002;47:449–469. doi: 10.1016/s0039-6257(02)00336-3. [DOI] [PubMed] [Google Scholar]
- 3.Mohan RR, Sharma A, Netto MV, Sinha S, Wilson SE. Gene therapy in the cornea. Prog Retin Eye Res. 2005;24:537–559. doi: 10.1016/j.preteyeres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 4.Acland GM, Aguirre GD, Ray J, et al. Gene therapy restores vision in a canine model of childhood blindness. Nat Genet. 2001;28:92–95. doi: 10.1038/ng0501-92. [DOI] [PubMed] [Google Scholar]
- 5.Acland GM, Aguirre GD, Bennett J, et al. Long-term restoration of rod and cone vision by single dose rAAV-mediated gene transfer to the retina in a canine model of childhood blindness. Mol Ther. 2005;12:1072–1082. doi: 10.1016/j.ymthe.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bainbridge JW, Ali RR. Keeping an eye on clinical trials in 2008. Gene Ther. 2008;15:633–634. doi: 10.1038/gt.2008.28. [DOI] [PubMed] [Google Scholar]
- 7.Bessis N, GarciaCozar FJ, Boissier MC. Immune responses to gene therapy vectors: influence on vector function and effector mechanisms. Gene Ther. 2004;11(Suppl 1):S10–S17. doi: 10.1038/sj.gt.3302364. [DOI] [PubMed] [Google Scholar]
- 8.Jooss K, Chirmule N. Immunity to adenovirus and adeno-associated viral vectors: implications for gene therapy. Gene Ther. 2003;10:955–963. doi: 10.1038/sj.gt.3302037. [DOI] [PubMed] [Google Scholar]
- 9.Provost N, Le Meur G, Weber M, et al. Biodistribution of rAAV vectors following intraocular administration: evidence for the presence and persistence of vector DNA in the optic nerve and in the brain. Mol Ther. 2005;11:275–283. doi: 10.1016/j.ymthe.2004.09.022. [DOI] [PubMed] [Google Scholar]
- 10.Walther W, Stein U. Viral vectors for gene transfer: a review of their use in the treatment of human diseases. Drugs. 2000;60:249–271. doi: 10.2165/00003495-200060020-00002. [DOI] [PubMed] [Google Scholar]
- 11.Han S, Mahato RI, Sung YK, Kim SW. Development of biomaterials for gene therapy. Mol Ther. 2000;2:302–317. doi: 10.1006/mthe.2000.0142. [DOI] [PubMed] [Google Scholar]
- 12.Bloquel C, Bourges JL, Touchard E, Berdugo M, BenEzra D, Behar-Cohen F. Non-viral ocular gene therapy: potential ocular therapeutic avenues. Adv Drug Deliv Rev. 2006;58:1224–1242. doi: 10.1016/j.addr.2006.07.023. [DOI] [PubMed] [Google Scholar]
- 13.Klausner EA, Peer D, Chapman RL, Multack RF, Andurkar SV. Corneal gene therapy. J Control Release. 2007;124:107–133. doi: 10.1016/j.jconrel.2007.05.041. [DOI] [PubMed] [Google Scholar]
- 14.Yusifov TN, Abduragimov AR, Narsinh K, Gasymov OK, Glasgow BJ. Tear lipocalin is the major endonuclease in tears. Mol Vis. 2008;14:180–188. [PMC free article] [PubMed] [Google Scholar]
- 15.Urtti A, Salminen L. Minimizing systemic absorption of topically administered ophthalmic drugs. Surv Ophthalmol. 1993;37:435–456. doi: 10.1016/0039-6257(93)90141-s. [DOI] [PubMed] [Google Scholar]
- 16.Bertelmann E, Jaroszewski J, Pleyer U. Corneal allograft rejection: current understanding. 2 Clinical implications. Ophthalmologica. 2002;216:2–12. doi: 10.1159/000048289. [DOI] [PubMed] [Google Scholar]
- 17.Poulaki V, Colby K. Genetics of anterior and stromal corneal dystrophies. Semin Ophthalmol. 2008;23:9–17. doi: 10.1080/08820530701745173. [DOI] [PubMed] [Google Scholar]
- 18.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–249. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 19.Carr DJ, Harle P, Gebhardt BM. The immune response to ocular herpes simplex virus type 1 infection. Exp Biol Med (Maywood) 2001;226:353–366. doi: 10.1177/153537020122600501. [DOI] [PubMed] [Google Scholar]
- 20.Williams KA, Coster DJ. Gene therapy for diseases of the cornea – a review. Clin Experiment Ophthalmol. 2010;38:93–103. doi: 10.1111/j.1442-9071.2009.02179.x. [DOI] [PubMed] [Google Scholar]
- 21.Jun AS, Larkin DF. Prospects for gene therapy in corneal disease. Eye (Lond) 2003;17:906–911. doi: 10.1038/sj.eye.6700565. [DOI] [PubMed] [Google Scholar]
- 22.Mishra GP, Gaudana R, Tamboli VM, Mitra AK. Recent advances in ocular drug delivery: role of transporters, receptors, and nanocarriers. In: Maharo RI, Narang AS, editors. Targeted Delivery of Small and Macromolecular Drugs. Taylor and Francis; NY, USA: 2010. pp. 421–456. [Google Scholar]
- 23.Hamalainen KM, Kananen K, Auriola S, Kontturi K, Urtti A. Characterization of paracellular and aqueous penetration routes in cornea, conjunctiva, and sclera. Invest Ophthalmol Vis Sci. 1997;38:627–634. [PubMed] [Google Scholar]
- 24.Kamata Y, Okuyama T, Kosuga M, et al. Adenovirus-mediated gene therapy for corneal clouding in mice with mucopolysaccharidosis type VII. Mol Ther. 2001;4:307–312. doi: 10.1006/mthe.2001.0461. [DOI] [PubMed] [Google Scholar]
- 25.Hornof M, Toropainen E, Urtti A. Cell culture models of the ocular barriers. Eur J Pharm Biopharm. 2005;60:207–225. doi: 10.1016/j.ejpb.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 26.Liaw J, Chang SF, Hsiao FC. In vivo gene delivery into ocular tissues by eye drops of poly(ethylene oxide)–poly(propylene oxide)–poly(ethylene oxide) (PEO–PPO–PEO) polymeric micelles. Gene Ther. 2001;8:999–1004. doi: 10.1038/sj.gt.3301485. [DOI] [PubMed] [Google Scholar]
- 27.Tong YC, Chang SF, Liu CY, Kao WW, Huang CH, Liaw J. Eye drop delivery of nano-polymeric micelle formulated genes with cornea-specific promoters. J Gene Med. 2007;9:956–966. doi: 10.1002/jgm.1093. [DOI] [PubMed] [Google Scholar]
- 28.de la Fuente M, Seijo B, Alonso MJ. Bioadhesive hyaluronan–chitosan nanoparticles can transport genes across the ocular mucosa and transfect ocular tissue. Gene Ther. 2008;15:668–676. doi: 10.1038/gt.2008.16. [DOI] [PubMed] [Google Scholar]
- 29.Hao J, Li SK, Kao WW, Liu CY. Gene delivery to cornea. Brain Res Bull. 2010;81:256–261. doi: 10.1016/j.brainresbull.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.George AJ, Arancibia-Carcamo CV, Awad HM, et al. Gene delivery to the corneal endothelium. Am J Respir Crit Care Med. 2000;162:S194–S200. doi: 10.1164/ajrccm.162.supplement_3.15tac11. [DOI] [PubMed] [Google Scholar]
- 31.Collins L, Fabre JW. A synthetic peptide vector system for optimal gene delivery to corneal endothelium. J Gene Med. 2004;6:185–194. doi: 10.1002/jgm.482. [DOI] [PubMed] [Google Scholar]
- 32.Duvvuri S, Majumdar S, Mitra AK. Drug delivery to the retina: challenges and opportunities. Expert Opin Biol Ther. 2003;3:45–56. doi: 10.1517/14712598.3.1.45. [DOI] [PubMed] [Google Scholar]
- 33.Naik R, Mukhopadhyay A, Ganguli M. Gene delivery to the retina: focus on non-viral approaches. Drug Discov Today. 2009;14:306–315. doi: 10.1016/j.drudis.2008.09.012. [DOI] [PubMed] [Google Scholar]
- 34.Bochot A, Fattal E, Boutet V, et al. Intravitreal delivery of oligonucleotides by sterically stabilized liposomes. Invest Ophthalmol Vis Sci. 2002;43:253–259. [PubMed] [Google Scholar]
- 35.Bennett J, Maguire AM, Cideciyan AV, et al. Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina. Proc Natl Acad Sci USA. 1999;96:9920–9925. doi: 10.1073/pnas.96.17.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bishop P. The biochemical structure of mammalian vitreous. Eye (Lond) 1996;10(Pt 6):664–670. doi: 10.1038/eye.1996.159. [DOI] [PubMed] [Google Scholar]
- 37.Pitkanen L, Ruponen M, Nieminen J, Urtti A. Vitreous is a barrier in nonviral gene transfer by cationic lipids and polymers. Pharm Res. 2003;20:576–583. doi: 10.1023/a:1023238530504. [DOI] [PubMed] [Google Scholar]
- 38.Peeters L, Sanders NN, Braeckmans K, et al. Vitreous: a barrier to nonviral ocular gene therapy. Invest Ophthalmol Vis Sci. 2005;46:3553–3561. doi: 10.1167/iovs.05-0165. [DOI] [PubMed] [Google Scholar]
- 39.Pitkanen L, Pelkonen J, Ruponen M, Ronkko S, Urtti A. Neural retina limits the nonviral gene transfer to retinal pigment epithelium in an in vitro bovine eye model. AAPS J. 2004;6:e25. doi: 10.1208/aapsj060325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Borras T. Recent developments in ocular gene therapy. Exp Eye Res. 2003;76:643–652. doi: 10.1016/s0014-4835(03)00030-7. [DOI] [PubMed] [Google Scholar]
- 41.Kang HC, Lee M, Bae YH. Polymeric gene carriers. Crit Rev Eukaryot Gene Expr. 2005;15:317–342. doi: 10.1615/critreveukargeneexpr.v15.i4.30. [DOI] [PubMed] [Google Scholar]
- 42.He CX, Tabata Y, Gao JQ. Non-viral gene delivery carrier and its three-dimensional transfection system. Int J Pharm. 2010;386:232–242. doi: 10.1016/j.ijpharm.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 43.Perales JC, Ferkol T, Molas M, Hanson RW. An evaluation of receptor-mediated gene transfer using synthetic DNA–ligand complexes. Eur J Biochem. 1994;226:255–266. doi: 10.1111/j.1432-1033.1994.tb20049.x. [DOI] [PubMed] [Google Scholar]
- 44.Pouton CW, Uduehi AN, Milroy DA, Lucas P. Cellular interaction and fate of polycationñ DNA complexes. In: Kabanov AV, Felgner P, Seymour LW, editors. Self-Assembling Complexes for Gene Delivery. Wiley; NY, USA: 1998. pp. 255–273. [Google Scholar]
- 45.Sun X, Zhang N. Cationic polymer optimization for efficient gene delivery. Mini Rev Med Chem. 2010;10:108–125. doi: 10.2174/138955710791185109. [DOI] [PubMed] [Google Scholar]
- 46.Perales JC, Ferkol T, Beegen H, Ratnoff OD, Hanson RW. Gene transfer in vivo: sustained expression and regulation of genes introduced into the liver by receptor-targeted uptake. Proc Natl Acad Sci USA. 1994;91:4086–4090. doi: 10.1073/pnas.91.9.4086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garcia-Valenzuela E, Rayanade R, Perales JC, Davidson CA, Hanson RW, Sharma SC. Axon-mediated gene transfer of retinal ganglion cells in vivo. J Neurobiol. 1997;32:111–122. doi: 10.1002/(sici)1097-4695(199701)32:1<111::aid-neu10>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 48.Mannisto M, Vanderkerken S, Toncheva V, et al. Structure-activity relationships of poly(L-lysines): effects of pegylation and molecular shape on physicochemical and biological properties in gene delivery. J Control Rel. 2002;83:169–182. doi: 10.1016/s0168-3659(02)00178-5. [DOI] [PubMed] [Google Scholar]
- 49.Mannisto M, Ronkko S, Matto M, et al. The role of cell cycle on polyplex-mediated gene transfer into a retinal pigment epithelial cell line. J Gene Med. 2005;7:466–476. doi: 10.1002/jgm.693. [DOI] [PubMed] [Google Scholar]
- 50.Farrell LL, Pepin J, Kucharski C, Lin X, Xu Z, Uludag H. A comparison of the effectiveness of cationic polymers poly-L-lysine (PLL) and polyethylenimine (PEI) for non-viral delivery of plasmid DNA to bone marrow stromal cells (BMSC) Eur J Pharm Biopharm. 2007;65:388–397. doi: 10.1016/j.ejpb.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 51.Midoux P, Monsigny M. Efficient gene transfer by histidylated polylysine/pDNA complexes. Bioconjug Chem. 1999;10:406–411. doi: 10.1021/bc9801070. [DOI] [PubMed] [Google Scholar]
- 52.Kwoh DY, Coffin CC, Lollo CP, et al. Stabilization of poly-L-lysine/DNA polyplexes for in vivo gene delivery to the liver. Biochim Biophys Acta. 1999;1444:171–190. doi: 10.1016/s0167-4781(98)00274-7. [DOI] [PubMed] [Google Scholar]
- 53.Tros de Ilarduya C, Sun Y, Duzgunes N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40:159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 54.Yamazaki M, Ito T. Deformation and instability in membrane structure of phospholipid vesicles caused by osmophobic association: mechanical stress model for the mechanism of poly(ethylene glycol)-induced membrane fusion. Biochemistry. 1990;29:1309–1314. doi: 10.1021/bi00457a029. [DOI] [PubMed] [Google Scholar]
- 55.Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS One. 2006;1:e38. doi: 10.1371/journal.pone.0000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cai X, Nash Z, Conley SM, Fliesler SJ, Cooper MJ, Naash MI. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS One. 2009;4:e5290. doi: 10.1371/journal.pone.0005290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wadhwa S, Paliwal R, Paliwal SR, Vyas SP. Chitosan and its role in ocular therapeutics. Mini Rev Med Chem. 2009;9:1639–1647. doi: 10.2174/138955709791012292. [DOI] [PubMed] [Google Scholar]
- 58.Alonso MJ, Sanchez A. The potential of chitosan in ocular drug delivery. J Pharm Pharmacol. 2003;55:1451–1463. doi: 10.1211/0022357022476. [DOI] [PubMed] [Google Scholar]
- 59.Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62:12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 60.Huang M, Fong CW, Khor E, Lim LY. Transfection efficiency of chitosan vectors: effect of polymer molecular weight and degree of deacetylation. J Control Release. 2005;106:391–406. doi: 10.1016/j.jconrel.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 61.Badawi AA, El-Laithy HM, El Qidra RK, El Mofty H, El Dally M. Chitosan based nanocarriers for indomethacin ocular delivery. Arch Pharm Res. 2008;31:1040–1049. doi: 10.1007/s12272-001-1266-6. [DOI] [PubMed] [Google Scholar]
- 62.De Campos AM, Sanchez A, Alonso MJ. Chitosan nanoparticles: a new vehicle for the improvement of the delivery of drugs to the ocular surface. Application to cyclosporin A. Int J Pharm. 2001;224:159–168. doi: 10.1016/s0378-5173(01)00760-8. [DOI] [PubMed] [Google Scholar]
- 63.Pathak A, Patnaik S, Gupta KC. Recent trends in non-viral vector-mediated gene delivery. Biotechnol J. 2009;4:1559–1572. doi: 10.1002/biot.200900161. [DOI] [PubMed] [Google Scholar]
- 64.Borchard G. Chitosans for gene delivery. Adv Drug Deliv Rev. 2001;52:145–150. doi: 10.1016/s0169-409x(01)00198-3. [DOI] [PubMed] [Google Scholar]
- 65.Sato T, Ishii T, Okahata Y. In vitro gene delivery mediated by chitosan. effect of pH, serum, and molecular mass of chitosan on the transfection efficiency. Biomaterials. 2001;22:2075–2080. doi: 10.1016/s0142-9612(00)00385-9. [DOI] [PubMed] [Google Scholar]
- 66.Mao HQ, Roy K, Troung-Le VL, et al. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70:399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 67.Nimesh S, Thibault MM, Lavertu M, Buschmann MD. Enhanced gene delivery mediated by low molecular weight chitosan/DNA complexes: effect of pH and serum. Mol Biotechnol. 2010;46:182–196. doi: 10.1007/s12033-010-9286-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lavertu M, Methot S, Tran-Khanh N, Buschmann MD. High efficiency gene transfer using chitosan/DNA nanoparticles with specific combinations of molecular weight and degree of deacetylation. Biomaterials. 2006;27:4815–4824. doi: 10.1016/j.biomaterials.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 69.de la Fuente M, Ravina M, Paolicelli P, Sanchez A, Seijo B, Alonso MJ. Chitosan-based nanostructures: a delivery platform for ocular therapeutics. Adv Drug Deliv Rev. 2010;62:100–117. doi: 10.1016/j.addr.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 70.Prow TW, Bhutto I, Kim SY, et al. Ocular nanoparticle toxicity and transfection of the retina and retinal pigment epithelium. Nanomedicine. 2008;4:340–349. doi: 10.1016/j.nano.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paolicelli P, de la Fuente M, Sanchez A, Seijo B, Alonso MJ. Chitosan nanoparticles for drug delivery to the eye. Expert Opin Drug Deliv. 2009;6:239–253. doi: 10.1517/17425240902762818. [DOI] [PubMed] [Google Scholar]
- 72.Mansouri S, Lavigne P, Corsi K, Benderdour M, Beaumont E, Fernandes JC. Chitosan–DNA nanoparticles as non-viral vectors in gene therapy: strategies to improve transfection efficacy. Eur J Pharm Biopharm. 2004;57:1–8. doi: 10.1016/s0939-6411(03)00155-3. [DOI] [PubMed] [Google Scholar]
- 73.Aragona P. Hyaluronan in the treatment of ocular surface disorders. In: Garga HG, Hales CA, editors. Chemistry and Biology of Hyaluronan. Elsevier Ltd; Oxford, UK: 2004. pp. 529–551. [Google Scholar]
- 74.Lerner LE, Schwartz DM, Hwang DG, Howes EL, Stern R. Hyaluronan and CD44 in the human cornea and limbal conjunctiva. Exp Eye Res. 1998;67:481–484. doi: 10.1006/exer.1998.0567. [DOI] [PubMed] [Google Scholar]
- 75.de la Fuente M, Seijo B, Alonso MJ. Novel hyaluronic acid-chitosan nanoparticles for ocular gene therapy. Invest Ophthalmol Vis Sci. 2008;49:2016–2024. doi: 10.1167/iovs.07-1077. [DOI] [PubMed] [Google Scholar]
- 76.Boussif O, Lezoualc’h F, Zanta MA, et al. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci USA. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ogris M. Gene delivery using polyethylenimine and copolymers. Polymeric gene delivery. In: Amiji MM, editor. Principles and Applications. CRC press; FL, USA: 2005. pp. 97–106. [Google Scholar]
- 78.Akinc A, Thomas M, Klibanov AM, Langer R. Exploring polyethylenimine-mediated DNA transfection and the proton sponge hypothesis. J Gene Med. 2005;7:657–663. doi: 10.1002/jgm.696. [DOI] [PubMed] [Google Scholar]
- 79.von Harpe A, Petersen H, Li Y, Kissel T. Characterization of commercially available and synthesized polyethylenimines for gene delivery. J Control Release. 2000;69:309–322. doi: 10.1016/s0168-3659(00)00317-5. [DOI] [PubMed] [Google Scholar]
- 80.Fischer D, Bieber T, Li Y, Elsasser HP, Kissel T. A novel non-viral vector for DNA delivery based on low molecular weight, branched polyethylenimine: effect of molecular weight on transfection efficiency and cytotoxicity. Pharm Res. 1999;16:1273–1279. doi: 10.1023/a:1014861900478. [DOI] [PubMed] [Google Scholar]
- 81.Oh YK, Suh D, Kim JM, Choi HG, Shin K, Ko JJ. Polyethylenimine-mediated cellular uptake, nucleus trafficking and expression of cytokine plasmid DNA. Gene Ther. 2002;9:1627–1632. doi: 10.1038/sj.gt.3301735. [DOI] [PubMed] [Google Scholar]
- 82.Lungwitz U, Breunig M, Blunk T, Gopferich A. Polyethylenimine-based non-viral gene delivery systems. Eur J Pharm Biopharm. 2005;60:247–266. doi: 10.1016/j.ejpb.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 83.Reinisalo M, Urtti A, Honkakoski P. Freeze-drying of cationic polymer DNA complexes enables their long-term storage and reverse transfection of post-mitotic cells. J Control Release. 2006;110:437–443. doi: 10.1016/j.jconrel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 84.Gomes dos Santos AL, Bochot A, Tsapis N, et al. Oligonucleotide-polyethylenimine complexes targeting retinal cells: structural analysis and application to anti-TGFβ-2 therapy. Pharm Res. 2006;23:770–781. doi: 10.1007/s11095-006-9748-0. [DOI] [PubMed] [Google Scholar]
- 85.Kuo CN, Yang LC, Wu PC, Kuo HK, Kuo CJ, Tai MH. Dehydrated form of plasmid expressing basic fibroblast growth factor-polyethylenimine complex is a novel and accurate method for gene transfer to the cornea. Curr Eye Res. 2005;30:1015–1024. doi: 10.1080/02713680500330512. [DOI] [PubMed] [Google Scholar]
- 86.Nguyen TH, Murakami A, Fujiki K, Kanai A. Transferrin-polyethylenimine conjugate, FuGENE6 and TransIT-LT as nonviral vectors for gene transfer to the corneal endothelium. Jpn J Ophthalmol. 2002;46:140–146. doi: 10.1016/s0021-5155(01)00491-9. [DOI] [PubMed] [Google Scholar]
- 87.Horbinski C, Stachowiak MK, Higgins D, Finnegan SG. Polyethyleneimine-mediated transfection of cultured postmitotic neurons from rat sympathetic ganglia and adult human retina. BMC Neurosci. 2001;2:2. doi: 10.1186/1471-2202-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.White JB, Taylor RE, Pittler SJ. Reproducible high efficiency gene transfer into Y79 retinoblastoma cells using adenofection. J Neurosci Methods. 2001;106:1–7. doi: 10.1016/s0165-0270(00)00368-x. [DOI] [PubMed] [Google Scholar]
- 89.Fattal E, De Rosa G, Bochot A. Gel and solid matrix systems for the controlled delivery of drug carrier-associated nucleic acids. Int J Pharm. 2004;277:25–30. doi: 10.1016/j.ijpharm.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 90.Hyon SH. Biodegradable poly (lactic acid) microspheres for drug delivery systems. Yonsei Med J. 2000;41:720–734. doi: 10.3349/ymj.2000.41.6.720. [DOI] [PubMed] [Google Scholar]
- 91.Zou W, Liu C, Chen Z, Zhang N. Preparation and characterization of cationic PLA-PEG nanoparticles for delivery of plasmid DNA. Nanoscale Res Lett. 2009;4:982–992. doi: 10.1007/s11671-009-9345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kimura H, Ogura Y, Moritera T, Honda Y, Tabata Y, Ikada Y. In vitro phagocytosis of polylactide microspheres by retinal pigment epithelial cells and intracellular drug release. Curr Eye Res. 1994;13:353–360. doi: 10.3109/02713689409167299. [DOI] [PubMed] [Google Scholar]
- 93.Giordano GG, Thomson RC, Ishaug SL, et al. Retinal pigment epithelium cells cultured on synthetic biodegradable polymers. J Biomed Mater Res. 1997;34:87–93. doi: 10.1002/(sici)1097-4636(199701)34:1<87::aid-jbm12>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 94.Moritera T, Ogura Y, Yoshimura N, et al. Biodegradable microspheres containing adriamycin in the treatment of proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 1992;33:3125–3130. [PubMed] [Google Scholar]
- 95.Bourges JL, Gautier SE, Delie F, et al. Ocular drug delivery targeting the retina and retinal pigment epithelium using polylactide nanoparticles. Invest Ophthalmol Vis Sci. 2003;44:3562–3569. doi: 10.1167/iovs.02-1068. [DOI] [PubMed] [Google Scholar]
- 96.Basarkar A, Devineni D, Palaniappan R, Singh J. Preparation, characterization, cytotoxicity and transfection efficiency of poly(DL-lactide-co-glycolide) and poly(DL-lactic acid) cationic nanoparticles for controlled delivery of plasmid DNA. Int J Pharm. 2007;343:247–254. doi: 10.1016/j.ijpharm.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Panyam J, Zhou WZ, Prabha S, Sahoo SK, Labhasetwar V. Rapid endo-lysosomal escape of poly(DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217–1226. doi: 10.1096/fj.02-0088com. [DOI] [PubMed] [Google Scholar]
- 98.Bejjani RA, BenEzra D, Cohen H, et al. Nanoparticles for gene delivery to retinal pigment epithelial cells. Mol Vis. 2005;11:124–132. [PubMed] [Google Scholar]
- 99.Luten J, van Nostrum CF, De Smedt SC, Hennink WE. Biodegradable polymers as non-viral carriers for plasmid DNA delivery. J Control Release. 2008;126:97–110. doi: 10.1016/j.jconrel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 100.Chen J, Tian B, Yin X, et al. Preparation, characterization and transfection efficiency of cationic PEGylated PLA nanoparticles as gene delivery systems. J Biotechnol. 2007;130:107–113. doi: 10.1016/j.jbiotec.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 101.Jeon O, Lim HW, Lee M, Song SJ, Kim BS. Poly(L-lactide-co-glycolide) nanospheres conjugated with a nuclear localization signal for delivery of plasmid DNA. J Drug Target. 2007;15:190–198. doi: 10.1080/10611860601143479. [DOI] [PubMed] [Google Scholar]
- 102.Rafat M, Cleroux CA, Fong WG, et al. PEG-PLA microparticles for encapsulation and delivery of Tat-EGFP to retinal cells. Biomaterials. 2010;31:3414–3421. doi: 10.1016/j.biomaterials.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 103.Zhang C, Wang YS, Wu H, et al. Inhibitory efficacy of hypoxia-inducible factor 1α short hairpin RNA plasmid DNA-loaded poly (DL-lactide-co-glycolide) nanoparticles on choroidal neovascularization in a laser-induced rat model. Gene Ther. 2009;17:338–351. doi: 10.1038/gt.2009.158. [DOI] [PubMed] [Google Scholar]
- 104.Jeong JH, Kim SW, Park TG. Biodegradable triblock copolymer of PLGA-PEG-PLGA enhances gene transfection efficiency. Pharm Res. 2004;21:50–54. doi: 10.1023/b:pham.0000012151.05441.bf. [DOI] [PubMed] [Google Scholar]
- 105.Chang CW, Choi D, Kim WJ, et al. Non-ionic amphiphilic biodegradable PEG–PLGA–PEG copolymer enhances gene delivery efficiency in rat skeletal muscle. J Control Release. 2007;118:245–253. doi: 10.1016/j.jconrel.2006.11.025. [DOI] [PubMed] [Google Scholar]
- 106.Lee M, Kim SW. Polyethylene glycol-conjugated copolymers for plasmid DNA delivery. Pharm Res. 2005;22:1–10. doi: 10.1007/s11095-004-9003-5. [DOI] [PubMed] [Google Scholar]